Abstract
Background:
The association between lithium and thyroid dysfunction has long been known. However, it remains unknown if lithium-associated hypothyroidism is reversible once lithium treatment has been stopped.
Aims:
To determine whether lithium-associated hypothyroidism was reversible in patients who subsequently discontinued lithium.
Methods:
A retrospective cohort study in the Swedish region of Norrbotten into the effects and side- effects of lithium treatment and other drugs for relapse prevention (Lithium – Study into Effects and Side Effects). For this particular study, we reviewed medical records between 1997 and 2015 of patients with lithium-associated hypothyroidism who had discontinued lithium.
Results:
Of 1340 patients screened, 90 were included. Of these, 27% had overt hypothyroidism at the start of thyroid replacement therapy. The mean delay from starting lithium to starting thyroid replacement therapy was 2.3 years (SD 4.7). In total, 50% of patients received thyroid replacement therapy within 10 months of starting lithium. Of 85 patients available for follow-up, 41% stopped thyroid replacement therapy after lithium discontinuation. Only six patients reinstated thyroid replacement therapy subsequently. Of these, only one had overt hypothyroidism.
Conclusions:
Lithium-associated hypothyroidism seems reversible in most patients once lithium has been discontinued. In such cases, thyroid replacement therapy discontinuation could be attempted much more often than currently done. Based on the limited evidence of our study, we can expect hypothyroidism to recur early after thyroid replacement therapy discontinuation, if at all.
Keywords: Bipolar disorder, lithium, hypothyroidism, thyroxine, adverse effect
Introduction
Lithium remains a first-line maintenance treatment for bipolar affective disorder (BPAD) (NICE, 2014). Several large cohort studies have suggested that lithium is most effective for the prevention of suicide, self-harm, and acute affective episodes (Hayes et al., 2016; Song et al., 2017). Lithium may even reduce the need for hospital admissions (Hayes et al., 2016; Joas et al., 2017; Song et al., 2017).
Lithium has also been proposed as a possible augmentation treatment for refractory depression (NICE, 2009). Yet, despite its therapeutic utility, lithium is not without adverse effects. Concerning endocrine adverse effects, the association between lithium and thyroid dysfunction has long been known. Historically, the first two cases of lithium-associated hypothyroidism were reported by Rogers and Whybrow (1971). An even earlier report of Schou et al. (1968) pertained to lithium-associated euthyroid goitre, which was reversible once lithium was discontinued. A later study, however, did not show any correlation between goitre and thyroid function in lithium-treated patients with BPAD (Kuman Tunçel et al., 2017). Soon, further cases of thyroid dysfunction associated with lithium were published, leading to speculations that myxoedema was a possible side effect of lithium treatment (Vestergaard and Poulsen, 1972). In the following decades, more evidence emerged, firmly establishing an association of lithium treatment with both subclinical and overt hypothyroidism (Gitlin, 2016; Grandjean and Aubry, 2009; McKnight et al., 2012). Prevalence estimates of lithium-associated thyroid dysfunction range from 14–17% for overt and from 19–35% for subclinical hypothyroidism (Aliasgharpour et al., 2005; Fagiolini et al., 2006; Kupka et al., 2002). One study in patients aged ⩾65 years reported a prevalence of 24% for overt hyperthyroidism (van Melick et al., 2010). Currently, it remains unknown if lithium-associated hypothyroidism is reversible once lithium treatment has been stopped. It has been suggested that patients can stop their thyroid replacement therapy (TRT) having discontinued lithium, although hypothyroidism may occasionally reoccur (Gitlin, 2016). Yet, the evidence remains mainly anecdotal (Amdisen and Andersen, 1982; Brandrup, 1972; Karlsson et al., 1975; Souza et al., 1991).
We set up this study to determine whether lithium-associated hypothyroidism was reversible in patients who subsequently discontinued lithium.
Method
Study design
This study is part of the Lithium – Study into Effects and Side Effects (LiSIE) research programme. LiSIE is set up as a retrospective cohort study, based on reviews of medical records, to explore the effects and potential adverse effects of lithium treatment compared to other drugs for relapse prevention. This way, LiSIE aims at identifying the best long-term treatment options for patients with BPAD and related conditions. The Regional Ethics Review Board at Umeå University, Sweden, approved this study (DNR 2010-227-31M, DNR 2011-228-32M, DNR 2014-10-32M).
LiSIE participants
LiSIE invited all individuals in the Swedish regions of Västerbotten and Norrbotten aged at least 18 years who had either received a diagnosis of BPAD (International Classification of Diseases (ICD) F31), schizoaffective disorder (ICD F25), or who had used lithium as a drug for relapse prevention between 1997 and 2011. The participants were informed about the nature of the study in writing and provided verbal informed consent. The consent was documented in our research files, dated and signed by the research worker who obtained the consent. In accordance with the ethics approval granted, for deceased patients no consent was obtained. Consent procedures concluded by the end of 2012. The cohort was locked at this point and no new patients were included in the study thereafter.
Patient selection and inclusion criteria
For this particular study, we included patients from Norrbotten who (a) had at least one prescription of lithium, (b) then discontinued lithium on at least one occasion at any time between 1997 and 2013, and (c) had received at least one prescription of thyroxine or liothyronine.
Exclusion criteria
We excluded patients who had received a lithium prescription, but never exceeded 0.2 mmol/L in lithium concentration (Ott et al., 2016). We further excluded patients who reinstated lithium within 3 months of lithium discontinuation. We chose a lithium-free period of 3 months based on the available experience with amiodarone (Narayana et al., 2011), assuming that any reversible effects of lithium on the thyroid would wear off within this time period. We also excluded patients for whom we could not clearly establish that the thyroid dysfunction was attributable to lithium. This concerned patients who had (a) started TRT before they started lithium or commenced on the same day as lithium, (b) used lithium only in the short term and discontinued within 3 months, (c) commenced TRT 3 months or more after lithium discontinuation, or (d) received TRT as an adjuvant treatment for depression rather than a correction of thyroid dysfunction. We finally excluded patients (e) who started and stopped TRT during lithium treatment.
