Abstract
A surprising yet highly practical approach to improve the performance of a TADF exciplex blend is reported. Using the TSBPA donor and PO-T2T acceptor to form an exciplex, we are able to blue shift the emission, increase PLQY from 58 to 80%, and increase the device EQE from 14.8 to 19.2% by simply diluting the exciplex with an inert high triplet energy host material—here either UGH-3 or DPEPO. These effects are explained in terms of an increasing donor–acceptor distance and associated charge separation, while different behaviors observed in the different hosts are attributed to different energy barriers to electron transfer through the host. We expect that the observed performance-enhancing effects of dilution will be general to different exciplex blends and host materials and offer a new way to optimize the electrical properties of exciplex emission layers with narrow blue emission.
In recent years, organic light-emitting diodes (OLEDs) based on thermally activated delayed fluorescence (TADF) have attracted much attention. Efficient TADF has been observed in both intermolecular (exciplex) and covalently linked (molecular) charge transfer (CT) systems.1,2 At present, reports investigating new structures, color tuning methods, and strategies for improving device performance of TADF molecules greatly outnumber similar reports for exciplexes.3−7 Nonetheless, existing studies show the potential of TADF exciplexes as high-performance emitters or triplet harvesting hosts for fluorescent/phosphorescent emitters. Additionally, the intermolecular nature of CT in exciplexes results in new phenomena without direct analogues in molecular systems.2,8−12
While the donor (D) and acceptor (A) spacing is rigidly set by the chemical structures of molecular TADF materials, D–A spacing in exciplexes has only gained limited attention as a design parameter until recently. Adachi et al. recently reported that inserting a spacer layer between an interfacial blue exciplex shifts its emission.10 Similar observation have also been reported by Monkman and Al’Attar for interfacial exciplexes through the effect of an applied electric field.12 Graves et al. initially described how sample inhomogeneity caused dispersion of the singlet–triplet energy gap (ΔEST) in exciplex films, resulting in nonexponential decay kinetics and time-dependent emission spectra.11 Kim et al. have recently used computational methods to show how critical the D–A distance and orientation are to the singlet and triplet energies in an exciplex blend, which ultimately control the effective ΔEST and rate of reverse intersystem crossing (rISC).9 Kim et al. have also recently reported the surprising result that a TADF exciplex OLED shows improved performance at low temperatures.8 Although low temperatures decrease the rISC rate, this is outcompeted by a simultaneous increase in the exciplex photoluminescence quantum yield (PLQY) as nonradiative singlet decay channels are also suppressed at low temperatures.
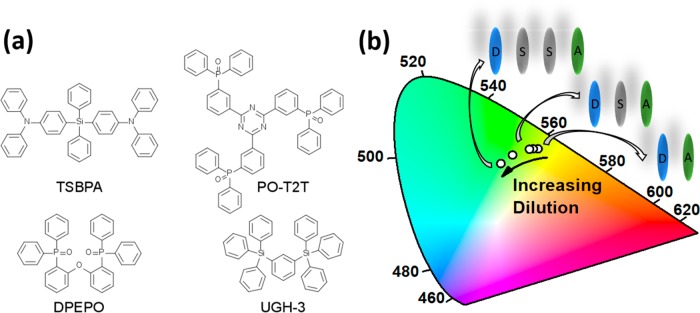
Here we present a similarly surprising yet highly practical approach to improve the performance of a recently reported TADF exciplex blend,13 with donor and acceptor structures shown in Figure 1a. Using a fixed 1:1 ratio of 4,4′-(diphenylsilanediyl)bis(N,N-diphenylaniline) (TSBPA) donor and 2,4,6-tris[3-(diphenylphosphinyl)phenyl]-1,3,5-triazine (PO-T2T) acceptor, we are able to blue shift the emission (summarized in Figure 1b), increase the PLQY (from 58 to 80%), and increase the device external quantum efficiency (EQE) from 14.8 to 19.2% by simply diluting the exciplex with an inert host material—either 1,3-bis(triphenylsilyl)benzene (UGH-3) or bis[2-(diphenylphosphino)phenyl]ether oxide (DPEPO). These hosts were selected because they are both optically and UV transparent (essential for photophysical measurements), have high triplet energies (crucial for device performance), while also allowing comparison of the effects of different host polarities and HOMO/LUMO levels.
