Abstract
Ischemic stroke, caused by a clot that blocks blood flow to the brain, can be severely disabling and sometimes fatal. We previously showed that transient focal ischemia in a rat model induces extensive temporal changes in the cerebral miRNAome, with a sustained decrease in the abundance of miR-7a-5p (miR-7), which represses the abundance of various proteins, including α-synuclein (α-Syn), that contribute to neurodegenerative disease. Here, we evaluated the therapeutic efficacy of a miR-7 mimic oligonucleotide after cerebral ischemia in rodents according to Stroke Treatment Academic Industry Roundtable (STAIR) criteria. Rodents were injected locally or systemically with miR-7 mimic either 2 hours before or 0.5–2 hours after transient middle cerebral artery occlusion. Decreased miR-7 expression was observed in both young and aged rats of both sexes after cerebral ischemia. Pre- or post-ischemic treatment with miR-7 mimic decreased the lesion volume in both sexes and ages studied. Furthermore, systemic injection of miR-7 mimic into mice at 30 min (but not 2 hours) after cerebral ischemia significantly decreased the lesion volume and improved motor and cognitive functional recovery with minimal peripheral toxicity. The miR-7 mimic treatment significantly reduced the post-ischemic induction of α-Syn, which has been shown to induce mitochondrial fragmentation, oxidative stress, and autophagy that promote neuronal cell death. Genetic deletion of α-Syn abolished miR-7 mimic-dependent neuroprotection and functional recovery in young male mice. Further analysis confirmed that the transcript encoding α-Syn was bound and repressed by miR-7. Our findings suggest that miR-7 mimics may therapeutically minimize stroke-induced brain damage and disability.
INTRODUCTION
Focal cerebral ischemia induces a complex series of biochemical and molecular events that include excitotoxicity, ionic imbalance, oxidative stress, endoplasmic reticulum (ER) stress, mitochondrial fragmentation, and apoptosis that synergistically jeopardize the cellular integrity and impair neurologic function (1–3). In addition to these, studies show that perturbations in noncoding RNAs (ncRNAs) and epigenetics also mediate some of the post-ischemic pathophysiologic changes. In particular, many microRNAs (miRNAs; a class of small ncRNAs) that arrest the translation by targeting the 3’-untranslated regions (UTRs) of mRNAs are extensively altered after cerebral ischemia and are implicated in mediating the secondary brain damage and plasticity after stroke (4–6). miR-7a-5p (miR-7) is one of the miRNAs that is decreased in a sustained manner during the acute phase (1 to 3 days of reperfusion – the return of blood flow to the brain) after transient cerebral ischemia in adult rodents (4).
The function of miR-7 reportedly ameliorates cellular stress under various conditions, including cancer (7) and Parkinson’s disease (PD) (8). In an animal model of PD, reduced abundance of miR-7 is implicated in the regulation of a purported target, the transcript encoding α-synuclein (α-Syn) (9, 10), the aggregation and accumulation of which is believed to contribute to the neurodegeneration in the disease (11). Incidentally, we have also previously shown that cerebral ischemia in rodents induces α-Syn expression and aggregation and that knockdown or knockout of α-Syn decreases infarction, mitochondrial fragmentation, oxidative stress, apoptosis, and autophagy and promotes better neurological recovery after cerebral ischemia (12). This suggests that abnormal aggregation of α-Syn promotes neuronal death not only in chronic neurodegenerative conditions like PD but also in acute conditions like ischemic stroke. Although α-Syn mRNA has been identified as a direct target of miR-7 in an in vitro model of PD (9), the underlying molecular mechanisms that are responsible for the induction of α-Syn after focal cerebral ischemia are not yet identified.
Here, following on from that work, we investigated whether reductions in miR-7 after cerebral ischemia is more generally associated with secondary ischemic brain damage in rodents, whether increasing its abundance by injection of miR-7–mimic oligonucleotides might protect the brain after stroke, and whether miR-7 is mechanistically connected to a-Syn in the cellular, tissue, and behavioral effects of stroke. To satisfy the Stroke Treatment Academic Industry Roundtable (STAIR) criteria (13), we assessed young and aged animals of both sexes, pre- and post-ischemic treatment, intracerebral (IC) and intravenous (retro-orbital; IV) routes of administration, central and peripheral toxicity, and multiple outcome parameters (histologic damage and motor function recovery).
RESULTS
miR-7 was decreased in the brain after cerebral ischemia in rats
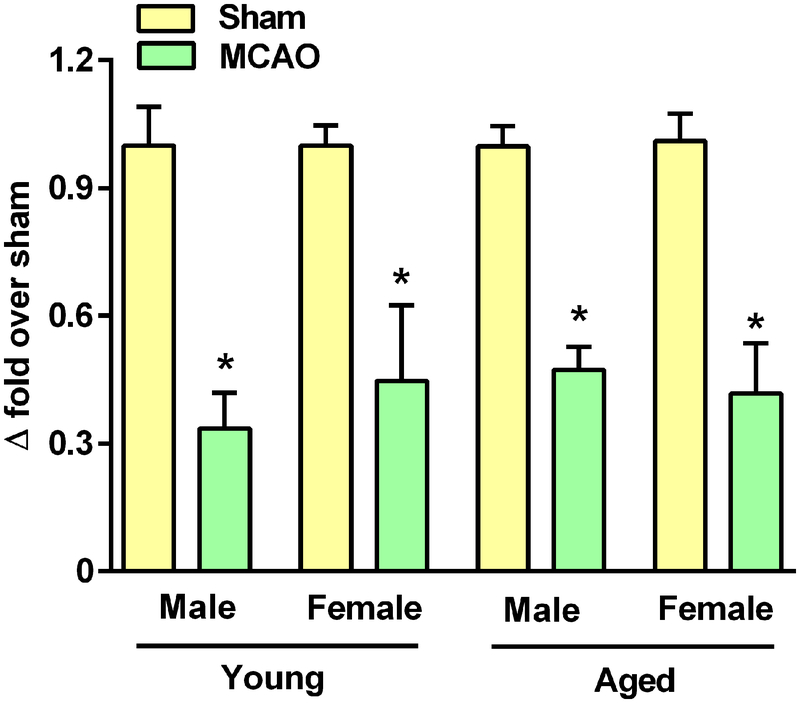
We previously detected a sustained decrease in miR-7 expression after experimental stroke in adult male rodents (4). However, age and sex have a complex and interactive effect on ischemic stroke risk and pathophysiology (14). After 60 min transient middle cerebral artery occlusion (MCAO) and 1 day of reperfusion, we detected a 2.1 to 3 fold decrease in miR-7 expression in the ipsilateral cortex of both young and aged rats of both sexes compared to the sham-operated controls in (Fig. 1). These data indicate that a decrease in miR-7 appears to be associated with stroke regardless of age or gender.
Fig. 1: Cerebral ischemia decreased miR-7 expression.
RT-PCR assessment of the abundance of miR-7 in the cortical peri-infarct region of 85–95–day-old (young) and 300–330–day-old (aged) male and female rats upon 1 day of reperfusion after 60 min transient focal cerebral ischemia relative to those that underwent a sham (control) procedure. Data are mean ± SD (n = 4 rats per group); *p<0.05 compared to the corresponding sham, by Mann-Whitney U test.
