Abstract
This study provides insights in patterns of distribution of abiotic and biotic stress resilience across Vigna gene pools to enhance the use and conservation of these genetic resources for legume breeding. Vigna is a pantropical genus with more than 88 taxa including important crops such as V. radiata (mung bean) and V. unguiculata (cowpea). Our results show that sources of pest and disease resistance occur in at least 75 percent of the Vigna taxa, which were part of screening assessments, while sources of abiotic stress resilience occur in less than 30 percent of screened taxa. This difference in levels of resilience suggests that Vigna taxa co-evolve with pests and diseases while taxa are more conservative to adapt to climatic changes and salinization. Twenty-two Vigna taxa are poorly conserved in genebanks or not at all. This germplasm is not available for legume breeding and requires urgent germplasm collecting before these taxa extirpate on farm and in the wild. Vigna taxa, which tolerate heat and drought stress are rare compared with taxa, which escape these stresses because of short growing seasons or with taxa, which tolerate salinity. We recommend prioritizing these rare Vigna taxa for conservation and screening for combined abiotic and biotic stress resilience resulting from stacked or multifunctional traits. The high presence of salinity tolerance compared with drought stress tolerance, suggests that Vigna taxa are good at developing salt-tolerant traits. Vigna taxa are therefore of high value for legume production in areas that will suffer from salinization under global climate change.
Subject terms: Plant breeding, Plant domestication, Conservation biology
Introduction
Legume crops are an important and cheap source of proteins and micronutrients in human diets1. These crops also fix nitrogen because of their symbiosis with Rhizobium bacteria, which makes them attractive crops for soil improvement in farming systems2. However, increased abiotic stress such as heat, drought, and salinity, and high pressure of diseases and insect pests under climate change decrease yield and quality of existing legume varieties3.
To develop varieties that can sustain legume production under climate change, crop wild relatives would be important sources to introgress traits in crop varieties, which help to tolerate, escape, or avoid abiotic stresses and to resist against pests and diseases4,5. So far, genetic improvement of legume crops lags behind compared with cereal crops1, and legume breeders have underutilized crop wild relatives in developing varieties because of limited information on traits of economic importance in wild species, linkage drag of undesired traits and crossing barriers6. A better understanding of trait distribution across legume gene pools will help exploiting legume crop diversity and identifying conservation gaps in legume crop gene pools. In combination with advances in functional genomics, gene editing, and phenotyping, this can broaden the currently narrow genetic basis in legume breeding1,5,6.
In this paper, we focus on Vigna, to understand patterns of distribution of abiotic and biotic stress resilience across legume gene pools. Vigna is a complex and pantropical genus of more than 88 taxa, which are principally diploid and selfing. The genus includes a number of important legume crops for food and nutrition security in tropical Asia and Africa such as V. radiata (mung bean) and V. unguiculata (cowpea, vegetable cowpea, and yard-long bean) as well as several neglected and underutilized crops such as V. aconitifolia (moth bean) and V. subterranea (Bambara groundnut).
The optimum temperature range for legume crops is between 10–36 °C and various climatic models predict that temperatures will increase on average with 4 °C by the end of this century7. A principal production challenge, which is considered in Vigna breeding is therefore heat stress >40 °C8–10. Other production challenges relate to drought stress, water logging, and salinity8–10, and in the case of cowpea also phosphorus-use inefficiency8. Vigna wild relatives could contain traits to respond to these abiotic-stress related production challenges.
Traits of breeding interest to escape heat and drought stress include early flowering and maturation. Important morphological and physiological traits in Vigna screening for drought tolerance are the number and diameter of xylem vessels, root suberization, stomatal leaf conductance, and low leaf hydraulic conductance, among others11. The development of long roots or tubers can help avoiding drought stress but may come at the costs of reduced leaf, pod, or bean productivity12. Traits to tolerate heat tolerance include membrane stability, pollen viability, and anther dehiscence, and decline in GABA (γ-aminobutyric acid) declination and increase in antioxidant enzymes, among other traits during plant development10,13,14. Traits related to salinity tolerance include relative quantum yield of photosystem II, leaf Na+ and K+ concentrations, malondialdehyde (MDA) levels, and antioxidant enzyme activities15,16.
Major production challenges related to biotic stress for several Vigna crops include legume pod borer and Yellow Mosaic Disease (YMD) (R. Nair, pers. comm., World Vegetable Center). Specific challenges for V. radiata include powdery mildew, Cercospora leaf spot; and resistance to the following five pests: bruchids, Thrips spp., bean flies (Ophiomyia spp. and Melanagromyza spp.), and whitefly (Bemisia tabaci)17. Vigna unguiculata is also susceptible to bacterial blight (Xanthomonas axonopodis pv phaseoli), the parasitic weed striga, viruses such as bean common mosaic virus (BCMV), and anthracnose (Colletotrichum destructivum)8. There are several traits involved in plant defence against these pests and diseases such as colour, texture, hardness, leaf age, size, spines, trichomes, and chemical constituents in both plants and storage seeds18,19.
Owing to the larger number of traits related to abiotic and biotic stress resilience, it is a challenge to carry out a systematic assessment to provide insights in trait distribution and evolution across crop gene pools. In this study, we therefore examine the distribution of traits related to abiotic stress resilience across four Vigna gene pools by carrying out a systematic ecogeographic analysis for heat and drought stress. Such an analysis indicates the adaptation of crop wild relatives to hot, dry, or humid growing conditions, independent of a specific trait20,21. We develop four Vigna gene pools by combining existing information on Vigna phylogenetics with information on crossing compatibility. The results of the ecogeographic analysis are compared with reports of systematic screening of Vigna taxa for root and shoot biomass accumulation under drought conditions and biomass accumulation under high salinity concentrations coupled to relative quantum yield of photosystem II and leaf Na+ and K+ concentrations11,16. Furthermore, we carry out a comprehensive literature review on biotic stress resilience for important pest and diseases, and compare the distribution of these traits with the distribution of traits related to abiotic stress resilience. Finally, we assess the ex situ conservation status of Vigna to check out germplasm availability for breeders and to target countries for missions to collect germplasm of Vigna taxa, which are not well conserved in situ and ex situ.
Methods
Four Vigna gene pools with 88 taxa from three subgenera and nine biosystematics sections were defined for nine domesticated Vigna taxa as listed in GRIN taxonomy22. We excluded other taxa because they are part from sections, which are remote to domesticated Vigna taxa. We delineated four gene pools following intercrossing studies and complemented with information from genomic, phylogenetic, and biosystematics studies (Text S1). Taxa names were revised according to GRIN taxonomy22 and Iseki et al.16 (Table S1). The Vigna ex situ conservation status was determined with data from the 2017 WIEWS database23 and the online platform Genesys24.