Figure 1.
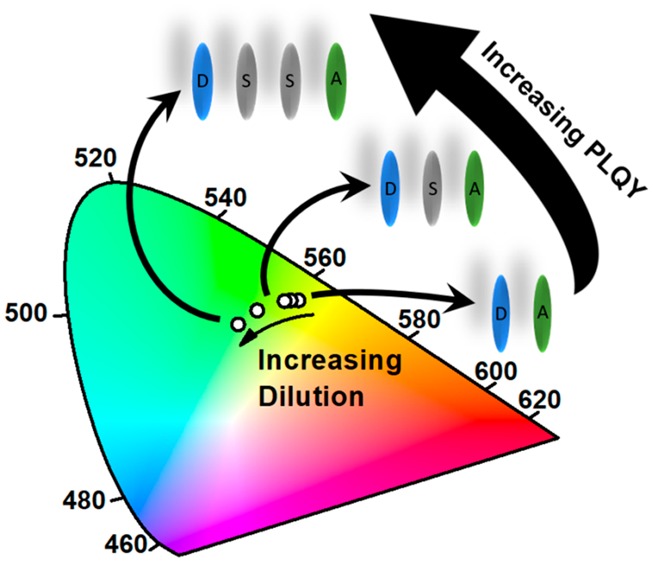
(a) Molecular structures of exciplex-forming molecules TSBPA and PO-T2T and the hosts molecules UGH-3 and DPEPO. (b) 1931 CIE chromaticity diagram schematically showing the blue shift of the exciplex emission with increasing dilution from the neat exciplex to 90 vol % UGH-3.
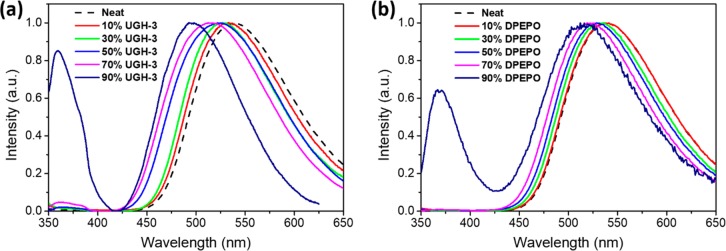
To investigate the effect of the solid-state dilution, the photophysical properties of 100 nm thick vacuum-deposited films were first investigated. Steady-state photoluminescence (PL) and absorption spectra of the exciplex formed between PO-T2T and TSBPA in two different hosts, UGH-3 or DPEPO, are shown in Figures 2 and S1–S3. In both hosts, a substantial blue shift of the exciplex emission is observed, with onset energies given in Table 1. The onset of the neat exciplex (without any dilution) is observed at 2.67 eV and increases with dilution in both hosts up to 2.85 eV in 90 vol % UGH-3 and 2.80 in 90 vol % DPEPO. In both 90% host films (i.e., exciplex highly diluted), TSBPA emission becomes clearly visible at wavelengths below 425 nm, indicating that exciplex formation is being hindered; however, it is extremely surprising that the exciplex is still so dominant even diluted at 90 vol % host.
Figure 2.
Steady-state PL spectra of vacuum-deposited TSBPA/PO-T2T films in (a) UGH-3 and (b) DPEPO. Legend percentages are vol % of the host material, with “neat” equivalent to 0% host.