Pre-ischemic intracerebral administration of miR-7 mimic improved motor function recovery and decreased lesion volume in young male rats
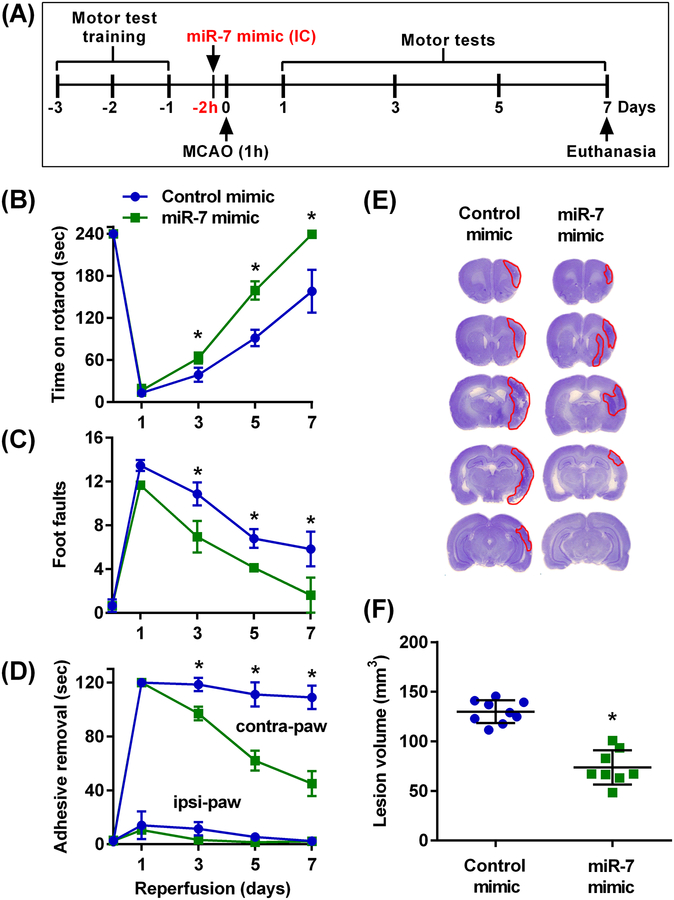
We then tested whether restoring miR-7 abundance by treating rats with mimic oligonucleotides improves functional recovery after cerebral ischemia. miR-7 mimic was first injected intracerebrally 2 hours before 60 min transient MCAO to ensure its immediate availability upon ischemic insult (Fig. 2A). Spontaneously hypertensive rats were used for this experiment, because hypertension is one of the major stroke comorbidities, and young males were used to initially minimize the confounding variables of hormones and old age on the results. Performance on a rotarod test, measured at the 3rd to 7th day of reperfusion, revealed that post-ischemic motor dysfunction that was seen in control young male rats was significantly curtailed in the miR-7 mimic pre-treated group (Fig. 2B). Similar results were seen on other motor function tests: the beam-walk test (Fig. 2C) and the adhesive removal test (Fig. 2D). The lesion volume measured at 7 days of reperfusion was also significantly smaller in the miR-7 mimic group compared to the control mimic group (by 43.2%) (Fig. 2, E and F).
Fig. 2: Pre-ischemic intracerebral miR-7 mimic treatment improved motor function recovery and decreased lesion volume in young male rats.
(A) Schematic diagram of the experimental design, wherein motor training was performed for 3 days prior to 60 min (1h) transient MCAO. Injection of the miR-7 or control mimics was initiated 2 hours before transient MCAO. Motor testing was then performed at days 1–7 of reperfusion. Brains were collected at 7 days of reperfusion for lesion volume assessment. (B to D) Recovery of functional performance on the rotarod test (B), beam-walk test (C) and adhesive removal test (D) by young male rats that received an intracerebral injection of either control or miR-7 mimic 2 hours before 60 min transient MCAO. The performance was assessed before treatment (time 0) and on days 1 through 7 of reperfusion. Data are mean ± SD (n = 8 to 9 rats per group). *p<0.05 compared to the respective control mimic group, by repeated measures ANOVA followed by Sidak’s multiple comparisons post-test. (E and F) Representative cresyl violet-stained serial sections (E) and quantified lesion volume (F) in brains from mice treated with the miR-7 mimic and those treated with the control mimic. Lesion volume was measured at 7 days of reperfusion. Data are mean ± SD (n = 8 to 9 rats per group). *p<0.05 compared to the control mimic group, by Mann-Whitney U test. Rats were randomly assigned to treatment groups and brains were obtained and analyzed by an investigator blinded to the study groups.
Post-ischemic intracerebral administration of miR-7 mimic decreased ischemic brain damage irrespective of sex and age in rats
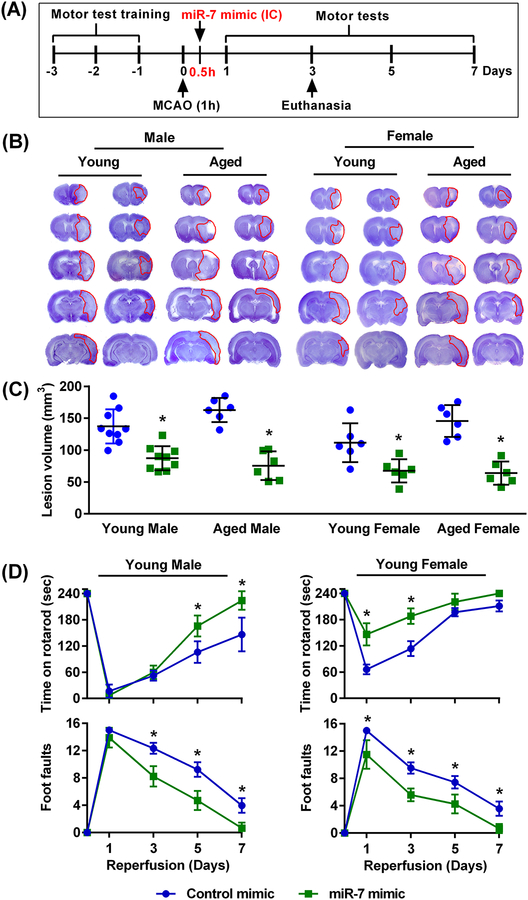
Given that we observed neuroprotection in young male rats by injecting miR-7 mimic intracerebrally before cerebral ischemia, we next examined whether miR-7-mediated neuroprotection can also be observed in both sexes, young and old, when treated after cerebral ischemia. For this experiment, we injected miR-7 mimic 30 min after 60 min transient MCAO (Fig. 3A), which is a clinically relevant time point. All 4 groups of rats (male and female, young and aged) treated with miR-7 mimic showed significantly decreased lesion volume compared to respective control mimic-treated groups. Whereas young male and female cohorts showed 36% to 40% smaller infarcts, aged male and female cohorts showed 54% to 56% smaller infarcts (Fig. 3, B and C). Furthermore, post-ischemic motor dysfunction was significantly curtailed in miR-7 mimic-treated young male and female rats compared to control mimic-treated rats as measured by the rotarod and beam-walk tests (Fig. 3D).
Fig. 3: Post-ischemic intracerebral treatment with miR-7 mimic protected the rat brain irrespective of age and sex.
(A) Schematic diagram of the experimental design, wherein rats were subjected to 60 min (1h) transient MCAO followed 30 min (0.5h) thereafter (during reperfusion) by injection of the miR-7 or control mimic. Brains were collected at 3 days of reperfusion for lesion volume assessment. Motor training was performed on separate cohorts of rats for 3 days prior to 60 min (1h) transient MCAO, followed 30 min (0.5h) thereafter (during reperfusion) by injection of the miR-7 or control mimic, and then motor testing at 1–7 days after. (B) Representative cresyl violet-stained serial sections (B) and lesion volume (C) in brains from the miR-7 mimic- and control mimic-treated groups of both sexes and ages. Lesion volume was measured at day 3 of reperfusion after 60 min transient MCAO. Data are mean ± SD (n = 6–9 rats per group). *p<0.05 compared to the control mimic group, by Mann-Whitney U test. (D) Recovery of functional performance assessed by the rotarod test and beam-walk test by young male and female rats that received an intracerebral injection of either control or miR-7 mimic 30 min after 60 min transient MCAO. The performance was assessed before treatment (time 0) and on days 1 through 7 of reperfusion. Data are mean ± SD (n = 4 rats per group). *p<0.05 compared to the respective control mimic group, by repeated measures ANOVA followed by Sidak’s multiple comparisons post-test.
Post-ischemic IV administration of miR-7 mimic decreased brain damage after cerebral ischemia in young male mice
Given that miR-7 treatment was neuroprotective in both sexes and ages, we then focused on improving the clinical relevance of miR-7 delivery by injecting it through an intravenous (IV) route, specifically through the retro-orbital venous sinus. For this experiment, we switched our animal model to mice due to both the amount of miR-7 mimic required to be injected in rats versus mice as well as experimental restrictions in the rat stroke model (such as laser speckle imaging).
