Abstract
Members of the COG0523 subfamily of candidate GTPase metallochaperones function in bacterial transition-metal homeostasis, but the nature of the cognate metal, mechanism of metal transfer, and identification of target protein(s) for metal delivery remain open questions. Here, we explore the multifunctionality of members of the subfamily linked to delivering ZnII to apoprotein targets under conditions of host-imposed transition-metal depletion. We examine two zinc-uptake repressor (Zur)-regulated COG0523 family members, each from a major human pathogen, Acinetobacter baumannii (AbZigA) and Staphylococcus aureus (SaZigA), in an effort to develop a model for ZnII metallochaperone activity. ZnII chelator competition experiments reveal one high-affinity (KZn1 ≈ 1010–1011 M−1) metal-binding site in each GTPase, while AbZigA and SaZigA are characterized by an additional one and two (lower-affinity) metal-binding sites, respectively. CoII titrations reveal that both metallochaperones have similar electronic absorption characteristics that indicate the presence of two tetrahedral metal coordination sites. High-affinity metal binding at the CXCC motif activates the GTPase activity of both enzymes, with ZnII more effective than CoII. Both GTPases bind the product, GDP, more tightly in the apoprotein than the ZnII-bound state and exhibit what is best described as a “locked” conformation around the GTP substrate. Negative thermodynamic linkage is observed between nucleotide binding and metal binding, leading to a new mechanistic model for COG0523-catalyzed metal delivery.
Graphical Abstract
INTRODUCTION
Nutritional Immunity.
Upon infection by bacterial pathogens, the vertebrate host restricts the availability of essential transition metals, notably ZnII, via the action of calprotectin (CP) and related S100 family calcium-activated transition-metal chelators in a process termed nutritional immunity.1,2 As such, bacteria have evolved adaptive mechanisms to overcome ZnII starvation via transcriptional derepression by the zinc uptake regulator, Zur.3-6 In Bacillus subtilis, Zur derepression occurs in a stepwise manner.5 The early stages of ZnII starvation consist of mobilizing ZnII from the ribosome through the expression of ZnII-independent ribosomal paralogues to replace ZnII-dependent ribosomal proteins.7 Additionally, ZnII is mobilized from small-molecule pools via degradation pathways.8 If insufficient, the middle stages of ZnII starvation attempt to bring more ZnII into the cell by increasing the expression of transporters. Under extreme starvation, ZnII is reallocated as essential ZnII-dependent enzymes fail and are replaced with ZnII-independent paralogues, such as the replacement of FolE by FolEB (also known as FolE2).5
Additional mechanisms to adapt to host-imposed metal restriction include the staphylopine metallophore system of Staphylococcus aureus, among other bacterial pathogens, which is capable of competing with CP for extracellular ZnII.6,9-11 In addition, genes encoding proteins from clusters of the orthologous group metallochaperone subfamily (COG0523) are sometimes Zur-regulated and induced under conditions of ZnII limitation.12 COG0523 proteins are, at least in one case, among the most abundant proteins in the cell under conditions of ZnII depletion,13 yet their physiological functions remain elusive. It is postulated that prioritized metalation of a small subset of ZnII metalloenzymes might require such a metallochaperone, but only under conditions of extreme ZnII limitation. In a similar manner, the CuI, ZnII-SOD does not require the CuI chaperone CCS in an excess of CuI.14 This subset of Zur-regulated GTPases that belong to the COG0523 subfamily are promising ZnII metallochaperone candidates.12
COG0523 Metallochaperone Family.
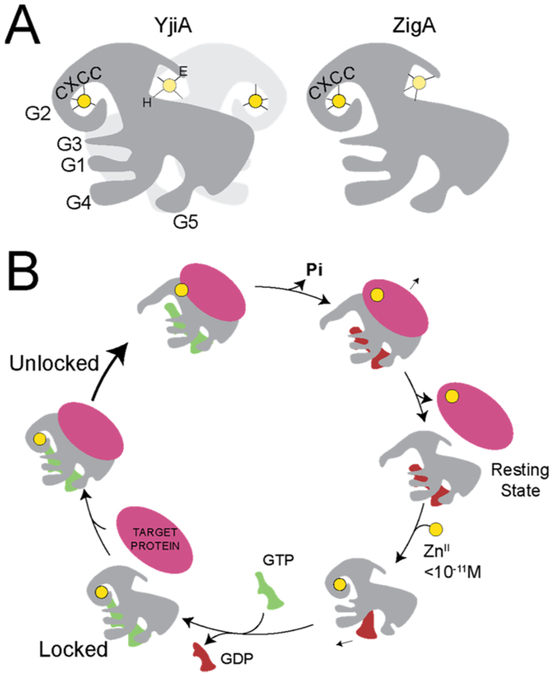
The COG0523 family of proteins belongs to the G3E GTPase superfamily, which includes MeaB, UreG, and HypB, a group of ubiquitous GTPases defined by an aspartate to glutamate (D-to-E) substitution in the G3 loop (Figure S1). These GTPases have five signature loops (G1–G5) involved in nucleotide binding and hydrolysis (Figure 1A) and consist of metallochaperones implicated in metal or metallocofactor maturation, where loops G2 and G3 act as molecular switches required for activity.15-17 Generally, G3E family members are thought not to deliver the metal directly to the target protein (Figure 1B,i) with a set of accessory proteins often required for metalloenzyme maturation. UreG and HypB indirectly activate urease and hydrogenase, respectively, by inserting NiII into an accessory complex 18-22 (Figure 1B,ii), while MeaB gates the transfer of adenosylcobalamin between methylmalonyl-CoA mutase and adenosyltransferase.23,24 While structural information available for G3E GTPases provides important details on possible mechanisms of metal or metallocofactor delivery,17,18,25-28 only one COG0523 structure, for Escherichia coli YjiA,29 exists to date. This structure provides valuable information on possible metal coordination sites; however, YjiA may not be a good model for all COG0523 enzymes because of low sequence identity beyond the conserved N-terminal G-domain 29,30 (Figure 2A).
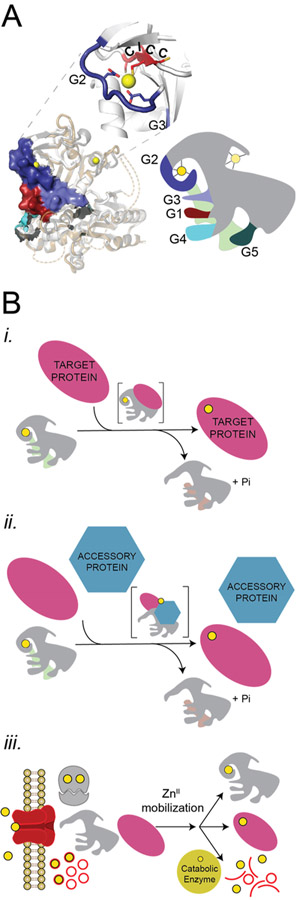
Figure 1.
Metallochaperone mechanisms of metal transfer. (A) Structural models of AbZigA (gray) and SaZigA (brown) overlaid with ZnII (yellow spheres)-bound YjiA (white; PDB 4IXM) used as a template for the modeling (left) and a generalized cartoon depiction of COG0523 members (right) with G1–G5 loops highlighted (for further details on the loops, refer to Figures S1 and S2) and bound to GTP (green, modeled in) and ZnII (yellow circles). Dashed lines represent insertions on the YjiA structure that are present in AbZigA and SaZigA. G1 (red) binds to the nucleotide phosphates, G2 (dark blue) and G3 (light blue) interact with the phosphates and MgII activating a water molecule for GTP hydrolysis, while G4 (cyan) and G5 (deep teal) help to enforce guanine nucleotide specificity. (B) (i) Direct ZnII transfer between metallochaperone and client apoprotein. (ii) Indirect ZnII transfer between metallochaperone, accessory protein(s) and client apoprotein. Metallochaperone directly interacts with the metal, and the accessory protein may or may not interact with the metal. (iii) Generalized cellular ZnII mobilization model, where metallochaperone activates trafficking of ZnII across the plasma membrane, from a small-molecule pool or from the ribosome so that a client apoprotein(s) can access the metal. The transporter and small-molecule pool are depicted as red shapes and lines, respectively, and the ribosome is depicted as gray.
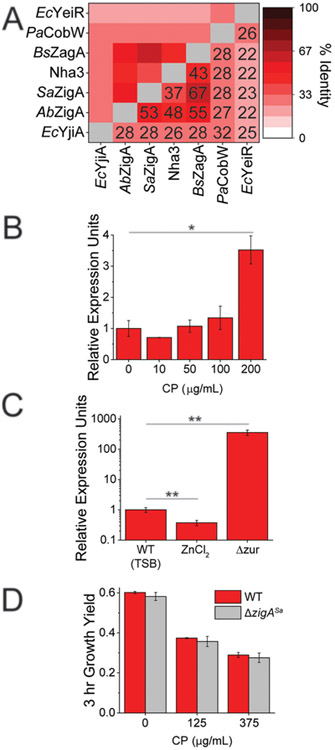
Figure 2.
Sequence and functional analysis of AbZigA and SaZigA. (A) Pairwise identity of characterized COG0523 GTPases. The numbers inside the boxes represent the percentage identity. (B) Expression of the zigASa gene with increasing amounts of CP added to the growth medium as measured by qRT-PCR. (C) Expression of zigASa in the WT strain under conditions of ZnII toxicity (200 μM ZnCl2), relative to growth in a TSB medium versus constitutive expression of zigASa in the Δzur strain by qRT-PCR (D) 3 h growth yields (OD at 600 nm) of WT S. aureus strain Newman and ΔzigASa with increasing concentrations of CP added to the growth media. (*) p < 0.05 and (**) p < 0.01 as determined by Student’s t test determined by quadruplicate (panel B) or triplicate (panel C) measurements.