Table 1. Exciplex Photophysical Properties at Different Dilutions in UGH-3 or DPEPO.
| dilution | vol % | average D–A distance (Å) | τDF (μs) | PL onset (eV) | PL fwhm (eV) | PLQY air/N2 |
|---|---|---|---|---|---|---|
| neat exciplex | 0 | 6.0 | 2.4 ± 0.1 | 2.67 | 0.51 | 0.45/0.58 |
| UGH-3 | 10 | 6.3 | 2.2 ± 0.1 | 2.68 | 0.52 | 0.50/0.63 |
| UGH-3 | 30 | 6.8 | 2.8 ± 0.1 | 2.70 | 0.51 | 0.51/0.65 |
| UGH-3 | 50 | 7.6 | 3.1 ± 0.1 | 2.79 | 0.58 | 0.67/0.80 |
| UGH-3 | 70 | 9.0 | 3.1 ± 0.2 | 2.83 | 0.58 | 0.49/0.70 |
| UGH-3 | 90 | 13 | 2.85 | 0.48 | 0.35/0.35 | |
| DPEPO | 10 | 6.3 | 2.4 ± 0.1 | 2.67 | 0.52/0.68 | |
| DPEPO | 30 | 6.8 | 2.5 ± 0.1 | 2.67 | 0.34/0.45 | |
| DPEPO | 50 | 7.6 | 2.3 ± 0.1 | 2.70 | 0.23/0.28 | |
| DPEPO | 70 | 9.0 | 2.9 ± 0.1 | 2.73 | 0.28/0.38 | |
| DPEPO | 90 | 13 | 2.8 ± 0.1 | 2.80 | 0.06/0.07 |
We assign the blue shift of the exciplex emission onset, hνmax, to the increase in the average D–A separation distance as the host vol % increases. Increasing the average D–A distance and therefore the separation, r, of the electron and hole in the CT state causes the Coulombic potential energy, EC(r), to rise (toward zero) as per eq 1. The average distances between nearest-neighbor D–A pairs has also been estimated in Table 1, using the molecular masses and volume fractions of the films and assuming a uniform cubic lattice, uniform orientation distribution, and density of 1.1 g/mL (approximated from tetraphenylsilane) for all materials.14,15 The total energy of the CT state thus increases with separation (eq 2), resulting in a higher-energy exciplex and emission, in accordance with the findings of Al’Attar12 and calculations reported by Kim et al.9 This observation is also consistent with previously mentioned recent work of Adachi et al.10
| 1 |
| 2 |
where e is the electron charge, F is the electric field, and ε0 and ε are, respectively, the permittivity of the vacuum and of the medium. ID and AA are the ionization potential of the donor and the electronic affinity of the acceptor. The electric field term in eq 1 has the effect of broadening the CT emission band as the field increases.12
The difference in the magnitude of the observed blue shift between the two hosts is rationalized in terms of their different polarities. Rich in polar O–C and O=P bonds, DPEPO is indeed known to be a very polar host,16 while UGH-3 is largely aromatic and nonpolar.15 Increased host polarity allows the exciplex CT state to relax further (by shielding the coulomb term through increased ε) before emission, resulting in a consistently smaller overall blue shift for DPEPO films than for UGH-3. The sensitivity of the exciplex emission to polarity even at high dilutions suggest that the exciplex maintains CT character even at the largest D–A distances examined here.16
To better understand how the presence of a host affects the kinetics of charge separation, exciplex formation, and emission, time-resolved PL decays of these films were measured and are shown in Figures S4–S6. The spectra at different times all display the same blue shift with dilution seen in Figure 2, while films of TSBPA in DPEPO or UGH-3 (without PO-T2T) show no sign of exciplex formation (Figure S9), confirming the inert nature of the hosts. Delayed fluorescence (DF) decay times (τDF) were obtained for the different exciplex samples by fitting the experimental data with monoexponential decay functions in the second cascade region of the log–log intensity time plots. A general increase in τDF was observed with increasing dilution for both hosts, with values given in Table 1. The increase in delayed lifetime can be indicative of a decrease in nonradiative decay from the exciplex triplet state, although this explanation cannot be asserted from lifetimes alone. The dependence of the delayed emission intensity upon excitation power was also checked for each sample (Figures S7 and S8) in order to confirm that the DF was due to TADF rather than triplet fusion.