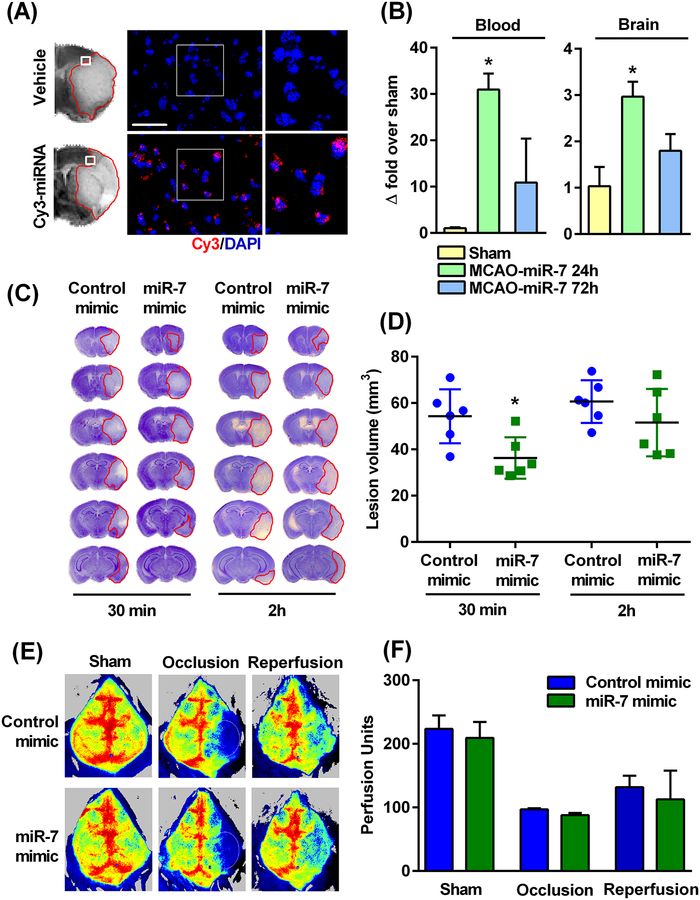
In the brains of mice injected IV with Cy3-tagged miR-7 mimic at 30 min of reperfusion after 90 min transient MCAO, several cell nuclei in the ipsilateral penumbral region showed significant fluorescence at 24h of reperfusion (Fig. 4A). Furthermore, the identical systemic injection of miR-7 mimic after 90 min transient MCAO significantly increased blood as well as brain miR-7 levels at 24h of reperfusion (Fig. 4B). Post-ischemic IV treatment with miR-7 mimic (injected at 30 min of reperfusion after 90 min transient MCAO) significantly decreased the lesion volume in young male mice (by 33%; measured at 3 days of reperfusion) compared to the control mimic-treated cohort (Fig. 4, C and D). However, post-ischemic IV treatment at 2 hours of reperfusion did not induce significant protection (Fig. 4, C and D). The in vivo laser speckle imaging revealed that the protective effects of IV miR-7 mimic administration were not due to changes in cerebral blood flow (Fig. 4, E and F).
Fig. 4: Post-ischemic IV injection of miR-7 mimic decreased ischemic brain damage in young male mice.
(A) A Cy3-labeled mimic injected IV (retro-orbital venous sinus) at 30 min of reperfusion following 90 min transient MCAO was observed to be present in the peri-infarct region of the ipsilateral cortex at 24h of reperfusion. (B) The abundance of miR-7, assessed by real-time PCR, in blood and the peri-infarct region of the ipsilateral cortex of young male mice at 24h and 72h of reperfusion after 90 min transient MCAO, relative to those that underwent a sham (control) procedure. Data are mean ± SD (n = 4 mice per group). *p<0.05 compared to sham, by Kruskal-Wallis one-way ANOVA followed by Dunn’s post-test. (C and D) Representative cresyl violet-stained serial sections (C) and lesion volume (D) in brains from the miR-7 mimic- and control mimic-treated groups injected at either 30 min or 2h of reperfusion. Lesion volume was measured at day 3 of reperfusion after the 90 min transient MCAO. Data are mean ± SD (n = 6 mice per group). *p<0.05 compared to the control mimic group, by Mann-Whitney U test. (E and F) Representative in vivo laser speckle imaging (E) showing changes in cerebral blood flow before, during, and 24h after 90 min transient MCAO from the miR-7 mimic- and control mimic-treated groups injected at 30 min of reperfusion. Data are mean ± SD (n = 4 mice per group). *p<0.05 compared to the corresponding control mimic group, by Mann-Whitney U test (G). Scale bar, 30 μm.
Post-ischemic IV administration of miR-7 mimic decreased the stroke-induced cognitive deficit and accelerated motor recovery in young male mice
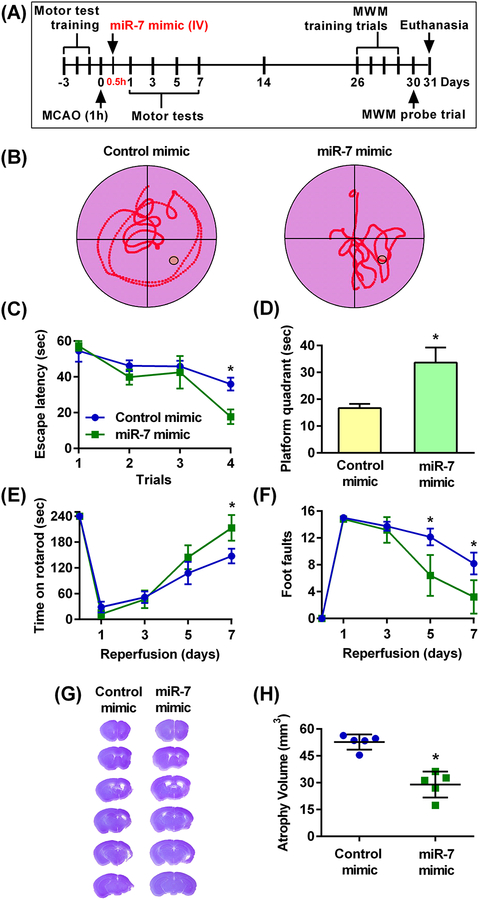
Because we found smaller lesion volume in mice treated with miR-7 mimic intravenously at 30 min of reperfusion, we further evaluated if miR-7 mimic treatment at this time point can also improve functional recovery. The substantial brain damage, mortality and profound functional deficits in mice subjected to 90 min of transient MCAO make it difficult to follow these mice for longer than 3 days. Therefore, we subjected an additional cohort of young male mice to 60 min transient MCAO followed by intravenous miR-7 mimic treatment at 30 min of reperfusion, allowing longer term follow-up. These mice were then assessed for cognitive deficits with the Morris water maze (MWM) test (Fig. 5A). Mice treated with miR-7 mimic showed a significantly improved memory retention compared to control mimic-treated mice (Fig. 5B). Specifically, miR-7 mimic-treated mice located the platform faster during the training trial (Fig. 5C) and stayed in the platform quadrant significantly longer during the probe trial (Fig. 5D). Motor function recovery assessed with the rotarod test (Fig. 5E) and the beam-walk test (Fig. 5F) was also significantly better between days 5 and 7 of reperfusion in the miR-7 mimic group compared to the control mimic group. In addition, miR-7 mimic-treated mice showed significantly decreased atrophy volume measured at day 31 of reperfusion following transient MCAO compared to control mimic-treated mice (Fig. 5, G and H).
Fig. 5: Post-ischemic IV administration of miR-7 mimic decreased the cognitive deficit and accelerated motor recovery in young male mice.