Variable definitions
Hypothyroidism was thought to be reversible if TRT could be stopped after lithium discontinuation without persistent thyroid stimulating hormone (TSH) elevation during follow-up. We explored the potential reversibility of hypothyroidism at several intervals, within 2, 5 and 10 years after lithium discontinuation. We categorized thyroid status into six categories: normal, overt hypothyroidism, subclinical hypothyroidism, low free serum T4, unclassified, and hyperthyroidism (Table 1). We classified these categories according to the laboratory methods and reference intervals used at the time. Laboratory methods were known for 91.6% of tests. Reference intervals were known for 100% of all tests, allowing accurate categorization of thyroid status in all cases (Appendix 1). Most laboratory values were analysed with an immunoassay from Roche Diagnostics Scandinavia with normal range reference values for thyroid function tests (TFT) of 0.27–4.20 IU/mL for TSH and 12.0–22.0 pmol/L for free serum thyroxine (fT4).
Table 1.
Categorization of thyroid status at start of TRT.
| Type of thyroid dysfunction | Laboratory values |
|---|---|
| Normal | Within the normal reference interval valid at the time (Appendix 1) |
| Hypothyroidism | |
| Overt | TSH elevated, fT4 lowered |
| Subclinical | TSH elevated, fT4 normal |
| Low fT4 only | TSH normal, fT4 lowered |
| Unclassified | TSH elevated, fT4 value unknown |
fT4: free serum thyroxine; TSH: thyroid-stimulating hormone; TRT: thyroid replacement therapy.
Validation of data
The date of the electronic prescription when lithium was started or discontinued was manually validated in the medical records for all patients. This way, we established the total time of lithium exposure. At this point, the psychiatric diagnosis at initiation of lithium treatment was excerpted (Öhlund et al., 2018). Equally, we validated the accuracy of the start and stop date of TRT in the medical records. If no specific date for discontinuation could be established, for example, if a patient stopped treatment without notifying health care personnel, we estimated the stop date from the doctors’ notes.
Chart review and analysis
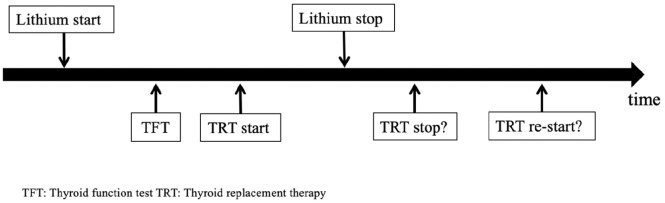
We retrospectively reviewed the medical records of all eligible patients from 1997 to 31 December 2015. We additionally checked for lithium, TRT and TFT in medical records dating back to 1965 to ensure we had not missed any previous treatment periods. We followed the clinical course of lithium-associated hypothyroidism and its treatment according to a predefined process flow chart (Figure 1).
Figure 1.
Clinical course of lithium-associated hypothyroidism and its treatment in patients who subsequently discontinued lithium.
Outcome parameters
We mapped the clinical course with the following parameters, which we then stratified further by age and sex: (a) time from lithium start to TRT start, (b) TSH and fT4 levels at which TRT was initiated, (c) proportion of patients who had discontinued TRT having stopped lithium, (d) time from stopping lithium to stopping TRT, and (e) proportion of patients requiring reinstatement of TRT. Age at the TRT start was stratified into two groups, <60 years and ⩾60 years in analogy to Shine et al., examining long-term effects of lithium in a retrospective analysis of laboratory data (Shine et al., 2015).
Control for bias
We controlled for selection bias in the whole retrospective cohort study (LiSIE). Age, sex, maximum recorded lithium and creatinine concentrations were key parameters, available in anonymized form. In accordance with the ethics approval granted, we compared these parameters for consenting and non-consenting patients. No significant difference was found between the two groups.
Statistics
All data were anonymized before analysis. We first analysed the data descriptively, establishing means and medians for continuous variables and frequencies for categorical variables. TSH at the time of TRT start was analysed as a continuous variable. The distribution of this variable was presented in a histogram using a logarithmic scale to normalize data. We used the t-test for determining any potential differences in mean TSH level at TRT start for men and women and patients <60 years and ⩾60 years. The Chi-squared test was applied to assess whether there were any age or sex differences. We used Kaplan-Meier plots to map the time periods between the following events, (a) from starting lithium to first elevated TSH value and (b) from starting lithium to instating TRT. For the latter, we created separate Kaplan-Meier curves for men and women and patients <60 years and ⩾60 years. We tested potential differences between the two respective groups using the log rank test, setting the significance to p ⩽ 0.05. For the statistical analysis, we used SPSS 25.0 (IBM, Armonk, NY, USA). We have summarized our method in a Strobe checklist (Appendix 2).