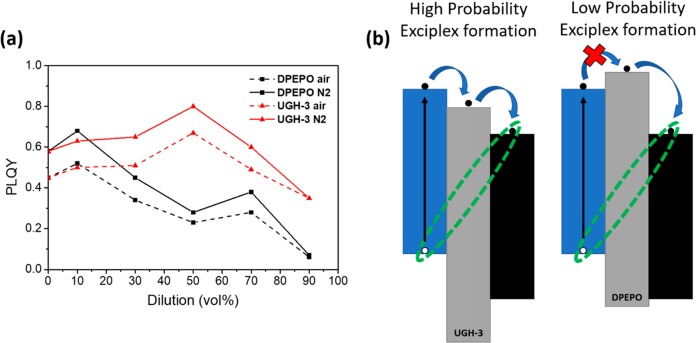
To better understand the cause of the observed increase in τDF, we measured the absolute PLQYs of the films, shown in Figure 3a and Table 1. We note that at the excitation wavelengths used (340 nm for PLQY, 355 nm for time-resolved PL) we only excite the exciplex donor. For all films, the PLQY was found to increase with removal of atmospheric oxygen, confirming that triplet harvesting is occurring. For the UGH-3 diluted films, we observed that the PLQY increased from 58% for the neat exciplex to a maximum of 80% when diluted with 50 vol % UGH-3.
Figure 3.
(a) PLQY of vacuum-deposited films in air and nitrogen atmospheres with different dilution in UGH-3 and DPEPO. (b) Schematic representation of the D (blue), A (black), and host (gray) HOMO and LUMO levels relevant to electron transport (blue arrows) and exciplex formation (green oval).
The PLQYs then decreased when the exciplex was further diluted. We assign this decrease to the fact that at very high dilution (and corresponding large D–A distances) the probability of exciplex formation must begin to decrease, so that eventually most of the excitons remain localized on the donor and therefore decay through low-efficiency pathways. This is supported by the growth of the 340 nm TSBPA donor emission band at high dilutions in Figure 2, as well as the fact that at 90% dilution the PLQYs in air and vacuum converge, indicating negligible contribution to emission from TADF. This is also seen in the time-resolved spectra, where a higher ratio of overall prompt (containing both exciplex and donor contributions) to delayed emission (exciplex only) is seen in higher-dilution time-resolved PL traces (Figures S5 and S6). This changing ratio of prompt to delayed emission manifests as an apparent lower level of DF when decays are normalized to initial time points.
Combined, the tandem increases in τDF and PLQY with dilution strongly suggest that some concentration quenching pathways are reduced for exciplexes with larger D–A distances. This observation may also indicate that PLQY is enhanced through reduction in the degree of charge separation in the excited state, for example, avoiding nonradiative radical ion pair formation.18,19 Despite the fact that the PL emission onsets blue shift upon dilution closer to the TSBPA and PO-T2T triplet energies (2.9 and 3.0 eV, respectively),13 we nonetheless rule out the possibility that the increase in PLQY arises from a distance-associated minimization of ΔEST (and improvement of triplet harvesting) as this would accelerate rISC, leading instead to a shorter τDF.7
Surprisingly, the trend in PLQY shown for the UGH-3 diluted exciplex is not reproduced for those diluted in DPEPO. Instead, the PLQY has an initial increase up to 68% for the 10 vol % (similar to what was measured for the sample diluted with 10 vol % UGH-3) but only decreases upon further dilution. The difference in optical behavior for different hosts demonstrates that the CT state formation in the diluted films must be mediated by the host as a tunneling or purely through-space mechanism would be independent of the choice of host. Furthermore, from the estimated D–A separations, it is clearly seen that electron transfer (ET) occurs strongly at separations greater than 0.6 nm, which again indicates that the host is acting as a mediator between the LUMO orbitals of TSBPA (−2.3 eV18) and PO-T2T (−3.2 eV19). As pictured in Figure 3b, the differences in the LUMO levels of the hosts then readily explain why the UGH-3 spacer (LUMO −2.8 eV17) still allows exciplex formation and high PLQY at larger distances whereas DPEPO (LUMO −2.0 eV3) does not.23,24 Comparing the fwhm for the CT emission band as a function of UGH-3 dilution (Table 1), we observe an increase as the dilution increases, until 90% UGH-3 where it instead narrows. This might imply that only certain orientations of D and A facilitate enhance hopping ET through the intermediate host molecule. The broadened spectra result from a distribution of emitting CT states having different conformations and ET routes,25 and at 90% dilution, only the highly blue-shifted and narrowed exciplexes can form. This then gives an indication of a possible way to produce narrow emission bands from exciplex systems. We again stress that in all photophysical measurements only the donor is excited, so that ET through the host is required for exciplex formation in the diluted films. This is not the case for electrical excitation where the CT state is formed directly from free charges, as discussed in the context of OLEDs below.