(A) Schematic diagram of the experimental design, wherein young male mice were subjected to 60 min (1h) transient MCAO followed 30 min (0.5h) thereafter (during reperfusion) by injection of the miR-7 or control mimics. Motor training was performed for 3 days prior to transient MCAO and motor performance was then assessed at days 1–7 of reperfusion. Training trials for the Morris water maze (MWM) test were initiated at 26 days of reperfusion for 4 days followed by the probe trial at 30 days of reperfusion. Brains were collected at 31 days of reperfusion for atrophy volume assessment. (B) Representative trace maps from the MWM tests assessing memory retention in the miR-7 mimic-treated and control mimic-treated cohorts during the probe trial. (C) Time taken by control- and miR-7 mimic-treated mice to reach the platform (”escape latency”) during the training trials. Data are mean ± SD (n = 5 mice per group). *p<0.05 compared to the corresponding control mimic group, by repeated measures ANOVA followed by Sidak’s multiple comparisons post-test. (D) Length of time mice treated with the control- or miR-7 mimic remained in the platform quadrant during the probe trial. Data are mean ± SD (n = 5 mice per group). *p<0.05 compared to the control mimic group, by Mann-Whitney U test. (E and F) Functional recovery assessed by the rotarod test (E) and the beam-walk test (F) in control- and miR-7 mimic-treated mice over 7 days of reperfusion. Data are mean ± SD (n = 5 mice per group). *p<0.05 compared to the corresponding control mimic group, by repeated measures ANOVA followed by Sidak’s multiple comparisons post-test. (G and H) Representative cresyl violet-stained serial sections (G) and atrophy volume (H) from miR-7 mimic or control mimic-treated mice. Data are mean ± SD (n = 5 mice per group). *p<0.05 compared to the control mimic group, by a Mann-Whitney U test.
Post-ischemic IV administration of miR-7 mimic did not reveal overt evidence of toxicity to peripheral organs in young male mice
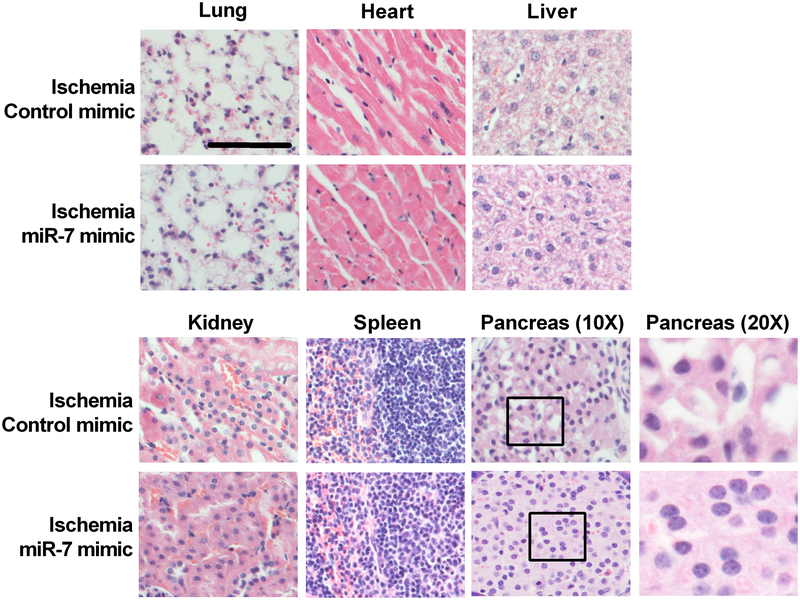
At approximately 60 days of reperfusion following 60 min transient MCAO, animals showed some degree of multifocal hepatic leukocyte infiltrates, sometimes with presumptively related oval cell hyperplasia, and some degree of renal interstitial nephritis, tubular degeneration, and peri-pelvic leukocyte infiltrates (Fig. 6). However, these changes were similar in both control mimic and miR-7 mimic groups. The islets of Langerhans (pancreas) in the control mimic-treated group had moderate cellular vacuolation, while the miR-7 mimic-treated group had relatively homogeneously granular cell cytoplasm with no vacuolation. In addition, one animal in the miR-7 group had robust lymphoid cuffing around several bronchioles that was not observed in the other two animals in the group.
Fig. 6: Post-ischemic IV administration of miR-7 mimic did not reveal overt evidence of toxicity to peripheral organs.
Representative H&E staining images of lung, heart, liver, kidney, spleen, and pancreas in ischemic mice injected with either miR-7 mimic or control mimic at 30 min of reperfusion following 60 min transient MCAO. Mice were euthanized at approximately 60 days of reperfusion for histopathologic assessment. Boxes and inserts show magnification of the pancreas to examine cellular vacuolation in the islets of Langerhans. Scale bar, 30 μm.
3’-UTR of α-Syn mRNA has a conserved binding site for miR-7
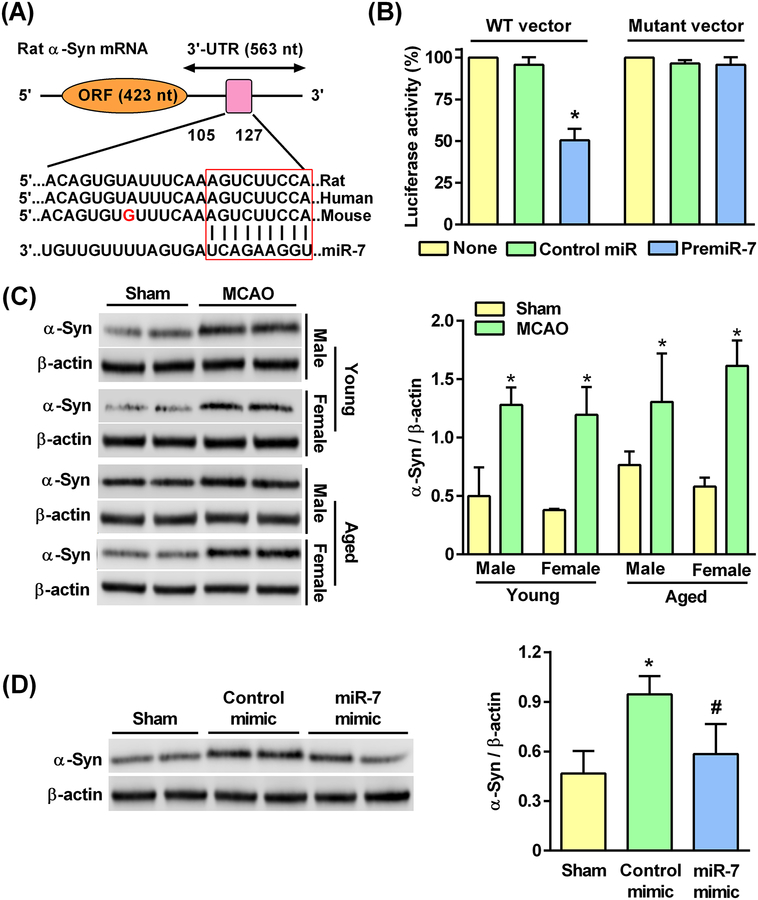
We used four miRNA target prediction algorithms with different target searching parameters (microRNA.org, TargetScan, miRDB, and miRanda) to identify the miRNAs that bind to the 3’-UTR of α-Syn mRNA. Four rat miRNAs (rno-miR-7a, rno-miR-7b, rno-miR-673, and rno-miR-153) were predicted to target α-Syn mRNA by all four algorithms. Furthermore, α-Syn is one of the top predicted targets for miR-7 by TargetScan (Version 7.1) with a cumulative weighted context++ score of 0.99 (CWCS; a computational model to predict the most effectively targeted mRNAs) for the interaction between miR-7 and α-Syn 3’-UTR binding site (15). Within the α-Syn 3’-UTR, the miR-7 target sequence was observed to be between bases 105 and 127, which is conserved in human, mouse, and rat (Fig. 7A).
Fig. 7: miR-7 mimic treatment prevented the post-MCAO induction of α-Syn protein.