Collection of presence records
In total, 28,313 georeferenced presence records for 84 of the 88 Vigna taxa were used for the detection of geographic patterns of taxonomic richness and gaps in genebank collections. Four Vigna taxa did not have any record reported. The presence records come from four data sources:
Presence records from herbaria, which were reported by Tomooka et al.25 were manually curated and where possible georeferenced in Google Earth or with support of www.geonames.org.
Presence records from herbaria and living collections, which were stored in the Global Biodiversity Information Facility (https://www.gbif.org/) were collected with the rgbif package26 and manually georeferenced if taxa had less than 30 georeferenced presence records.
Presence records from genebanks listed in the 2017 WIEWS database23.
Presence records from the Vigna genebank collection of the World Vegetable Center (WorldVeg) were manually curated and where possible georeferenced in Google Earth or with support of www.geonames.org.
Cleaning of presence records
Presence records with inconsistencies between countries as reported in the passport data and at the projected locations outside a border buffer zone of 10 arc minutes were removed following van Zonneveld et al.27. Coordinates of presence records located in coastal waters within a 10-arc minute buffer zone to the coastline were relocated to the nearest point in the coastline. Presence records with coordinates from country centroids were removed because these points are likely georeferenced at country level with low precision. For each taxon, duplicate records with the same coordinates were removed to reduce sample bias.
Outlier presence records with climate values far beyond taxa’ niche margins were removed from our dataset because these are likely errors in coordinates or taxonomy. We removed outliers when the values of three or more of 19 bioclimatic variables were outside a threshold of 2.5 times the interquartile range below the first quartile or above the third quartile following van Zonneveld et al.27. Climate data were derived from the 2.5-arc minute environmental layers of the 2.0 WorldClim database28.
Gap analysis
We identified taxonomic and geographic gaps in genebank collections in two steps. First, to target germplasm from taxa and countries underrepresented in ex situ collections, we compared sampled taxonomic richness reported by genebanks and living collections with sampled taxonomic richness reported by herbaria. Second, to detect geographic areas where taxa have not been reported before, we applied ecological niche modelling with Maxent, a widely used modelling algorithm to detect areas where climate conditions are suitable for plant species29. We modelled the distribution of each Vigna taxon under current climate conditions (1970–2000) using a selection of seven bioclimatic variables available from the WorldClim 2.0 database28 (Text S2): These bioclimatic variables were selected after the removal of correlated variables with a Pearson correlation coefficient of more than 0.7. We used the threshold value of maximum specificity + sensitivity to differentiate suitable from not-suitable areas for taxa presence30. To reduce sampling bias, we first took the average Maxent results for each taxa from three runs each time, using 80% of the randomly resampled records from grid cells with a size corresponding to 10% of the longest inter-point distance following Fourcade et al.31. Second, to allow Maxent to discriminate areas with presence records from the areas with no data, we randomly extracted ten times more background points from the area enclosed by the convex hull polygon based on the presence records, and extended with a buffer corresponding to 10% of the longest inter-point distance following van Zonneveld et al.27. Third, to reduce the risk of including modelled areas where the taxa do not occur in reality, we limited the modelled distribution range by the area enclosed by the convex hull polygon based on the presence records and extended with the buffer around it following van Zonneveld et al.27.
Assessment of abiotic stress and biotic stress resilience
We did a literature review to assess biotic stress resistance of Vigna taxa with a focus on bruchids (Callosobruchus spp.) and Yellow Mosaic Disease (YMD), both of which are important across all Vigna, and other pests and diseases as indicated in the introduction. For the assessment of abiotic stress resilience, we carried out an ecogeographic analysis. This analysis was complemented with scores on a 1–5 Likert scale of high to low tolerance of Vigna taxa to hydratation and salinity on the basis of biomass accumulation during early growth stages under drought and saline conditions in pot experiments11,16. These experiments were carried out by the Genetic Resources Center of the Japanese Agriculture and Food Science Organization. All taxa, which scored a “1” for at least one accession in the pot experiments, were identified in this study as taxa with a high level of tolerance. Taxa, which scored a “2” for at least one accession, were identified as taxa with an intermediate level of tolerance. Taxa with poorer scores were identified as taxa with a low level of tolerance.
The ecogeographic analysis consisted of one-way ANOVAs with ranked climate data to identify Vigna taxa, which occur in harsh climate conditions, and which therefore would have acquired traits related to abiotic stress resilience. Climate data was extracted from the WorldClim 2.0 database28. We identified four types of harsh climate conditions:
Permanently hot climate conditions with high annual mean temperature;
Seasonally hot climate conditions with high mean temperatures in the wettest quarter;
Permanently dry climate conditions with low annual rainfall; and
Seasonally dry climate conditions with low rainfall in the wetter quarter.
We selected temperature and rainfall in the wettest quarter as indicators for seasonally hot and seasonally dry climate conditions because these are critical variables for flowering and seed development of annual plants such as Vigna taxa (pers. comm., Ken Naito, Genetic Resources Center, Japanese Agriculture and Food Science Organization). Post-hoc Tukey Honest Significant Difference (HSD) tests were carried out to identify Vigna taxa, which occur in harsh climate conditions; these taxa belonged to the HSD group with highest temperature or lowest rainfall values. We then identified a second group of Vigna taxa, which occur in less harsh climate conditions; these taxa belonged to the HSD group with second-highest temperature or second-lowest rainfall values. Finally, all other Vigna taxa were assigned to a third group because they occur in less harsh climate conditions. For nine taxa, less than five georeferenced records were available to extract climate data. Because of this lack of sufficient data, these taxa were excluded from the ecogeographic analysis.
Software
We performed all analyses in R version 3.3.3. Taxonomic richness maps were developed with R version 3.3.3 and the ggplot2 version 3.1.032, and with the use of a freely available global political map33. The code and datasets are available at https://figshare.com/articles/R_Script_and_dataset_of_Vigna_ecogeographic_analysis/7551011. Text S3 lists all R packages, which were used in this study.