Results
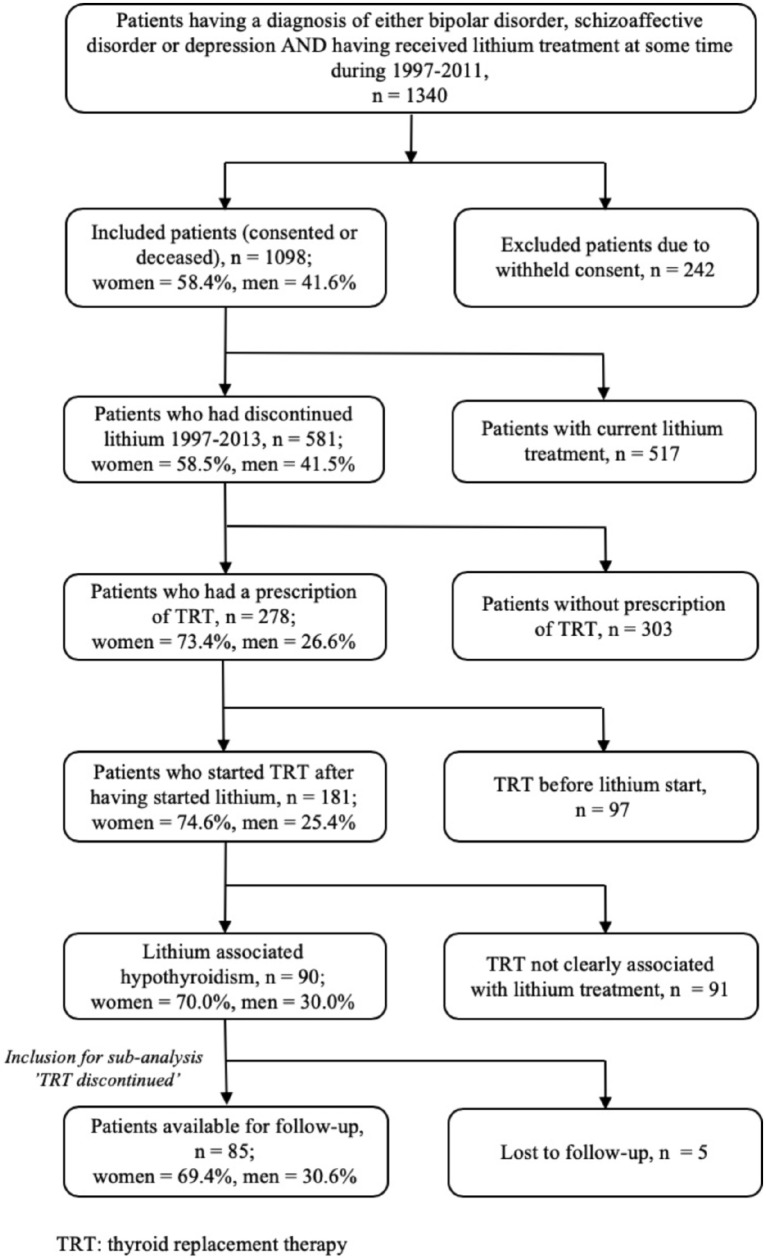
For this study, 1340 patients were potentially eligible, meeting the sampling requirements. According to our consent procedures, we could include 1098 patients, 58% of whom were women. We identified 181 patients who had received an electronic prescription for TRT after starting lithium, 75% of whom were women (p < 0.01). Of these 181 patients, 91 patients were excluded according to our procedures. Thus, the final sample consisted of 90 patients (Figure 2).
Figure 2.
Selection of study sample.
Sample characteristics
Of the final sample, women accounted for 70% of patients who received TRT in the context of lithium treatment. Of all patients, 70% were younger than 60 years. More patients had subclinical than overt hyperthyroidism at the point of starting TRT. For 17% of patients, TFT were either normal or difficult to interpret at the start of TRT (Table 2).
Table 2.
Baseline characteristics.
| Total sample, n = 90 | |
|---|---|
| Type of disorder (%) | |
| BPAD type 1 | 14.4 |
| BPAD type 2 | 60.0 |
| Recurrent depression / unspecified BPAD | 16.7 |
| Schizoaffective disorder | 8.9 |
| Sex (%) | |
| Male | 30.0 |
| Female | 70.0 |
| Age at lithium initiation (years) | |
| Mean (SD) | 48.9 (15.8) |
| Median (min., max.) | 51.0 (18–81) |
| Age at TRT initiation (%) | |
| <60 years | 70.0 |
| ⩾60 years | 30.0 |
| Total time on lithium (years) | |
| Mean (SD) | 5.3 (7.4) |
| Median (min., max.) | 2.0 (0–38) |
| TSH elevated at any time during lithium treatment episode at which TRT was initiated (%) | |
| Yes | 94.4 |
| No | 5.6 |
| Thyroid function at start of TRT (%) | |
| Hypothyroidism | |
| Overt | 26.7 |
| Subclinical | 56.7 |
| Normal | 10.0 |
| Other (unclassified, low fT4) | 6.6 |
BPAD: bipolar affective disorder SD: standard deviation; TSH: thyroid stimulating hormone;
TRT: thyroid replacement therapy; fT4: free serum thyroxine.
Time from starting lithium to starting TRT
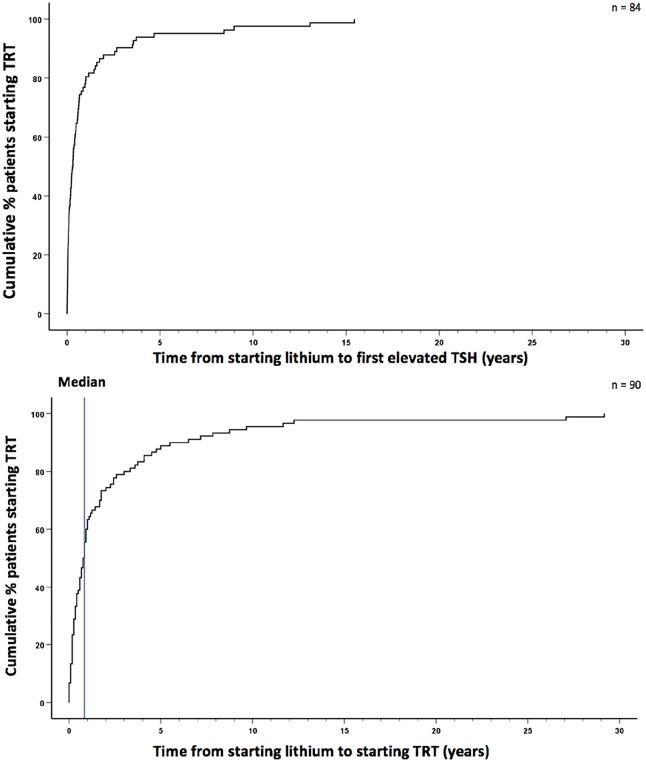
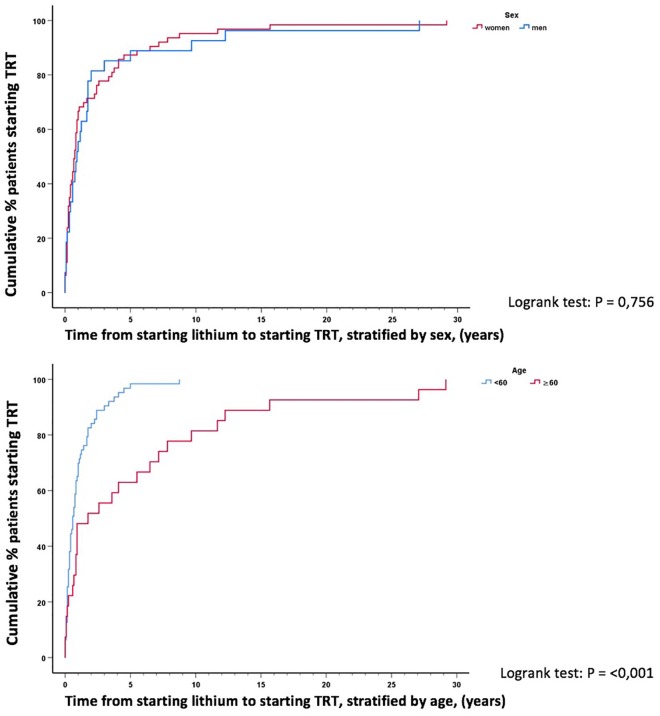
The mean delay from lithium to TRT start was 2.3 years (SD 4.7) with a median of 10 months (9.5 months, min. 15 days, max. 29 years). In total, 90% started within 5.5 years of lithium initiation. TRT initiation closely followed the first elevated TSH (Figure 3). Men and women took approximately the same time from starting lithium to starting TRT (log rank test p = 0.76) (Figure 4). However, patients <60 years started TRT significantly faster than patients ⩾60 years (log rank test p < 0.001) (Figure 4). Mean time from lithium to TRT start was 1.1 years (SD = 1.5, min. 14 days, max. 8.8 years) for patients <60 years and 5.1 years (SD = 7.6, min. 29 days, max. 29.2 years) for patients ⩾60 years.