We estimate the distance dependence of ET by plotting the ratio of residual donor emission to exciplex emission from the spectra in Figure 2 (normalizing for changes in exciplex PLQY upon dilution). For UGH-3, we find an exponential increase in donor emission with average D–A spacing (Figure S9D), from which we estimate a distance decay constant βf = 1.1 Å–1, indicating efficient hopping ET with similar distance dependence as that found in other organic systems.24 For DPEPO, the mechanism for long-range ET cannot proceed exothermically and is not yet understood, but based on the PLQY rolloff, it is not as efficient as that in UGH-3.
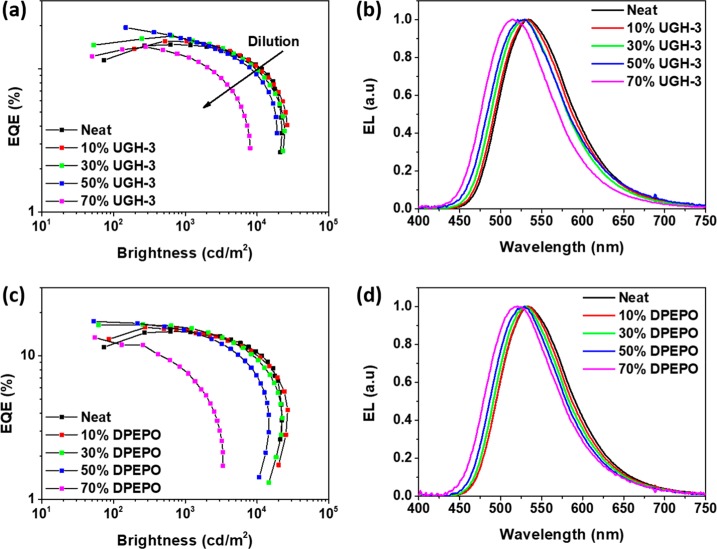
To assess the practical application of the dilution-enhanced exciplex, OLEDs with different host vol % DPEPO and UGH-3 were produced. The structure used was NPB (40 nm)|TSBPA (10 nm)|TSBPA:PO-T2T 1:1 in X vol % Host (40 nm)|PO-T2T (50 nm)|LiF (1 nm)|Al (100 nm), where X was varied from 0 to 70 vol %. In both device series, the maximum EQE (shown in Figure 4a,c) increases with dilution up to 50 vol %, matching the trend seen for UGH-3 film PLQYs in Figure 3a. The maximum device brightness decreases with increasing dilution as this reduces the total amount of exciplex D and A in the device. The electroluminescence (EL) spectra of both UGH-3 (Figure 4b) and DPEPO (Figure 4d) OLEDs blue shift with dilution, consistent with the trend observed in the steady-state PL spectra (Figure 2), although the EL spectra are each slightly red-shifted with respect to their PL counterparts. This red shift of EL compared to PL is commonly seen in TADF systems and is likely due to the differences between direct charge carrier recombination to form the CT state in devices, compared to photoexcitation, and ET to form the CT state in photophysics.20−
Figure 4.
EQE vs brightness and EL spectra of OLEDs with different dilution vol %, respectively, in UGH-3 (a,b) and DPEPO (c,d).
