Abstract
Despite their importance in mammalian reproduction, substances in the oxytocin-prostaglandins pathways have not been investigated in the horse placenta during most of pregnancy and parturition. Therefore, we quantified placental content of oxytocin (OXT), oxytocin receptor (OXTR), and prostaglandin E2 and F2 alpha during days 90–240 of pregnancy (PREG), physiological parturition (PHYS), and parturition with fetal membrane retention (FMR) in heavy draft horses (PREG = 13, PHYS = 11, FMR = 10). We also quantified OXTR and prostaglandin endoperoxide synthase-2 (PTGS2) mRNA expression and determined the immunolocalization of OXT, OXTR, and PTGS2. For relative quantification of OXT and OXTR, we used western blotting with densitometry. To quantify the prostaglandins, we used enzyme immunoassays. For relative quantification of OXTR and PTGS2, we used RT-qPCR. For immunolocalization of OXT, OXTR, and PTGS2, we used immunohistochemistry. We found that OXT was present in cells of the allantochorion and endometrium in all groups. PTGS2 expression in the allantochorion was 14.7-fold lower in FMR than in PHYS (p = 0.007). These results suggest that OXT is synthesized in the horse placenta. As PTGS2 synthesis is induced by inflammation, they also suggest that FMR in heavy draft horses may be associated with dysregulation of inflammatory processes.
Subject terms: Zoology, Reverse transcription polymerase chain reaction, Immunoblotting, Immunohistochemistry, ELISA
Introduction
In the placenta, a delicate balance of hormones helps to support pregnancy and initiate parturition1–3. Parturition is a proinflammatory event4–8, and delivery of the fetus and the placenta are accompanied by coordinated contractions, pain, and swelling, which are mostly mediated by prostaglandins9.
Exposure of the placenta to endogenous or exogenous oxytocin (OXT) causes it to release prostaglandin E2 (PGE2) and prostaglandin F2 alpha (PGF2ɑ) within a few hours10–21 (see Supplementary Fig S1 for the pathways that start with OXT release and end with the prostaglandins binding to their receptors). OXT is released from the posterior pituitary gland22, and during pregnancy and parturition in humans, from the placenta23. Given that, of all domestic and laboratory species, horses may be the most similar to humans in terms of the endocrinology of pregnancy and parturition24, it seems likely that horses also release OXT from the placenta. Moreover, human placental OXT is not believed to play a role in myometrial contractions, unlike pituitary OXT13. Thus, it seems likely that horse placental OXT would be a signal for prostaglandin release in the placenta itself, and not a signal for myometrial contractions. However, the presence of OXT in horse placental cells needs to be verified.
OXT exerts its effects by binding with its receptor (OXTR), which is expressed in the myometrium, mammary gland, and placenta23. During pregnancy and parturition in horses, OXTR has only been studied in the nonpregnant uterus, the endometrium in early pregnancy (<21 days)16,25,26, and the endometrium and allantochorion within 3 hours of foal delivery27. Thus, OXTR levels in the horse placenta during the majority of pregnancy and parturition remain unknown.
The chemical precursor of prostaglandins, arachidonic acid, is abundant in cell membranes. This means that the rate limiting step in production of PGE2 and PGF2α is the conversion of arachidonic acid to prostaglandin H2 by prostaglandin endoperoxide synthase-1 and prostaglandin endoperoxide synthase-2 (PTGS1 and PTGS2, formerly referred to as cyclooxygenase-1 and 2). PTGS2 is the predominant isoform in human, bovine, and ovine placentas18,19,28. PTGS2 synthesis is induced by inflammation29–34, and during pregnancy in the horse, its synthesis should be suppressed by high levels of progestagens, as it is suppressed by high levels of progesterone in other species35. Similarly, it seems likely that PTGS2 synthesis would be upregulated at parturition in the horse, as it is in other species. However, neither PTGS2 enzyme nor PTGS2 mRNA have been quantified in the horse placenta after day 22 of pregnancy or at parturition.
During pregnancy and parturition, PGE2 and PGF2α are synthesized by the placenta35,36 and immediately secreted28. In epitheliochorial placentas, like that of the mare, they are believed to be synthesized mostly in the allantochorion and quickly degraded in the endometrium35. Both prostaglandins are released to placental fluids, but only small quantities are released to maternal peripheral blood35. Thus, for better insight into pregnancy and parturition in horses, it would be helpful to directly measure the content of PGE2 and PGF2α in the placenta.
The current thinking on PGE2 and PGF2α in reproduction emphasizes not only their uterotonic roles, but also their proinflammatory effects. In addition to being a uterotonic7, PGE2 causes cervical ripening in humans37, and it has been effective as a drug for opening the cervix in horses38. It increases pain, swelling, and temperature. It also increases the migration of immune cells toward placental tissues, and these cells play important roles in tissue remodelling, including activation of matrix metalloproteinases4,7,37,39,40. PGF2α is not only the main uterotonic agent in many species23,35, but it also augments the inflammatory cascade in the placenta by increasing output of interleukins and chemokines40.
Dysregulation of tissue remodelling and inflammatory processes may play roles in fetal membrane retention (FMR) in horses, as indicated by our previous findings that extensive placental fibrosis41, and differential placental content and activity of matrix metalloproteinases42 are associated with FMR in heavy draft horses. This led us to hypothesize that, in the placenta at foal delivery, FMR in this breed is associated with differential content of substances in the OXT-prostaglandins pathways and differential expression of genes coding for these substances. Given that FMR is the most common peripartum condition in horses, particularly in heavy draft horses41,43, and that FMR can be life-threatening in this species, it seemed worthwhile to test this hypothesis.
Therefore, we had two primary objectives: first, to quantify the content of four key substances in these pathways (OXT, OXTR, PGE2, and PGF2α) in the allantochorion and the endometrium of heavy draft horses during days 90–240 of pregnancy, physiological parturition, and FMR. Second, to provide further information about these pathways by quantifying mRNA expression of OXTR and PTGS2 in these tissues in these groups. For further context, we also determined the immunolocalization of OXT, OXTR, and PTGS2. To quantify mRNA, proteins/peptides, and prostaglandins, we used RT-qPCR, western blotting with densitometry, and enzyme immunoassays, respectively. For immunolocalization of OXT, OXTR, and PTGS2, we used immunohistochemistry.
Results
OXT
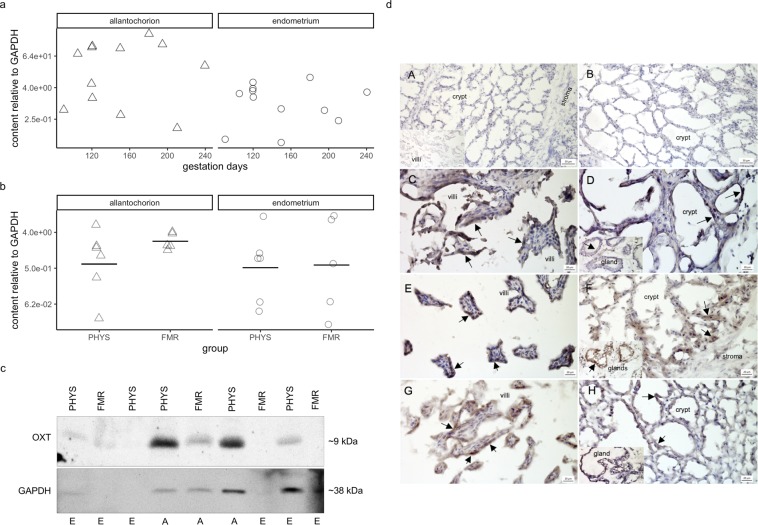
In pregnancy, there was no apparent correlation between OXT peptide content and gestation days (allantochorion: τ = 0.08, p = 0.89; endometrium: τ = −0.05, p = 0.92) (Fig. 1a).
Figure 1.