Mechanistically, metallochaperones can function in one of four potential mechanisms to enhance or maintain the metalation status of the proteome. They can act directly by inserting metal into a client apoprotein (Figure 1B,i),31,32 indirectly by inserting metal into a client apoprotein as part of an accessory complex (as in UreG and HypB)21,22 (Figure 1B,ii), indirectly by gating the transfer of metal between an additional metal-bound chaperone and a client apoprotein (as in MeaB, analogous to Figure 1B,ii with a metallochaperone that does not contain a metal or a metallocofactor binding site),23,24 or indirectly by gating metal transport into the bulk cytoplasm from either transport across the plasma membrane from ribosomal stores5,7,33 or from the breakdown of potential small-molecule chelators, e.g., histidine,8 in cells (Figure 1B,iii). With no unambiguously identified COG0523 metallochaperone client protein described as yet, mechanistic details remain unknown.
Biological Functions of COG0523 Family GTPases.
The biological functions of COG0523 GTPases are not fully understood. COG0523 GTPases are distinct relative to other G3E family GTPases in that each contains a CXCC (C, cysteine; X, any amino acid, often hydrophobic) metal-binding motif in the β5 strand (Figure S1), yet the cognate metal is not necessarily the same for all of the family members. Non-Zur-regulated members are linked to cobalamin biosynthesis34 and Fe-type nitrile hydratase activation,35 while the subset of Zur-regulated members is linked to ZnII homeostasis. Characterized Zur-regulated COG0523 members include Acinetobacter baumannii Zur-induced GTPase A (AbZigA) and B. subtilis ZTP-activated GTPase A (BsZagA), a close AbZigA homologue formerly known as YciC.33 Although linked to ZnII homeostasis through Zur regulation, the genomic context of COG0523 members is often not informative, and definitive partner target proteins (see Figure 1B) remain undefined. AbZigA has been linked to both mobilization of ZnII through histidine catabolism and ZnII-dependent de novo flavin biosynthesis, yet thus far, there is no physical interaction described between AbZigA and a client apoenzyme.13 Recently, a bacterial two-hybrid experiment identified an interaction between BsZagA and ZnII-dependent FolE in cells. FolE is a GTP cyclohydrolase IA that catalyzes the rate-determining step in de novo folate biosynthesis, but evidence to support ZagA-catalyzed metal transfer to FolE is not yet available.33 It is likely that the exact COG0523 function is organism-dependent, as the ZnII requirement under CP-induced starvation will vary from cell to cell.
Our previous work8 on AbZigA motivated us to characterize a closely related enzyme from another organism with distinct physiology. Because the biological importance of the metallochaperone likely differs from organism to organism, we evaluated what physical and chemical properties might be conserved. The S. aureus proteome contains three members of the COG0523 subfamily. With 53% identity and 69% similarity to AbZigA, NWMN_0417 emerges as a ZigA-like metallochaperone candidate, henceforth named SaZigA (Figure 2A). Of the characterized COG0523 members, SaZigA, AbZigA, BsZagA, and a putative nitrile hydratase activator from Rhodococcus (Nha3) exhibit the highest degree of pairwise sequence similarity and all possess ≈400 amino acids (Figures 2A and S2), considerably larger than the structurally characterized E. coli YjiA.29 Increased sequence similarity often implies similar biological functions, yet we note that these COG0523 GTPases with extended C-termini domains are diverse: some are Zur-regulated while others are not, including the Fe-type nitrile hydratase activator protein that shares the CXCC motif.35 This observation suggests that the metal specificity of these GTPases may not be the same, and ZnII, FeII, and CoII can all be considered candidate cognate metals. Thus, we aimed to better characterize the COG0523 subfamily by investigating two Zur-regulated members; AbZigA8 and SaZigA. Here, we present a comparative characterization of the metal selectivity, nucleotide hydrolysis activity, nucleotide binding, and thermodynamic linkage in order to develop a minimal mechanism of metal delivery to a target protein. Overall, we conclude that ZnII-specific binding to the CXCC motif promotes product release through allosteric inhibition and, thus, facilitates nucleotide hydrolysis on multiple turnovers. These results provide the basis for understanding the role of GTP hydrolysis in metal delivery.
RESULTS
NWNM_0417 as a ZigA-like Protein in S. aureus.
We first determined if the gene encoding SaZigA is expressed under conditions of CP-mediated ZnII depletion. As was previously shown for AbZigA, SaZigA transcription is significantly upregulated under CP treatment relative to untreated cells, as well as in the Δzur strain relative to a wild-type (WT) strain (Figure 2B,C).8 These results reveal that SaZigA functions in the response of S. aureus to CP-induced ZnII starvation and remains fully repressed under ZnII toxicity (Figure 2C). To evaluate a functional role for SaZigA in resisting the impact of CP-dependent ZnII starvation in S. aureus, we examined the growth of WT S. aureus strain Newman and the deletion of zigA (ΔzigA) strains in the presence of increasing concentrations of CP. Unlike the measurable growth phenotype observed with the ΔzigA A. baumannii strain,8 we could observe no significant growth rate or growth yield phenotype for the S. aureus ΔzigA strain under conditions of CP stress (Figure 2D). Although AbZigA and SaZigA have a high degree of sequence identity and both are induced by ZnII depletion, the lack of a growth phenotype reveals a difference in the essentiality of each gene during CP-imposed ZnII starvation. We note that the S. aureus genome encodes three candidate COG0523 genes relative to two in A. baumannii, and thus one or both may be partially redundant to SaZigA. Alternatively, the lack of a growth phenotype could be derived from CP-mediated induction of the S. aureus staphylopine metallophore biosynthesis and uptake genes 9-11 that are not known to be present in A. baumannii. Because this metallophore (KZn = 1015 M−1)6 is likely able to compete with CP (KZn = 1014 M−1)36 for extracellular ZnII, the bioavailable pool may not be as greatly perturbed in S. aureus as in A. baumannii.13,37
Metal-Binding Properties.
In order to explore the metalbinding properties for AbZigA and SaZigA, we first employed ZnII chelator competition experiments to evaluate the ZnII binding and stoichiometry for WT and variant proteins in absence at pH 7.4 (Figure 3A-D and Table 1). The data obtained for AbZigA are consistent with that published previously8 and reveal one high-affinity site that outcompetes the fluorescent ZnII chelator mag-fura-2 (mf2; Ka = 8.7 × 1011 M−1 measured previously with a higher-affinity competitor dye, quin-28), with an additional lower-affinity site (Ka = 2.6 × 107 M−1; Figure 3A).8 This binding model (2:1 ZnII/ZigA) was used because the experimental data presented here reveal that AbZigA is predominantly monomeric under these solution conditions (vide infra). SaZigA binds 3 equiv of ZnII with one high-affinity site (Figure S4) and two lower-affinity sites (Ka = 5.0 × 107 and 1.1 × 107 M−1; Figure 3B), consistent with the multiple binding sites observed in EcYjiA and EcYeiR.29,38
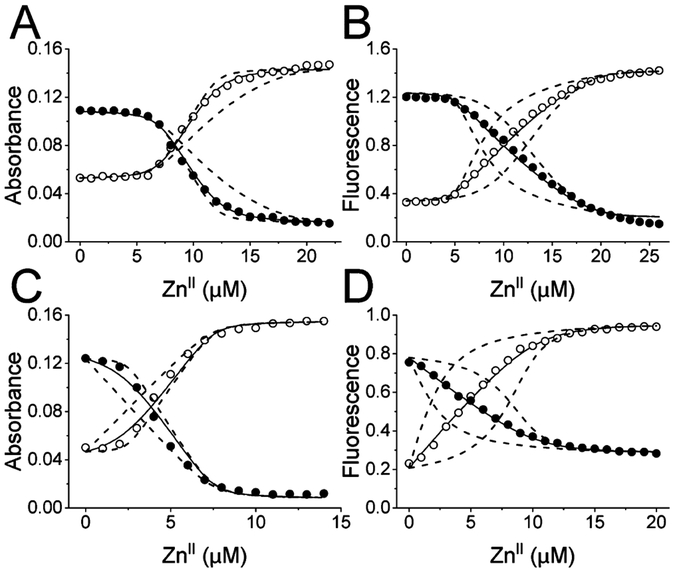
Figure 3.
Representative binding isotherms of titrations of ZnII into mixtures of (A) 7.0 μM AbZigA and 5.0 μM mf2, (B) 5.0 μM SaZigA and 5.0 μM mf2, (C) 5.0 μM SISS-AbZigA and 5.0 μM mf2, and (D) 7.5 μM SISS-SaZigA and 2.5 μM mf2. For absorbance-based experiments, filled symbols indicate absorbance at 366 nm and open symbols indicate absorbance at 324 nm, corresponding to the unmetalated and metalated mf2, respectively. For fluorescence-based experiments, the symbols indicate the emission intensity at the corresponding excitation wavelength. The solid line represents a global nonlinear, least-squares fit of duplicate experiments. The dashed lines represent simulations of 10-fold higher and lower affinities. WT AbZigA was fit to a 2:1 ZnII/AbZigA model. WT SaZigA was fit to a 3:1 ZnII/SaZigA model. SISS-AbZigA was fit to a 0.5:1 ZnII/AbZigA model. SISS-SaZigA was fit to a 2:1 ZnII/SaZigA model.
Table 1.
Metal Equilibrium Association Constants Determined from Chelator Competition Experimentsa
|
Ka (106 M−1) |
||||
|---|---|---|---|---|
| site 1 | site 2 | site 3 | ||
| ZnII | AbZigA | 870000 (±35000)b,c | 26 (±4)d | |
| SISS-AbZigA | 720 (±100)d,e | |||
| SaZigA | 13700 (±3400)b | 50 (±4)d | 12 (±1)d | |
| SISS-SaZigA | 42 (±2)d | 1.7 (±0.2)d | ||
| CoII | AbZigA | NDf | 0.03 (±0.01) | |
| SISS-AbZigA | <0.05 | |||
| SaZigA | NDf | <0.05 | ||
| SISS-SaZigA | <0.05 | |||
Globally optimized parameters from at least triplicate measurements, with standard deviations shown in parentheses. ND, not determined. Measured in 25 mM HEPES, pH 7.4, 0.15 M NaCl, 2.5 mM TCEP, chelexed buffer.
