Abstract

Particle tracking microrheology was used to investigate the viscoelasticity of Staphylococcus aureus biofilms grown in microfluidic cells at various flow rates and when subjected to biofilm-degrading enzymes. Biofilm viscoelasticity was found to harden as a function of shear rate but soften with increasing height away from the attachment surface in good agreement with previous bulk results. Ripley’s K-function was used to quantify the spatial distribution of the bacteria within the biofilm. For all conditions, biofilms would cluster as a function of height during growth. The effects of proteinase K and DNase-1 on the viscoelasticity of biofilms were also investigated. Proteinase K caused an order of magnitude change in the compliances, softening the biofilms. However, DNase-1 was found to have no significant effects over the first 6 h of development, indicating that DNA is less important in biofilm maintenance during the initial stages of growth. Our results demonstrate that during the preliminary stages of Staphylococcus aureus biofilm development, column-like structures with a vertical gradient of viscoelasticity are established and modulated by the hydrodynamic shear caused by fluid flow in the surrounding environment. An understanding of these mechanical properties will provide more accurate insights for removal strategies of early-stage biofilms.
1. Introduction
Following attachment to a boundary, such as the glass interface in a flow chamber or a catheter surface in vivo, many bacteria species produce a complex extracellular matrix of polymeric material collectively called a biofilm.1 Biofilms provide a number of benefits for the proliferating bacterial community, ranging from facilitating communication via quorum-sensing2 to acting as physical barriers against phagocytic cells and antibiotics, leading to increases in antibiotic resistance by factors of 100.3 Additional physiological roles for biofilms include nutrient reservoirs,4 water-resisting protein “raincoats”,5 and to facilitate horizontal gene transfer between cells.6 It is expected that this is just a small fraction of the roles performed by biofilms; since it is one of the earliest biological structures formed by evolution with fossilized biofilms dating back to 3.3–3.5 billion years ago.7
Biofilm-bound bacteria are thought to be associated with ∼80% of chronic infections, with 2 million cases collectively costing the US healthcare system up to $10 billion each year.8,9 Staphylococcal strains are of particular clinical relevance due to their resistance to widely used antibiotics, most notably methicillin-resistant S. aureus (MRSA). Effective antibiofilm strategies require a complete understanding of the mechanical and rheological responses during biofilm development to environmental conditions, from the earliest stages of surface colonization to later dispersal stages, where entire sections of biofilm are shed from the bulk to colonize other environments.10 Indeed, mechanical cleaning is one of the principal mechanisms for biofilm prevention, be it the brushing of teeth, the scouring of sewerage pipes, or the removal of debris on the hulls of ships. Previous authors have examined the mechanical response of microscale biofilms to differing hydrodynamic shear stresses using magnetic force modulation atomic force microscopy,11 but in general the microscale effects of shear have been relatively little studied with biofilms and never with S. aureus or using particle tracking microrheology.
The majority of the extracellular components of biofilms grown in nutrient-rich conditions are categorized as extracellular polysaccharides (for S. aureus these are partially deacetylated polymer residues of poly-β-1-6-linked N-acetylglucosamine, or PNAG), nucleic acids, and proteinaceous adhesins.12,13 Numerous studies indicate that the addition of enzymes targeting these components of the biofilm can effectively remove or substantially inhibit biofilm growth.14−16
Passive microrheology is a reasonably well-established technique that can be used to investigate the viscoelasticity of biofilms.17 We employed the passive microrheological technique of particle tracking using the bacteria as probes to infer the viscoelastic properties of monoculture S. aureus biofilms. This work builds on the previous investigations of our group whereby individual bacteria were used as tracers in particle tracking experiments.18 This methodology provided a convenient noninvasive method to investigate the viscoelasticity of biofilms during their development. We have thus extended the passive particle tracking microrheology technique to study biofilms grown under different shear flow conditions. We find that shear flow tends to harden the viscoelastic shear moduli of the biofilms, presumably as a response of the bacteria to less favorable attachment conditions. A detailed statistical analysis of images from bright-field microscopy using a fast CMOS camera shows that the spatial heterogeneity of the bacteria within biofilms decreases over time using an analysis based on Ripley’s K-function (not to our knowledge previously used with biofilms). This indicates that biofilms grow in tapered columns, which could be a precursor to the filamentary structure exploited by biofilms to increase dispersal efficiency or as a response to local nutrient concentrations.