Oxytocin peptide content and immunolocalization. (a) Oxytocin peptide content relative to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) during pregnancy. Protein content was quantified by western blotting with densitometry. Symbols correspond to tissues from individual mares (Δ, allantochorion; ○, endometrium). Note the logarithmic scales in panels a and b. (b) Oxytocin peptide content relative to that of GAPDH in physiological parturition (PHYS) and parturition with fetal membrane retention (FMR). Horizontal lines indicate geometric means. (c) Representative blots of oxytocin and GAPDH. Uncropped blots are presented in Supplementary Fig S4. Image Lab version 5.2.1 software was used for visualization and densitometry (Bio-Rad Laboratories, https://www.bio-rad.com/en-pl/product/image-lab-software?ID=KRE6P5E8Z). Abbreviations: OXT, oxytocin; A, allantochorion; E, endometrium. (d) Tissue expression and localization of oxytocin in horse placenta. Oxytocin peptide was visible as dark brown to black cytoplasmic staining (arrows). Images of negative controls (A,B): (A) endometrium with primary antibody omitted (no staining visible); inset in (A), allantochorion with primary antibody omitted (no staining visible); (B) endometrium stained with antibody incubated with blocking peptide (no staining visible). Images of the pregnancy group (C,D): (C) allantochorion with positively stained epithelial cells on villi; (D) endometrium with positively stained epithelial cells in crypts; inset in (D), endometrial glands with positively stained endothelial cells. Images of the physiological parturition group (E,F): (E) allantochorion with positively stained epithelial cells on villi; (F) endometrium with positively stained epithelial cells in crypts; inset in (F), endometrial glands with positively stained endothelial cells. Images of the fetal membrane retention group (G,H): (G) allantochorion with positively stained epithelial cells on villi; (H) endometrium with positively stained epithelial cells in crypts; inset in (H), endometrial glands with positively stained endothelial cells. Micrographs were made with Zen 2012 (blue edition) software (Zeiss, https://www.zeiss.com/microscopy/int/products/microscope-software/zen-lite.html).
Mean OXT content was higher in FMR than in physiological parturition in both tissues, but the differences were not statistically significant (allantochorion: 3.7-fold, 95% confidence interval for the fold-change (CI) = −1.9 to 27.0-fold, p = 0.44; endometrium: 1.2-fold, CI = −30.1 to 41.1-fold, p = 0.92) (Fig. 1b). The variation in OXT content within groups was large relative to the difference between group means, with the exception of the allantochorion in FMR, in which there was relatively little difference between horses.
Interestingly, immunohistochemical staining for OXT was visible in the cytoplasm in both tissues in all groups (Fig. 1d). It was present mostly in the epithelial cells of villi and endometrial crypts. In the endometrium, OXT was also visible in the endothelial cells of the glands.
OXTR
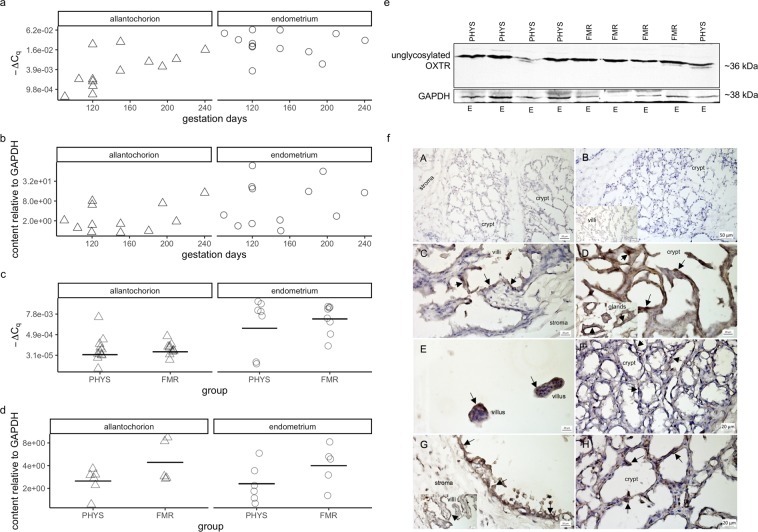
In the allantochorion in pregnancy, OXTR mRNA expression tended to increase with gestation days (Fig. 2a). This correlation was moderately strong but not statistically significant (τ = 0.54, p = 0.13). However, there was little correlation between gestation days and OXTR expression in the endometrium, or between gestation days and OXTR protein content in the allantochorion and endometrium (τ = −0.18, p = 0.68; τ = 0.18, p = 0.68; τ = 0.08, p = 0.89; respectively) (Fig. 2a,b).
Figure 2.
Oxytocin receptor (OXTR) mRNA expression, protein content, and immunolocalization. (a) OXTR mRNA expression during pregnancy. mRNA expression was quantified by RT-qPCR. ∆Cq is the difference between the quantification cycle value of OXTR and that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) after weighting those values by the genes’ amplification efficiencies. Symbols correspond to tissues from individual mares (Δ, allantochorion; ○, endometrium). Note the logarithmic scales in panels a–d. (b) OXTR protein content relative to that of GAPDH during pregnancy. Protein content was quantified by western blotting with densitometry. (c) OXTR mRNA expression in physiological parturition (PHYS) and parturition with fetal membrane retention (FMR). Horizontal lines indicate geometric means. (d) OXTR protein content relative to that of GAPDH in PHYS and FMR. (e) Representative blots of OXTR and GAPDH. Uncropped blots are presented in Supplementary Fig S5. Quantity One 1-D version 4.6.6 software was used for visualization and densitometry (Bio-Rad Laboratories, https://www.bio-rad.com/en-pl/product/quantity-one-1-d-analysis-software?ID=1de9eb3a-1eb5-4edb-82d2-68b91bf360fb). Abbreviation: E, endometrium. (f) Tissue expression and localization of OXTR in horse placenta. Oxytocin receptor protein was visible as dark brown to black membrane staining (arrows). Hematoxylin was used as a counterstain. Images of positive and negative controls (A,B): (A) endometrium with primary antibody omitted (no staining visible); (B) endometrium stained with antibody incubated with blocking peptide (no staining visible); inset in (B), allantochorion stained with antibody incubated with blocking peptide (no staining visible). Images of the pregnancy group (C,D): (C) allantochorion with positively stained epithelial cells on villi; (D) endometrium with positively stained epithelial cells in crypts; inset in (D), endometrial glands with positively stained endothelial cells. Images of the physiological parturition group (E,F): (E) allantochorion with positively stained epithelial cells on villi; (F) endometrium with positively stained epithelial cells in crypts. Images of the fetal membrane retention group (G,H): (G) allantochorion with positively stained epithelial cells; inset in (G), allantochorion with positively stained epithelial cells on villi; (H) endometrium with positively stained epithelial cells in crypts. Micrographs were made with Zen 2012 (blue edition) software (Zeiss, https://www.zeiss.com/microscopy/int/products/microscope-software/zen-lite.html).
In both tissues, mean OXTR expression was higher in FMR than in physiological parturition, but the differences were not significant (allantochorion: 1.5-fold, CI = −1.4 to 3.2-fold, p = 0.59; endometrium: 3.5-fold, CI = −8.3 to 102.1, p = 0.68) (Fig. 2c). Similarly, in both tissues, OXTR content was higher in FMR, although the differences were not significant (allantochorion: 1.8-fold, CI = −1.2 to 3.9-fold, p = 0.89; endometrium: 1.7-fold, CI = −1.3 to 4.0-fold, p = 0.44) (Fig. 2d). The variation in OXTR expression and OXTR content within groups was large relative to the difference between group means.
Immunohistochemical staining for OXTR was visible on the cell membrane (Fig. 2f). In the allantochorion, it was present on the epithelial cells of the villi, and also on the endothelial cells of some vessels. In the endometrium, it was present on the epithelial cells of crypts and on the endothelial cells of glands and some vessels.