Results
Vigna gene pools
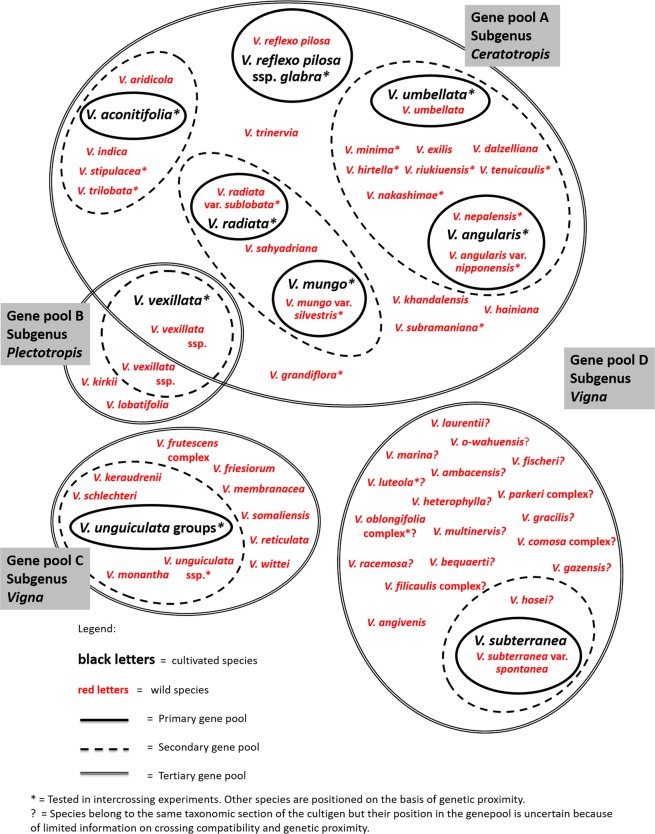
Gene pool A consists of Asian Vigna taxa of the subgenus Ceratotropis, including V. radiata (mung bean), V. mungo (urd bean), V. angularis (azuki bean), V. umbellata (rice bean), V. reflexo-pilosa var. glabra (creole bean), and V. aconitifolia (moth bean) (Fig. 1). Vigna radiata is the economically most important Vigna of gene pool A. Vigna radiata can produce mature seeds within two months, which makes it popular for crop diversification of rice and wheat systems34. The domestication centre of V. radiata is thought to be in current India. Vigna mungo is also thought to be domesticated in current India and is genetically close to mung bean, and is especially popular in southern India and Pakistan. Vigna angularis is mainly cultivated in Japan and Korea, and is thought to be domesticated in Japan35. Other domesticated taxa are minor crops with important features. Vigna umbellata can grow on poor lands and is mostly cultivated in the tropical Asian highlands36. Vigna reflexo-pilosa var. glabra, which is the only known tetraploid Vigna taxa25, is cultivated in Viet Nam, the Philippines, Mauritius, and Tanzania25. Vigna aconitifolia, another legume crop is thought to be domesticated in current India is cultivated where it is too hot for mung bean cultivation36. In addition, several wild taxa are locally consumed and cultivated in Southeast Asia including V trilobata (jungle bean) and Vigna trinervia (tooapee)37,38.
Figure 1.
Vigna gene pools.
Gene pool B comprises taxa from subgenus Plectotropis to which the domesticated V. vexillata (tuber cowpea) and its wild relatives belong (Fig. 1). There are two types of domesticated V. vexillata. The tuber type is domesticated in Asia while the pea type was domesticated in Africa39.
Gene pool C comprises taxa of the sections Catiang, Macrodontae, and Reticulatae of subgenus Vigna (Fig. 1). The domesticated taxa in this gene pool are V. unguiculata group sesquipedalis (yard-long bean), which is mostly cultivated in Asia, and V. unguiculata group unguiculata (grain and vegetable cowpea), which was domesticated in West and Central Africa. Vigna unguiculata group sesquipedalis (yard-long bean) distinguishes itself from the V. unguiculata group unguiculata by its long pod (~1 m). People harvest and eat young pods of V. unguiculata group sesquipedalis, rather than the beans.
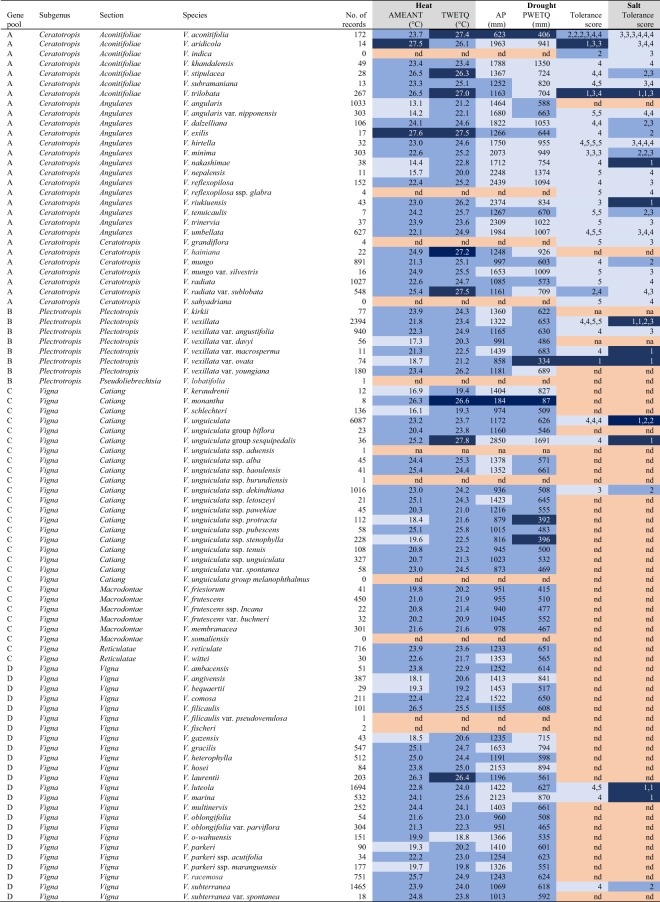
Figure 2.
Abiotic stress tolerance of 88 Vigna species. The tolerance of these species was assessed for six variables: (1) Mean value of Annual Mean Temperature across the distribution of presence records (AMEANT); (2) Mean value of Temperature in the Wettest Quarter (TWEQ); (3) Mean value of Annual Precipitation (AP); (4) Mean value of Precipitation in the Wettest Quarter (PWETQ); (5) Drought tolerance score after lab screening carried out by Iseki et al.11 in which 1 indicates high tolerance while 5 indicates low tolerance; (6) Salinity tolerance score after lab screening carried out by Iseki et al.16 in which 1 indicates high tolerance while 5 indicates low tolerance. The four climate variables were calculated with data from WorldClim28. The colour of the cells indicates the level of tolerance for a specific variable. Darkblue indicates presence in harsh climate conditions or a high tolerance score in pot experiments. Intermediate blue indicates presence in intermediate climates or a suboptimal tolerance score in pot experiments. Light blue indicates presence in temperate or humid climate conditions or a poor tolerance score in pot experiments. Salmon indicates no data (nd).
Finally, gene pool D comprises the domesticated V. subterranea (Bambara groundnut) and other taxa of section Vigna of subgenus Vigna (Fig. 1). Vigna subterranea is thought to be domesticated in West Africa40. Farmers cultivate several wild taxa from this gene pool for local consumption, such as V. marina and V. luteola41,42. Several taxa from this genus are grown widely in the tropics as forages including V. luteola, V. hosei, and V. parkeri42. This pantropical section includes taxa from Africa such as Bambara groundnut, from the Americas such as V. luteola, and one taxa endemic to Hawaii in the Pacific: V. o-wahuensis. Gene pool D is the least investigated of all four gene pools. Many taxa of this gene pool have not yet been included in crossing compatibility experiments.