(A) The miR-7 seed sequence in the 3’-UTR of α-Syn mRNA is outlined with the red box. (B) The expression of α-Syn 3’-UTR luciferase vector when co-transfected with premiR-7 compared to a mutant vector in PC12 cells. Luciferase activities were normalized to Renilla luciferase activities and shown as percent of control miR. Data are mean ± SD (n = 4 batches of cells per group). *p<0.05 compared to the control miR group, by Kruskal-Wallis one-way ANOVA followed by Dunn’s post-test. (C) Western blot analysis of α-Syn protein levels in the cortical peri-infarct region of rats induced at 1 day of reperfusion after 60 min transient MCAO. Blots were representative of 4 independent experiments. Data are mean ± SD (n = 4 rats per group). *p<0.05 compared to corresponding sham, by Mann-Whitney U test. (D) Western blot analysis of α-Syn protein levels induced at 1 day of reperfusion after 60 min transient MCAO in the cortical peri-infarct region of young male rats treated with miR-7 mimic at 2 hours prior to 60 min MCAO. Data are mean ± SD (n = 4 rats per group). *p<0.05 compared to sham, and #p<0.05 compared to the control mimic group, by Kruskal-Wallis one-way ANOVA followed by Dunn’s post-test.
miR-7 targets and suppresses α-Syn expression
To conclusively show the miR-target relationship, we co-transfected α-Syn 3’-UTR vector or a mutant α-Syn 3’-UTR vector (in which the miR-7 seed sequence was mutated) with premiR-7 or a control premiR in PC12 cells. We observed a significant reduction of α-Syn 3’-UTR expression (by 49.9%) in the premiR-7-treated cells compared to control premiR-treated cells, and disruption of miR-7 binding site abrogated the premiR-7-mediated inhibition of luciferase activity of α-Syn vector by >98% (Fig. 7B). We then explored this connection in rats. At 1 day of reperfusion following 60 min transient MCAO, α-Syn protein levels were significantly increased in all the 4 groups of rats (young: by ~3.1 fold in males and by ~3.2 fold in females, and aged: by ~1.7 fold in males and by ~2.4 fold in females) compared to their respective sham controls (Fig. 7C). In young male rats, miR-7 mimic treatment (2 hours before transient MCAO) significantly reduced the post-ischemic α-Syn protein induction (by 38.2%) compared to the control mimic-treated group (Fig. 7D).
Post-ischemic IV administration of miR-7 mimic failed to improve cognitive function and motor recovery in young male α-Syn−/− mice
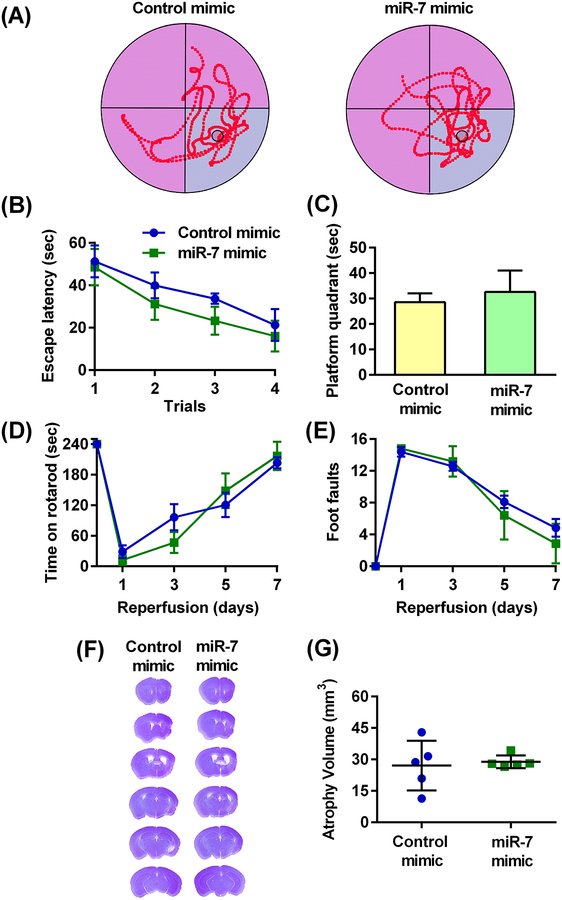
To examine if α-Syn is a major target of miR-7-mediated neuroprotection in the post-ischemic brain, we induced 60 min transient MCAO in α-Syn−/− mice (C57BL/6N-Sncatm1Mjff/J) followed by IV injection of miR-7 at 30 min of reperfusion. These mice were then assessed for cognitive and motor deficits as described in Fig. 5A. α-Syn−/− mice treated with miR-7 mimic failed to show any improvement in the MWM test compared to control mimic-treated α-Syn−/− mice (Fig. 8A). Specifically, there was no difference in escape latency during the training trial (Fig. 8B), and both groups spent the similar time in the platform quadrant during the probe trial (Fig. 8C). Motor function recovery assessed with the rotarod test (Fig. 8D) and the beam-walk test (Fig. 8E) was also found to be similar between the 2 groups. In addition, miR-7 mimic-treated and control miR-treated α-Syn−/− mice showed similar atrophy volume measured at day 31 of reperfusion following transient MCAO (Fig. 8, F and G).
Fig. 8: Post-ischemic IV miR-7 mimic administration failed to decrease cognitive deficits and motor dysfunction in young male α-Syn-deficient mice.
(A) Representative trace maps of the MWM showing the swim patterns of miR-7 mimic-treated and control mimic-treated α-Syn−/− mice during the probe trial. (B) Escape latency, or time to reach the platform, in control or miR-7-treated mice during the training trials. Data are mean ± SD (n = 5 mice per group). *p<0.05 compared to the corresponding control mimic group, by repeated measures ANOVA followed by Sidak’s multiple comparisons post-test. (C) Length of time mice treated with the control- or miR-7 mimic remained in the platform quadrant during the probe trial. Data are mean ± SD (n = 5 mice per group). *p<0.05 compared to the control mimic group, by Mann-Whitney U test. (D and E) Functional recovery assessed by the rotarod test (D) and beam-walk test (E) in control- and miR-7 mimic-treated mice over 7 days of reperfusion. Data are mean ± SD (n = 5 mice per group). *p<0.05 compared to the corresponding control mimic group, by repeated measures ANOVA followed by Sidak’s multiple comparisons post-test. (F and G) Representative cresyl violet-stained serial sections (F) and atrophy volume (G) from miR-7 mimic or control mimic-treated mice. Data are mean ± SD (n = 5 mice per group). *p<0.05 compared to the control mimic group, by Mann-Whitney U test.
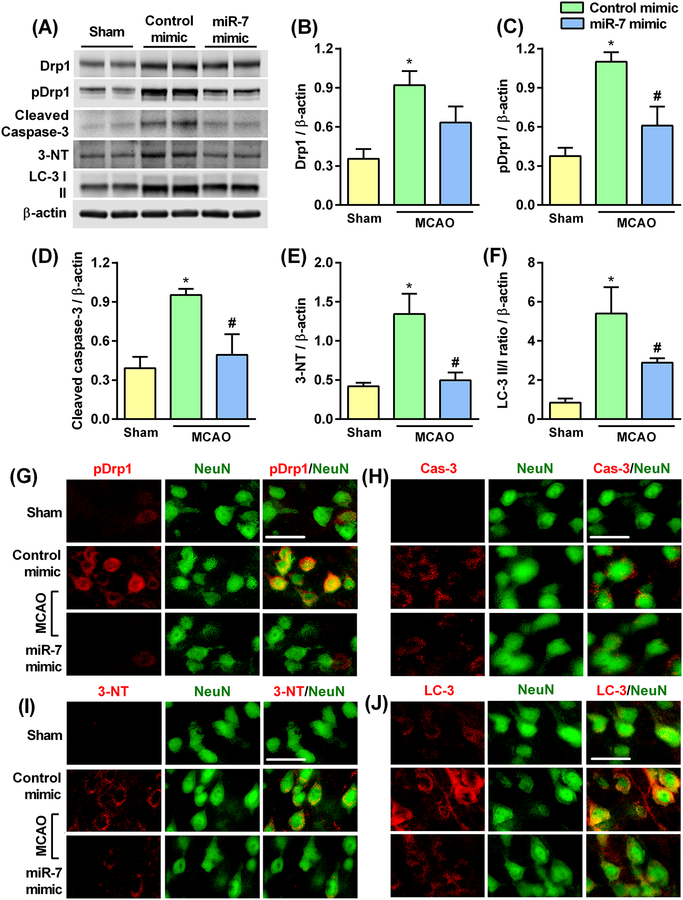
miR-7 treatment curtailed post-ischemic mitochondrial dysfunction, apoptosis, oxidative stress and autophagy in young male rats
In young male rats pre-treated with miR-7 mimic, there was significant reduction in post-ischemic protein abundance of Drp1 and phospho-Drp1 (markers of mitochondrial fragmentation; Fig. 9, A to C), cleaved caspase-3 (marker for apoptosis) (Fig. 9, A and D), 3-NT (a marker of oxidative stress; Fig. 9A and E), and LC3-II/I ratio (a marker of autophagy; Fig. 9A and F), compared to the control mimic-treated group at 3 days of reperfusion following transient MCAO. Immunohistochemical analysis confirmed the Western blot results and further showed colocalization of pDrp1 (Fig. 9G), cleaved caspase-3 (Fig. 9H), 3-NT (Fig. 9I) and LC-3 II/I (Fig. 9J) with the neuronal marker NeuN after transient MCAO. This data indicates that miR-7 treatment is associated with suppression of known ischemic pathological markers.