DNase-1 and proteinase K were used to disrupt the structural integrity of the biofilms. An order of magnitude change in viscoelasticity was observed using proteinase K (the biofilms soften by this amount), whereas DNase-1 had a negligible effect on the biofilms. Overall, these data suggest that for S. aureus initial biofilm growth away from the surface occurs in narrowing columns, rather than homogeneously, that there is a response to higher shear stresses to produce more rigid early-stage biofilms and that in the early-stages extracellular DNA plays a minor role in the structural integrity of the biofilm.
2. Experimental Section
2.1. Bacteria Preparation
All experiments were carried out with the S. aureus clinical strain, ATCC 25923. To ensure, bacteria concentrations were consistent across all experiments, 0.5 mL of batches of a 3 h culture is grown in tryptic soy broth, TSB (Sigma-Aldrich, Gillingham, UK) were frozen in a sterile 25% glycerol solution. For every repeat experiment, a separate sample was thawed, centrifuged for 5 min at 5000 rpm, and resuspended in fresh sterile TSB twice before incubation at 37 °C for 30 min. Prior to the deposition of the bacteria in the flow cell, 0.1 mL of a 10–5 dilution was plated on TSB agar (Sigma-Aldrich, Gillingham, UK), and the subsequent colonies were counted after an overnight incubation. From this, the number of colony forming units per mL (CFU/mL) was calculated. All bacteria inoculants used in this study contained 5 × 107 CFU/mL within one standard deviation (from three plates).
2.2. Biofilm Cultivation
Biofilms were grown in a chemostat, as shown in Figure 1. The chemostat primarily consisted of an IBI 3-channel flow cell (purchased through Sigma-Aldrich, Gillingham, UK) that was modified to operate in conjunction with an Ismatec REGLO ICC digital peristaltic pump (Cole-Parmer). Each channel had dimensions equal to 1 mm × 4 mm × 40 mm (height, h, width, w, and length, respectively). To sustain incubation temperatures, a Grant JB Aqua 18 Plus water bath was maintained at 37 °C, which housed a reservoir of media that were sealed with aluminum foil pierced with an air filter. Media were pumped out of the reservoir into the flow cell through a bubble trap to prevent the passage of bubbles into the cell, which would disrupt the fluid flow consistency and the biofilm growth. The microscope and flow cell were encapsulated in a custom-made incubator that was sustained at 37 °C using an Air-ThERM ATX (World Precision Instruments Ltd.), coupled to a thermometer with a feedback control loop. All waste media were collected in a separate container.
Figure 1.
Schematic diagram of the chemostat used to grow the bacterial biofilms (a flow cell fed with tryptic soya broth by a peristaltic pump) mounted around the tracking microscope with a vibration isolation unit to reduce erroneous signals. A closed system was employed to maintain sterility.
For each experiment, the chemostat was sterilized by first pumping through with a 3% Virkon-water solution for 12 h, then evacuated entirely of liquid and pumped through with a 5% Decon-water solution for a further 3 h. Following this, the chemostat was again evacuated of liquid and finally flushed with autoclaved deionized water to ensure no sterilization chemicals remained in the tubing or the flow cell. Despite the proficiency of this technique to adequately remove all bacteria from the interior of the flow cell, regular replacements of the flow cell chamber and tubing were made to ensure sterility.
Before inoculation with bacteria, the flow cell was primed with an initial passage of media. Biofilm development was initiated by injecting a sufficient dose of the bacterial culture (as described in Section 2.1) to fill the entire volume of the flow cell. Any bubbles that had formed during this step were removed by vigorous shaking, before the bacteria were left to deposit onto the surfaces of the flow cell for 1 h. Any planktonic bacteria remaining in the cell rapidly left the flow chamber when the flow was started. Shear stresses, τ were calculated from the pump flow rates in the parallel plate microfluidic chamber using
| 1 |
where Q is the media flow rate and μ is the dynamic viscosity of the TSB media, which is assumed to be equal to water.19 Flow rates were set at 0.15 and 1.50 mL/min to generate equivalent hydrodynamic shear stresses of 1 and 10 mPa, respectively, with a comparative dataset being produced for no flow (categorized as “stationary”). Experiments at 37 °C were conducted for 6 h, which allowed for significant proliferation of the bacteria.