Abiotic stress resilience
Nine taxa from three gene pools occur in seasonally hot climate conditions and five taxa from three gene pools occur in seasonally dry climate conditions (Tables 1, 2). In contrast, only two taxa from just one gene pool occur in permanently hot climate conditions and two other taxa from two gene pools occur in permanently dry climate conditions. Six taxa from three gene pools tolerated high levels of salinity in contrast to only three taxa from two gene pools, which tolerated high levels of dehydration.
Table 1.
Patterns of abiotic and biotic stress resilience across the Vigna genus.
| Main region of distribution | Sections and gene pools | Total no. of taxa | Presence in extreme climates compared with other taxaa | Abiotic stress toleranceb | Biotic stress resistancec | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of taxa evaluated | High AMEANTd | High TWETQe | Low APf | Low PWETQg | No. of taxa screened | Dehydration | Salinity | No. of taxa reviewed | YMDh | No. of taxa reviewed | Bruchids | |||
| Asia | Section Aconitifoliae | 7 | 6 | 1 | 3 | 1 | 1 | 7 | 1 | 0 | 2 | 2 | 3 | 1 |
| Asia | Section Angulares | 14 | 13 | 1 | 1 | 0 | 0 | 13 | 1 | 3 | 4 | 4 | 11 | 9 |
| Asia | Section Ceratotropis | 7 | 5 | 0 | 2 | 0 | 0 | 6 | 0 | 0 | 3 | 3 | 5 | 3 |
| Asia | Total gene pool A | 28 | 24 | 2 | 6 | 1 | 1 | 26 | 2 | 3 | 9 | 9 | 19 | 13 |
| Africa | Section Plectotropis | 7 | 7 | 0 | 0 | 0 | 1 | 4 | 1 | 3 | 0 | na | 1 | 1 |
| Africa | Section Pseudoliebrechtsia | 1 | 0 | na | na | na | na | 0 | na | na | 0 | na | 0 | na |
| Africa | Total gene pool B | 8 | 7 | 0 | 0 | 0 | 1 | 4 | 1 | 3 | 0 | na | 1 | 1 |
| Africa | Section Catiang | 20 | 17 | 0 | 2 | 1 | 3 | 3 | 0 | 2 | 1 | 1 | 1 | 1 |
| Africa | Section Macrodontae | 6 | 5 | 0 | 0 | 0 | 0 | 0 | na | na | 0 | na | 0 | na |
| Africa | Section Reticulatae | 2 | 2 | 0 | 0 | 0 | 0 | 0 | na | na | 0 | na | 1 | 1 |
| Africa | Total gene pool C | 28 | 24 | 0 | 2 | 1 | 3 | 3 | 0 | 2 | 1 | 1 | 2 | 2 |
| Africa | Section Vigna | 24 | 22 | 0 | 1 | 0 | 0 | 3 | 0 | 2 | 0 | na | 2 | 2 |
| Africa | Total gene pool D | 24 | 22 | 0 | 1 | 0 | 0 | 3 | 0 | 2 | 0 | na | 2 | 2 |
aData analysis obtained in the ecogeographic analysis of this study; bScreening for drought and salinity tolerance carried out by Iseki et al.11,16; cLiterature review carried out in this study; dAMEANT: Annual Mean Temperature; eTWETQ: Temperature in the Wettest Quarter; fAP: Annual Precipitation; gPWETQ: Precipitation in the Wettest Quarter; hYMD: Yellow Mosaic Disease; na: not applicable.
Table 2.
Percentages of taxa, sections, and gene pools of Vigna, which harbor traits related to abiotic or biotic stress resilience.
| Type of Evolutionary Significant Unit (ESU) | Taxa | Sections | Gene pools |
|---|---|---|---|
| No. of ESUs evaluated in ecogeographic analysis | 77 | 8 | 4 |
| % of ESUs in climates with high annual mean temperature | 3% | 25% | 25% |
| % of ESUs in climates with high temperature in the wettest quarter | 12% | 58% | 75% |
| % of ESUs in climates with low annual precipitation | 3% | 25% | 50% |
| % of ESUs in climates with low precipitation in the wettest quarter | 6% | 38% | 75% |
| No. of ESUs screened for abiotic stress tolerance | 36 | 6 | 4 |
| % of ESUs with reported dehydration tolerance | 8% | 50% | 50% |
| % of ESUs with reported salinity tolerance | 27% | 67% | 100% |
| No. of ESUs reviewed for resistance against Yellow Mosaic Disease (YMD) | 10 | 4 | 2 |
| % of ESUs with reported YMD resistance | 100% | 100% | 100% |
| No. of ESUs reviewed for bruchid resistance | 24 | 7 | 4 |
| % of ESUs with reported bruchid resistance | 75% | 100% | 100% |
The wild V. trilobata, V. vexillata var. ovata, and V. monantha and the domesticated V. aconitifolia returned highest scores for abiotic stress resilience (Fig. 2). These four taxa showed high levels of abiotic stress resilience for three of the six variables. Vigna trilobata occurs in seasonally hot climate conditions and tolerated high levels of dehydration and salinity. Vigna vexillata var. ovata occurs in seasonally dry climate conditions and tolerated high levels of dehydration and salinity. Vigna monantha and V. aconitifolia occur in permanently dry and seasonally hot climate conditions with low rainfall during the wettest quarter. While V. monantha was not screened for dehydration or salinity tolerance, V. aconitifolia accessions did not tolerate high levels of dehydration or salinity.
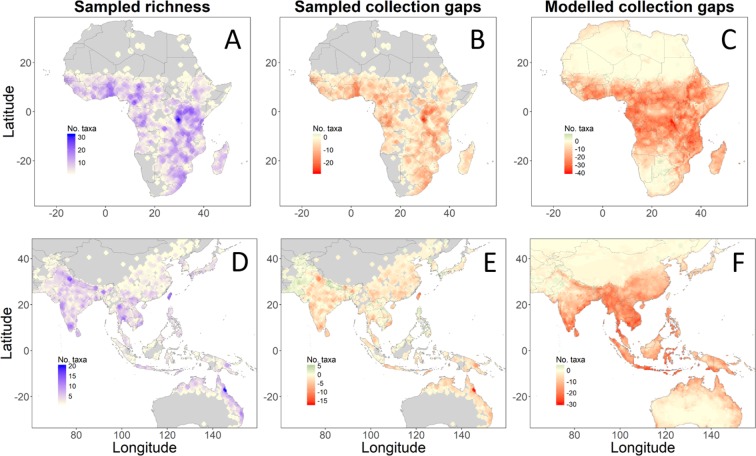
Figure 3.