Fig. 9: miR-7 treatment curtailed post-ischemic mitochondrial dysfunction, apoptosis, oxidative stress, and autophagy.
(A to F) Western blotting and quantification of the protein abundance of markers of mitochondrial fragmentation (Drp1 and pDrp1) (A, B and C), apoptosis (cleaved caspase-3) (A and D), oxidative stress (3-NT) (A and E) and autophagy (LC-3 II/I ratio) (A and F) in the peri-infarct region of the ipsilateral cortex from miR-7 mimic- and control mimic-treated rats, assessed at 3 days of reperfusion after 60 min transient MCAO. Blots are representative of 4 independent experiments. Data are mean ± SD (n = 4 rats per group). *p<0.05 compared to sham and #p<0.05 compared to the control mimic group, by Kruskal-Wallis one-way ANOVA followed by Dunn’s post-test. (G to J) Immunostaining of pDrp1 (G), cleaved caspase-3 (H), 3-NT (I), and LC-3 (J) in the NeuN+ cells (neuronal) in the cortical peri-infarct region of rats treated with miR-7 mimic or the control mimic assessed at 7 days of reperfusion after 60 min transient MCAO. Representative immunofluorescence images were taken from the cortical peri-infarct region where neurons were still relatively intact and viable. Scale bar, 30 μm.
DISCUSSION
We found that focal cerebral ischemia decreased miR-7 and increasing its basal levels reduced ischemic brain damage in both sexes irrespective of age. We also showed that miR-7 mimic was efficacious when administered centrally or peripherally and whether given pre- or post-ischemia. Furthermore, miR-7 mimic protected the brain across species and has no peripheral or central toxicity. miR-7 mimic also promoted better neurological recovery after stroke in young male and female rodents. Mechanistically, miR-7 administration repressed the expression of the α-Syn protein and genetic deletion of α-Syn ablated miR-7-mediated neuroprotection after cerebral ischemia. Lastly, miR-7 administration resulted in curtailed mitochondrial fission, apoptosis, oxidative stress and autophagy in the post-ischemic brain.
Experimental and clinical studies show that sex and age play an important role in deciding the outcome after ischemic stroke (14, 16). At younger ages, males tend to have a higher risk of stroke and higher brain damage after stroke than females. However, this trend reverses, particularly when females reach menopause. Older females show higher stroke prevalence as well as the higher amount of post-stroke brain damage than younger males and females, and older males. In addition, studies indicate that miRNAs play important roles in mediating sex and/or age-specific outcome after cerebral ischemia by regulating stroke-related genes (17, 18). This suggests the importance of either using tailor-made miRNA therapies based on sex or finding a miRNA therapy that is universally efficacious in both sexes. We observed that miR-7 down-regulation after cerebral ischemia was neither age nor sex-dependent. Increasing the basal miR-7 levels reduced the post-ischemic lesion volume not only in male but also in the female of young and aged rats. In addition, miR-7 mimic administration accelerated motor function recovery in young male and female rats, and ameliorated learning and memory deficits in young male mice. This indicates that miR-7 mimic is an attractive therapeutic candidate to consider for translation to stroke patients.
As per STAIR criteria, efficacy when given peripherally is preferred for the future stroke therapeutic. Using young male mice, our studies showed that miR-7 mimic satisfies this. When Cy3-tagged miR-7 mimic was administered IV, neurons in the ipsilateral peri-infract cortex showed the fluorescence indicating the presence of the mimic at 1 day of reperfusion. Real-time PCR analysis further confirmed that post-stroke peripheral miR-7 mimic injection robustly increases blood and brain miR-7 levels at 1 day of reperfusion. Furthermore, miR-7 mimic given peripherally at 30 min, but not at 2h, of reperfusion significantly decreased the lesion volume as well as functional recovery after transient MCAO. Laser speckle imaging showed that this effect is not due to differential cerebral blood flow between groups. Significant reduction in the lesion volume when miR-7 mimic was injected at 30 min, but not at 2h, of reperfusion time suggests that an early treatment more effectively suppresses post-ischemic induction of α-Syn expression. Targeting α-Syn mRNA as early as possible after stroke seems to reduce toxic accumulation of α-Syn and downstream pathological process.
Any new therapeutic needs to be safe before translating to humans. Hence, we examined the long-term pathology in peripheral organs following IV administration of miR-7 in mice subjected to transient MCAO. At approximately 60 days after the treatment, no major histologic abnormalities were observed in the lung, heart, liver, kidney, and spleen of either miR-7 mimic or control mimic-treated animals. Although animals of both groups exhibited moderate multifocal leukocyte infiltration in the liver and renal interstitial nephritis and tubular degeneration and regeneration in kidneys, these changes are similar in both groups and probably reflect stroke-induced effects.
One notable exception was in the endocrine pancreas which showed moderate cellular vacuolation among the islets of Langerhans in the control mimic group, but homogenously granular cell cytoplasm in the miR-7 mimic group. Previous studies show that pancreatic transcription factors Ngn3 and NeuroD/Beta2 induce miR-7 expression in the pancreas which might regulate the development and function of the pancreas (19). In adult mice, miR-7 is enriched in pancreatic islets where it targets the mTOR signaling pathway and thus retards β-cell proliferation (20). The miR-7 knockout mice show altered expression of the genes that control late stages of insulin granule fusion with the plasma membrane and ternary SNARE complex activity indicating the role of miR-7 in regulating β-cell function (21). Stroke is known to alter pancreatic function. NGF synthesized in the β cells is a known promoter of insulin, and transient MCAO in adult mice is shown to increase NGF expression and insulin content altered pancreatic function (22). Furthermore, levels of pancreatic enzymes amylase and lipase in the serum are shown to increase in stroke patients, probably due to altered autonomic nervous system function (23, 24). At this time, it is not conclusively known if miR-7 is mechanistically related to this post-ischemic disturbance of pancreas or has any functional implications in pro-survival signaling in the pancreatic islet cells, but evidence suggests possible links among various pathological conditions including PD, stroke, and diabetes.
As miRNAs do not code for proteins, their effects are assumed to be mediated by modulation of their targets. Our previous study showed that stroke increases α-Syn abundance, oligomerization, and translocation into neuronal nuclei in both rodents and humans, and that blocking these α-Syn effects is neuroprotective after cerebral ischemia in rodents (12). In this study, in silico analysis predicted, and we experimentally confirmed, that α-Syn transcripts are a target of miR-7. α-Syn is one of the most abundantly expressed proteins in the mammalian brain (25). The normal cellular functions of α-Syn are not known, but its aggregation and accumulation over years promote neurodegeneration observed in PD and Alzheimer’s disease (26–28). In contrast, the role of α-Syn in neuronal death after acute CNS insults like stroke is not well understood. Our study indicates that miR-7 minimizes post-ischemic brain damage by directly suppressing the abundance of α-Syn.
Previous studies show the therapeutic potential of miRNAs in protecting the brain after acute as well as chronic conditions and an apparent lack of adverse effects by miRNA therapies (29). As miR-7 also targets several mRNAs associated with growth and metastasis, its modulation is thought to suppress various types of malignant tumors (7). In addition, miR-7 is also thought to be a potential clinical diagnostic marker of cancer recurrence (30). Promoting miR-7 levels is also shown to suppress α-Syn toxicity in PD (9, 10). Our findings here suggest that miR-7 is a promising therapeutic option to mitigate α-Syn-mediated secondary brain damage in stroke and might be a potential multi-faceted – and minimally toxic – compound that acts on multiple downstream targets within apoptosis, autophagy, oxidative stress, and mitochondrial damage to prevent neurological pathology in stroke and possibly also synucleinopathies like PD.