Determined by titrations with quin-2.
Obtained from ref 8.
Determined by titrations with mf2.
Determined by a 0.5:1 ZnII/AbZigA model because of poor fitting of the 1:1 model (see Figure S3).
The CoII/ZnII competition experiments could not be used to determine a CoII binding constant at site 1.
Because other non-COG0523 G3E family proteins do not possess a high-affinity (1010–1011 M−1) metal-binding site, we anticipated that the CXCC motif might define the COG0523 high-affinity metal site.27,39 To probe this specific site, variants of AbZigA and SaZigA were generated, converting CICC to SISS (S, Ser), and we again performed mf2 competition experiments. SISS-AbZigA has completely lost the high-affinity site, leaving only a lower-affinity site that appears to bind substoichiometric ZnII (Figures 3C and S3C). Substitution of the CXCC motif in the SISS-SaZigA variant also eliminates the high-affinity site but does not result in perturbation of the affinities of the remaining two lower-affinity sites (Figure 3D and Table 1). These data confirm that the CXCC motif is the high-affinity ZnII binding site in AbZigA and SaZigA, and we speculate that this is also the case for the high-affinity sites previously observed in EcYjiA and EcYeiR.29,38
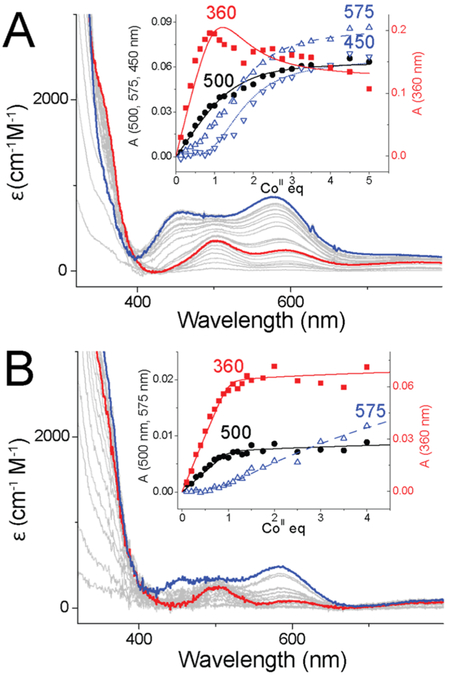
The low degree of sequence similarity among the ZigA-like proteins studied here and EcYjiA, coupled with limited structural information around the CXCC site (Figures S1 and S2), makes it difficult to predict the metal coordination sites in AbZigA and SaZigA. We therefore performed CoII titrations of WT and SISS variants to investigate the selectivity between CoII and ZnII as well as the first coordination shell of ligands around the CoII d7 ion because the resulting CoII electronic spectrum is a sensitive function of the coordination number and geometry. These spectra reveal that AbZigA and SaZigA harbor one Cys-containing high-affinity tetrahedral site [d–d transition envelope at 500 nm and ligand-to-metal charge transfer (LMCT) with ε ≈ 2000 M−1 cm−1 at 360 nm] and one lower-affinity tetrahedral site that lacks thiolate ligands (d–d transition at 600 nm; Figure 4). This assignment of the absorption bands was confirmed by CoII titrations of the SISS variants, where the d–d transition envelope centered at 500 nm is not present and the LMCT absorption between 300 and 400 nm is greatly decreased (Figure S5). The CoII affinities for the second site could be fit by direct titrations (Table 1; Ka = 3.3 × 104 M−1 for AbZigA and Ka ≤ 5 × 103 M−1 for SaZigA), both of which are ≈ 100-fold lower than the ZnII affinity for the same site. Competition with ZnII for WT proteins gives rise to an increase in the occupancy of the Cys-free site and a decrease in the absorption bands that correspond to the Cys-to-metal LMCT, suggesting a higher ZnII affinity for the Cys-containing site (Figure S6). Overall, the CoII titration experiments provide evidence for two tetrahedral or pseudotetrahedral sites with distinct affinities and confirm that the high-affinity site contains at least one Cys ligand and that both sites bind ZnII preferentially over CoII.40
Figure 4.
Titrations of CoII into (A) 100 μM apoprotein AbZigA and (B) 25 μM apoprotein SaZigA. Electronic absorption bands at 360 and 500 nm correspond to the first metal-binding event, while absorption bands at 450 and 575 nm correspond to the second metal-binding event. Insets: Changes in the absorbance as a function of the CoII concentration. The solid lines represent a nonlinear, least-squares fit to a 2:1 CoII/ZigA binding model. Red lines indicate spectra obtained with 1 equiv of CoII added, while blue lines indicate that obtained with 4 equiv of CoII.
Given the high sequence similarity of AbZigA and SaZigA with the Fe-type nitrile hydratase activator (Nha3)41 (Figures 2A and S2) and the fact that ΔzigAAb exacerbates both ZnII and FeII starvation induced by CP,13 we explored the possibility that these GTPases also bind FeII. We used the same ZnII chelator, which, given its lower affinity for FeII, would allow us to evaluate FeII binding affinities in the 104–106 M−1 affinity range. However, neither AbZigA nor SaZigA binds FeII tightly enough to outcompete the chelator, providing a lower limit of KFe ≥ 104 M−1 (Figure S7). Thus, we predict that none of these metal sites are expected to bind FeII under typical cellular conditions.42
GTPase Activity in the Absence of a Target Protein.
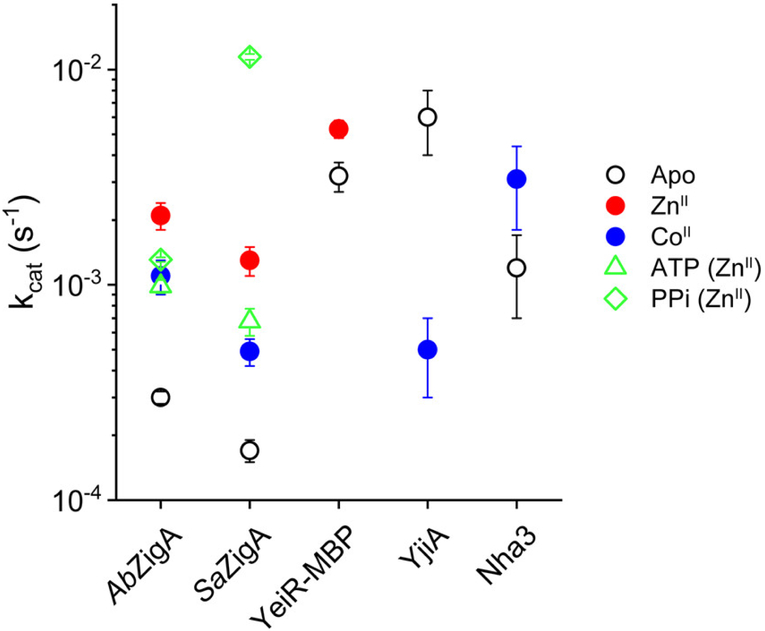
We next measured the GTPase activity of SaZigA in the metal-free and different metalation states and compared these data to AbZigA,8 as well as previously published data for other G3E GTPases.29,38,41 Like AbZigA, SaZigA has intrinsically low GTPase activity in the apoprotein state that is stimulated by ZnII. ZnII-bound SaZigA has a slightly lower kcat compared to AbZigA (Table S1 and Figure 5A,B). This low intrinsic activity of the apoprotein state is consistent with all characterized members of the COG0523 subfamily (Figure 6). The putative role of AbZigA and SaZigA as ZnII-chaperones suggests that the metal-induced GTPase activation would be ZnII-specific. Thus, we studied the activity of AbZigA and SaZigA in the presence of CoII to understand if the metal activation was specific for ZnII. Both CoII-bound GTPases exhibit enhanced activity relative to the apoprotein state but not to the extent of the ZnII-bound state (Table S1 and Figure 5A,B).
Figure 5.
Steady-state GTPase activities of WT and variant ZigA members. (A) AbZigA and (B) SaZigA GTPase activity in the apoprotein (open circles and dot-dashed black line) and CoII-bound (blue circles, dashed blue line) and ZnII-bound (red circles, solid red line) states. (C) SISS-AbZigA and (D) SISS-SaZigA GTPase activity in the apoprotein (open circles, dot-dashed black line) and ZnII-bound (red circles, solid red line) states. Lines represent fittings to the Michaelis–Menten equation, and the parameter values from these fitted curves are compiled in Figure 6 and Table 1.
Figure 6.
Comparison of the GTPase (circles), ATPase (triangles), and pyrophosphatase (diamonds) activities of AbZigA and SaZigA in the absence and presence of the indicated metal ion, compared to published values for other COG0523 members (EcYeiR-MBP,38 EcYjiA,29 and Nha341).
In order to obtain an understanding of the structural basis of this metal-induced activation, we next evaluated the role of the CXCC motif in activation of the GTPase activity by determining the GTPase activities of the SISS variants. SISS-AbZigA has similar GTPase activities in both the apoprotein and metal-bound states, suggesting that a coordination event that includes one or more of the cysteine residues in the CXCC motif is the likely origin of the metal-induced activation (Table S1 and Figure 5C). Surprisingly, metal-free SISS-AbZigA has catalytic properties of the WT ZnII-bound state, instead of that of the metal-free WT protein, although ZnII leads to a small decrease in Km as well (Table S1 and Figure 5C; see Methods). One possible explanation is that SISS substitution mimics some structural characteristics of the ZnII-bound state and, thus, has enhanced catalytic activity. Like SISS-AbZigA, metal-free SISS-SaZigA has activity more similar to that of the metal-bound WT enzyme rather than the metal-free state; however, substitution of the CXCC motif again leads to a loss of ZnII-stimulated activity (Table S1 and Figure 5D), with SISS-SaZigA characterized by a lower specific activity in the presence of ZnII. In any case, a loss of the high affinity CXCC site clearly eliminates ZnII-induced activation of the GTPase activity and effectively uncouples these two activities.