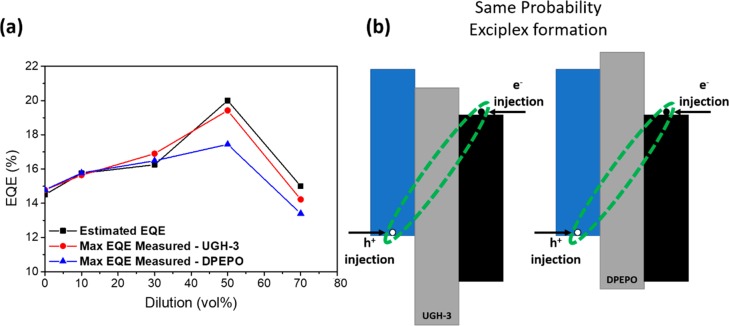
In Figure 5a, we compare the values of the maximum measured EQE for the devices produced with estimated based on the PLQYs using EQE = ηout·PLQY·γ·ηfr, where ηout is the outcoupling factor (assumed here to be 0.25), the PLQY values taken from Table 1 are those from the UGH-3 samples, γ is the charge balance factor (assumed to be 1), and ηfr is the fraction of excited states that can radiatively decay, which is 1 for TADF exciplexes that can harvest both singlets and triplets. We use the UGH-3 PLQYs here, having identified that DPEPO blocks ET and exciplex formation after optical excitation. In contrast, in electrically driven devices, there is no need for ET through the diluting host material, as shown schematically in Figure 5b. As such, we did not expect to observe the same early decrease in DPEPO device EQEs as was observed for PLQYs.
Figure 5.
(a) Comparison between the measured maximum EQE values of OLED devices with different dilutions in UGH-3 and DPEPO, along with estimated EQE values calculated from UGH-3 nitrogen PLQYs. (b) Schematic representation of the exciplex formation mechanism under electrical excitation in OLED devices.
Indeed, the estimated EQE values in Figure 5a are in very good agreement with the measured maximum EQE values for both UGH-3 and DPEPO based devices for dilution up to 30 vol %. At 50 vol % dilution, the UGH-3 device still shows very good agreement with an estimated EQE of 20% and a measured one of 19.2% (the highest measured EQE in this study, and a 20% enhancement of the neat exciplex), while the DPEPO device qualitatively follows the estimated trend but quantitatively underperforms. At higher dilutions, the maximum EQE decreases, again in line to what was observed for PLQYs and again thought to be due to extreme D–A distances inhibiting exciplex formation.
It is not trivial to explain the discrepancy between the estimated EQEs and those measured in hosts at 50 vol % and above. This might be caused by the different polarities and dielectric constants of DPEPO and UGH-3, large values of which would electrostatically screen the donor and acceptor, reducing their coupling and the probability of exciplex formation. If this is the case, significant care must be taken to choose appropriate hosts for future studies in exciplex dilution. Similar to the requirements for molecular TADF hosts, these hosts will need to balance both polarity and electrical transport properties to achieve the best devices. Alternatively, it could be that the discrepancy is a simple problem of charge balance in these specific devices and that further optimization of the organic stack could increase efficiency closer to the estimated values. Nonetheless, both device series and the estimates show the same qualitative trend.
In conclusion, we diluted the exciplex formed by TSBPA and PO-T2T into two different hosts with different polarity. Rather than immediately preventing exciplex formation, we found that dilution blue shifts the emission of the exciplex due to the reduction of the electrostatic interaction between the CT state’s D and A over greater distances. The exciplex maintains strong CT character despite the D–A separation, with films diluted in DPEPO systematically red-shifted compared to those in UGH-3 due to the effect of polarity on CT state relaxation. In addition to the emission blue shift, we demonstrated an increase in the PLQY of the exciplex passing from 58 to 80% before further dilution began to hinder exciplex formation. The increase in PLQY is directly transferable to OLEDs, where the maximum EQE increases from 14.8% for the neat exciplex to 19.2% for the device diluted with 50 vol % UGH-3, in good agreement with the estimated EQE values.