Sampled taxonomic richness and sampled and modelled collection gaps. Panel A and D show sampled taxonomic richness; Panel B and E show sampled taxonomic richness, which is not conserved ex situ; Panel C and F show gaps where a high number of taxa are modelled to occur but are not reported in herbaria, living collections, and genebanks. Maps were made by Maarten van Zonneveld in R version 3.3.3 with ggplot2 version 3.1.0.
The wild V. aridicola, V. exilis, V. laurentii and the domesticated V. unguiculata group sesquipedalis showed high levels of abiotic stress resilience for two of the six variables (Fig. 2). Vigna aridicola occurs in permanently hot climate conditions and tolerated high levels of dehydration. Vigna exilis occurs in permanently and seasonally hot climate conditions. This species, however, did not tolerate high levels of dehydration or salinity. Vigna laurentii occurs in seasonally hot and seasonally dry climate conditions. This species was not tested for dehydration and salinity tolerance. Finally, V. unguiculata group sesquipedalis occurs in seasonally hot climate conditions and tolerated high levels of salinity.
Thirteen taxa showed high levels of abiotic stress resilience for one of the six variables. Vigna hainiana, V. radiata var. sublobata, and V. stipulacea occur in seasonally hot climate conditions. Vigna heterophylla, V. kirkii, and V. unguiculata subsp. stenophylla, occur in seasonally dry climate conditions. Finally, high levels of salinity tolerance were reported for the domesticated V. unguiculata and V. vexillata, and the wild V. luteola, V. marina, V. nakashimae, V. riukiuensis, and V. vexillata var. macrosperma.
Biotic stress resistance
Bruchid resistance was reported in 18 of the 24 taxa (75%), which were evaluated in literature. These 18 taxa included representatives from all four gene pools (Table 2; S2). However, no resistance against bruchids was reported for mung bean. YMD resistance was reported in all ten taxa (100%), which were evaluated, and which belonged to gene pool A of V. radiata and other Asian Vigna crops and gene pool C of V. unguiculata group unguiculata and unguiculata group sesquipedalis.
For the other pest and diseases, remarkably less research has been conducted. In gene pool A, only for germplasm of V. radiata and V. mungo, resistance was reported against legume pod borer, whiteflies, stem borer and Thrips spp. In gene pool A, no reports were found for resistance against powdery mildew, bacterial blight, or Cercospora leaf spot. In gene pool C, germplasm of the primary gene pool of V. unguiculata group unguiculata and unguiculata group sesquipedalis was reported to resist against bacterial blight, Thrips spp., and legume pod borer. No reports were found on resistance against anthracnose. Little research was done on biotic stress resistance in gene pools B and D, except for bruchid resistance and cowpea mottle carmovirus (CPMoV).
Ex situ conservation status
In 2017, 96 institutions conserved ex situ 89,288 accessions of the targeted Vigna taxa. Eight institutes maintained more than 53,756 of these accessions (60%) (Table S3). As a safety duplicate, in 2018, 31,500 accessions from 25 taxa were stored in the Svalbard Global Seed Vault (SGSV, 2018, https://www.nordgen.org/sgsv/), mainly from the International Institute of Tropical Agriculture (IITA), the Centro Internacional de Agricultura Tropical (CIAT), and WorldVeg. From the domesticated taxa, IITA held the largest collections of V. unguiculata (cowpea) and V. subterranea (Bambara groundnut). The status of V. unguiculata group sesquipedalis (yard-long bean) collection is unclear because not all genebanks provided taxonomic data below species level, which is necessary for this crop. WorldVeg held the largest collection of V. radiata (mung bean) and V. angularis (azuki bean). The Indian Bureau of Plant Genetic Resources (NBPGR) held the largest collections of V. mungo (urd bean), V. umbellata (rice bean), and V. aconitifolia (moth bean). CIAT held the largest collection of V. vexillata (tuber cowpea). The Genetic Resources Center of the Japanese Agriculture and Food Science Organization held the largest collection of V. reflexo-pilosa (creole bean). The Australian Grains genebank and IITA maintained important collections of African wild Vigna while the Japanese Agriculture and Food Science Organization and NBPGR held an important collection of wild Asian Vigna. The national botanic garden of Belgium, Meise held the most diverse Vigna collection but only keeps a limited number of accessions per taxon.
Collection gaps
Two Asian Vigna and four African Vigna were not represented in any of the genebanks, which reported to WIEWS: Vigna sahyadriana, V. indica, V. keraudrenii, V. monantha, V. somaliensis, and V. gazensis. Genebanks maintained less than 10 accessions for nine other Asian Vigna and seven other African Vigna (Table S3). Priority countries for germplasm collecting missions of these 22 poorly- conserved taxa in Asia are Thailand, India, Sri Lanka, and Myanmar (Table S4). In Africa, priority countries are Madagascar, DRC Congo, South Africa, Benin, Burundi, Somalia, Namibia, and Tanzania. In the Pacific, V. o-wahuensis requires urgent collection in Hawaii.
Geographic gaps in Asia and the Pacific with high taxonomic richness reported by herbaria but low coverage reported by genebanks and living collections are Taiwan, Northeast Australia, and India (Fig. 3). When comparing the reported overall taxonomic richness with the modelled taxonomic richness, our analysis showed modelled gaps in West Cambodia, Central Thailand, South Viet Nam, and coastal India (Fig. 3). Geographic gaps in Africa with high reported taxonomic richness but low coverage by genebank collections are Burundi, Benin, and Uganda. In addition, East DRC Congo is a collection gap following the modelled richness analysis.
Discussion
Genus-wide screening of Vigna reveals an evolutionary pattern of high biotic vs. low abiotic stress resilience
Our findings show that sources of pest and disease resistance occur in at least 75 percent of the taxa, which were part of screening assessments. In contrast, sources of abiotic stress resilience occur in less than 30 percent of the taxa, which were part of screening assessments. We therefore hypothesize that during evolution, Vigna taxa have been conservative in acquiring traits related to abiotic stress resilience compared with pest and diseases resistance. To verify the pattern of high biotic vs. low abiotic stress resilience for the whole Vigna it is necessary to screen more Vigna taxa from gene pools C and D, which belong both to Vigna subgenus Vigna because these are under-represented in the screening studies, which were reported in this study.
Fifteen percent of the taxa screened in the ecogeographic analysis occur in seasonally hot or seasonally dry climate conditions, where it is possible to escape heat and drought stress through rapid flowering and maturation rather than to tolerate heat and drought stress. In contrast, five percent of the taxa occurs in permanently hot or permanently dry climate conditions, and requires traits to tolerate continuous heat or drought stress. This finding suggests that during evolution Vigna taxa more easily acquire phenological traits for short life cycles to escape drought and heat stresses compared with acquiring physiological traits to tolerate these stresses continuously.