The low degree of conservation on the G4 and G5 loops (Table S1 and Figure S1A) might suggest that AbZigA and SaZigA are not necessarily specific for GTP relative to other NTPs. We therefore investigated the nucleotide specificity of both enzymes by evaluating hydrolysis of adenosine triphosphate (ATP) as well as inorganic pyrophosphate, PPi, by quantifying the release of inorganic phosphate, as with GTP. Both AbZigA and SaZigA show no detectable hydrolysis of ATP in the metal-free state (data not shown). However, the addition of ZnII results in an ATP hydrolysis rate that is just ≈2-fold lower in kcat relative to GTP, suggesting only a slight preference for GTP over ATP under these conditions (Table S1 and Figure 6). ZnII-stimulated PPi hydrolysis shows maximal activity of SaZigA, with 10-fold higher activity compared to the ZnII-stimulated GTPase activity. Together, these data suggest that GTP may not be the only physiological substrate for ZigA-like GTPases.
Nucleotide Binding.
Our results provide support for the conclusion that metal-dependent GTPase activation of AbZigA and SaZigA requires metal binding to the CXCC motif. It is likely that allosteric activation of GTP hydrolysis is due to thermodynamic coupling between the metal and nucleotidebinding events. We next studied the effect of metal binding on nucleotide binding to AbZigA and SaZigA.
We first used 2′ (or 3′)-O-[N-methylanthraniloyl (mant)]-derivatized substrate GTP and product GDP as probes of nucleotide binding because the mant fluorescence increases when bound to protein (Figure 7 and Table 2). Surprisingly, direct titrations of each show very little allosteric activation by metal for either enzyme. This observation prompted us to investigate the kinetics of nucleotide-mediated mant-GTP or mant-GDP dissociation to ensure that these titrations represent equilibrium titrations. Dissociation of mant-GTP by unlabeled GTP is very slow, requiring more than 5 h for complete dissociation with a 100-fold excess of unlabeled GTP; in contrast, GDP-mediated dissociation of mant-GDP is rapid (Figures 8A and S8A). In addition, GDP-dependent displacement of mant-GTP appears far weaker than anticipated on the basis of their relative apparent affinities, suggesting that these are not equilibrium measurements (Figures 8B and S8B). These results suggest that the substrate GTP is kinetically trapped by the enzyme, unlike the product GDP. Moreover, experiments with an AbZigA variant that is able to bind but not hydrolyze GTP43 (a Walker A mutant, K25R) show slow kinetics of dissociation of mant-GTP by GDP, and the GDP-displacement titrations are even more perturbed relative to what is anticipated on the basis of equilibrium values (Figure S8A,B). These data suggest that the slow hydrolysis of mant-GTP to mant-GDP by WT AbZigA may be at least partly responsible for the slow decrease in the mant-GTP fluorescence by unlabeled GTP or GDP (Figure S8A,B).
Figure 7.
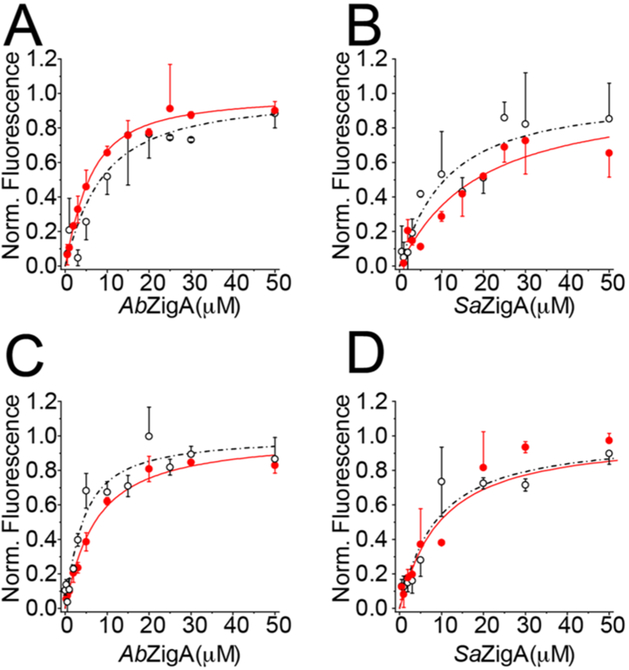
mant-GTP and mant-GDP binding to ZigA members. Duplicate direct titrations of (A) AbZigA and (B) SaZigA into 0.75 μM mant-GTP and (C) AbZigA and (D) SaZigA into 0.75 µM mant-GDP. Open symbols indicate metal-free apoprotein, fitted to a 1:1 binding model, as defined by the broken dot-dashed lines. Filled, red symbols indicate ZnII-loaded protein, fitted to a 1:1 binding model, as defined by solid red lines. Optimized parameter values for mant-GDP and GDP binding from these fits are compiled in Table 2. mant-GTP binding parameters are complicated by the slow kinetics of GTP dissociation and are thus indicative of nonequilibrium experiments (see Figure 8). Apparent Ka values for mant-GTP defined by the dot-dashed lines are 0.16 (±0.03), 0.28 (±0.03), 0.11 (±0.02), and 0.053 (±0.008) μM−1 for apoprotein- and ZnII-bound AbZigA and SaZigA, respectively.
Table 2.
Nucleotide Product Association Equilibrium Constants for ZigA Membersa
|
Ka (×106 M−1) |
|||||
|---|---|---|---|---|---|
| state | AbZigA | SISS- AbZigA |
SaZigA | SISS- SaZigA |
|
| mant-GDP | apo | 0.32 (±0.04) | 0.54 (±0.11) | 0.14 (±0.02) | 0.14 (±0.08) |
| ZnII | 0.17 (±0.01) | 1.10 (±0.20) | 0.12 (±0.05) | <0.05 | |
| GDP | apo | 10.2 (±6.1)b | 0.23 (±0.05) | >50.0 | 0.27 (±0.06) |
| ZnII | 1.4 (±0.6)b | 0.43 (±0.09) | 3.0 (±1.7) | NDc | |
Optimized parameter values are shown, with the standard deviation given in parentheses.
These values are statistically different (p < 0.1) from at least triplicate measurements.
Not determined means that it could not be determined by indirect titration due to the low affinity of mant-GDP. Conditions: 25 mM HEPES, 150 mM NaCl, 2.5 mM MgCl2, 2.5 mM TCEP, pH 7.4.
Figure 8.
(A) Time course of the apparent displacement of mant-GTP (filled circles) and mant-GDP (open circles) by unlabeled nucleotide (500 μM GTP or GDP added to 10.0 μM SaZigA; 0.75 μM mant-GTP or mant-GDP, respectively). (B) Displacement of mant-GTP via titrations of GDP (50.0 μM SaZigA and 2.0 μM mant-GTP). The solid line represents a fit of the experimental data, and the dashed line represents what is anticipated on the basis of the experimentally determined GDP binding affinity (see Table 2). (C) Displacement of mant-GDP via titrations of GDP (3.0 μM apoprotein AbZigA and 2.0 μM mant-GDP). The continuous black and dashed black lines represent fits to the experimental data and simulations of 10-fold higher and lower affinities, respectively. (D) Displacement of mant-GDP via titrations of GDP (3.0 μM ZnII-bound AbZigA and 2.0 μM mant-GDP). The continuous red and dashed red lines represent fits to the experimental data and simulations of 10-fold higher and 10-fold affinities, respectively. (E) Displacement of mant-GDP via titrations of GDP (15.0 μM apoprotein SISS-AbZigA and 2.0 μM mant-GDP). The continuous black and dashed red lines represent fits to the experimental data and the fitted data for ZnII-bound SISS AbZigA from panel F, respectively. (F) Displacement of mant-GDP via titrations of GDP (15.0 μM ZnII-bound SISS-AbZigA and 2.0 μM mant-GDP). The continuous red and dashed black lines represent fits to the experimental data and the fitted data for apoprotein SISS AbZigA from panel C, respectively. Parameters from these fitted curves are compiled in Table 2.
To avoid complications of kinetic trapping of the substrate mant-GTP, we used a competition strategy to elucidate equilibrium binding constants by dissociating the product mant-GDP with unlabeled GDP in the presence and absence of ZnII for both ZigAs (Figures 8C,D and S8A,B). Strikingly, both AbZigA and SaZigA bind the GDP product with much higher affinity in the absence of ZnII. The addition of ZnII results in ≈10-fold weaker GDP affinities for both AbZigA and SaZigA compared to those of the metal-free GTPase members. The nucleotide specificity was also probed through ATP binding, but up to 1 mM ATP is unable to displace mant-GDP so these binding constants could not be quantified (data not shown, Ka < 1 × 104 M−1).
To understand the effect of the CXCC metal-binding site on the thermodynamic coupling to nucleotide binding, we returned to the SISS variants. In this case, both apoprotein- and ZnII-bound SISS-AbZigA have low and comparable GDP affinities, consistent with their comparable GTPase activities (Figure 8E,F). Metal-free SISS-SaZigA, on the other hand, also binds GDP weakly, while the functionally inactive ZnII-bound form does not show significant binding to any tested nucleotides (Figure S8C and Table 2). Overall, these findings show that substitution of the CXCC motif completely disrupts or inverts the thermodynamic linkage between the metal binding and nucleotide binding/hydrolysis observed in the WT enzymes. We conclude that the high-affinity CXCC site activates steady-state GTPase activity primarily by enhancing product GDP release by lowering its equilibrium affinity for the enzyme.
Oligomerization State and Global Conformational Changes.