PTGS2
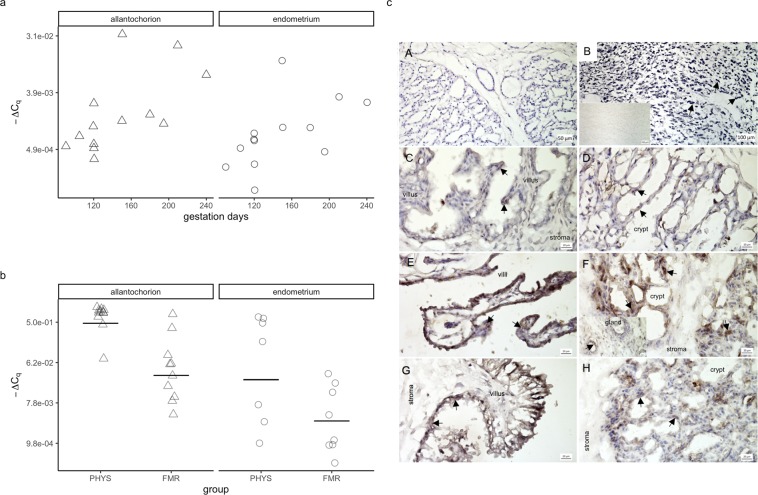
In both tissues in pregnancy, PTGS2 expression tended to increase with greater gestation length (Fig. 3a). Although these correlations were not statistically significant, they were moderately strong (allantochorion: τ = 0.48, p = 0.19; endometrium: τ = 0.54, p = 0.13).
Figure 3.
Prostaglandin endoperoxide synthase-2 (PTGS2) mRNA expression and immunolocalization. (a) PTGS2 mRNA expression during pregnancy. mRNA expression was quantified by RT-qPCR. ∆Cq is the difference between the quantification cycle value of PTGS2 and that of glyceraldehyde-3-phosphate dehydrogenase after weighting those values by the genes’ amplification efficiencies. Symbols correspond to tissues from individual mares (Δ, allantochorion; ○, endometrium). Note the logarithmic scales in panels a and b. (b) PTGS2 mRNA expression in physiological parturition (PHYS) and parturition with fetal membrane retention (FMR). Horizontal lines indicate geometric means. (c) Tissue expression and localization of PTGS2 in horse placenta. PTGS2 protein was visible as dark brown to black cytoplasmic or nuclear staining (arrows). Hematoxylin was used as a counterstain. Images of positive and negative controls (A,B): (A) endometrium stained with antibody incubated with blocking peptide (no staining visible); (B) mature corpus luteum with positively stained luteal cells; inset in (B), the same corpus luteum stained with primary antibody omitted (no staining visible). Images of the pregnancy group (C,D): (C) allantochorion with some positively stained epithelial cells on villi; (D) endometrium with some positively stained epithelial cells in crypts. Images of the physiological parturition group (E,F): (E) allantochorion with positively stained epithelial cells on villi; (F) endometrium with positively stained epithelial cells in crypts; inset in (F), endometrial glands with some positively stained endothelial cells. Images of the fetal membrane retention group (G,H): (G) allantochorion with positively stained epithelial cells; (H) endometrium with positively stained epithelial cells in crypts. Micrographs were made with Zen 2012 (blue edition) software (Zeiss, https://www.zeiss.com/microscopy/int/products/microscope-software/zen-lite.html).
In the allantochorion, mean PTGS2 expression was substantially lower in FMR than in physiological parturition, and the difference was statistically significant (–14.7-fold, CI = −49.8 to –4.3-fold, p = 0.007) (Fig. 3b). In the endometrium, although the difference was not significant, PTGS2 expression was also substantially lower in FMR than in physiological parturition (–8.4-fold, CI = −105.7 to 1.5-fold, p = 0.32).
In both physiological parturition and FMR, staining for PTGS2 protein was mostly visible in the cytoplasm in the form of granules, and some nuclei were also stained (Fig. 3c). Some cytoplasmic staining was also visible in pregnancy. In all groups, staining was observed in epithelial cells in these locations: the endometrial crypts, the surface of the allantochorion, and the villi. Additionally, it was visible in the endothelium of endometrial glands.
PGE2
In the allantochorion in pregnancy, PGE2 content tended to decrease with gestation days. This correlation was moderately strong but not statistically significant (τ = −0.40, p = 0.68) (Fig. 4a). In the endometrium, PGE2 content showed a strong but non-significant tendency to increase with gestation days (τ = 0.80, p = 0.32).
Figure 4.
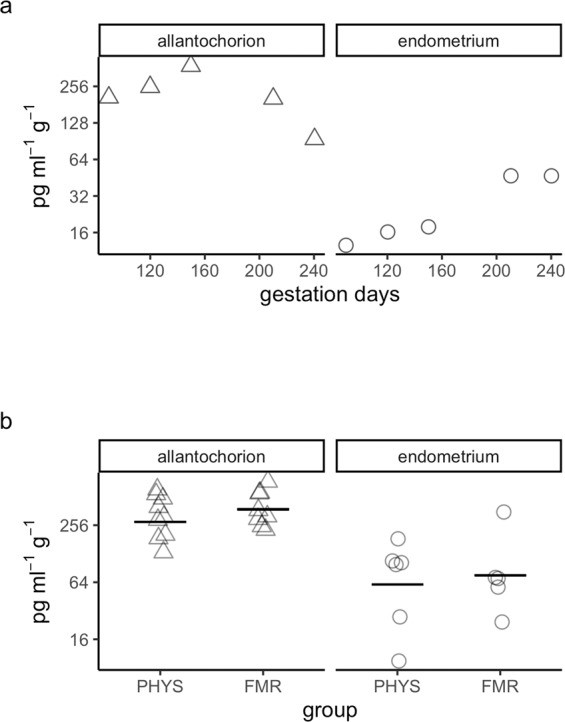
Prostaglandin E2 content. (a) Prostaglandin E2 content during pregnancy. Hormone content was quantified by enzyme immunoassay. Symbols correspond to tissues from individual mares (Δ, allantochorion; ○, endometrium). Note the logarithmic scales in panels a and b. (b) Prostaglandin E2 content in physiological parturition (PHYS) and parturition with fetal membrane retention (FMR). Horizontal lines indicate geometric means.
In the allantochorion in FMR, mean PGE2 content was 1.4-fold higher than in this tissue in physiological parturition (Fig. 4b, Supplementary Table S2). Although this difference was not significant, the CI included values up to 2.4-fold higher in FMR (CI = −1.3 to 2.4-fold, p = 0.57). In the endometrium, PGE2 content was 1.2-fold higher in FMR. This difference was not significant, but the CI included values up to 5.1-fold higher in FMR (CI = −3.3 to 5.1-fold, p = 0.89).
PGF2α
In the allantochorion in pregnancy, there was a moderately strong but non-significant tendency for PGF2α content to decrease with greater gestation length (τ = −0.40, p = 0.68) (Fig. 5a, Supplementary Table S2). In the endometrium, there was a strong but non-significant tendency for its content to increase with gestation length (τ = 0.80, p = 0.32).
Figure 5.
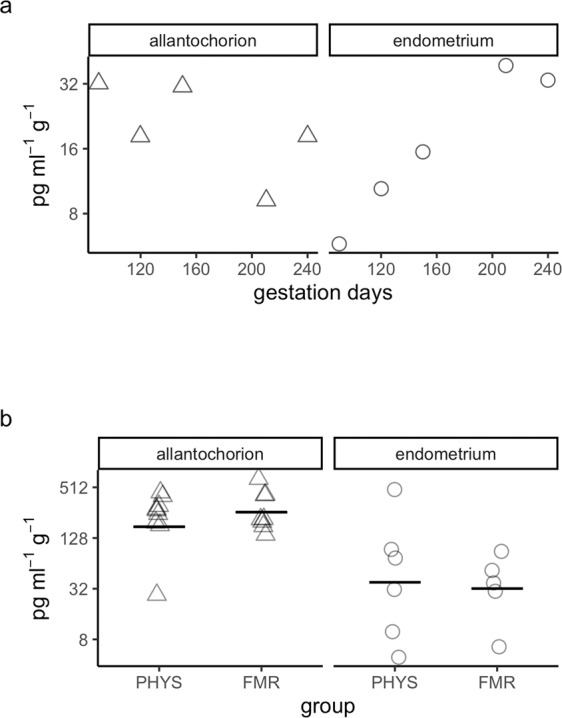
Prostaglandin F2 alpha content. (a) Prostaglandin F2 alpha content during pregnancy. Hormone content was quantified by enzyme immunoassay. Symbols correspond to tissues from individual mares (Δ, allantochorion; ○, endometrium). Note the logarithmic scales in panels a and b. (b) Prostaglandin F2 alpha content in physiological parturition (PHYS) and parturition with fetal membrane retention (FMR). Horizontal lines indicate geometric means.