Co-evolution can explain the high percentage of taxa, which possesses biotic stress resistance compared with heat and drought stress resilience. Resistance against pest and diseases has often been a result from continuous and recent co-evolution in the geographic areas of occurrence and pressure of pest and diseases43. Pest pressure can change genotype frequencies in a plant population within just a few generations44,45. For example for wild tomato relatives, the density of trichomes and levels of acylsugar concentrations, related to direct pest defence, correspond with pest pressure and the geographic distribution of these pests46,47.
The presence of salinity tolerance in 27 percent of taxa compared with eight percent drought-tolerant taxa in the pot experiments, suggest that Vigna taxa are good at developing salt-tolerant traits compared with drought-tolerant traits16. Several wild Vigna taxa occur naturally in coastal areas and are adapted to a saline environment41. These Vigna genetic resources are of high value for legume production in geographic areas, which suffer from salinization.
Our findings suggest that the section Aconitifolia contains high levels of heat and drought stress resilience. This section includes V. aconitifolia (moth bean), which occurs in permanently dry and seasonally hot climate conditions. This finding contrasts with the low dehydration tolerance of V. aconitifolia, which was reported in the pot experiments11. Two possible reasons can explain this discrepancy. First, the pot experiment measured dehydration tolerance at an early growth stage while plants can have traits to tolerate or escape drought stress during all growth stages. The ecogeographic analysis captures this broad range of traits with presence records of populations of V. aconitifolia in harsh environments. Second, the limited number of accessions of V. aconitifolia in the pot experiments may not reflect the full intra-specific genetic variation of this species.
Vice versa, V. aridicola from section Aconitifoliae tolerated dehydration while the ecogeographic analysis indicated that this species occurs in humid regions. It could be that our analysis does not reflect the complete climatic ranges because we only were able to collect 14 presence records of this species.
Numerous Vigna taxa from all four gene pools occur in marginal conditions, on poor sandy soils, or geographic areas that are regularly inundated41, 42. We therefore anticipate that many Vigna taxa, which have not been phenotyped for abiotic stress resilience yet, have traits related to abiotic stress resilience. The biggest phenotyping gap is in the Vigna taxa of gene pools C and D, which belong both to Vigna subgenus Vigna.
To test if the pattern of high biotic vs. low abiotic stress resilience is common in gene pools of other legume crops, more screening studies are required that simultaneously assess abiotic or biotic stress resilience. We only found one study, which simultaneously screened legume wild relatives for abiotic stress and biotic stress resilience48. This study with eight chickpea wild relatives (Cicer spp.) focuses on cold stress rather than heat stress in combination with biotic stress resistance. Nevertheless, the study’s results are in line with our findings of high biotic vs. low abiotic stress resilience because the researchers identified four or more Cicer taxa resisting bruchids and other pests and diseases compared with only two wild Cicer taxa tolerating cold stress.
Identification of multifunctional traits
Because of the high frequency of taxa with traits related to biotic stress resilience, we expect that taxa with traits related to abiotic stress resilience have also a high chance to include traits related to biotic stress resilience. Our analysis suggests that many taxa from the section Aconitifoliae show high levels of abiotic stress resilience, but only a limited number of accessions from this section were screened for resistance against pests and diseases. These and other Vigna taxa with high levels of abiotic stress resilience would require further evaluation under different abiotic and biotic stress combinations.
To date, screening and understanding of the ability of Vigna taxa to adapt against combined biotic and abiotic stresses is largely unknown. Taxa, which are adapted to hot, drought, or extreme-rainfall conditions could have stacked different traits over time to cope with both abiotic and biotic stresses. In addition, multifunctional traits, such as high levels of antioxidant capacity, could help these taxa to tolerate abiotic and biotic stresses at the same time49. In fact, exaptation, which is the evolutionary process of changes in trait functions50, predicts that a trait, which initially responded to a single stress, is likely to generate several functions over evolutionary time to eventually respond to multiple stresses.
We propose trichomes as a promising multifunctional trait in Vigna taxa for further screening because trichomes help plants to cope with both abiotic and biotic stresses51. High density of trichomes on the pods of V. radiata and the V. unguiculata complex complicates the mobility of the adult of bruchids and pod borers over the pods and decrease pest infestations18,52. Numerous studies show that glandular trichomes in tomato wild relatives produce secondary metabolites including acylsugars, methylketones, and sesquiterpenes, which intoxicate, repel, or trap pests19,53. At the same time, trichomes may increase abiotic stress tolerance by reducing leaf radiation absorbance and facilitate condensation of air moisture onto the plant surface, among other functions54. Further research will reveal the relationships between trichomes types and densities, trichrome evolution, and abiotic and biotic stress resilience in Vigna taxa.
Cross-compatibility between Vigna taxa is poorly understood
For many Vigna taxa, genetic relationships and crossing compatibility are still poorly understood. This is especially true for the pantropical Vigna section of subgenus Vigna, which includes taxa from Africa, the Americas, and the Pacific. Domestication and origin of Vigna taxa is largely unknown. For example, where did the Vigna genus originate and how did the genus spread across Africa, Asia, the Americas, and the Pacific?
Only few studies reported on biotic stress resilience of wild relatives of V. unguiculata (cowpea) compared with wild relatives of V. radiata (mung bean). It could be that cowpea breeders do not yet need to use wild relatives of the V. unguiculata complex for biotic stress resilience because the many botanical varieties in the primary gene pool of the V. unguiculata complex provide sufficient variation for finding traits. Another possibility could be that V. unguiculata is difficult to cross with close relatives compared with V. radiata and its close relatives, which requires further crossing studies.
Quarter of Vigna taxa requires urgent conservation actions
Twenty-six percent of the 88 Vigna taxa, which were considered in this study, requires urgent germplasm collecting efforts because they are not- or under-represented in genebanks. Two Asian Vigna taxa and four African Vigna taxa require urgent efforts of germplasm collecting because no genebank had reported the maintenance of these taxa. Nine other Asian Vigna taxa and seven other African Vigna taxa also require urgent efforts of germplasm collecting because genebanks reported less than 10 accessions of these taxa. Targeted Asian and Pacific countries for germplasm collecting efforts include India, Thailand, Myanmar, Sri Lanka, Australia, and Taiwan. In Africa, our sampled and modelled richness analyses indicate Burundi, Benin, DRC Congo, and Madagascar as priority countries for Vigna germplasm collecting efforts..
Vigna taxa with high levels of heat and drought stress tolerance are rare. We therefore propose to use the presence of these type of traits as a criterion to prioritize taxa for conservation. The section Aconitifoliae requires urgent conservation efforts when considering this criterion in combination with poor genebank coverage. Five out of the seven Aconitifoliae taxa are poorly conserved ex situ with 10 or fewer reported accessions in genebanks while this section includes several traits related to abiotic stress tolerance. Taxa from this section mainly occur in India and Sri Lanka, which are priority countries for germplasm collecting. In addition to the taxa in this section, Vigna exilis, which occurs in permanently hot climate conditions and V. monantha, which occurs in permanently dry conditions, also require urgent conservation because these taxa were not reported in genebanks, except for one accession of V. exilis.