The structural comparison between the COG0523 structure and other G3E GTPase members suggests that the C-terminal domain creates a significant perturbation of the dimerization interface observed in the structure of homodimeric YjiA (Figures S1 and S9). It has previously been shown that G3E GTPase members exist in a monomer–dimer equilibrium that is impacted by metal and nucleotide binding, as well as an interaction with a partner protein.8,25 Structural predictions of AbZigA and SaZigA suggest that a conserved insertion between the β7 strand and α5 helix potentially interferes in an EcYjiA-like dimerization process (Figures 1A and S9). However, AbZigA in the unligated state was previously suggested to exist in a monomer–dimer equilibrium, with the presence of nucleotide displacing this equilibrium to that of an asymmetric monomer (observed apparent molecular weight of 66 kDa vs a predicted value of 46 kDa).8
In order to further investigate the oligomeric state and hydrodynamic properties of AbZigA and SaZigA as a function of the ligation (metal, nucleotide) state, we performed a series of gel filtration (Figure S10) and small-angle X-ray scattering (SAXS) experiments (Figures S11 and S12). The gel filtration experiments with AbZigA and SaZigA strongly suggest that both proteins exist predominantly as asymmetric monomers over a 20-fold range in the protein concentration. In these experiments, the previously described dimeric assembly state was not observed.8 Moreover, the SAXS data show that the radii of gyration of the various liganded states of the proteins do not differ significantly from one another (Table 3), supporting the conclusion that both ZigA members are predominantly or exclusively monomeric when bound to either or both nucleotide and metal (Figures S11 and S12). These findings provide further structural insights into allosteric activation of these GTPase members as a result of transition-metal binding to the CXCC site.
Table 3.
Hydrodynamic Parameters Obtained from Analysis of the SAXS Data for SaZigA and AbZigAa
| protein | state | RG(Guinier) Å | RG(GNOM) Å |
|---|---|---|---|
| SaZigA | apo | 24 (±1) | 30.7 (±0.4) |
| ZnII | ND | 32.8 (±0.2) | |
| GDPNP | 27 (±1) | 32.1 (±0.4) | |
| GDPNP + ZnII | 25 (±1) | 29.9 (±0.5) | |
| GDP | 25 (±1) | 31.3 (±0.4) | |
| GDP + ZnII | 27 (±1) | 32.1 (±0.1) | |
| AbZigA | apo | 30 (±1) | 28.9 (±0.1) |
ND, not determined. The optimized value is shown with the standard deviation provided in parentheses. The theoretical monomer molecular weight is 45.0 kDa for SaZigA and 46.1 kDa for AbZigA. The average molecular weight from GNOM is 75.8 kDa for SaZigA and 69.5 kDa for AbZigA, while the average molecular weights from the linear region of the Guinier plot are 47.0 kDa for SaZigA and 70.3 kDa for AbZigA.44 These findings are consistent with the results from size-exclusion chromatography (Figure S10), which reveals an apparent molecular weight of ≈68 kDa, or consistent with a nonspherical or elongated monomeric particle.
DISCUSSION
Model for Metal Insertion by a COG0523 Metallochaperone.
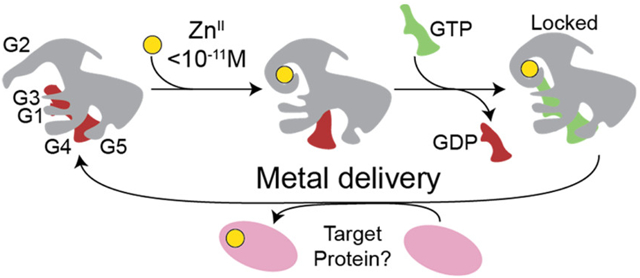
A subset of the COG0523 subfamily proteins have been described as ZnII metallochaperones responsible for metalating high-priority ZnII-dependent enzymes under conditions of severe ZnII restriction. Here, Zur-regulated AbZigA and SaZigA have been used to further elucidate the fundamental features of two closely related COG0523 proteins, thus providing new insights into the molecular mechanism of metal insertion into a target apoprotein. Both GTPase members preferentially bind ZnII with one high-affinity, tetrahedral site at the CXCC motif (Figures 3 and S4) and a second site devoid of thiolate ligation located elsewhere. This high-affinity site is additionally identified as the site involved in metal-stimulated activation of the GTPase activity (Figure 5). Moreover, nucleotide binding is thermodynamically coupled to the occupancy of this site because ZnII binding to the CXCC motif results in a lower GDP affinity compared to that of the apoprotein (Figure 8C,D). A loss of the high-affinity CXCC site abrogates the metal-dependent activation of the GTPase activity and the metal-dependent change in the GDP affinity. Additionally, these larger COG0523 proteins with extended C termini are likely functioning as monomers, whereas the structurally characterized EcYjiA exists as a homodimer. Thus, we postulate that the extended Trp-rich, C-terminal region in each case interferes with the dimerization interface (Figure S9) and may instead be used to recruit target proteins.
Combining this ZnII-activated GTP hydrolysis activity with the thermodynamic coupling of metal and nucleotide binding, we propose a mechanistic cycle of metal transfer to a target apoprotein from metallochaperone (Figure 9). Under ZnII-restricted conditions where AbZigA and SaZigA are operating in the cell, we hypothesize that the resting state of the enzyme is a GDP-bound state with no metal. Once bioavailable ZnII is coordinated to the CXCC motif (Figure 3), allosteric inhibition of GDP binding observed here will facilitate GDP release by mass action to allow for binding of the nucleotide substrate, GTP (Figure 8C,D and Table 2). Although ZnII-bound metallochaperone exhibits higher GTPase activity relative to the apoenzyme, the enzyme will turn over very slowly in the absence of a client protein. Additionally, the kinetic trapping of GTP observed here supports the idea that the enzyme will be “locked” into this conformation until it interacts with a target apoprotein (Figure 8A). Upon interaction with a target protein, a conformational change is likely to occur to allow for enhanced GTPase activity and metal insertion from the CXCC motif into the apoprotein substrate. After hydrolysis and metal delivery, metallochaperone will dissociate from its now metalated target protein, and the cycle restarts from the GDP-bound, apoenzyme state.
Figure 9.
Proposed metallochaperone mechanism. (A) Schematic representation of the structures of YjiA and ZigA (representative of both AbZigA and SaZigA; see Figure S1 for actual structures) with the approximate positions of the five loops (G1–G5) shown, along with bound metals at the CXCC and interfacial sites shown (yellow, ZnII), and the approximate position of GTP (green object). Note that both AbZigA and SaZigA are illustrated as monomeric on the basis of extensive hydrodynamic experiments over a wide range of ligation states presented here (Table 3 and Figures S10-S12). (B) Proposed metallochaperone catalytic cycle consistent with the data presented here. Magenta, client protein; other symbols as in panel A.
These data taken collectively reveal that Zur-regulated COG0523 proteins like AbZigA and SaZigA share a similar mechanism of Zn-dependent activation of GTP hydrolysis. This ZnII-induced GTPase activity of AbZigA, SaZigA, and other Zur-regulated COG0523 GTPase members supports the contention that these enzymes function as ZnII chaperones (see Figure 1B), while differentially regulated members of the family may function with different metals under different physiological conditions. For example, the GTPase activity of E. coli YjiA is inhibited by metal binding (CoII, NiII, and ZnII), while that of TG328-2 Rhodococcus equi Nha3 is slightly activated by metal (CoII;29,41 Figure 6). Moreover, E. coli YeiR also shows ZnII-stimulated GTPase activity,38 although yeiR is not Zur-regulated and the effect is smaller than those for AbZigA and SaZigA (Figure 6). Additionally, other G3E members (HypB of E. coli) show decreased GTPase activity in the presence of metal compared to the apoprotein when binding either cognate metal (NiII) or noncognate metal (ZnII).45 Because G3E family proteins have been shown to require one or more partner proteins to become maximally active in the presence of additional target proteins,25,45,46 the intrinsically low GTPase activity of COG0523 GTPase members suggests that a partner protein(s) will stimulate hydrolysis in a similar way. We note that these affinities for the GDP product are 1000-fold larger than the Km values (Tables 2 and S1), providing additional support for a kinetic model of the GTPase activity that is limited by “trapping” of the bound GTP, yielding extremely slow enzymatic turnover in the absence of a target protein. It is interesting to put this finding in the context of the differences between ATPases and GTPases. Unlike ATPases, GTPases typically function as molecular switches in cellular processes, and GTP hydrolysis and product dissociation is often facilitated by accessory proteins such as GTPase-activating proteins (GAP) or guanine nucleotide exchange factors (GEFs), respectively.47 In this case, the metal-induced allosteric activation of AbZigA and SaZigA suggests that ZnII itself may function as a GEF to exchange GDP for GTP, while also providing a metal cofactor for metalation of an apoenzyme target. This proposal is consistent with ZnII coordination adjacent to the G2 motif, which defines the switch 1 (Walker A) region that is known to stabilize the transition state necessary for GTP hydrolysis in other P-loop GTPases.15,16
Nucleotide Specificity and Hydrolysis.
Although classically annotated as GTPases, the nucleotide specificity and functional impact of NTP hydrolysis of COG0523 proteins are not yet fully defined. Here, we report weak ATP binding affinity, although ATP hydrolysis is only ≈2-fold lower than the steady-state rates of GTP hydrolysis, which suggests that ATP may not be kinetically trapped in the same way as GTP (Figure 6). In addition, more recent work reveals that BsZagA is capable of hydrolyzing both the alarmone ZTP [5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) triphosphate] and GTP at similar rates,17 while EcYjiA was pulled down from cell lysates through binding of a probe that harbors a second alarmone, (p)ppGpp,48 as part of the global nutrient stress response. The observation that COG0523 family members are activated by an alarmone further suggests an alternative regulatory model in which nucleotide hydrolysis may not be critical for metallochaperone activity. In this model, simple binding of a specific nucleotide, which itself serves as a proxy for the cellular status of a particular metabolite, will be capable of catalyzing metal delivery into a cognate target protein; this hypothesis can be directly tested with nonhydrolyzable nucleotide analogues once a specific target protein is identified. It is unknown if either AbZigA or SaZigA binds or hydrolyzes alarmones, although it is important to recognize that AbZigA is unable to substitute for the ZTP-binding BsZagA in support of cellular folate biosynthesis.33 This inability suggests that binding of ZTP might not activate AbZigA or that AbZigA simply functions in a different metabolic pathway, e.g., flavin biosynthesis.33 It is interesting to note that the committed step of both de novo folate and flavin biosynthesis is catalyzed by a ZnII metalloenzyme (FolE and RibA, respectively), each of which opens up the purine ring of GTP.