Our attribution of these effects to simple increases in D–A distance and reduction in concentration quenching suggests that the performance-enhancing effects of dilution are likely to be general to other exciplex blends and host materials. Optimization of exciplex dilution—previously assumed to only inhibit exciplex formation—will likely allow exciplex based TADF devices to rapidly match and exceed the performance of leading molecular TADF materials.
Acknowledgments
The authors would like to acknowledge the EXCILIGHT and HyperOLED projects funded by the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreements No. 674990 and No. 732013.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jpclett.8b03646.
Experimental methods; absorption spectra of PO-T2T, TSBPA, and neat and diluted exciplex films; time-resolved emission decays, spectra, and DF power dependences of neat and diluted exciplexes; time-resolved emission decays and spectra of TSBPA in DPEPO or UGH-3 hosts showing no exciplex formation in the absence of PO-T2T, and distance dependence of residual donor emission (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Uoyama H.; Goushi K.; Shizu K.; Nomura H.; Adachi C. Highly Efficient Organic Light-Emitting Diodes from Delayed Fluorescence. Nature 2012, 492, 234–238. 10.1038/nature11687. [DOI] [PubMed] [Google Scholar]
- dos Santos P. L.; Dias F. B.; Monkman A. P. Investigation of the Mechanisms Giving Rise to TADF in Exciplex States. J. Phys. Chem. C 2016, 120, 18259–18267. 10.1021/acs.jpcc.6b05198. [DOI] [Google Scholar]
- dos Santos P. L.; Ward J. S.; Bryce M. R.; Monkman A. P. Using Guest–Host Interactions To Optimize the Efficiency of TADF OLEDs. J. Phys. Chem. Lett. 2016, 7, 3341–3346. 10.1021/acs.jpclett.6b01542. [DOI] [PubMed] [Google Scholar]
- dos Santos P. L.; Ward J. S.; Batsanov A. S.; Bryce M. R.; Monkman A. P. Optical and Polarity Control of Donor – Acceptor Conformation and Their Charge-Transfer States in Thermally Activated Delayed- Fluorescence Molecules. J. Phys. Chem. C 2017, 121, 16462–16469. 10.1021/acs.jpcc.7b03672. [DOI] [Google Scholar]
- Higginbotham H. F.; Yi C.; Monkman A. P.; Wong K. Effects of Ortho-Phenyl Substitution on the RISC Rate of D–A Type TADF Molecules. J. Phys. Chem. C 2018, 122, 7627–7634. 10.1021/acs.jpcc.8b01579. [DOI] [Google Scholar]
- Zhang Q.; Li B.; Huang S.; Nomura H.; Tanaka H.; Adachi C. Efficient Blue Organic Light-Emitting Diodes Employing Thermally Activated Delayed Fluorescence. Nat. Photonics 2014, 8, 326–332. 10.1038/nphoton.2014.12. [DOI] [Google Scholar]
- Dias F. B.; Bourdakos K. N.; Jankus V.; Moss K. C.; Kamtekar K. T.; Bhalla V.; Santos J.; Bryce M. R.; Monkman A. P. Triplet Harvesting with 100% Efficiency by Way of Thermally Activated Delayed Fluorescence in Charge Transfer OLED Emitters. Adv. Mater. 2013, 25, 3707–3714. 10.1002/adma.201300753. [DOI] [PubMed] [Google Scholar]
- Kim K.-H.; Yoo S.-J.; Kim J.-J. Boosting Triplet Harvest by Reducing Nonradiative Transition of Exciplex toward Fluorescent Organic Light-Emitting Diodes with 100% Internal Quantum Efficiency. Chem. Mater. 2016, 28, 1936–1941. 10.1021/acs.chemmater.6b00478. [DOI] [Google Scholar]