The pacific V. o-wahuensis, endemic to Hawaii, is critically endangered according to the U.S. Endangered Species Act56. Fortunately, 27 accessions have been safeguarded in the Lyon Arboretum, Hawaiian Rare Plant Program (pers. comm. Marian Chau, Lyon Arboretum). Strengthening collaboration between genebanks and botanical gardens such as the Lyon Arboretum and the National Botanic Garden of Belgium in Meise, would further enhance ex situ conservation and germplasm availability of Vigna.
Vigna monantha and another African Vigna, V. keraudrenii require urgent in situ and ex situ conservation efforts because these taxa are endangered according to the IUCN Red List55. Until now, the IUCN has evaluated only six Vigna taxa as part of the Red List. The inclusion of more Vigna taxa on the IUCN Red List will help to understand their in situ conservation status better.
Supplementary information
Acknowledgements
Funding for the World Vegetable Center’s general research activities is provided by core donors: Republic of China (Taiwan), UK aid from the UK government, United States Agency for International Development (USAID), Australian Centre for International Agricultural Research (ACIAR), the Federal Ministry for Economic Cooperation and Development of Germany, Thailand, the Philippines, Korea, and Japan.
Author contributions
M.v.Z., M.R., Y.-Y.C., and S.Ø.S. conceived the ideas; M.v.Z., M.R., S.Y.T., R.N., C.-H.C., J.-Y.Y., K.N., and S.Ø.S. contributed to data and information collection; M.v.Z. and S.Y.T. analysed the data; M.v.Z. and M.R. wrote the paper; R.S. provided ideas and critique in the analysis and writing process.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-58646-8.
References
- 1.Foyer CH, et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants. 2016;2:16112. doi: 10.1038/nplants.2016.112. [DOI] [PubMed] [Google Scholar]
- 2.Snapp, S. S., Blackie, M. J., Gilbert, R. A., Bezner-Kerr, R. & Kanyama-Phiri, G. Y. Biodiversity can support a greener revolution in. Africa. Proc. Natl. Acad. Sci107, 20840–20845 (2010). [DOI] [PMC free article] [PubMed]
- 3.Scheelbeek PFD, et al. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc. Natl. Acad. Sci. USA. 2018;115:6804–6809. doi: 10.1073/pnas.1800442115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dwivedi SL, et al. Landrace germplasm for improving yield and abiotic stress adaptation. Trends Plant Sci. 2016;21:31–42. doi: 10.1016/j.tplants.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Sharma, S., Upadhyaya, H. D., Varshney, R. K. & Gowda, C. L. L. Pre-breeding for diversification of primary gene pool and genetic enhancement of grain legumes. Front. Plant Sci. 4 (2013). [DOI] [PMC free article] [PubMed]
- 6.Prohens J, et al. Introgressiomics: a new approach for using crop wild relatives in breeding for adaptation to climate change. Euphytica. 2017;213:158. doi: 10.1007/s10681-017-1938-9. [DOI] [Google Scholar]
- 7.Farooq M, et al. Heat stress in grain legumes during reproductive and grain-filling phases. Crop and Pasture Sci. 2017;68:985–1005. doi: 10.1071/CP17012. [DOI] [Google Scholar]
- 8.Boukar, O. et al. Cowpea (Vigna unguiculata): Genetics, genomics and breeding. Plant Breed.138, 415–424 (2018).
- 9.Shanmugasundaram, S., Keatinge, J. & Hughes, J. The mungbean transformation: Diversifying crops, defeating malnutrition. International Food Policy Research Institute00922 (2009).
- 10.Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 11.Iseki K, Takahashi Y, Muto C, Naito K, Tomooka N. Diversity of drought tolerance in the genus Vigna. Front. Plant Sci. 2018;9:729. doi: 10.3389/fpls.2018.00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cane S. Australian Aboriginal subsistence in the Western desert. Hum. Ecol. 1987;15:391–434. doi: 10.1007/BF00887998. [DOI] [Google Scholar]
- 13.Priya, M. et al. GABA (γ-aminobutyric acid), as a thermo-protectant, to improve the reproductive function of heat-stressed mungbean plants. Sci. Rep. 9 (2019). [DOI] [PMC free article] [PubMed]
- 14.Harsh A, et al. Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia) Ann. Agric. Sci. 2016;61:57–64. doi: 10.1016/j.aoas.2016.02.001. [DOI] [Google Scholar]
- 15.Hanumantharao, B., Nair, R. M. & Nayyar, H. Salinity and high temperature tolerance in mungbean [Vigna radiata (L.) Wilczek] from a physiological perspective. Front. Plant Sci. 7 (2016). [DOI] [PMC free article] [PubMed]
- 16.Iseki K, Takahashi Y, Muto C, Naito K, Tomooka N. Diversity and evolution of salt tolerance in the genus Vigna. PLoS One. 2016;11:e0164711. doi: 10.1371/journal.pone.0164711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair, R. M. et al. Biotic and abiotic constraints in mungbean production—Progress in genetic improvement. Front. Plant Sci. 10 (2019). [DOI] [PMC free article] [PubMed]
- 18.War AR, Murugesan S, Boddepalli VN, Srinivasan R, Nair RM. Mechanism of Resistance in Mungbean [Vigna radiata (L.) R. Wilczek var. radiata] to bruchids, Callosobruchus spp. (Coleoptera: Bruchidae) Front. Plant Sci. 2017;8:1031. doi: 10.3389/fpls.2017.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakha M, Hanson P, Ramasamy S. Identification of resistance to Bemisia tabaci Genn. in closely related wild relatives of cultivated tomato based on trichome type analysis and choice and no-choice assays. Genet. Resour. Crop Evol. 2017;64:247–260. doi: 10.1007/s10722-015-0347-y. [DOI] [Google Scholar]
- 20.Khoury, C. K. et al. Distributions, conservation status, and abiotic stress tolerance potential of wild cucurbits (Cucurbita L.). Plants, People, Planet 1–15, doi:10.1002/ppp3.10085 (2019).
- 21.Khoury CK, et al. Crop wild relatives of pigeonpea [Cajanus cajan (L.) Millsp.]: Distributions, ex situ conservation status, and potential genetic resources for abiotic stress tolerance. Biol. Conserv. 2015;184:259–270. doi: 10.1016/j.biocon.2015.01.032. [DOI] [Google Scholar]
- 22.USDA, ARS & NPGS. GRIN-Taxonomy. (2018). Available at: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomysearch.aspx. (Accessed: 10th December 2018)
- 23.FAO. WIEWS - World information and early warning system on plant genetic resources for food and agriculture. http://www.fao.org/wiews/en/ (2018).