Coevolution of Cognate Zinc Metalloenzyme–COG0523 Pairs?
Most of what we know about the COG0523 family metallochaperones and their interactions with other proteins is focused on obligatorily ZnII-dependent enzymes that are paired with Zur-regulated, non-ZnII-dependent paralogues in cells. For example, cellular interaction between BsZagA and BsFolE also occurs for the Acinetobacter baylyi homologues, and both A. baylyi and B. subtilis express a Zur-regulated, non-ZnII-dependent paralogue, FolEB. In contrast, A. baumannii does not encode a back-up FolEB, AbZigA and AbFolE do not interact, and neither AbZigA nor BsFolE forms a noncognate complex in B. subtilis cells.33 The S. aureus genome, on the other hand, uses only a non-ZnII-dependent paralogue, FolEB, to drive folate biosynthesis and lacks FolE altogether. Consistent with this observation, our previous work connects AbZigA to de novo flavin biosynthesis rather than folate biosynthesis, while quantitative proteomic experiments implicate other possible proteins as AbZigA client proteins, including RibA and QueD (QueD2), with the latter involved in queuosine–tRNA biosynthesis.13
We find it fascinating that FolE, FolEB, QueD, and QueD2 all belong to the oligomeric tunnel (T)-fold metalloenzyme superfamily.49 This structural similarity suggests the possibility that a specific T-fold family member(s) may have evolved in a common cytoplasm alongside a specific COG0523 protein to enable rescue when that enzyme starts to fail as a result of severe ZnII restriction. Although structural and functional similarities might provide additional information on possible apoprotein partners, it is critical to recognize that the bona fide partner(s) of a specific COG0523 protein is likely to be organism-specific. Indeed, the S. aureus metal starvation response is not likely connected to histidine catabolism because the hut (histidine utilization) gene cluster is repressed under CP treatment,50 contrasting with findings in A. baumannii; these data suggest that the function of SaZigA is likely to be altogether different from those of both AbZigA and BsZagA. Taken together, the current data support our contention of significant functional diversity among COG0523 subfamily members superimposed on a common mechanistic model for apoenzyme metal insertion and cellular transition-metal reallocation that is required under conditions of severe zinc restriction.
EXPERIMENTAL SECTION
Strain Construction and Growth Conditions.
S. aureus clinical isolate Newman was used in all experiments unless stated otherwise. The S. aureus USA300 JE2 strain carrying an insertion of the bursa aurealis Tn in zigASa (NE278) was acquired from the Nebraska transposon library (Table S3).51 The Tn was then transduced into the S. aureus Newman background using Φ11. The successful integration of the Tn in zigASa was confirmed by polymerase chain reaction (PCR) using gene-specific primers AW1–27 and AW1–28 (Tables S2 and S3). S. aureus growth was performed in tryptic soy broth (TSB) supplemented with 10 μg mL−1 erythromycin if required. To assess Zur-dependent expression, WT and Δzur overnight cultures were subcultured 1:100 into fresh TSB and grown to the exponential phase (Table S3). These cultures were used to inoculate fresh TSB at OD600 = 0.05 with or without supplementation of 200 μM ZnCl2. After 2 h of growth, cultures were pelleted and stored at −80 °C for subsequent RNA isolation. CP-dependent growth and SaZigA expression was assessed in CP growth media, consisting of 60% TSB and 40% CP buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM β-mercaptoethanol, and 3 mM CaCl2). Briefly, cultures were grown overnight in TSB, used to seed (1:100) fresh CP media, and grown for 3 h before inoculation of fresh CP media with or without CP at OD600 = 0.05. For RNA isolation, exponentially growing cultures (3 h) were harvested by centrifugation and pellets stored at −80 °C.
Quantitative Reverse Transcription PCR (RT-qPCR).
Gene expression was monitored by RT-qPCR. Briefly, RNA was isolated from bacterial cultures using an RNeasy kit (Qiagen). gDNA was depleted by employing the TURBO DNA-free kit (Invitrogen) and cDNA generated using the iScript cDNA synthesis kit (Bio-Rad). Subsequently, qPCR was performed using iQ SYBR Green Supermix (Bio-Rad) and the Bio-Rad CFX96 Real-Time system. Transcript quantification of SaZigA was performed using primer pairs AW1-42/43 (Table S2). 16S rRNA was used as a reference and amplified using primers 16S rRNA-F and 16S rRNA-R (Table S2). At least three biological replicates were included in each experiment.
Structural Modeling.
The structural models of AbZigA and SaZigA were built using YjiA (4IXM) as the template using Swiss-Model (https://swissmodel.expasy.org/interactive) running with default settings.
Protein Purification.
AbZigA was purified as previously described.8 SaZigA was cloned into the NdeI and BamHI sites in the pHis vector (primers listed in Table S2) via ligation. E. coli BL21(DE3) cells transformed with the SaZigA-containing pHis vector were grown in LB media with 100 μg mL−1 ampicillin at 37 °C to OD600 = 0.6. Protein expression was initiated with the addition of 1 mM isopropyl β-D-1-thiogalactopyranoside, the temperature was lowered to 18 °C, and the cells were allowed to grow for 14 h. Cells were harvested by centrifugation at 5000 rpm for 15 min at 4 °C and resuspended in 25 mM Tris (pH 8.0), 500 mM NaCl, 2.5 mM ethylenediaminetetraacetic acid (EDTA), and 5 mM tris(2-carboxyethyl)phosphine (TCEP). Cells were lysed by sonication for 15 min, and the resulting lysate was centrifuged at 10000 rpm for 20 min at 4 °C to remove cellular debris. The resulting supernatant was treated with 0.015% poly(ethyleneimene) on ice to precipitate nucleic acids, which were removed by centrifugation at 10000 rpm for 20 min at 4 °C. The supernatant was treated with 70% ammonium sulfate on ice for 30 min to precipitate SaZigA. The mixture was separated by centrifugation at 10000 rpm for 20 min at 4 °C, and the resulting pellet containing SaZigA was resuspended in 25 mM Tris (pH 8.0), 150 mM NaCl, 2.5 mM EDTA, and 5 mM TCEP. This solution was dialyzed into 25 mM Tris (pH 8.0), 50 mM NaCl, 2.5 mM EDTA, and 5 mM TCEP overnight, injected onto a preequilibrated Sepharose Q column, and eluted with a gradient from 50 to 500 mM NaCl. Fractions of >90% purity were combined for additional purification by size-exclusion chromatography (G200 16/60) in 25 mM Tris (pH 8.0), 150 mM NaCl, 2.5 mM EDTA, and 5 mM TCEP. All fractions of >95% purity (by inspection of sodium dodecyl sulfate polyacrylamide gel electrophoresis gels) were collected and buffer-exchanged 106-fold into chelexed 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.4), 150 mM NaCl, and 5 mM TCEP to create the final prep of SaZigA. SaZigA prepared in this way was devoid of bound metal [<0.01 mol equiv of ZnII as measured by inductively coupled plasma mass spectrometry (ICP-MS)] and contained the expected number of reduced thiols (7.0 measured; 8 expected, 87% reduced) as measured by Ellman’s reagent.52 SaZigA was stored at −80 °C until use. K25R AbZigA, SISS-AbZigA, and SISS-SaZigA were generated using a QuickChange mutagenesis protocol (Table S2). K25R AbZigA, SISS-AbZigA, and SISS-SaZigA were purified in the same way as that of SaZigA and characterized as to residual metal content and number of reduced thiols (<0.01 mol equiv of ZnII as measured by ICP-MS). Molar extinction coefficients for AbZigA and SaZigA of 63940 and 50920 M−1 cm−1 at 280 nm were used to calculate the protein concentration.
GTPase Activity.
The GTPase activity was measured by the Malachite green assay.53 Briefly, purified protein (see supplemental experimental procedures) was incubated with 0–1 mM GTP in assay buffer [25 mM HEPES, 100 mM KCl, 3 mM TCEP (pH 7.5), 2 mM MgCl2] in a volume of 90 μL. After 90 min, 35 μL of a Malachite green mixture [1.2 mL of 0.045% Malachite green in 4 N HCl (Fluka), 400 μL of 7.5% ammonium molybdate (Sigma-Aldrich), and 25.6 μL of 11% Tween 20 in 4 N HCl] was added and incubated for 3 min, and the reaction was stopped by the addition of 15 μL of 35% citric acid (Sigma) in 4 N HCl. After 30 min, the absorbance at 680 nm was correlated to the concentration of free phosphate using a standard curve (Fluka). The same method was used to measure the ATP and PPi phosphatase activity. The obtained data were fit to the Michaelis–Menten equation to calculate the kinetic parameters.
Nucleotide Binding Assays.
Experiments were performed using an ISS PC1 spectrofluorometer as well as a BioTek Synergy Neo2Microplate Reader at 25 °C. For mant-GTP experiments, λex= 360 nm and λem = 430 nm. For mant-GDP experiments, λex = 360 nm and λem = 450 nm. For time-course assays, protein and mant-GTP or mant-GDP were first incubated for 10 min in chelexed buffer [25 mM HEPES, 150 mM NaCl, and 2.5 mM TCEP (pH 7.4)]. Unlabeled nucleotide was then added at t = 0 min, and the fluorescence was monitored over time. For direct titration assays, increasing concentrations of protein were added into different wells of a 96-well plate containing 2 μM mant-GDP and 2 mM MgCl2 in chelexed buffer. For metal-bound titrations, proteins were preloaded with 5 mol equiv of metal and added to wells containing 2 μM mant-GDP, 2 mM MgCl2, and 10 μM metal in chelexed buffer. The equilibration time was 5 min before measurements. For nucleotide competition experiments, protein and mant-GDP were incubated in chelexed buffer in a 96-well plate. After 10 min, increasing concentrations of GDP were added into the different wells and incubated 10 min prior to measurements. Metal-bound experiments were done in the presence of 5 mol equiv of metal. The peak emission intensities were globally fit to a 1:1 binding model derived from the displacement of mant-GDP using Dynafit.54
mf2 ZnII and FeII Competition Assays.