Antibiofilm enzymes were added to the initial sterile TSB. To target the main components of the biofilm extracellular matrix, proteinase K from Tritirachium album and deoxyribonuclease 1 (DNase-1) from bovine pancreas (purchased through Sigma-Aldrich, Gillingham, UK) were added to final concentrations of 60 and 100 μg/mL, respectively.14,15 Proteinase K is a broad-spectrum enzyme that digests extracellular proteins in the biofilm by cleaving the peptide bond next to the carboxylic group of hydrophobic amino acids. DNase-1 was chosen as it selectively cleaves extracellular DNA, which is believed to be an important structural component of the Staphylococcal biofilm.15
2.3. Microscopy
The flow cell and incubator chamber were mounted on an Olympus IX70 inverted microscope fitted with a 100× oil-immersion objective lens illuminated by a pE-100 LED (CoolLED, UK). An AVI350M dynamic vibration isolation system (Table Stable Ltd., Switzerland) was employed to counteract unwanted vibrations. Videos of the bacteria motion were captured every hour on a Photron Fastcam PCI camera (Photron Ltd., Bucks, UK) operating in the bright-field mode. All videos were recorded at 1000 frames per second, over a field of view of 1024 × 1024 pixels (or ∼116 μm2). As the biofilm grew, bacterial motion was recorded at different heights with 5 ± 1 μm increments using the built-in micrometer focusing scale. A large gap (relative to the diameter of an individual bacterium, 1 μm) was used to ensure that bacteria from adjacent height layers were not recorded. The pump and air provider were temporarily turned off, whereas videos were recorded to avoid vibrations that could detrimentally alter the particle tracks. The LED was turned to a low-medium power setting, and all external sources of light in the lab were turned off during image capture to limit the amount of flicker appearing on videos due to AC mains electric input. The camera occasionally measured harmonics in the oscillatory circuit used to modulate the power of the LED (the duty ratio of LEDs is modulated, rather than the voltage, to vary the effective power, otherwise the spectral balance of the LED can change), which was not intended for such fast camera applications. This intermittent problem was corrected for using Fourier filtering (described later).
2.4. Particle Tracking
Individual bacteria were tracked with a MATLAB-based software package called PolyParticleTracker, which employs a polynomial-fit, Gaussian-weight algorithm to distinguish particles from the background noise.20 The software is particularly effective with biofilms, because the polynomial fit to the background makes it relatively robust to changes in the background intensity (a constant threshold is not used for identifying particles in the heterogeneous biofilms). Passive particle tracking microrheology of the spherical nonmotile bacteria was used based on the generalized Stokes–Einstein equation.17,21,22 The mean-square displacement, ⟨Δr2(t)⟩, was calculated and converted to the shear creep compliance, J(t), using the proportionality constant
| 2 |
where a is the hydrodynamic
radius of a bacterium, T is the temperature, kB is Boltzmann’s constant, and t is the time interval over which the bacterial displacements
were considered.23Figure 2a shows an example of a biofilm image which
highlights some of the identified bacteria and tracks (Figure 2b,c). An example of the MSD
signals as a function of time interval is also shown in Figure 2d. Creep compliances corresponding
to displacements of less than 10 nm over a time interval of 1 ms were
considered to be due to firmly attached bacteria, either to the microscope
slide surface or the surrounding biofilm, as any displacement would
be indistinguishable from noise due to the resolution of the camera.20 If a bacterium had a compliance value less than
this resolution value, it was set to a value equal to the noise limit.
Erroneous vibrations occurring in the bacteria tracks at frequencies
greater than 50 Hz (likely caused by electrical noise in the LED)
were removed using the Fourier-based low-pass filter. The smooth monotonic
compliance curves meant that it was relatively easy to isolate the
oscillatory noise using the narrow band Fourier filter. To compare
compliance values, a reference time interval of 10 ms was used. The
compliance values that were most representative of the data sets were
found by performing a log-normal fit to the distribution at this reference
time interval, and the mean values were found. All data are presented
with the associated standard errors, produced by taking the standard
deviations from the log-normal fits and weighting by a factor of 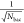 (where Nbac is equal to the number of successfully identified and tracked
bacteria,
which typically was in the hundreds for each observation).
(where Nbac is equal to the number of successfully identified and tracked
bacteria,
which typically was in the hundreds for each observation).
Figure 2.