Figure 3.
Times from starting lithium to first elevated thyroid stimulating hormone (TSH) and to starting thyroid replacement therapy (TRT).
Figure 4.
Time from starting lithium to starting thyroid replacement therapy (TRT) stratified by sex and age.
TSH and fT4 levels at which the TRT was initiated
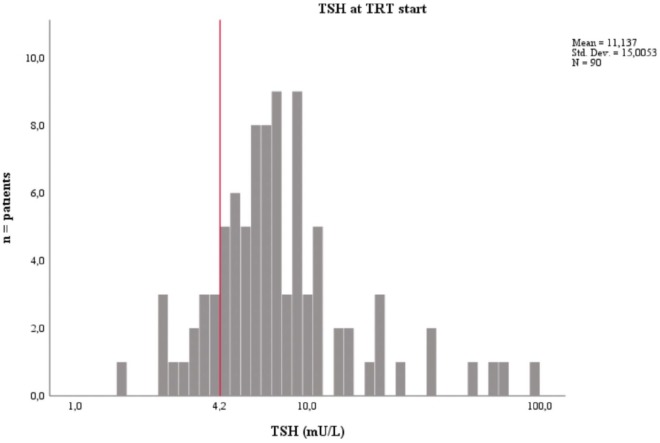
The mean TSH at TRT start was 11.1 IU/mL (SD 15.0) with a median of 6.9 IU/mL and a range of 1.6–100 IU/mL. The mean fT4 at TRT start was 12.4 pmol/L (SD 3.0) with a median of 12.5 pmol/L and a range of 4.3–18.7 pmol/L (Figure 5). There was no age (t = -0.08; df = 88; p = 0.939) or sex difference (t = 1.15; df = 88; p = 0.252) regarding TSH levels at TRT start.
Figure 5.
Mean thyroid stimulating hormone (TSH) level at which thyroid replacement therapy (TRT) was started.
Stopping TRT after discontinuing lithium
Of the 85 patients available for follow-up, 35 (41.2%) had stopped TRT at some point. Of these, 34.3% stopped TRT within 3 months and 71.4% within 1 year of discontinuing lithium. The mean time between discontinuing lithium to stopping TRT was 9.2 (SD 10.5) months with a median of 6.2 months and a range of 3.7 years. There was no difference in TSH (t = -1.193; df = 83; p = 0.236) or fT4 (t = 0.837; df = 80; p = 0.405) at time of starting TRT between patients who continued TRT after discontinuing lithium and those who did not.
Reinstating TRT
Of the 35 patients who stopped TRT, we identified six who reinstated TRT within the available follow-up period. The mean time to reinstatement was 1.8 years (SD 1.8), with a range from 11 days to 4.5 years. All six patients were female (Table 3). One female patient, with subclinical hypothyroidism, developed overt hypothyroidism within 11 days after TRT was stopped. This patient was treated with lithium alone before it was discontinued and then with zuclopenthixol. In another woman, TRT was restarted after childbirth based on elevated TSH, which was still below 10 IU/mL. For the remaining four patients, thyroid status was normal, subclinical or not recorded at the time of TRT reinstatement. Of these, two patients had concomitant treatment with quetiapine and one patient had concomitant treatment with valproate, both at the point of lithium discontinuation and at the point of TRT reinstatement.
Table 3.
Cases with reinstatement of TRT.
| Case | TFT at start of TRT | Antibodies | Time from starting lithium to initiating TRT | Time from discontinuing lithium to stopping TRT | Time from stopping TRT to reinstating TRT | TFT at or near time of TRT reinstatement | Reason for TRT reinstatement as recorded in the medical notes |
|---|---|---|---|---|---|---|---|
| 1 | Subclinical | N/A | 4.3 months | 2 days | 11 days | Overt | Increasing TSH and decreasing fT4 |
| 2 | Subclinical | N/A | 3.2 months | 0 days | 16 days | Normal | Reinstated via primary care, no comment in journal |
| 3 | Subclinical | N/A | 5.3 months | 1.9 years | 1.2 years | TSH and fT4 not taken | Reinstated via primary care, no comment in journal |
| 4 | Overt | N/A | 2.3 months | 0 days | 1.6 years | Low fT4 only | Reinstated via psychiatry clinic, low fT4 |
| 5 | Subclinical | TPO negative | 4.5 years | 2.5 years | 3.4 years | Subclinical | Reinstated via primary care, no comment in journal |
| 6 | Overt | N/A | 9.7 months | 10 days | 4.5 years | TSH 7.5 IU/mL fT4 not taken | Postpartum |
N/A: not available; TFT: thyroid function tests; TRT: thyroid replacement therapy; TPO: thyroid peroxidase antibodies; fT4: free serum thyroxine; TSH: thyroid stimulating hormone.