Experiments were performed as described previously55,56 using an HP8453 UV–vis spectrophotometer or a ISS PC1 spectrofluorometer at 25.0 °C for ZnII experiments and monitoring the absorbance in the visible region at room temperature for FeII experiments. For fluorescence-based mf2 titrations, λex = 324 or 366 nm, while λem = 505 nm, where the emission at 505 nm increases at 324 nm and decreases at 366 nm as ZnII binds mf2. For absorbance-based mf2 titrations, the absorbance at 324 nm increases and the absorbance at 366 nm decreases when mf2 binds ZnII. Different [protein]/[mf2] values were used for replicate experiments to better calculate the stoichiometry and analyzed globally. In a typical experiment, 5 μM monomer and 2.5 μM mf2 in 2.5 mL were incubated for 10 min in chelexed buffer [25 mM HEPES, 150 mM NaCl, and 2.5 mM TCEP (pH 7.4)], and aliquots of ZnII and Feu were added. The equilibrium time was 2–5 min between subsequent additions of metal. The peak intensities at 324 and 366 nm from two or more experiments were globally fit to 3:2 or 2:1 binding models for AbZigA and 3:1 binding models for SaZigA with the KZn value of mf2 fixed to 5 × 108 M−1 and the KFe value of mf2 fixed to 1.9 × 105 M−1 using Dynafit.54 All FeII titrations were done under anaerobic conditions, with an FeII stock solution prepared by dissolving ammonium ferrous sulfate hexahydrate (Alfa Aesar) in an acidic solution in a glovebox with O2 < 5 ppm. The concentration of the FeII stock solution was verified by atomic absorption spectroscopy (PerkinElmer AAnalyst 400). In a typical experiment, 10–25 μM monomer and 10–15 μM mf2 in 300 μL were prepared in the glovebox in chelexed buffer and incubated in a capped cuvette. Aliquots of FeII were added with an equilibration time of 2–5 min between measurements.
quin-2 ZnII Competition Assays.
Experiments were performed as described previously55,56 using a ISS PC1 spectrofluorometer at 25.0 °C. ZnII was titrated into a mixture of protein and quin-2 and fluoresced (λex = 265 and λem = 490 nm) and the fluorescence monitored upon ZnII binding to quin-2. Different [protein]/[quin-2] values were used in duplicate experiments to better calculate the stoichiometry and the resulting data analyzed globally. The equilibrium time was 2–5 min between subsequent additions of metal. The peak intensity at 490 nm was globally fit to a 1:1 binding model for SaZigA, with the KZn value of quin-2 fixed to 2 × 1011 M−1 using Dynafit.54
CoII Titrations.
Proteins were titrated with increasing molar equivalents of CoII, with monitoring of the absorbance in the visible region. After 4 equiv, ZnII was added to outcompete the bound CoII. Spectra were corrected to account for additional scattering upon the addition of metal.57
Analytical Gel Filtration Chromatography.
A Superdex 300 (Sepax SRT-10, 10 × 300 mm, 10 μm particle size) GL column was run in buffer [25 mM HEPES, 150 mM NaCl, and 5 mM TCEP (pH 7.4)] at a flow rate of 0.5 mL min−1 and calibrated with the Gel Filtration Calibration Kit HMW (GE Healthcare). A linear-fit plot of Kav versus log Mr gave R2 = 0.99. Varying concentrations of protein (5–100 μM) were incubated with ZnII on ice for 1 h, and 100 μL samples were injected onto a preequilibrated column.
SAXS Experiments.
SAXS data of the apoprotein, ZnII, and nucleotide-bound states of AbZigA and SaZigA were collected at three different protein concentrations [5 mg mL−1 (110 μM), 2 mg mL−1 (44 μM), and 1 mg mL−1 (22 μM)] in buffer [25 mM HEPES, 150 mM NaCl, and 5 mM TCEP (pH 7.4)]. The AbZigA and SaZigA apoprotein samples were devoid of bound metal (<0.01 mol equiv of ZnII as measured by ICP-MS). To prepare ZnII samples, 150 μM SaZigA was preloaded with ZnII and buffer-exchanged into buffer containing 10 μM ZnII. To prepare nucleotide-loaded samples, 300 μM SaZigA was buffer-exchanged into buffer containing 0.5 mM nucleotide. For each protein concentration and matching background buffer, 30 images were collected and averaged using the NCI-SAXS program package. The scattering profile at each concentration was manually adjusted with the scale factor to remove the effect of the concentration prior to subtraction of the scattering profile of the buffer. Scattering profiles of each protein concentration were then merged for further analysis. The Guinier region was plotted as ln I(q) versus q2 to confirm the monodispersity of the sample and to obtain I0 and the radius of gyration (Rg) within the range of qmaxRg < 1.3. The compaction of each state was estimated using the Kratky plot for q < 0.3 Å−. Scattering profiles were then Fourier-transformed using GNOM of the ATSAS package to obtain the normalized pairwise distance distribution function and an independent measure of the molecular weight and Rg.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge support from the U.S. National Institutes of Health from Grant R01 AI101171 (to E.P.S. and D.P.G.) and R35 GM118157 (to D.P.G.) and the Pew Charitable Trusts and Fundación Williams (to D.A.C.). M.R.J. acknowledges receipt of a predoctoral fellowship from the Quantitative and Chemical Biology Training Program at Indiana University (T32 GM109825). A.W. gratefully acknowledges support from an American Heart Association Postdoctoral Fellowship (18POST33990262). We also thank Yu-Chen Huang for her help with protein purification and Dr. Lixin Fan of the Small-Angle X-ray Scattering Core Facility, National Cancer Institute, Frederick, MD, for acquiring the SAXS data.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.inorgchem.9b01173.
Supplementary Figures S1-S12 and Tables S1-S3 (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Zygiel EM; Nolan EM Transition Metal Sequestration by the Host-Defense Protein Calprotectin. Annu. Rev. Biochem 2018, 87 (1), 621–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hood MI; Skaar EP Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol 2012, 10 (8), 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Patzer SI; Hantke K The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol 1998, 28 (6), 1199–1210. [DOI] [PubMed] [Google Scholar]
- (4).Mortensen BL; Rathi S; Chazin WJ; Skaar EP Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. J. Bacteriol 2014, 196 (14), 2616–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Shin JH; Helmann JD Molecular logic of the Zur-regulated zinc deprivation response in Bacillus subtilis. Nat. Commun 2016, 7 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Ghssein G; Brutesco C; Ouerdane L; Fojcik C; Izaute A; Wang S; Hajjar C; Lobinski R; Lemaire D; Richaud P; Voulhoux R; Espaillat A; Cava F; Pignol D; Borezée-Durant E; Arnoux P Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science 2016, 352 (6289), 1105–1109. [DOI] [PubMed] [Google Scholar]
- (7).Panina EM; Mironov AA; Gelfand MS Comparative genomics of bacterial zinc regulons: Enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. U. S. A 2003, 100 (17), 9912–9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Nairn BLL; Lonergan ZRR; Wang J; Braymer JJJ; Zhang Y; Calcutt MWW; Lisher JPP; Gilston BAA; Chazin WJJ; De Crécy-Lagard V; Giedroc DPP; Skaar EPP The Response of Acinetobacter baumannii to Zinc Starvation. Cell Host Microbe 2016, 19 (6), 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Grim KP; San Francisco B; Radin JN; Brazel EB; Kelliher JL; Párraga Solórzano PK; Kim PC; Mcdevitt CA; Kehl-Fie TE The Metallophore Staphylopine Enables Staphylococcus aureus To Compete with the Host for Zinc and Overcome Nutritional Immunity. mBio 2017, 8 (5), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Song L; Zhang Y; Chen W; Gu T; Zhang S-Y; Ji Q Proc. Natl. Acad. Sci. U. S. A 2018, 115 (15), 3942–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Fojcik C; Arnoux P; Ouerdane L; Aigle M; Alfonsi L; Borezée-Durant E Independent and cooperative regulation of staphylopine biosynthesis and trafficking by Fur and Zur. Mol. Microbiol 2018, 108, 159–177. [DOI] [PubMed] [Google Scholar]
- (12).Haas CE; Rodionov DA; Kropat J; Malasarn D; Merchant SS; de Crécy-Lagard V A subset of the diverse COG0523 family of putative metal chaperones is linked to zinc homeostasis in all kingdoms of life. BMC Genomics 2009, 10, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wang J; Lonergan ZR; Gonzalez-Gutierrez G; Nairn BL; Maxwell CN; Zhang Y; Andreini C; Karty JA; Chazin WJ; Trinidad JC; Skaar EP; Giedroc DP Multi-metal Restriction by Calprotectin Impacts De Novo Flavin Biosynthesis in Acinetobacter baumannii. Cell Chem. Biol 2019, 26, 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Rae TD; Schmidt PJ; Pufahl RA; Culotta VC; O’Halloran TV Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 1999, 284 (5415), 805–808. [DOI] [PubMed] [Google Scholar]
- (15).Martínez-Vicente M; Yim L; Villarroya M; Mellado M; Pérez-Payá E; Björk GR; Armengod ME Effects of mutagenesis in the switch I region and conserved arginines of Escherichia coli MnmE protein, a GTPase involved in tRNA modification. J. Biol. Chem 2005, 280 (35), 30660–30670. [DOI] [PubMed] [Google Scholar]