In the allantochorion, mean PGF2α content was 1.5-fold higher in FMR than in physiological parturition (Fig. 5b). This difference was not significant, but the CI included values up to 3.0-fold higher in FMR (CI = −1.4 to 3.0-fold, p = 0.57). In the endometrium, its content was 1.2-fold lower in FMR than in physiological parturition. Here too, the difference was not significant, although the CI included values as low as 7.3-fold lower in FMR (CI = −7.3 to 5.2-fold, p = 0.91).
Discussion
Our results indicate that OXT is present within the cells of the horse allantochorion and endometrium during pregnancy, physiological parturition, and FMR. They also indicate that PTGS2 expression in the allantochorion is markedly lower in FMR than in physiological parturition.
When considering the results of our study, there are three general limitations that should be taken into account. First, we studied heavy draft horses and not other breeds, and all of our horses in physiological parturition and FMR lived in the vicinity of our practice. Thus, some of our findings may not apply to other horse populations. Nevertheless, our study is a step towards better understanding of the endocrinology of equine reproduction in general and of FMR, which is particularly common in heavy draft horses.
Second, many of our estimates of differences between pregnancy, physiological parturition, and FMR have wide CIs. This is due in part to the small number of horses in our study. This small number also meant that we could not estimate the influence of herd- and farm-level confounders on our results. However, many studies of these large and expensive-to-maintain animals have similar numbers, and combining such studies in meta-analyses should provide more precise estimates.
Third, in the parturition groups, we took samples immediately after foal delivery to avoid unnecessary risks to our patients. Therefore, our results are representative of the placental levels of substances at that time, but they do not necessarily reflect levels of those substances at earlier or later stages of parturition. Although differential protein/hormone content or gene expression that is associated with FMR at foal delivery may not be a cause of the condition, these associations can provide information about the aetiology of FMR, as we discuss below in the context of the PTGS2 results.
The presence of OXT in the cytoplasm of cells in the allantochorion and endometrium of all three groups strongly suggests that OXT is synthesized in the horse placenta. Proof of this hypothesis awaits a confirmed horse OXT sequence so that OXT expression in the placenta can be verified. However, the fact that humans, whose reproductive endocrinology is very similar to that of horses24, synthesize OXT in the placenta13,23 suggests that OXT is also synthesized in the horse placenta.
The presence of additional OXTR bands in some of our western blots, and the difference between the molecular weight of the un-glycosylated OXTR band in our study (~36 kDa) and the weights reported by other studies (~70 kDa26, ~55 kDa27) is likely due to the accuracy of molecular weight estimation with SDS-PAGE (±5–10%44) and differences in the glycosylation status of OXTR. Glycosylation of OXTR is common, and as a result, multiple bands are often detected45. The protein has three glycosylation sites46, and each additional glycosylation core adds ~10 kDa45. So far, no effect of glycosylation on OXTR function has been found47,48; thus, differences in its glycosylation status seem unlikely to be physiologically relevant.
Although OXTR expression and OXTR content in both tissues were higher in FMR than in physiological parturition, the differences were not statistically significant. More importantly, the within-group variation in OXTR expression, and OXT and OXTR content was large relative to the difference between group means. This suggests that most of the variation in the levels of these substances is due to factors other than the presence or absence of FMR.
Our present results differ from those of our previous study of the placenta in horses, in which we observed substantially less OXTR content in FMR associated with secondary atony of the uterus than in physiological parturition27. The difference between studies is attributable to two factors: first, the FMR horses in our present study did not have secondary atony of the uterus. Second, the horses in our present study were sampled immediately after foal delivery, not up to three hours after, as they were in our previous study.
After adjustment for multiple comparisons, the correlations between gestation days and PTGS2 expression in the allantochorion and endometrium were no longer significant at p < 0.05. However, this does not mean our data indicate that, in the population of all such horses, there is no correlation. Rather, these results should be considered inconclusive49,50. Some evidence from sheep suggests that PTGS2 expression in horses would tend to increase as gestation progresses, as Wimsatt18 found that PTGS2 activity increased close to term in maternal parts of the sheep placenta.
Our results indicate that PTGS2 expression in the allantochorion at foal delivery is markedly lower in FMR than in physiological parturition. All of the values in the CI for the difference (49.8 to 4.3-fold lower in FMR) are large enough that this difference seems likely to be biologically relevant.
PTGS2 production is induced by inflammation, and the enzyme is generally only found in inflamed tissues29–34. Thus, lower PTGS2 expression in FMR suggests that the condition is associated with dysregulation of inflammatory processes. The inflammatory response plays a key role in both physiological tissue repair/remodelling and in fibrosis51,52, and growing evidence indicates its importance in parturition6,53. Tissue remodelling may have particular relevance to FMR in horses. It has been hypothesized that, in this species, shrinkage of the allantochorion villi allows them to slide out of their corresponding endometrial crypts, thus releasing the fetal membranes54. This shrinkage would involve tissue remodelling. Previously, we found that extensive fibrosis41, and differential content and activity of matrix metalloproteinases42 are associated with FMR in heavy draft horses. Thus, evidence from this study and others suggests that, in this breed of horse, FMR is linked to dysregulation of inflammation and tissue remodelling.
Although the results were statistically inconclusive (p ≥ 0.32), the correlations between gestation days and content of PGE2 and PGF2a ranged from moderately strong to strong. Interestingly, the content of both prostaglandins in the allantochorion tended to decrease with gestation days, whereas their content in the endometrium tended to increase. The data on prostaglandin content in FMR and physiological parturition were also inconclusive, but the CIs contained differences large enough to be physiologically relevant. Moreover, because PTGS2 expression in the allantochorion was lower in FMR than in physiological parturition, the content of prostaglandins in this tissue may decrease faster in FMR than in physiological parturition as the third stage of parturition progresses. Thus, it would be worthwhile to conduct further investigations of prostaglandin content during pregnancy, physiological parturition, and FMR, and to combine the results of all such studies in a meta-analysis.
In summary, our study indicates that OXT is present in the cells of the horse placenta during pregnancy, physiological parturition, and FMR, and that PTGS2 expression in the allantochorion is lower in FMR than in physiological parturition. The presence of OXT in these cells suggests that the horse placenta synthesizes OXT. Lower PTGS2 expression in FMR suggests a link between this condition and dysregulation of inflammatory processes in heavy draft horses.
Material and Methods
Settings, animals, and sample collection
All animals were heavy draft horses. Material from some of the samples that were taken for this study was also used to describe the expression of major histocompatibility complex class I in the equine placenta55.
To standardize sampling, we took all samples from the same place in the body of the pregnant uterus, either immediately post-mortem in pregnancy or intra-vitality in physiological parturition and FMR. We took samples from this location because it was the furthest that we could reach with equine biopsy forceps. The Local Ethics Committee for Experiments on Animals in Olsztyn (Poland) approved all sample collection protocols (permission no. 05/2014/DTN for slaughterhouse samples and 18/2015/DTN for patient samples), and we followed guidance in EU Directive 2010/63/EU for animal experiments.
Pregnancy
Samples were obtained post-mortem from 13 horses in a slaughterhouse (the pregnancy status of the mares was not known until after slaughter). See Table 1 for the number of horses assayed with each technique. The duration of pregnancy (3–8 months) was determined based on fetal development56, but the ages of the mares were not available to us. Three biopsies of allantochorion were taken, and separately, three biopsies of endometrium. The horses were healthy before slaughter, and placental inflammation was not detected.