- Moon C.-K.; Huh J.-S.; Kim J.-M.; Kim J.-J. Electronic Structure and Emission Process of Excited Charge Transfer States in Solids. Chem. Mater. 2018, 30, 5648–5654. 10.1021/acs.chemmater.8b02011. [DOI] [Google Scholar]
- Nakanotani H.; Furukawa T.; Morimoto K.; Adachi C. Long-Range Coupling of Electron-Hole Pairs in Spatially Separated Organic Donor-Acceptor Layers. Sci. Adv. 2016, 2, e1501470–e1501470. 10.1126/sciadv.1501470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D.; Jankus V.; Dias F. B.; Monkman A. Photophysical Investigation of the Thermally Activated Delayed Emission from Films of M-MTDATA:PBD Exciplex. Adv. Funct. Mater. 2014, 24, 2343–2351. 10.1002/adfm.201303389. [DOI] [Google Scholar]
- Al Attar H. A.; Monkman A. P. Electric Field Induce Blue Shift and Intensity Enhancement in 2D Exciplex Organic Light Emitting Diodes; Controlling Electron-Hole Separation. Adv. Mater. 2016, 28, 8014–8020. 10.1002/adma.201600965. [DOI] [PubMed] [Google Scholar]
- Chapran M.; Pander P.; Vasylieva M.; Wiosna-Salyga G.; Ulanski J.; Dias F. B.; Data P.. Realizing 20% External Quantum Efficiency in Electroluminescence with Efficient Thermally Activated Delayed Fluorescence from an Exciplex. ACS Appl. Mater. Interfaces; Under Revision [DOI] [PubMed] [Google Scholar]
- Aesar A.Density of Tetraphenylsilane. https://www.alfa.com/en/catalog/L04857/ (accessed Feb 1, 2019).
- Davis N. J. L. K.; MacQueen R. W.; Roberts D. A.; Danos A.; Dehn S.; Perrier S.; Schmidt T. W. Energy Transfer in Pendant Perylene Diimide Copolymers. J. Mater. Chem. C 2016, 4, 8270. 10.1039/C6TC02555B. [DOI] [Google Scholar]
- Reed L. H.; Allen L. C. Bond Polarity Index : Application to Group Electronegativity. J. Phys. Chem. 1992, 96, 157–164. 10.1021/j100180a032. [DOI] [Google Scholar]
- Gordon M.The Exciplex, 1st ed.; Academic Press, 1975. [Google Scholar]
- Hoang H. M.; Pham T. B. Van; Grampp G.; Kattnig D. R. Exciplexes versus Loose Ion Pairs: How Does the Driving Force Impact the Initial Product Ratio of Photoinduced Charge Separation Reactions?. J. Phys. Chem. Lett. 2014, 5, 3188–3194. 10.1021/jz501575r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-C.; Sarma M.; Chen Y.-T.; Liu S.-H.; Lin K.-T.; Chiang P.-Y.; Chuang W.-T.; Liu Y.-C.; Hsu H.-F.; Hung W.-Y.; et al. Probe Exciplex Structure of Highly Efficient Thermally Activated Delayed Fluorescence Organic Light Emitting Diodes. Nat. Commun. 2018, 9, 3111. 10.1038/s41467-018-05527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook K. S.; Lee J. Y. Organic Materials for Deep Blue Phosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2012, 24, 3169–3190. 10.1002/adma.201200627. [DOI] [PubMed] [Google Scholar]
- Natali M.; Campagna S.; Scandola F. Photoinduced Electron Transfer across Molecular Bridges: Electron- and Hole-Transfer Superexchange Pathways. Chem. Soc. Rev. 2014, 43, 4005–4018. 10.1039/C3CS60463B. [DOI] [PubMed] [Google Scholar]
- Wenger O. S. Photoinduced Electron Tunneling between Randomly Dispersed Donors and Acceptors in Frozen Glasses and Other Rigid Matrices. Phys. Chem. Chem. Phys. 2013, 15, 10673–10685. 10.1039/c3cp00011g. [DOI] [PubMed] [Google Scholar]
- Dinnocenzo J. P.; Feinberg A. M.; Farid S. Multiple Intermolecular Exciplexes in Highly Polar Solvents. J. Phys. Chem. A 2017, 121, 3662–3670. 10.1021/acs.jpca.7b01461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.