Discussion
The decision of whether to stop TRT after discontinuing lithium remains clinically challenging. If TRT was continued but lithium-associated hypothyroidism was reversible, patients would receive unnecessary treatment. If TRT was stopped but lithium-associated hypothyroidism was not reversible, patients might run the risk of hypothyroidism and a deterioration of mental health.
Reversibility of lithium-associated hypothyroidism
Our study suggests lithium-associated hypothyroidism is reversible in the majority of patients who discontinue lithium. In our study, 41% of 85 patients available for follow-up stopped TRT after discontinuing lithium treatment. Only 17% subsequently restarted TRT. Just one patient had overt hypothyroidism at the time of TRT reinstatement. In this patient, TRT had been stopped two days after lithium discontinuation. TRT was then reinstated 11 days later. Most likely, this was too short a time interval for the thyroid gland to recover. One patient restarted TRT postpartum. In this patient, the time between stopping TRT and reinstatement exceeded 4 years; TRT reinstatement may have been more likely due to post-partum thyroiditis than permanent lithium-associated damage to the thyroid gland. In the other four patients, judging on TFT alone, it remains unclear whether TRT reinstatement was really necessary.
In 1972, Brandrup published the case of a woman who developed a goitre and difficulties swallowing within half a month of starting lithium. Her serum T4 concentration was 11 nmol/L (normal range 66–139 nmol/L). Her protein bound iodine (PBI), an indirect measurement of thyroid function used at the time, was 0.3 µg/100ml (normal range 3.5–7.5 µg/100ml). After discontinuation of lithium, T4 concentration increased to 115 nmol/L and PBI to 4.2 µg/100ml. The patient’s mental state deteriorated after lithium discontinuation. Therefore, lithium reinstatement with concomitant thyroxine substitution was considered (Brandrup, 1972).
Amdisen and Andersen (1982) cross-sectionally examined a cohort of 237 patients treated with lithium for at least 6 months. Of these, 7.6% had TSH values ⩾ 10 IU/mL. At review after 3 years, there were three patients who had their lithium treatment discontinued. In two of these patients, one female and one male, TFT normalized within 1 and 2.5 years respectively. The third, female, patient was still considered in need of TRT 2 years after stopping lithium.
A randomized cross-over study investigated the impact of lithium discontinuation in 14 euthyroid patients with TSH and fT4 in the reference interval. The patients with BPAD had been on lithium for an average of 9.8 years. After stopping lithium, TSH decreased and fT4 increased significantly during the 4-week study period (Souza et al., 1991).
Possibly the BPAD itself could also constitute a risk factor for hypothyroidism. This could then also increase the risk of recurrence of hypothyroidism after stopping TRT. In our study, patients acted as their own controls, before and after lithium discontinuation. This makes it unlikely the underlying BPAD affected the reversibility of hypothyroidism after lithium discontinuation. The emergence of lithium-induced hypothyroidism during the initial period of lithium therapy may point towards a genetic susceptibility. This could increase the risk of recurrence of hypothyroidism in patients stopping TRT after stopping lithium. Genes have been identified that modify thyroid function directly, the TSH receptor or immune regulation. However, they may only account for a small proportion of hypothyroidism (Panicker, 2011). At present, our understanding of genetic factors remains limited. Family history of thyroid dysfunction in patients treated with lithium is rarely explored in scientific studies. One cross-sectional study found a positive association between family history and clinical hypothyroidism (Ozpoyraz et al., 2002). In our study, information on family history of thyroid dysfunction was mostly absent in the medical records. Clinicians should be encouraged to record a family history of thyroid disorder more systematically than is currently done.
Concomitant treatment with other drugs for relapse prevention and other psychotropic medications
Concomitant treatment with other drugs for relapse prevention or other psychotropic drugs could also affect the reversibility of hypothyroidism after lithium discontinuation. A large cohort of about 25,000 US patients with BPAD treated with mood stabilisers in monotherapy examined the cumulative incidence of hypothyroidism over a 4-year period (Lambert et al., 2016). Hypothyroidism occurred in 7.5% of the sample. The 4-year cumulative risk was highest for lithium (8.8%) and quetiapine (8.3%). Some thyroid-inhibiting action has also been suggested for valproate, carbamazepine, drugs for depression and phenothiazine-derived drugs (Bou Khalil and Richa, 2011; Park et al., 2011). In the six patients who subsequently reinstated TRT in our study, only one developed overt hypothyroidism. At the time of TRT reinstatement, this patient was only treated with zuclopenthixol, a drug not usually associated with hypothyroidism. In another patient, TRT was restarted much later postpartum. For the other four patients, thyroid status was normal, subclinical or not available at the time of TRT reinstatement. In these four patients, other concomitantly given drugs for relapse prevention did not seem to affect thyroid function substantially. Yet, although lithium-associated hypothyroidism seems reversible in the majority of patients who discontinue lithium, concomitant medication with other drugs for relapse prevention or other psychotropic drugs may still affect thyroid function. Currently, our knowledge regarding such interactions remains limited. We intend to study the impact of various drugs for relapse prevention and their combinations in more detail in future work based on the LiSIE cohort.