Table 1.
Number of horses assayed with each technique.
| Group | PREG | PHYS | FMR |
|---|---|---|---|
| Method | allantochorion/ endometrium | allantochorion/ endometrium | allantochorion/ endometrium |
| RT-qPCR | 13/13 | 11/7 | 10/8 |
| EIA | 5/5 | 9/6 | 8/5 |
| Western blotting | 12/12 | 6/6 | 5/5 |
| Immunohistochemistry | 6/6 | 6/6 | 6/6 |
Abbreviations: PREG, pregnancy; PHYS, physiological parturition; FMR, fetal membrane retention; RT-qPCR, reverse transcription quantitative polymerase chain reaction; EIA, enzyme immunoassay.
Physiological parturition and FMR
All mares in these groups (physiological parturition, 11 mares; FMR, 10 mares; see Table 1 for the number of horses assayed with each technique) were monitored during their entire pregnancy; none of them developed placentitis. They weighed approximately 800–900 kg; physiological parturition mares had a mean age of 7.6 years (range 3–15); FMR mares had a mean age of 10.0 years (range 4–16). All mares delivered foals within the timeframe for term delivery57, and the second phase of parturition lasted for 10-20 minutes.
Directly after foal delivery, uterine biopsies were taken as described in detail in Rapacz-Leonard42 and immediately fixed (fixation details are below). Because some owners feared that taking samples from the endometrium might decrease fertility, fewer horses were biopsied in the endometrium than in the allantochorion (Table 1). The myometrium was not biopsied to avoid unnecessary risks to our patients. To control for a possible effect of sampling on placenta retention, each horse had four biopsies taken from each sampled tissue. In general, mares with a history of physiological delivery delivered physiologically, and mares with a history of FMR retained the placenta, but two mares with a history of FMR did not retain after endometrial sampling.
Physiological parturition mares delivered fetal membranes within 20–120 minutes. If fetal membranes were not expelled within 180 minutes of foal delivery, mares were classified as having FMR43,58–60, then manually examined and treated.
RT-qPCR
Samples were fixed in RNAlater Stabilization Solution (ThermoFisher Scientific) and stored at −80 °C until assay. 50 mg of tissue per sample was used. RNA was isolated with a Total RNA Mini Kit (A&A Biotechnology). RNA concentration and quality were determined using a NanoVue Plus Spectrophotometer (GE Healthcare). After DNase treatment, reverse transcription was performed with a Maxima First Strand cDNA Synthesis Kit (ThermoFisher Scientific).
Primers were designed using Primer-BLAST and manufactured by Sigma-Aldrich (Table 2). No secondary structures were detected (Supplementary Fig S2 and S3); no pseudogenes were amplified. To confirm primer specificity, PCR products were sequenced (Supplementary Table S3). As negative controls, reactions without template, DNA polymerase, or reverse transcriptase were used, as well as a reaction with only ultrapure water.
Table 2.
Primers for RT-qPCR analysis.
| Gene name and abbreviation | Sequence accession number | Primers | Sequence (5′ → 3′) | Amplicon length | Location by exon | Concentration | Efficiency |
|---|---|---|---|---|---|---|---|
| GAPDH as a reference gene75,76 | NM_001163856.1 |
Forward Reverse |
GTCAAGCTCATTTCCTGGTATGAC TTGCTGGGTGATTGGTGGTC |
117 |
Yes, exon-exon junction by exons no. 11 and 12 |
F: 5μM R: 5 μM |
86.4% |
| OXTR |
XM_023620040.1 variant X1 XM_023620041.1 variant X2 XM_014731360.2 variant X3 |
Forward Reverse |
TTCATCATCGTGCTGGCCTT TGAAAGCCGAGGCTTCCTTG |
103 |
Yes, exon-exon junction by exons no. 3 and 4 (for X1), and exons no. 2 and 3 (for X2 and X3) |
F: 5μM R: 5 μM |
114.3% |
| PTGS2 | NM_001081775.2 |
Forward Reverse |
TGGGTCACGGGGTGGATTTA GCGGATACACCTCGCCATTA |
120 |
Yes, exon-exon junction by exons no. 5, 6, and 7 |
F: 5μM R: 5 μM |
115.2% |
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; OXTR, oxytocin receptor; PTGS2, prostaglandin-endoperoxide synthase 2.
RT-qPCR was performed with SYBR Select Master Mix (ThermoFisher Scientific) using a 7500 Fast Real-Time PCR System (Applied Biosystems). The reaction volume was 20 µL (10 µL Master Mix, 8 µL ultrapure water, 1 µL cDNA (~75 ng), 1 µL primer mix). The efficiency-corrected expression of the investigated genes relative to that of GAPDH was calculated61.
Western blotting
Samples were fixed in liquid nitrogen and stored at −80 °C until assay. 100 µg of protein were loaded in each well.
For quantification of OXT, anti-OXT antibody (Table 3) was used. Because OXT was present in small amounts, chemiluminescent western blotting with horseradish peroxidase was employed44. The procedure in Rękawiecki62 was followed, with three modifications: Mini-Protean TGX Stain-Free Precast Gels 4–20% (456–8093, Bio-Rad) were used, as well as semi-dry transfer with Sequi-Blot PVDF Membrane (162–0184, Bio-Rad) and Prestained SDS-PAGE Standards (161–0318, Bio-Rad). Briefly, after electrophoresis, the transfer was performed with Tris-glycine methanol buffers at 0.2 A for 45 min. After the transfer, non-specific binding was blocked with 5% nonfat dry milk in TBST buffer. The membranes were then left overnight to incubate with primary antibody.
Table 3.
List of antibodies.
| Name of the antibody | Supplier and catalogue no. | Antibody ID | Host | Clonality | Antigen | Validated by western blot | Used at concentration | Available blocking peptide | Supplier and catalogue no. for blocking peptide | Used at ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| Anti- OXT |
LifeSpan BioScience LS-C145973 |
AB_10969170 | rabbit | polyclonal | aa11-60 of human OXT (P01178, NP_000906) | yes | 1ug/1 ml TBST | yes |
LifeSpan BioScience LS-E15388 |
4:1 |
| Anti- OXTR |
Abcam ab87312 |
AB_10674457 | goat | polyclonal |
aa 355–367 of human OXTR (C terminal) Sequence: C-RRLGETSASKKSN |
yes | 5ug/1 ml TBST | yes |
Abcam ab173096 |
3:1 |
| Anti-PTGS2 |
Santa Cruz Biotechnology sc-1746 |
AB_631310 | goat | polyclonal |
peptide mapping near the N-terminus of Cox −2 of rat origin |
yes by77,78 | 1:100 | yes |
Santa Cruz Biotechnology sc-1746P |
4:1 |
| Anti-GAPDH |
Sigma-Aldrich G8795 |
AB_1078991 | mouse | monoclonal | hybridoma GAPDH-71.1 produced by the fusion of mouse NS1 cells and splenocytes from BALB/c mice immunized with rabbit GAPDH | yes | 2ul/24 ml TBST | no |
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; OXT, oxytocin; OXTR, oxytocin receptor; PTGS2, prostaglandin-endoperoxide synthase 2.
For OXT visualization, (Sigma-Aldrich Cat# A6154, RRID:AB_258284, 0.5ul/25 ml TBST) was used. Then blots were stripped and incubated with anti-GAPDH antibody (Table 3). For GAPDH visualization, (Santa Cruz Biotechnology Cat# sc-2318, AB_641171, 0.75ul/15 ml TBST) was used.