- 24.Trust, C. Genesys - the global gateway to genetic resources. https://www.genesys-org (2018).
- 25.Tomooka, N., Vaughan, D. A., Moss, H. & Maxted, N. The Asian Vigna: Genus Vigna subgenus Ceratotropis genetic resources. (Springer Netherlands, 2002).
- 26.Chamberlain, S., Ram, K., Barve, V. & Mcglinn, D. rgbif: Interface to the Global Biodiversity Information Facility. R package version 0.4. 0. (2016).
- 27.van Zonneveld M, et al. Tree genetic resources at risk in South America: A spatial threat assessment to prioritize populations for conservation. Divers. Distrib. 2018;24:718–729. doi: 10.1111/ddi.12724. [DOI] [Google Scholar]
- 28.Fick SE, Hijmans RJ. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- 29.Elith J, et al. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011;17:43–57. doi: 10.1111/j.1472-4642.2010.00725.x. [DOI] [Google Scholar]
- 30.Liu C, White M, Newell G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013;40:778–789. doi: 10.1111/jbi.12058. [DOI] [Google Scholar]
- 31.Fourcade Y, Engler JO, Rödder D, Secondi J. Mapping species distributions with MAXENT using a geographically biased sample of presence data: A performance assessment of methods for correcting sampling bias. PLoS One. 2014;9:e97122. doi: 10.1371/journal.pone.0097122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wickham, H. ggplot2: elegant graphics for data analysis. (Springer New York, 2009).
- 33.Tapiquén, C. E. P. World Countries. (2015). Available at, http://tapiquen-sig.jimdo.com. (Accessed: 11th December 2018).
- 34.Weinberger, K. Impact analysis of mungbean research in South and Southeast Asia. Final report of GTZ Project. (2003).
- 35.Kaga A, Isemura T, Tomooka N, Vaughan DA. The genetics of domestication of the azuki bean (Vigna angularis) Genetics. 2008;178:1013–1036. doi: 10.1534/genetics.107.078451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomooka, N., Pandiyan, M., Senthil, N., Ramamoorthi, N. & Kaga, A. Collection and conservation of crops and their wild relatives in Tamil Nadu, India. Annual Report on exploration and introduction of plant genetic resources. (2009).
- 37.Aitawade MM, et al. Section Ceratotropis of subgenus Ceratotropis of Vigna (Leguminosae - Papilionoideae) in India with a new species from northern Western Ghats. Rheedea. 2012;22:20–27. [Google Scholar]
- 38.Tomooka, N., Kaga, A., Isemura, T. & Vaughan, D. Vigna. in Wild Crop Relatives: Genomic and Breeding Resources: Legume Crops and Forages 291–311 (Springer Berlin Heidelberg, 2011).
- 39.Garba, M. & Pasquet, R. S. The Vigna vexillata (L.) A. Rich. gene pool. in Proceedings of the 2nd International Symposium on Tuberous Legumes (eds. Soerensen M., Estrella, E., Hamann, O. & Rios, R.) 61–71 (Copenhague MacKensie, 1998).
- 40.Somta P, Chankaew S, Rungnoi O, Srinives P. Genetic diversity of the Bambara groundnut (Vigna subterranea (L.) Verdc.) as assessed by SSR markers. Genome. 2011;54:898–910. doi: 10.1139/g11-056. [DOI] [PubMed] [Google Scholar]
- 41.Tomooka N, et al. Evolution, domestication and neo-domestication of the genus Vigna. Plant Genet. Resour. C. 2014;12:S168–S171. doi: 10.1017/S1479262114000483. [DOI] [Google Scholar]
- 42.Maxted, N. et al. An ecogeographic study: African Vigna. (IPGRI, 2004).
- 43.Hare JD. How insect herbivores drive the evolution of plants. Science. 2012;338:50–51. doi: 10.1126/science.1228893. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal AA, Hastings AP, Johnson MTJ, Maron JL, Salminen J-P. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science. 2012;338:113–116. doi: 10.1126/science.1225977. [DOI] [PubMed] [Google Scholar]
- 45.Züst T, et al. Natural enemies drive geographic variation in plant defenses. Science. 2012;338:116–119. doi: 10.1126/science.1226397. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro JA, Steffens JC, Mutschler MA. Acylsugars of the wild tomato Lycopersicon pennellii in relation to geographic distribution of the species. Biochem. Syst. Ecol. 1994;22:545–561. doi: 10.1016/0305-1978(94)90067-1. [DOI] [Google Scholar]
- 47.Lucatti AF, van Heusden AW, de Vos RC, Visser RG, Vosman B. Differences in insect resistance between tomato species endemic to the Galapagos Islands. BMC Evol. Biol. 2013;13:175. doi: 10.1186/1471-2148-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh KB, Ocampo B, Robertson LD. Diversity for abiotic and biotic stress resistance in the wild annual Cicer species. Genet. Resour. Crop Evol. 1998;45:9–17. doi: 10.1023/A:1008620002136. [DOI] [Google Scholar]
- 49.Kissoudis C, van de Wiel C, Visser RGF, van der Linden G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014;5:207. doi: 10.3389/fpls.2014.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gould SJ, Vrba ES. Exaptation—a missing term in the science of form. Paleobiology. 1982;1:4–15. doi: 10.1017/S0094837300004310. [DOI] [Google Scholar]
- 51.Harada E, et al. Expression profiling of tobacco leaf trichomes identifies genes for biotic and abiotic stresses. Plant Cell Physiol. 2010;51:1627–1637. doi: 10.1093/pcp/pcq118. [DOI] [PubMed] [Google Scholar]
- 52.Oghiakhe S, Jackai LEN, Makanjuola WA, Hodgson CJ. Morphology, distribution, and the role of trichomes in cowpea (Vigna unguiculata) resistance to the legume pod borer, Maruca testulalis (Lepidoptera: Pyralidae) Bull. Entomol. Res. 1992;82:499. doi: 10.1017/S0007485300042577. [DOI] [Google Scholar]
- 53.Bleeker PM, et al. Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. USA. 2012;109:20124–20129. doi: 10.1073/pnas.1208756109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalin, P., Ågren, J., Björkman, C., Huttunen, P., & Kärkkäinen, K. Leaf trichome formation and plant resistance to herbivory. Induced plant resistance to herbivory (Springer Netherlands, 2008).
- 55.IUCN. The IUCN Red List of threatened species. Version 2018-1. Available at: www.iucnredlist.org. (Accessed: 25th September 2018). (2018).
- 56.NatureServe. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. Available at: http://explorer.natureserve.or. (Accessed: 25th September 2018). (2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.