Role of anti-thyroid antibodies
Bocchetta et al. (2016) conducted a comprehensive literature review and report of 14 cases. Mood disorders and circulating thyroid antibodies were ‘very prevalent’ in the general population. However, they might have concurred by chance. Yet, based on the evidence, the authors proposed thyroid antibodies were of relevance in bipolar spectrum disorders. Antibodies were not systematically available in our study. Thus, we could not assess thyroid antibodies as a predictor of the reversibility of thyroid status. In one prospective study, the occurrence of thyroid antibodies in lithium-treated patients predicted the subsequent need of TRT (Bocchetta et al., 2007). In another prospective study, there was no association between lithium treatment and thyroid autoimmunity (Ozpoyraz et al., 2002). The presence of antibodies is unlikely to affect the decision of whether to start lithium. Hypothyroidism is an adverse effect that can easily be managed clinically. BPAD in contrast is a severe psychiatric condition that requires maintenance treatment to prevent suicide and relapse (Werneke et al., 2012). As the thyroid function is monitored anyway, the incremental costs of routine thyroid antibodies testing may outweigh the gains.
Time between lithium and TRT start
In our study, 50% started TRT within 10 months, 75% within 2.5 years and 90% within 5.5 years. We found the time interval between lithium and TRT start was significantly longer in older patients. Our results are comparable to the findings of another study, exploring the time interval between lithium start and diagnosis of hypothyroidism (van Melick et al., 2010). This study compared thyroid status in 79 lithium and 85 non-lithium patients ⩾65 years. Of all lithium patients, 28 (33%) had developed clinical or subclinical hypothyroidism while on lithium treatment. For 25 patients, the time interval could be determined. In these, 25% had developed thyroid dysfunction within 1 year, 52% within 2 years and 80% within 7 years. Another previous study explored the development of clinical hypothyroidism in 695 patients treated with lithium. In this study, the prevalence of clinical hypothyroidism was 10.4%. For a subsample of 548 patients with continuous lithium treatment, the survival curves suggest that about 3% of men and 9% of women developed clinical hypothyroidism within 2 years, 4% of men and 14% of women develop it within 5 years and 6% of men and 20% of women develop hypothyroidism within 10 years (Johnston and Eagles, 1999).
Natural course of subclinical hypothyroidism
In the general non-lithium-exposed population, the annual rate of progression from subclinical to overt hypothyroidism lies between 2–6%. The risk of progression is higher in women and individuals with higher TSH concentrations (Vanderpump et al., 1995). But subclinical hypothyroidism can also revert to normal. In one-third of affected individuals, this occurs within 1 year. In two-thirds, this occurs within 2 years (Diez et al., 2005). We do not know whether spontaneous recovery of thyroid function is slower in lithium-treated patients.
Subclinical hypothyroidism and mental health
Although thyroid status and mood disorders have been associated, the relationship remains poorly understood and controversial. A large cohort study followed 7323 middle-aged adults in South Korea without depression at the starting point. This study failed to find a significant association between subclinical hypothyroidism and incident depressive symptoms (Kim et al., 2018). Yet, in another study, Amann et al. (2017) found that higher TSH concentrations increased the risk of manic relapse in patients with BPAD 1 disorder, whereas fT4 or fT3 concentrations did not increase that risk. Even if an association between subclinical hypothyroidism and mood disorders was confirmed, TRT might not necessarily improve affective symptoms. Current meta-analytic evidence does not support the use of TRT in patients with subclinical hypothyroidism and depressive symptoms (Feller et al., 2018). TRT has been suggested to have some role as an augmentation treatment for resistant depression. The evidence, however, remains inconclusive (NICE, 2009; Nierenberg et al., 2006; Rush et al., 2009; Strawbridge et al., 2019; Zhou et al., 2015). Ultimately, we do not know when TRT should be used in patients with subclinical hypothyroidism. Watchful waiting may be an option, because many patients may revert to normal TSH spontaneously. In a study of 40 patients with spontaneous subclinical hypothyroidism, 68% had recovered within 2 years. Only four patients had not reverted to normal after 5 years (Diez et al., 2005). The available evidence does not allow us to predict the impact of TRT discontinuation on mood.
It is equally unclear whether patients with BPAD 2 disorder may be more likely than patients with BPAD 1 disorder to discontinue lithium because of associated hypothyroidism. In a previous study based on the LiSIE cohort, we identified the reasons of lithium discontinuation for 561 episodes of lithium discontinuation recorded between 1997 and 2015. Overall, 62% of reasons for lithium discontinuation related to adverse effects. But only 2% of episodes of lithium discontinuation related to TSH increase or hypothyroidism. There was no significant difference in patients with BPAD 1 or schizoaffective disorder and BPAD 2 or other BPAD (Öhlund et al., 2018).
Strengths
To our knowledge, this study is unique in looking explicitly into the potential reversibility of hypothyroidism after lithium discontinuation. The study was observational, reflecting real-life clinical practice. Due to its retrospective nature, it did not influence the decisions on treatment of the studied subjects. This study provided an in-depth analysis of clinical data over a 19-year period in a large sample of patients who had been exposed to lithium. The participation rate was high at 75%. For all included patients, psychiatric diagnoses were validated. Access to both prescription and laboratory data enabled us to check whether a patient had followed the prescription. Access to laboratory data across specialties permitted follow-up of thyroid function. Above all, access to the original medical records allowed us to determine whether TRT was causally related to lithium exposure or just associated. This way, we could eliminate association bias inherent in register studies or studies limited to laboratory and/or prescription data alone.
Limitations
In 60% of our patients, TRT continued despite stopping lithium. In many patients, thyroid function could have recovered without continuing TRT. This we could not assess. Hence, our study may underestimate the likelihood of reversibility. Bias can arise when patients who have agreed to participate systematically differ from patients who have not. We controlled for such selection bias in the whole LiSIE study. The TRT starting dates were not always clearly documented. Neither were the reasons for starting or restarting TRT always recorded. TSH and fT4 values at TRT start varied considerably. Periods available for follow-up also varied. The more time that passed between discontinuation of lithium and reinstatement of TRT, the less likely it was that hypothyroidism could be attributed to lithium. Ultimately, the quality of our results depended on the quality of the documented clinical information. This way, our study reflected real life and provided an opportunity to derive implications for practice. Due to the retrospective nature of our material, we could not equate association with causation, which reflects clinical practice. We only included patients for whom we could reasonably assume that TRT had indeed been attributable to lithium. This reduced our final sample size to only 90 patients. Still, at present, our study is the largest available on this subject.