To quantify OXTR, which was present in abundance, colorimetric western blotting with alkaline phosphatase was used44, following the procedure in Kowalik63 except for the use of 10% polyacrylamide gel electrophoresis with SDS (SDS–PAGE). Briefly, after electrophoresis with PageRuler Prestained Protein Ladder (SM0671, Fermentas), wet transfer was performed with Tris-glycine methanol buffer on ice at 60 V for 90 min. Blocking and incubation with antibody were performed as for OXT. To detect OXTR, anti-OXTR antibody (Table 3) was used with (Sigma-Aldrich Cat# A4187, RRID:AB_258141, 1ul/30 ml TBST). After visualization, blots were incubated with anti-GAPDH antibody (Table 3) with (Sigma-Aldrich Cat# A3562, RRID:AB_258091, 0.5ul/15 ml TBST).
OXT was observed at ~9 kDa (Fig. 1c), the core of un-glycosylated OXTR at ~36 kDa (Fig. 2e), and GAPDH at ~38 kDa. The molecular weights of OXT and GAPDH are in agreement with those predicted by the manufacturer. The molecular weight of un-glycosylated OXTR is within the range of 22–42 kDa predicted with ExPASy Server64,65 and NCBI66. Occasionally, OXTR bands were visible at ~48 and ~55 kDa, indicating glycosylation at one or two sites, which is common45.
After removing background density, the normalized expression was calculated by dividing the average intensity of the OXT or un-glycosylated OXTR band by that of the GAPDH band from the same horse that was run on the same gel.
Immunohistochemistry on frozen samples
Samples were fixed and stained as described in Rapacz-Leonard55, except for the use of 0.5% BSA (AM2616, Thermo Fisher Scientific) for blocking non-specific binding. Briefly, Bloxall (SP-6000, Vector Laboratories) was used for blocking endogenous peroxidase, then 0.5% BSA and slides were incubated overnight with primary antibodies (Table 3: anti-OXT, anti-OXTR, anti-PTGS2). For detection, an ImmPRESS HRP Universal Antibody (Anti-Mouse IgG/Anti-Rabbit IgG, Peroxidase) Polymer Detection Kit (MP-7500, Vector Laboratories) for rabbit antibody and an ImmPRESS HRP Anti-Goat IgG (Peroxidase) Polymer Detection Kit (MP-7405, Vector Laboratories) for goat antibodies were used. Slides were photographed using a Zeiss microscope (Axio Imager.M2, Zeiss) with an AxioCam MRC 5 (Zeiss).
As positive controls, equine corpus luteum and the endometrium from a non-pregnant mare in late luteal phase were used. For negative controls, blocking peptides were used. Omission controls were performed with only antibody diluent (ab64211, Abcam). Replacement controls were performed with normal serum instead of primary antibody, using either rabbit serum (R9133, Sigma Aldrich) or goat serum (PK-4010, Vector Laboratories). No isotype control was performed because of the polyclonality of the antibodies.
Enzyme immunoassays for PGE2 and PGF2ɑ
Samples were frozen in liquid nitrogen and stored at −80 °C. Extraction was performed as described in Siemieniuch67, who followed Tsang68. Briefly, the prostaglandins were extracted from samples with ethyl petroleum, and the supernatants were evaporated to dryness under nitrogen and reconstituted with 400 µL EIA buffer with 0.1% BSA. The extraction efficiencies were 74% for PGF2ɑ and 72% for PGE2, as determined by performing the extraction with PGF2α standard (ADI-931-069; ENZO Life Sciences Inc.) and PGE2 standard (ADI-931-001; ENZO Life Sciences Inc.), then measuring the concentration as below.
To measure the concentration of PGE2, a species-independent PGE2 EIA kit (ADI-931-001; ENZO Life Sciences Inc.) was used, which has intra- and inter-assay coefficients of variation of 6.7% and 12.2%, respectively. Because of the high concentration of PGE2, a 1:20 dilution of the samples in buffer solution was performed. To measure the concentration of PGF2ɑ, a species-independent PGF2ɑ EIA kit (ADI-931-069; ENZO Life Sciences Inc.) was used, which has intra- and inter-assay coefficients of variation of 6.8% and 11.2%, respectively. With both kits, the manufacturer’s instructions were followed. For PGE2, the sensitivity was 8.26 pg/ml (range, 7.8–1,000 pg/ml); for PGF2ɑ, it was 0.98 pg/ml (range, 1.95–2,000 pg/ml). The content of PGE2 and PGF2ɑ is reported as pg ml−1 g−1, adjusted for the dilution of PGE2.
Statistical analyses
Full details of statistical methods are in Supplementary Note - Statistical analyses. Briefly, all data were log-transformed before further calculations and analyses61,69. For reporting, data was back-transformed to the original scale; thus, geometric means are reported.
Correlations between the amount of mRNA/protein/hormone and gestation days were assessed with Kendall’s tau (two-sided). Differences between mean expression of mRNA/protein/hormone in physiological parturition and FMR were tested with Welch’s t-test (two-tailed). Sensitivity analysis70 by bootstrapping the difference between medians71 indicated that potential deviations from normality did not affect the interpretation of Welch’s test (Supplementary Table S1).
To account for multiple comparisons, all 24 p-values were adjusted to maintain the false discovery rate below 5%72. After adjustment, results were considered significant at p < 0.05. Unadjusted 95% CIs for fold-changes are provided.
All statistical calculations were performed with R (v. 3.5.273), employing the WRS package for R (v. 3550) for bootstrapping.
Acknowledgements
This study was supported by a National Science Centre (NCN) grant (2012/07/D/NZ5/04290) and co-supported by Minister of Science and Higher Education in the range of the program entitled “Regional Initiative of Excellence” for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Supplementry information
Author contributions
All authors contributed to data analysis and all approved the final version of the manuscript. ARL designed the study with the help of TJ. ARL collected samples and performed western blotting and immunohistochemistry. MCK supervised RT-qPCR. MS performed EIA. ML performed statistical analyses. ARL and ML interpreted the data and wrote the manuscript.
Data availability
The dataset analysed during the current study is available from Dryad Digital Repository (10.5061/dryad.fbg79cnr4)74.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59085-1.
References
- 1.Ousey JC. Hormone Profiles and Treatments in the Late Pregnant Mare. Vet. Clin. North Am. - Equine Pract. 2006;22:727–747. doi: 10.1016/j.cveq.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Kota SK, et al. Endocrinology of parturition. Indian J. Endocrinol. Metab. 2013;17:50–9. doi: 10.4103/2230-8210.107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front. Physiol. 2018;9:1–39. doi: 10.3389/fphys.2018.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keelan JA, et al. Cytokines, Prostaglandins and Parturition — A Review Cytokine Production by Gestational. Placenta. 2003;17:S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 5.Kindahl H. Placenta functions with special emphasis on endocrine changes – a comparative overview. Acta Vet. Scand. 2007;49:S15. doi: 10.1186/1751-0147-49-S1-S15. [DOI] [Google Scholar]
- 6.Bollopragada S, et al. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am. J. Obstet. Gynecol. 2009;200:104.e1–104.e11. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Sykes L, MacIntyre DA, Teoh TG, Bennett PR. Anti-inflammatory prostaglandins for the prevention of preterm labour. REPRODUCTION. 2014;148:R29–R40. doi: 10.1530/REP-13-0587. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, et al. A Role for the Inflammasome in Spontaneous Labor at Term. Am. J. Reprod. Immunol. 2018;79:1–20. doi: 10.1111/aji.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott, W. H. (B) & Elliott, D. C. Biochemistry and molecular biology. (Oxford University Press, 2009).