MATERIALS AND METHODS
All experimental protocols using animals were approved by the University of Wisconsin Research Animal Resources and Care Committee, and animals were cared in accordance with the Guide for the Care and Use of Laboratory Animals, U.S. Department of Health and Human Services Publication Number. 86–23 (revised). In all experiments, animals were randomly assigned to groups. Behavioral and histological analyses were performed by an investigator blinded to the study groups. Experiments were conducted in compliance with the “Animal Research: Reporting of In Vivo Experiments (ARRIVE)” guidelines.
Animals
For experiments in Fig. 1, 2, 3, 7 and 9, young (85 to 95 days) and/or aged (300 to 330 days) spontaneously hypertensive rats (SHR) of both sexes (Charles River USA) were used. The body weights of various groups were as follows: young male, 250–275g; aged male, 300–350g; young female, 180–210g; and aged female, 200–225g. For experiments in Fig. 4–6, male C57BL/6 mice (3 months old; 25–28g; Jackson Labs USA) were used. For experiments in Fig. 8, male homozygous α-Syn−/− mice (C57BL/6N-Sncatm1Mjff/J; 3–4 months old; 27–30g; Jackson Labs USA) were used. Animals were housed in a standard pathogen-free environment with free access to food and water.
Focal cerebral ischemia
Under isoflurane anesthesia, middle cerebral artery (MCA) was occluded (60 min for rats and 60–90 min for mice) using a silicone coated nylon monofilament (4–0 for rats and 6–0 for mice; Doccol USA) followed by 1 day, 3 days or 7 days of reperfusion as described previously (4, 12). Sham-operated animals served as control. Regional cerebral blood flow and physiological parameters (pH, PaO2, PaCO2, hemoglobin, and blood glucose) were monitored, and rectal temperature was maintained at 37.0 ± 0.5°C during surgery. To minimize the impact of the estrous cycle, female rats were randomly assigned to groups. Rodents with no evidence of neurological deficit were excluded. Upon euthanasia, rodents that showed hemorrhage were also excluded.
microRNA injections
Rats were injected intracerebrally with miR-7 mimic (Cat#: 4464070; Mature miRNA Sequence: UGGAAGACUAGUGAUUUUGUUGUU) targeting 3’-UTR of α-Syn mRNA or non-targeting negative control miR mimic (Cat#: 4464061; Life Technologies USA) at either 2h prior to transient MCAO (Fig. 2, 7, 9) or 30 min (Fig. 3) of reperfusion after transient MCAO as described earlier (31). In brief, miR-7 mimic (8 nmol for pre-MCAO injection and 16 nmol for post-MCAO injection) in buffer was mixed with 2 μl invivofectamine (Cat#: 1377501; Life Technologies USA), incubated at 50°C for 1h and injected stereotaxically (1 μl/5min) into cerebral cortex (bregma: −0.2 mm posterior, 3 mm dorsoventral, 4.5 mm lateral). To test whether miRNA injected IV (retro-orbital sinus) enters into the post-ischemic brain, mice were injected with 25 nmol of Cy3-labeled pre-miR Negative Control #1 (Cat#: AM17120; Life Technologies USA) mixed with PEG-Liposome In Vivo Transfection Reagent (Cat#: 5041; Altogen Biosystems USA) at 30 min of reperfusion after transient MCAO (Fig. 4). For IV injections, mice were injected at either 30 min (Fig. 4, 5, 6, 8) or 2h (Fig. 4) of reperfusion after transient MCAO with miR-7 mimic (50 nmol) mixed with PEG-Liposome.
Motor function and lesion volume determination
Post-ischemic motor function was evaluated by the rotarod test (4 min on a cylinder rotating at 8 RPM), beam-walk test (number of foot faults while crossing a tapered 120 cm-long beam) and/or adhesive removal test (time taken to remove a small adhesive sticker placed on each forepaw) at days 1, 3, 5 and 7 of reperfusion as described earlier (12, 32). On day 7, animals were euthanized by transcardiac 4% phosphate-buffered paraformaldehyde (PFA) perfusion. Each brain was post-fixed, cryoprotected and sectioned (coronal; 40 μm thickness). Serial sections were stained with cresyl violet and scanned using the NIH ImageJ software. For brains collected at 7 days of reperfusion or earlier, the ischemic lesion volume was estimated by numeric integration of data from 5 serial coronal sections in respect to the sectional interval as described earlier (31, 33). The lesion volume was corrected to account for edema and differential shrinkage during tissue processing using the Swanson formula (34). For brains collected at 30 days of reperfusion, following formula was used to estimate the brain atrophy volume: volume of a contralateral hemisphere – volume of an ipsilateral hemisphere.
Morris water maze test
Both wild-type C57BL/6 (Fig. 5) and α-Syn−/− mice (Fig. 8), all 3–4–month-old males, were subjected to the Morris water maze test to examine post-stroke spatial learning and memory capabilities (32). The test consists of 8 blocks of testing over 4 days, followed by a probe trial. Each mouse received 2 blocks of testing per day, each block was comprised of 4 sequential trials, for a total of 32 trials over 4 days. For each trial, the mouse was placed into the pool at a different start location and was allowed to swim until it either located the hidden platform or reached the end of the 60-second trial. Following completion of the 8th block of testing, mice were exposed to the pool for a probe trial where the platform was removed, and swimming behavior was monitored for 60 seconds. The mouse’s learning of the platform location was evaluated by escape latency (meaning the duration to reach the platform) during the training trials and behavior during the probe trial measured as time spent in the platform quadrant.
Pathologic evaluation of peripheral organs
To examine if miR-7 mimic does not result in long-term peripheral organ toxicity, mice were injected IV with 150 nmol of miR-7 mimic or control mimic (3x higher dose than the most efficacious dose identified; n=3/group) at 30 min of reperfusion after 60 min transient MCAO. At approximately 60 days of reperfusion, animals were euthanized by transcardiac perfusion, and peripheral organs (lung, heart, liver, kidney, spleen, and pancreas) were post-fixed, sectioned (10 μm thickness) and stained with hematoxylin and eosin (H&E). Organ pathology assessment was performed by a board-certified veterinary pathologist from the UW-Madison Department of Comparative Biosciences. Displayed representative regions of interest were selected from images taken at 10X magnification (and 20X for pancreas).
In vivo laser speckle imaging
Cerebral blood flow was assessed by laser speckle analysis on a subset of mice subjected to 90 min transient MCAO followed by IV injection of either miR-7 mimic or control mimic at 30 min of reperfusion. Readings were obtained prior to transient MCAO, at 40 to 55 minutes of occlusion and at 24h of reperfusion. For each reading, mice were anesthetized with isoflurane, fixed into a stereotaxic frame and cranium was exposed via a midline incision. A laser speckle imager (high-resolution mode) was positioned 10 cm above the skull, and light-based readings were collected at an effective rate of 2.1 images/sec. PIMSoft analysis software (Perimed USA) was used to establish an arbitrary index of cerebral blood flow (Perfusion Units) in the ischemic hemisphere at each time point.
Western blotting
Protein samples (40 μg protein equivalent) were electrophoresed, transferred to nitrocellulose membranes, incubated with 0.4% PFA (30 min at room temperature) (35) before blocking with 5% BSA in TBS-T. Blots were then probed with antibodies against α-Syn (Cell Signaling Technology (CST); Cat#: 2642S; 1:1,500) dynamin-related protein-1 (Drp1; CST; Cat#: 8570S; 1:1,000), phospho-Drp1 (pDrp1; CST; Cat#: 6319S; 1:1,000), cleaved caspase-3 (CST; Cat#: 9661S; 1:1,000), 3-nitrotyrosine (3-NT; Abcam; Cat#: ab61392; 1:1,400), and LC-3 (CST; Cat#: 12741S; 1:1,000) followed by HRP-conjugated anti-rabbit (CST; Cat#: 7074S) or anti-mouse IgG (CST; Cat#: 7076S; 1:5,000). Blots were stripped and reprobed with antibodies against β-actin (CST; Cat#: 3700S; 1:3,000) followed by HRP-conjugated anti-mouse IgG. Blots were developed using enhanced chemiluminescence (Life Technologies; Cat#: 34076) and quantified with Image Studio software (LI-COR Biotechnology USA).