Conclusion
In most patients lithium-associated hypothyroidism seems reversible once lithium has been discontinued. Therefore, in such cases, TRT discontinuation could be attempted much more often than is currently done. It seems prudent to allow some weeks for the thyroid gland to recover before stopping TRT. Based on the limited evidence of our study, we can expect hypothyroidism to recur early after TRT discontinuation, if at all. Thus, it is advisable to monitor thyroid function for 3 to 6 months. TRT reinsertion should then only be reconsidered if there are unambiguous signs of hypothyroidism, such as persistently high TSH and low fT4. Judging from the fading significance of TRT as an augmentation for treatment-resistant depression, TRT discontinuation may not adversely affect mood by default. Shared care between psychiatry, endocrinology and primary care can help to optimize treatment decisions to ensure that patients who do not need TRT can safely discontinue.
Supplemental Material
Supplemental material, Appendix_1 for Lithium-associated hypothyroidism and potential for reversibility after lithium discontinuation: Findings from the LiSIE retrospective cohort study by Ingrid Lieber, Michael Ott, Louise Öhlund, Robert Lundqvist, Mats Eliasson, Mikael Sandlund and Ursula Werneke in Journal of Psychopharmacology
Supplemental material, Appendix_2 for Lithium-associated hypothyroidism and potential for reversibility after lithium discontinuation: Findings from the LiSIE retrospective cohort study by Ingrid Lieber, Michael Ott, Louise Öhlund, Robert Lundqvist, Mats Eliasson, Mikael Sandlund and Ursula Werneke in Journal of Psychopharmacology
Acknowledgments
We wish to thank Dr Rebecca Johansson of the clinical chemistry laboratory at Sunderby Hospital, Luleå, Sweden for collating all current and historical methods and reference intervals for TFT having been used in Norrbotten Region since 1980.
Footnotes
Availability of data and material: The datasets generated and/or analysed during the current study are not publicly available due to lack of ethics committee permission and not having been part of the consent process. The structure of the dataset and the coding specification are available from the authors. Any other reasonable request will be raised with the regional ethics committee and healthcare provider.
Declaration of Conflicting Interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: IL, LÖ, RL, MS and ME declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant of the Research & Development Fund and the Department of Psychiatry, Sunderby Hospital, both Norrbotten Region, Sweden. UW has received funding for educational activities on behalf of Norrbotten Region (Masterclass Psychiatry Programme, EAPM 2016 Luleå, Sweden): Astra Zeneca, Janssen, Eli Lilly, Novartis, Otsuka/Lundbeck, Servier, Shire and Sunovion. MO has been scientific advisory board member for AstraZeneca AB, Sweden, 2018.
ORCID iDs: Ingrid Lieber  https://orcid.org/0000-0002-3536-6227
https://orcid.org/0000-0002-3536-6227
Ursula Werneke  https://orcid.org/0000-0002-5023-3254
https://orcid.org/0000-0002-5023-3254
Supplemental material: Supplemental material for this article is available online.
References
- Aliasgharpour M, Abbassi M, Shafaroodi H, et al. (2005) Subclinical hypothyroidism in lithium-treated psychiatric patients in Tehran, Islamic Republic of Iran. East Mediterr Health J 11: 329–333. [PubMed] [Google Scholar]
- Amann BL, Radua J, Wunsch C, et al. (2017) Psychiatric and physical comorbidities and their impact on the course of bipolar disorder: A prospective, naturalistic 4-year follow-up study. Bipolar Disord 19: 225–234. [DOI] [PubMed] [Google Scholar]
- Amdisen A, Andersen CJ. (1982) Lithium treatment and thyroid function. A survey of 237 patients in long-term lithium treatment. Pharmacopsychiatria 15: 149–155. [DOI] [PubMed] [Google Scholar]
- Bocchetta A, Cocco F, Velluzzi F, et al. (2007) Fifteen-year follow-up of thyroid function in lithium patients. J Endocrinol Invest 30: 363–366. [DOI] [PubMed] [Google Scholar]
- Bocchetta A, Traccis F, Mosca E, et al. (2016) Bipolar disorder and antithyroid antibodies: Review and case series. Int J Bipolar Disord 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou Khalil R, Richa S. (2011) Thyroid adverse effects of psychotropic drugs: A review. Clin Neuropharmacol 34: 248–255. [DOI] [PubMed] [Google Scholar]
- Brandrup FO. (1972) Et tilfaelde af lithiuminduceret reversibelt myksodem. Ugeskr Laeger 134: 2710–2712. [PubMed] [Google Scholar]
- Diez JJ, Iglesias P, Burman KD. (2005) Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 90: 4124–4127. [DOI] [PubMed] [Google Scholar]
- Fagiolini A, Kupfer DJ, Scott J, et al. (2006) Hypothyroidism in patients with bipolar I disorder treated primarily with lithium. Epidemiol Psichiatr Soc 15: 123–127. [DOI] [PubMed] [Google Scholar]
- Feller M, Snel M, Moutzouri E, et al. (2018) Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: A systematic review and meta-analysis. JAMA 320: 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin M. (2016) Lithium side effects and toxicity: Prevalence and management strategies. Int J Bipolar Disord 4: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean EM, Aubry JM. (2009) Lithium: Updated human knowledge using an evidence-based approach: Part III: Clinical safety. CNS Drugs 23: 397–418. [DOI] [PubMed] [Google Scholar]
- Hayes JF, Pitman A, Marston L, et al. (2016) Self-harm, unintentional injury, and suicide in bipolar disorder during maintenance mood stabilizer treatment: A UK population-based electronic health records study. JAMA Psychiatry 73: 630–637. [DOI] [PubMed] [Google Scholar]
- Joas E, Karanti A, Song J, et al. (2017). Pharmacological treatment and risk of psychiatric hospital admission in bipolar disorder. Br J Psychiatry 210: 197–202. [DOI] [PubMed] [Google Scholar]