- 10.Fuchs A-R, Husslein P, Fuchs F. Oxytocin and the initiation of human parturition II. Stimulation of prostaglandin production in human decidua by oxytocin. Am. J. Obstet. Gynecol. 1981;141:694–697. doi: 10.1016/S0002-9378(15)33313-5. [DOI] [PubMed] [Google Scholar]
- 11.Husslein P, Fuchs AR, Fuchs F. Oxytocin and the initiation of human parturition. I. Prostaglandin release during induction of labor by oxytocin. Am. J. Obstet. Gynecol. 1981;141:688–93. doi: 10.1016/S0002-9378(15)33312-3. [DOI] [PubMed] [Google Scholar]
- 12.Ealy AD, Eroh ML, Sharp DC. Prostaglandin H synthase Type 2 is differentially expressed in endometrium based on pregnancy status in pony mares and responds to oxytocin and conceptus secretions in explant culture. Anim. Reprod. Sci. 2010;117:99–105. doi: 10.1016/j.anireprosci.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Terzidou V, Blanks AM, Kim SH, Thornton S, Bennett PR. Labor and Inflammation Increase the Expression of Oxytocin Receptor in Human Amnion1. Biol. Reprod. 2011;84:546–552. doi: 10.1095/biolreprod.110.086785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.PASHEN RL. Maternal and foetal endocrinology during late pregnancy and parturition in the mare. Equine Vet. J. 1984;16:233–238. doi: 10.1111/j.2042-3306.1984.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson T, Whittaker DJ, Hospital NW. Oxytocin stimulates the release of arachidonic acid and prostaglandin f2a from human decidual cells. Prostaglandins. 1988;35:771–780. doi: 10.1016/0090-6980(88)90149-9. [DOI] [PubMed] [Google Scholar]
- 16.Starbuck GR, Stout TAE, Lamming GE, Allen WR, Flint APF. Endometrial oxytocin receptor and uterine prostaglandin secretion in mares during the oestrous cycle and early pregnancy. Reproduction. 1998;113:173–179. doi: 10.1530/jrf.0.1130173. [DOI] [PubMed] [Google Scholar]
- 17.Hinko A, Soloff MS. Characterization of Oxytocin Receptors in Rabbit Amnion Involved in the Production of Prostaglandin E2. Endocrinology. 1992;130:3547–3553. doi: 10.1210/endo.130.6.1317789. [DOI] [PubMed] [Google Scholar]
- 18.Wimsatt J, Nathanielsz PW, Sirois J. Induction of prostaglandin endoperoxide synthase isoform-2 in ovine cotyledonary tissues during late gestation.pdf. Endocrinology. 1993;133:1068–1073. doi: 10.1210/endo.133.3.8365354. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs A-R, Rust W, Fields MJ. Accumulation of Cyclooxygenase-2 Gene Transcripts in Uterine Tissues of Pregnant and Parturient Cows: Stimulation by Oxytocin. Biol. Reprod. 1999;60:341–348. doi: 10.1095/biolreprod60.2.341. [DOI] [PubMed] [Google Scholar]
- 20.Molnár M, Rigó J, Romero R, Hertelendy F. Oxytocin activates mitogen-activated protein kinase and up-regulates cyclooxygenase-2 and prostaglandin production in human myometrial cells. Am. J. Obstet. Gynecol. 1999;181:42–49. doi: 10.1016/S0002-9378(99)70434-5. [DOI] [PubMed] [Google Scholar]
- 21.Pavan B, et al. Influence of oxytocin on prostaglandin E2, intracellular calcium, and cyclic adenosine monophosphate in human amnion-derived (WISH) cells. Am. J. Obstet. Gynecol. 2000;183:76–82. [PubMed] [Google Scholar]
- 22.Campbell, N. A. et al. Biology. (Pearson Benjamin Cummings, 2008).
- 23.Johnson, M. H. Essential reproduction. (Wiley-Blackwell Pub, 2013).
- 24.Conley AJ. Review of the reproductive endocrinology of the pregnant and parturient mare. Theriogenology. 2016;86:355–365. doi: 10.1016/j.theriogenology.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 25.Sharp DC, Thatcher M, Salute ME, Fuchs A-R. Relationship between endometrial oxytocin receptors and oxytocin induced prostaglandin F2 alpha release during the oestrous cycle and early oregnancy in pony mares. J. Reprod. Fertil. 1997;109:137–144. doi: 10.1530/jrf.0.1090137. [DOI] [PubMed] [Google Scholar]
- 26.De Ruijter-Villani M, Van Tol HTA, Stout TAE. Effect of pregnancy on endometrial expression of luteolytic pathway components in the mare. Reprod. Fertil. Dev. 2014;27:834–845. doi: 10.1071/RD13381. [DOI] [PubMed] [Google Scholar]
- 27.Rapacz-Leonard A, Raś A, Całka J, Janowski TE. Expression of oxytocin receptors is greatly reduced in the placenta of heavy mares with retained fetal membranes due to secondary uterine atony. Equine Vet. J. 2015;47:623–626. doi: 10.1111/evj.12426. [DOI] [PubMed] [Google Scholar]
- 28.Gibb W. The role of prostaglandins in human parturition. Ann. Med. 1998;30:235–241. doi: 10.3109/07853899809005850. [DOI] [PubMed] [Google Scholar]
- 29.Thorén S, Jakobsson P-J. Coordinate up- and down-regulation of glutathione-dependent prostaglandin E synthase and cyclooxygenase-2 in A549 cells. Eur. J. Biochem. 2000;267:6428–6434. doi: 10.1046/j.1432-1327.2000.01735.x. [DOI] [PubMed] [Google Scholar]
- 30.Mancini JA, et al. Cloning, Expression, and Up-regulation of Inducible Rat Prostaglandin E Synthase during Lipopolysaccharide-induced Pyresis and Adjuvant-induced Arthritis. J. Biol. Chem. 2001;276:4469–4475. doi: 10.1074/jbc.M006865200. [DOI] [PubMed] [Google Scholar]
- 31.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/S1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 32.Slater DM, Zervou S, Thornton S. Prostaglandins and Prostanoid Receptors in Human Pregnancy and Parturition. J. Soc. Gynecol Investig. 2002;9:118–124. doi: 10.1177/107155760200900302. [DOI] [PubMed] [Google Scholar]
- 33.Ricciotti E. Prostaglandins and Inflamation. Art. Thromb Vas. Biol. 2012;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkby NS, et al. Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-κB and NFAT transcriptional pathways. Proc. Natl. Acad. Sci. 2016;113:434–439. doi: 10.1073/pnas.1517642113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ousey JC, Fowden AL. Prostaglandins and the regulation of parturition in mares. Equine Vet. J. 2012;44:140–148. doi: 10.1111/j.2042-3306.2011.00506.x. [DOI] [PubMed] [Google Scholar]
- 36.Jarabak J. Human Placental 15-Hydroxyprostaglandin Dehydrogenase. Proc. Natl. Acad. Sci. 1972;69:533–534. doi: 10.1073/pnas.69.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly RW. Inflammatory mediators and cervical ripening. J. Reprod. Immunol. 2002;57:217–24. doi: 10.1016/S0165-0378(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 38.Witkowski M, Pawłowski K. Clinical observations on the course of oxytocinor prostaglandin E2/oxytocin-induced parturition in mares. Pol. J. Vet. Sci. 2014;17:347–351. doi: 10.2478/pjvs-2014-0047. [DOI] [PubMed] [Google Scholar]
- 39.Olson DM, Ammann C. Role of the prostaglandins in labour and prostaglandin receptor inhibitors in the prevention of preterm labour. Front. Biosci. 2007;12:1329–43. doi: 10.2741/2151. [DOI] [PubMed] [Google Scholar]
- 40.Ricciotti E, FitzGerald GA. Prostaglandins and Inflamation. Art Thromb Vas Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rapacz A, Paździor K, RaŚ A, Rotkiewicz T, Janowski TE. Retained Fetal Membranes in Heavy Draft Mares Associated with Histological Abnormalities. J. Equine Vet. Sci. 2012;32:38–44. doi: 10.1016/j.jevs.2011.06.015. [DOI] [Google Scholar]
- 42.Rapacz-Leonard A, et al. Differences in extracellular matrix remodeling in the placenta of mares that retain fetal membranes and mares that deliver fetal membranes physiologically. Placenta. 2015;36:1167–1177. doi: 10.1016/j.placenta.2015.07.126. [DOI] [PubMed] [Google Scholar]
- 43.Sevinga M, Barkema HW, Stryhn H, Hesselink JW. Retained placenta in Friesian mares: incidence, and potential risk factors with special emphasis on gestational length. Theriogenology. 2004;61:851–859. doi: 10.1016/S0093-691X(03)00260-7. [DOI] [PubMed] [Google Scholar]
- 44.Bio-Rad. A Guide to Polyacrylamide Gel Electrophoresis and Detection Part I: Theory and Product Selection Part II: Methods Part III: Troubleshooting Part IV: Appendices. Bio-Rad47, doi:Bulletin 6040 Rev B US/EG 13-0891 0513 (2012).