Immunostaining
Brain sections were immunostained with antibodies against α-Syn (CST; Cat#: 4179S; 1:200), NeuN (Millipore; Cat#: MAB377; 1:300), pDrp-1 (CST; Cat#: 3455S; 1:400), 3-NT (Abcam; Cat#: ab61392; 1:500), cleaved caspase-3 (CST; Cat#: 9661S; 1:400), and LC-3 (CST; Cat#; 12741S; 1:100) as described earlier (36). To ensure that the homologous areas of injury were samples between animals, sections between the coordinates +1 to +1.5 from Bregma were used in all cases. Analyses were performed by an investigator blinded to the study groups.
Luciferase reporter assay
The Firefly/Renilla Duo-Luciferase α-Syn 3’-UTR reporter vector (Cat#: RmiT049927-MT01) and a mutant vector with the miR-7 seed sequence within the 3’-UTR of α-Syn mutated (Cat#: CS-RmiT049927-MT01–01) were purchased from GeneCopoeia, USA. On the day of the experiment, Rat pheochromocytoma (PC12) cells were transfected with either wild-type or mutant 3’-UTR plasmid (200 ng) together with 25 nM premiR-7 or control premiR (Cat#: AM17100; Invitrogen, USA) using Lipofectamine 2000 (Cat#:11668027; Invitrogen USA). 1 day following transfection, cells were lysed and subjected to a dual luciferase assay using Luc-Pair miR Luciferase Assay Kit (Cat#: LPFR-M030; GeneCopoeia) according to the manufacturer’s instructions. Each transfection was conducted in triplicate and repeated four times.
Real-time PCR
Real-time PCR was performed for rat α-Syn (NM_019169.2) and miR-7 with the SYBR-green method as described earlier using LNA PCR primer sets for α-Syn (product No. 309999; proprietary sequence) and miR-7 (product No. 205877; Exiqon USA) (12). Relative quantification of gene expression was normalized to 18S mRNA and calibrated to the appropriate control sample by comparative CT method (2−ΔΔCt).
Statistical Analyses
For analyzing data which was collected repeatedly from the same set of subjects at different time points (such as rotarod and beam-walk tests), a nonparametric two-way repeated measures ANOVA with Sidak’s multiple-comparisons test was used. For comparing two groups (e.g. lesion volume, RT-PCR and Western blots), a nonparametric Mann-Whitney U test was used. For comparing three groups (e.g. data in Fig. 9), a nonparametric Kruskal-Wallis one-way ANOVA followed by Dunn’s post-test was used. Specific statistical tests used were specified in the corresponding figure legends. GraphPad Prism 6 software was used for the statistical analysis.
FUNDING
This work was supported, in part, by US Department of Veterans Affairs Merit Review Grant I01 BX002985, NIH grants RO1 NS101960 and NS099531 and American Heart Association grant 15PRE23230002.
Footnotes
COMPETING INTEREST
Authors declare that they have no competing interests.
DATA AND MATERIALS AVAILABILITY
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Mehta SL, Manhas N, Raghubir R, Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 54, 34–66 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Hu BR, Martone ME, Jones YZ, Liu CL, Protein aggregation after transient cerebral ischemia. J Neurosci 20, 3191–3199 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge P, Luo Y, Liu CL, Hu B, Protein aggregation and proteasome dysfunction after brain ischemia. Stroke 38, 3230–3236 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dharap A, Bowen K, Place R, Li LC, Vemuganti R, Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 29, 675–687 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeyaseelan K, Lim KY, Armugam A, MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39, 959–966 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Xin H et al. , MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31, 2737–2746 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J et al. , MicroRNA-7: a promising new target in cancer therapy. Cancer Cell Int 15, 103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S et al. , MicroRNA-7 inhibits neuronal apoptosis in a cellular Parkinson’s disease model by targeting Bax and Sirt2. Am J Transl Res 8, 993–1004 (2016). [PMC free article] [PubMed] [Google Scholar]
- 9.Junn E et al. , Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A 106, 13052–13057 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillan KJ et al. , Loss of MicroRNA-7 Regulation Leads to alpha-Synuclein Accumulation and Dopaminergic Neuronal Loss In Vivo. Mol Ther 25, 2404–2414 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dauer W, Przedborski S, Parkinson’s disease: mechanisms and models. Neuron 39, 889–909 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Kim T et al. , Poststroke Induction of alpha-Synuclein Mediates Ischemic Brain Damage. J Neurosci 36, 7055–7065 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albers GW et al. , Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke 42, 2645–2650 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Kim TH, Vemuganti R, Effect of sex and age interactions on functional outcome after stroke. CNS Neurosci Ther 21, 327–336 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal V, Bell GW, Nam JW, Bartel DP, Predicting effective microRNA target sites in mammalian mRNAs. Elife 4, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson CL, Cerebral ischemic stroke: is gender important? J Cereb Blood Flow Metab 33, 1355–1361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lusardi TA et al. , MicroRNA responses to focal cerebral ischemia in male and female mouse brain. Front Mol Neurosci 7, 11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvamani A, Sohrabji F, Mir363–3p improves ischemic stroke outcomes in female but not male rats. Neurochem Int 107, 168–181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kredo-Russo S, Ness A, Mandelbaum AD, Walker MD, Hornstein E, Regulation of pancreatic microRNA-7 expression. Exp Diabetes Res 2012, 695214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Liu J, Liu C, Naji A, Stoffers DA, MicroRNA-7 regulates the mTOR pathway and proliferation in adult pancreatic beta-cells. Diabetes 62, 887–895 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latreille M et al. , MicroRNA-7a regulates pancreatic beta cell function. J Clin Invest 124, 2722–2735 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyagi K, Harada S, Tokuyama S, Pancreatic Changes in Nerve Growth Factor/TrkA Associated with Insulin Secretion in Cerebral Ischemia. Biol Pharm Bull 38, 1747–1751 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Pezzilli R et al. , Serum pancreatic enzymes in patients with coma due to head injury or acute stroke. Int J Clin Lab Res 27, 244–246 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Larson GM, Sullivan HW, O’Dorisio T, Surgical sympathectomy increases pancreatic polypeptide response to food. Surgery 98, 236–242 (1985). [PubMed] [Google Scholar]
- 25.Iwai A et al. , The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 14, 467–475 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Bendor JT, Logan TP, Edwards RH, The function of alpha-synuclein. Neuron 79, 1044–1066 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavarria C, Souza JM, Oxidation and nitration of alpha-synuclein and their implications in neurodegenerative diseases. Arch Biochem Biophys 533, 25–32 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Kim T, Vemuganti R, Mechanisms of Parkinson’s disease-related proteins in mediating secondary brain damage after cerebral ischemia. J Cereb Blood Flow Metab 37, 1910–1926 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott AM, Heneghan HM, Miller N, Kerin MJ, The therapeutic potential of microRNAs: disease modulators and drug targets. Pharm Res 28, 3016–3029 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Duncavage E, Goodgame B, Sezhiyan A, Govindan R, Pfeifer J, Use of microRNA expression levels to predict outcomes in resected stage I non-small cell lung cancer. J Thorac Oncol 5, 1755–1763 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Mehta SL, Kim T, Vemuganti R, Long Noncoding RNA FosDT Promotes Ischemic Brain Injury by Interacting with REST-Associated Chromatin-Modifying Proteins. J Neurosci 35, 16443–16449 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandran R et al. , A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J Cereb Blood Flow Metab, 271678X17738701 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakka VP et al. , Increased cerebral protein ISGylation after focal ischemia is neuroprotective. J Cereb Blood Flow Metab 31, 2375–2384 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanson RA et al. , A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10, 290–293 (1990). [DOI] [PubMed] [Google Scholar]
- 35.Lee BR, Kamitani T, Improved immunodetection of endogenous alpha-synuclein. PLoS One 6, e23939 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan YP et al. , Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab 27, 1213–1224 (2007). [DOI] [PubMed] [Google Scholar]