- Johnston AM, Eagles JM. (1999) Lithium-associated clinical hypothyroidism. Prevalence and risk factors. Br J Psychiatry 175: 336–339. [DOI] [PubMed] [Google Scholar]
- Karlsson K, Lindstedt G, Lundberg PA, et al. (1975) Letter: Transplacental lithium poisoning: Reversible inhibition of fetal thyroid. Lancet 1: 1295. [DOI] [PubMed] [Google Scholar]
- Kim JS, Zhang Y, Chang Y, et al. (2018) Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab 103: 1827–1833. [DOI] [PubMed] [Google Scholar]
- Kuman Tunçel Ö, Akdeniz F, Özbek SS, et al. (2017) Thyroid function and ultrasonography abnormalities in lithium-treated bipolar patients: A cross-sectional study with healthy controls. Noro Psikiyatri Ars 54: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupka RW, Nolen WA, Post RM, et al. (2002) High rate of autoimmune thyroiditis in bipolar disorder: Lack of association with lithium exposure. Biol Psychiatry 51: 305–311. [DOI] [PubMed] [Google Scholar]
- Lambert CG, Mazurie AJ, Lauve NR, et al. (2016) Hypothyroidism risk compared among nine common bipolar disorder therapies in a large US cohort. Bipolar Disord 18: 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight RF, Adida M, Budge K, et al. (2012) Lithium toxicity profile: A systematic review and meta-analysis. Lancet 379: 721–728. [DOI] [PubMed] [Google Scholar]
- Narayana SK, Woods DR, Boos CJ. (2011) Management of amiodarone-related thyroid problems. Ther Adv Endocrinol Metab 2: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (NICE) (2009) Bipolar Disorder: Assessment and Management. Clinical guideline 90. Available at: https://www.nice.org.uk/guidance/cg90/ (accessed 2018). [PubMed]
- National Institute for Health and Care Excellence (NICE) (2014) Bipolar Disorder: Assessment and Management. Clinical guideline 185. Available at: https://www.nice.org.uk/guidance/cg185/ (accessed 6 June 2018). [PubMed]
- Nierenberg AA, Fava M, Trivedi MH, et al. (2006) A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: A STAR*D report. Am J Psychiatry 163: 1519–1530. [DOI] [PubMed] [Google Scholar]
- Ott M, Stegmayr B, Salander Renberg E, et al. (2016) Lithium intoxication: Incidence, clinical course and renal function - a population-based retrospective cohort study. J Psychopharmacol 30: 1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozpoyraz N, Tamam L, Kulan E. (2002) Thyroid abnormalities in lithium-treated patients. Adv Ther 19: 176–184. [DOI] [PubMed] [Google Scholar]
- Panicker V. (2011) Genetics of thyroid function and disease. Clin Biochem 32: 165–175. [PMC free article] [PubMed] [Google Scholar]
- Park YM, Kang SG, Lee BH, et al. (2011) Decreased thyroid function in Korean women with bipolar disorder receiving valproic acid. Gen Hosp Psychiatry 33: 200.13–200.15 [DOI] [PubMed] [Google Scholar]
- Rogers MP, Whybrow PC. (1971) Clinical hypothyroidism occurring during lithium treatment: Two case histories and a review of thyroid function in 19 patients. Am J Psychiatry 128: 158–163 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Warden D, Wisniewski SR, et al. (2009) STAR*D: Revising conventional wisdom. CNS Drugs 23: 627–647. [DOI] [PubMed] [Google Scholar]
- Schou M, Amdisen A, Eskjaer Jensen S, et al. (1968) Occurrence of goitre during lithium treatment. Br Med J 3: 710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine B, McKnight RF, Leaver L, et al. (2015) Long-term effects of lithium on renal, thyroid, and parathyroid function: A retrospective analysis of laboratory data. Lancet 386: 461–468. [DOI] [PubMed] [Google Scholar]
- Song J, Sjolander A, Joas E, et al. (2017) Suicidal behavior during lithium and valproate treatment: A within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry 174: 795–802. [DOI] [PubMed] [Google Scholar]
- Souza FG, Mander AJ, Foggo M, et al. (1991) The effects of lithium discontinuation and the non-effect of oral inositol upon thyroid hormones and cortisol in patients with bipolar affective disorder. J Affect Disord 22: 165–170. [DOI] [PubMed] [Google Scholar]
- Strawbridge R, Carter B, Marwood L, et al. (2019) Augmentation therapies for treatment-resistant depression: Systematic review and meta-analysis. Br J Psychiatry 214: 42–51. [DOI] [PubMed] [Google Scholar]
- Vanderpump MP, Tunbridge WM, French JM, et al. (1995) The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 43: 55–68. [DOI] [PubMed] [Google Scholar]
- van Melick EJ, Wilting I, Meinders AE, et al. (2010) Prevalence and determinants of thyroid disorders in elderly patients with affective disorders: Lithium and nonlithium patients. Am J Geriatr Psychiatry 18: 395–403. [DOI] [PubMed] [Google Scholar]
- Vestergaard PA, Poulsen JC. (1972) Myxoedema: Possible side-effect of lithium? Lancet 2: 427–428. [DOI] [PubMed] [Google Scholar]
- Werneke U, Ott M, Renberg ES, et al. (2012) A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand 126: 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ravindran AV, Qin B, et al. (2015) Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: Systematic review and network meta-analysis. J Clin Psychiatry 76: e487–e498. [DOI] [PubMed] [Google Scholar]
- Öhlund L, Ott M, Oja S, et al. (2018) Reasons for lithium discontinuation in men and women with bipolar disorder: A retrospective cohort study. BMC Psychiatry 18: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_1 for Lithium-associated hypothyroidism and potential for reversibility after lithium discontinuation: Findings from the LiSIE retrospective cohort study by Ingrid Lieber, Michael Ott, Louise Öhlund, Robert Lundqvist, Mats Eliasson, Mikael Sandlund and Ursula Werneke in Journal of Psychopharmacology
Supplemental material, Appendix_2 for Lithium-associated hypothyroidism and potential for reversibility after lithium discontinuation: Findings from the LiSIE retrospective cohort study by Ingrid Lieber, Michael Ott, Louise Öhlund, Robert Lundqvist, Mats Eliasson, Mikael Sandlund and Ursula Werneke in Journal of Psychopharmacology