- 45.Gimpl G, Fahrenholz F, Gene C. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 46.Breton C, et al. Direct identification of human oxytocin receptor-binding domains using a photoactivatable cyclic peptide antagonist: Comparison with the human V 1a vasopressin receptor. J. Biol. Chem. 2001;276:26931–26941. doi: 10.1074/jbc.M102073200. [DOI] [PubMed] [Google Scholar]
- 47.Wheatley M, Hawtin SR. Glycosylation of G-protein-coupled receptors for hormones central to normal reproductive functioning: Its occurrence and role. Hum. Reprod. Update. 1999;5:356–364. doi: 10.1093/humupd/5.4.356. [DOI] [PubMed] [Google Scholar]
- 48.Kimura T, et al. The role of N-terminal glycosylation in the human oxytocin receptor. Mol. Hum. Reprod. 1997;3:957–963. doi: 10.1093/molehr/3.11.957. [DOI] [PubMed] [Google Scholar]
- 49.Greenland S, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur. J. Epidemiol. 2016;31:337–350. doi: 10.1007/s10654-016-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilcox, R. R. Understanding and applying basic statistical methods using R. (John Wiley & Sons, 2017).
- 51.Galli SJ, Tsai M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 2008;49:7–19. doi: 10.1016/j.jdermsci.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stramer BM, Mori R, Martin P. The Inflammation–Fibrosis Link? A Jekyll and Hyde Role for Blood Cells during Wound Repair. J. Invest. Dermatol. 2007;127:1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- 53.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell. Mol. Immunol. 2014;11:571–581. doi: 10.1038/cmi.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts S.J. Veterinary obstetrics and genital diseases (Theriogenology). Theriogenology, 10.1016/0093-691X(86)90160-3 (Published by the author, Woodstock, VT 05091., 1986). [DOI] [PubMed]
- 55.Rapacz-Leonard A, Leonard M, Chmielewska-Krzesińska M, Paździor-Czapula K, Janowski T. Major histocompatibility complex class I in the horse (Equus caballus) placenta during pregnancy and parturition. Placenta. 2018;74:36–46. doi: 10.1016/j.placenta.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Brinsko, S. P., Blanchard, T. L., Schumacher, J. & Love, C. C. Pregnancy: Physiology and Diagnosis. in Manual of Equine Reproduction. 85–93, 10.1016/B0-32-301713-4/50008-X (2011).
- 57.Blanchard, T. L. et al. Management of the Pregnant Mare. in Manual of Equine Reproduction 93–105, 10.1016/B0-32-301713-4/50010-8 (Elsevier, 2003).
- 58.Provencher R, Threlfall WR, Murdick PW, Wearly WK. Retained fetal membranes in the mare: A retrospective study. Can. Vet. J. = La Rev. Vet. Can. 1988;29:903–10. [PMC free article] [PubMed] [Google Scholar]
- 59.Frazer GS. Post partum complications in the mare. Part 2: Fetal membrane retention and conditions of the gastrointestinal tract, bladder and vagina. Equine Vet. Educ. 2003;15:91–100. doi: 10.1111/j.2042-3292.2003.tb00223.x. [DOI] [Google Scholar]
- 60.Platt, M. K., Walker, A. J. & Gunn, A. In the mare, does manual removal of fetal membranes negatively affect fertility? Equine Vet. Educ., 10.1111/eve.13046 (2019).
- 61.Ganger MT, Dietz GD, Ewing SJ. A common base method for analysis of qPCR data and the application of simple blocking in qPCR experiments. BMC Bioinformatics. 2017;18:534. doi: 10.1186/s12859-017-1949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rekawiecki R, Kowalik MK, Kotwica J. The expression of progesterone receptor coregulators mRNA and protein in corpus luteum and endometrium of cows during the estrous cycle. Anim. Reprod. Sci. 2017;183:102–109. doi: 10.1016/j.anireprosci.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 63.Kowalik MK, Rekawiecki R, Kotwica J. Expression and localization of progesterone receptor membrane component 1 and 2 and serpine mRNA binding protein 1 in the bovine corpus luteum during the estrous cycle and the first trimester of pregnancy. Theriogenology. 2014;82:1086–1093. doi: 10.1016/j.theriogenology.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 64.ExPASy - Compute pI/Mw tool. Available at, https://web.expasy.org/compute_pi/.
- 65.Gasteiger, E. et al. Protein Identification and Analysis Tools on the ExPASy Server. in The Proteomics Protocols Handbook 571–607, 10.1385/1-59259-890-0:571 (Humana Press, 2005).
- 66.National Center for Biotechnology Information. Available at, https://www.ncbi.nlm.nih.gov/.
- 67.Siemieniuch MJ, et al. Steroidogenic capacity of the placenta as a supplemental source of progesterone during pregnancy in domestic cats. Reprod. Biol. Endocrinol. 2012;10:89. doi: 10.1186/1477-7827-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsang PC, Walton JS, Hansel W. Oxytocin-specific RNA, oxytocin and progesterone concentrations in corpora lutea of heifers treated with oxytocin. J. Reprod. Fertil. 1990;89:77–84. doi: 10.1530/jrf.0.0890077. [DOI] [PubMed] [Google Scholar]
- 69.Limpert E, Stahel WA. Problems with Using the Normal Distribution – and Ways to Improve Quality and Efficiency of Data Analysis. PLoS One. 2011;6:e21403. doi: 10.1371/journal.pone.0021403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Field AP, Wilcox RR. Robust statistical methods: A primer for clinical psychology and experimental psychopathology researchers. Behav. Res. Ther. 2017;98:19–38. doi: 10.1016/j.brat.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 71.Wilcox, R. R. & Rousselet, G. A. A Guide to Robust Statistical Methods in Neuroscience. in Current Protocols in Neuroscience82, 8.42.1–8.42.30 (John Wiley & Sons, Inc., 2018). [DOI] [PubMed]
- 72.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- 73.R Core Team. R: A language and environment for statistical computing. (2018).
- 74.Rapacz-Leonard, A., Leonard, M., Chmielewska-Krzesińska, M., Siemieniuch, M. & Janowski, T. The oxytocin-prostaglandins pathways in the horse (Equus caballus) placenta during pregnancy, physiological parturition, and parturition with fetal membrane retention. Dryad Dataset, 10.5061/dryad.fbg79cnr4 (2019). [DOI] [PMC free article] [PubMed]
- 75.Zhang, Y. W., Davis, E. G. & Bai, J. Determination of internal control for gene expression studies in equine tissues and cell culture using quantitative RT-PCR. Veterinary Immunology and Immunopathology130 (2009). [DOI] [PubMed]
- 76.Klein C, Rutllant J, Troedsson MH. Expression stability of putative reference genes in equine endometrial, testicular, and conceptus tissues. BMC Res. Notes. 2011;4:120. doi: 10.1186/1756-0500-4-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qin Y, et al. Lipopolysaccharide Preconditioning Induces an Anti-inflammatory Phenotype in BV2 Microglia. Cell. Mol. Neurobiol. 2016;36:1269–1277. doi: 10.1007/s10571-015-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ismail, Y., Lee, H., Riordan, S. M., Grimm, M. C. & Zhang, L. The Effects of Oral and Enteric Campylobacter concisus Strains on Expression of TLR4, MD-2, TLR2, TLR5 and COX-2 in HT-29 Cells. PLoS One8 (2013). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analysed during the current study is available from Dryad Digital Repository (10.5061/dryad.fbg79cnr4)74.