- (16).Campanello GC; Lofgren M; Yokom AL; Southworth DR; Banerjee R Switch I-dependent allosteric signaling in a G-protein chaperone–B12enzyme complex. J. Biol. Chem 2017, 292 (43), 17617–17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Lofgren M; Koutmos M; Banerjee R Autoinhibition and signaling by the switch II motif in the G-protein chaperone of a radical B12 enzyme. J. Biol. Chem 2013, 288 (43), 30980–30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Yuen MH; Fong YH; Nim YS; Lau PH; Wong K Structural insights into how GTP-dependent conformational changes in a metallochaperone UreG facilitate urease maturation. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (51), E10890–E10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Merloni A; Dobrovolska O; Zambelli B; Agostini F; Bazzani M; Musiani F; Ciurli S Molecular landscape of the interaction between the urease accessory proteins UreE and UreG. Biochim. Biophys. Acta, Proteins Proteomics 2014, 1844 (9), 1662–1674. [DOI] [PubMed] [Google Scholar]
- (20).Douglas CD; Ngu TT; Kaluarachchi H; Zamble DB Metal Transfer within the Escherichia coli HypB–HypA Complex of Hydrogenase Accessory Proteins. Biochemistry 2013, 52 (35), 6030–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cheng T; Li H; Xia W; Jin L; Sun H Exploration into the nickel ’microcosmos ’ in prokaryotes. Coord. Chem. Rev 2016, 311, 24–37. [Google Scholar]
- (22).Lacasse MJ; Zamble DB [NiFe]-Hydrogenase Maturation. Biochemistry 2016, 55 (12), 1689–1701. [DOI] [PubMed] [Google Scholar]
- (23).Jost M; Cracan V; Hubbard PA; Banerjee R; Drennan CL Visualization of a radical B 12 enzyme with its G-protein chaperone. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (8), 2419–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Capdevila DA; Edmonds KA; Giedroc DP Metallochaperones and metalloregulation in bacteria. Essays Biochem. 2017, 61 (2), 177–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Yang X; Li H; Lai T; Sun H UreE-UreG Complex Facilitates Nickel Transfer and Preactivates GTPase of UreG in Helicobacter pylori. J. Biol. Chem 2015, 290 (20), 12474–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Sydor AM; Lebrette H; Ariyakumaran R; Cavazza C; Zamble DB Relationship between Ni(II) and Zn(II) coordination and nucleotide binding by the helicobacter pylori [NiFe]-hydrogenase and urease maturation factor HypB. J. Biol. Chem 2014, 289 (7), 3828–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Xia W; Li H; Yang X; Wong KB; Sun H Metallo-GTPase HypB from helicobacter pylori and its interaction with nickel chaperone protein HypA. J. Biol. Chem 2012, 287 (9), 6753–6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lacasse MJ; Douglas CD; Zamble DB Mechanism of Selective Nickel Transfer from HypB to HypA, Escherichia coli [NiFe]-Hydrogenase Accessory Proteins. Biochemistry 2016, 55 (49), 6821–6831. [DOI] [PubMed] [Google Scholar]
- (29).Sydor AM; Jost M; Ryan KS; Turo KE; Douglas CD; Drennan CL; Zamble DB Metal binding properties of escherichia coli YjiA, a member of the metal homeostasis-associated COG0523 family of GTPases. Biochemistry 2013, 52 (10), 1788–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Khil PP; Obmolova G; Teplyakov A; Howard AJ; Gilliland GL; Camerini-Otero RD Crystal Structure of the Escherichia Coli YjiA Protein Suggests a GTP-Dependent Regulatory Function. Proteins: Struct., Funct., Genet 2004, 54, 371–374. [DOI] [PubMed] [Google Scholar]
- (31).Chacón KN; Mealman TD; McEvoy MM; Blackburn NJ Tracking metal ions through a Cu/Ag efflux pump assigns the functional roles of the periplasmic proteins. Proc. Natl. Acad. Sci. U. S. A 2014, 111 (43), 15373–15378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Fu Y; Tsui H-CTH; Bruce KE; Sham L-TL; Higgins K. a; Lisher JP; Kazmierczak KM; Maroney MJ; Dann CE; Winkler ME; Giedroc DP A new structural paradigm in copper resistance in Streptococcus pneumoniae. Nat. Chem. Biol 2013, 9 (3), 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Chandrangsu P; Huang X; Gaballa A; Helmann JD Bacillus subtilis FolE is sustained by the ZagA zinc metallochaperone and the alarmone ZTP under conditions of zinc deficiency. Mol. Microbiol 2019, DOI: 10.1111/mmi.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Crouzet J; Levy-Schil S; Cameron B; Cauchois L; Rigault S; Rouyez MC; Blanche F; Debussche L; Thibaut D Nucleotide sequence and genetic analysis of a 13.1-kilobase-pair Pseudomonas denitrificans DNA fragment containing five cob genes and identification of structural genes encoding Cob(I)alamin adenosyltransferase, cobyric acid synthase, and bifunctio. J. Bacteriol 1991, 173 (19), 6074–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lu J; Zheng Y; Yamagishi H; Odaka M; Tsujimura M; Maeda M; Endo I Motif CXCC in nitrile hydratase activator is critical for NHase biogenesis in vivo. FEBS Lett. 2003, 553 (3), 391–396. [DOI] [PubMed] [Google Scholar]
- (36).Nakashige TG; Stephan JR; Cunden LS; Brophy MB; Wommack AJ; Keegan BC; Shearer JM; Nolan EM The Hexahistidine Motif of Host-Defense Protein Human Calprotectin Contributes to Zinc Withholding and Its Functional Versatility. J. Am. Chem. Soc 2016, 138, 12243–12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Radin JN; Zhu J; Brazel EB; McDevitt CA; Kehl-Fie TE Synergy between Nutritional Immunity and Independent Host Defenses Contributes to the Importance of the MntABC Manganese Transporter during Staphylococcus aureus Infection. Infect. Immun 2019, 87 (1). DOI: 10.1128/IAI.00642-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Blaby-Haas CE; Flood JA; de Crécy-Lagard V; Zamble DB YeiR: a metal-binding GTPase from Escherichia coli involved in metal homeostasis. Metallomics 2012, 4 (5), 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zambelli B; Turano P; Musiani F; Neyroz P; Ciurli S Zn2+-linked dimerization of UreG from Helicobacter pylori, a chaperone involved in nickel trafficking and urease activation. Proteins: Struct., Funct., Genet 2009, 74 (1), 222–239. [DOI] [PubMed] [Google Scholar]
- (40).Irving BH; Williams RJP The Stability of Transition-metal Complexes. J. Chem. Soc 1953, 0, 3192–3210. [Google Scholar]
- (41).Gumataotao N; Lankathilaka KPW; Bennett B; Holz RC The iron-type nitrile hydratase activator protein is a GTPase. Biochem. J 2017, 474 (2), 247–258. [DOI] [PubMed] [Google Scholar]
- (42).Foster AW; Osman D; Robinson NJ Metal Preferences and Metallation. J. Biol. Chem 2014, 289 (41), 28095–28103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Wiese C; Hinz JM; Tebbs RS; Nham PB; Urbin SS; Collins DW; Thompson LH; Schild D Disparate requirements for the Walker A and B ATPase motifs of human RAD51D in homologous recombination. Nucleic Acids Res. 2006, 34 (9), 2833–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Smilgies D-M; Folta-Stogniew E Molecular weight–gyration radius relation of globular proteins: a comparison of light scattering, small-angle X-ray scattering and structure-based data. J. Appl. Crystallogr 2015, 48 (5), 1604–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Cai F; Ngu TT; Kaluarachchi H; Zamble DB Relationship between the GTPase, metal-binding, and dimerization activities of E. coli HypB. JBIC, J. Biol. Inorg. Chem 2011, 16 (6), 857–868. [DOI] [PubMed] [Google Scholar]
- (46).Zambelli B; Musiani F; Savini M; Tucker P; Ciurli S Biochemical Studies on Mycobacterium tuberculosis UreG and Comparative Modeling Reveal Structural and Functional Conservation among the Bacterial UreG. Biochemistry 2007, 46, 3171–3182. [DOI] [PubMed] [Google Scholar]
- (47).Bos JL; Rehmann H; Wittinghofer A GEFs and GAPs: critical elements in the control of small G proteins. Cell 2007, 129 (5), 865–877. [DOI] [PubMed] [Google Scholar]
- (48).Wang B; Dai P; Ding D; Del Rosario A; Grant RA; Pentelute BL; Laub MT Affinity-based capture and identification of protein effectors of the growth regulator ppGpp. Nat. Chem. Biol 2019, 15 (2), 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Phillips G; Grochowski LL; Bonnett S; Xu H; Bailly M; Blaby-Haas C; El Yacoubi B; Iwata-Reuyl D; White RH; De Crécy-Lagard V Functional promiscuity of the COG0720 family. ACS Chem. Biol 2012, 7 (1), 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Peng H; Shen J; Edmonds KA; Luebke JL; Hickey AK; Palmer LD; Chang FJ; Bruce KA; Kehl-Fie TE; Skaar EP; Giedroc DP Sulfide Homeostasis and Nitroxyl Intersect via Formation of Reactive Sulfur Species in Staphylococcus aureus. MSphere 2017, 2 (3), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Fey PD; Endres JL; Yajjala VK; Widhelm TJ; Boissy RJ; Bose JL; Bayles KW A Genetic Resource for Rapid and Comprehensive Phenotype Screening of Nonessential Staphylococcus aureus Genes. mBio 2013, 4 (1), 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Ellman GL Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82 (1), 70–77. [DOI] [PubMed] [Google Scholar]
- (53).Attin T; Becker K; Hannig C; Buchalla W; Wiegand A Suitability of a malachite green procedure to detect minimal amounts of phosphate dissolved in acidic solutions. Clin. Oral Investig 2005, 9 (3), 203–207. [DOI] [PubMed] [Google Scholar]
- (54).Kuzmic P Program DYNAFIT for the Analysis of Enzyme Kinetic Data: Application to HIV Proteinase. Anal. Biochem 1996, 237, 260–273. [DOI] [PubMed] [Google Scholar]
- (55).Capdevila DA; Braymer JJ; Edmonds KA; Wu H; Giedroc DP Entropy redistribution controls allostery in a metalloregulatory protein. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (17), 4424–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Capdevila DA; Huerta F; Edmonds KA; Le MT; Wu H; Giedroc DP Tuning site-specific dynamics to drive allosteric activation in a pneumococcal zinc uptake regulator. eLife 2018, 7 DOI: 10.7554/eLife.37268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Mäntele W; Deniz E UV–VIS absorption spectroscopy: Lambert-Beer reloaded. Spectrochim. Acta, Part A 2017, 173, 965–968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.