Example data from the tracking analysis. (a) A bright-field microscopy image of a S. aureus biofilm grown in the flow cell after an incubation time of 3 h at 37 °C, at an elevation of 5 μm from the flow cell bottom surface. (b) A magnified section from (2a) with an overlay showing individual bacteria that have been identified, their respective radii and tracks over 1000 frames, equivalent to 1 s. Each color represents a unique bacterium that has been identified and tracked. (c) An enlarged rendering of an example “track” constructed from the displacements of a single bacterium position between adjacent frames, showing the subpixel localization precision attainable with the fitting protocol. (d) All mean-square displacements, (MSDs, ⟨Δr2(t)⟩), shown as a function of time interval corresponding to all bacteria identified in (a). The scale bars are equal to 10 μm.
2.5. Ripley’s K-Function for Image Analysis
The heterogeneity of the early-stages of biofilm growth was quantified by applying Ripley’s K-function. Briefly, the function is proportional to the expected number of bacteria found within a circle of radius, r, centered on a randomly chosen bacterium normalized to the overall number density of bacteria.24 For bacteria that are distributed randomly across the region of interest, the expected value for K(r) is πr2. For a total of N bacteria in a region of interest of area, AROI, Ripley’s K-function can be written as
| 3 |
where rij is the Euclidean distance between bacteria i and j, Ain is the area
of the circle centered on the ith bacterium with
a radius, r that lies within the bounds of the region
of interest, and I(rij < r) is an indicator function
that is equal to 1 when the condition is satisfied (and equal to 0
otherwise). Significant clustering was indicated by comparing K(r) to the expected Ripley-K-function value for complete spatial randomness, (E[K(r)]) and subtracting 1, in the
form: 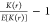 . Any
value above 0 signifies some spatial
clusterings, and conversely any value less than 0 can be attributed
to spatial regularity i.e., spatial anticorrelation.
. Any
value above 0 signifies some spatial
clusterings, and conversely any value less than 0 can be attributed
to spatial regularity i.e., spatial anticorrelation.
To visualize the amount of clustering as a function of the radius, an alternative way to display this is to reformulate, K(r), in the form L(r) – r, where
| 4 |
which has a value of 0 when bacteria are spaced completely randomly and is positive when there are some degrees of clustering.25 Upper and lower 97.5% critical values for significance testing were computed by sampling 50 hard-shell Monte Carlo simulations with the same number of bacteria of a fixed radius.
3. Results and Discussion
Figure 3a shows the mean creep compliance of all S. aureus bacteria in the field of view at a reference time interval of 10 ms as a function of height (in 5 μm increments) for biofilms grown under three different hydrodynamic shear regimes after 4 h. A time point of 4 h was chosen, as it represents significant biofilm proliferation compared to the initial number of bacteria following deposition. This can be observed in Figure S1, which shows the average number of bacteria for each hydrodynamic regime at each time point and each height. Hydrodynamic shear stresses of 0 mPa (stationary), 1 mPa, and 10 mPa were applied to the biofilm during development. Error bars are presented as the standard deviation of a log-normal fit of a probability density histogram containing all creep compliances observed in the field of view, weighted by the square root of the number of bacteria (Figure 3b–d shows these histograms for the biofilm-bound bacteria at a height of 15 μm at the different hydrodynamic shears). Significant differences arose between equivalent heights depending on the environmental flow conditions. For example, in a stationary biofilm after 4 h at 10 μm above the flow cell surface, the mean creep compliance was 0.708 ± 0.014 Pa–1. For comparison, biofilms grown under shear stresses of 1 and 10 mPa had mean creep compliances of 0.247 ± 0.003 and 0.251 ± 0.004 Pa–1, respectively, indicating a biofilm ∼3 times as rigid as that grown in a static environment. Variations in the identified bacteria radii are not significant enough to account for the differences in creep compliance observed throughout this experiment (Figure S2 shows the distributions of bacteria radii measured for all time points and heights for the three hydrodynamic regimes). Creep compliances for all time points over the 6 h observation period are shown in Figure S3, with similar relationships occurring at all time points.
Figure 3.
(a) At a reference time point of 10 ms, the mean creep compliance was calculated for biofilms after 4 h of sustained flow at 37 °C at incremental heights (represented by color) above the flow cell surface, subject to varying hydrodynamic shears. (b)–(d) show probability distributions for compliances at tref = 10 ms for a height of 15 μm subject to different hydrodynamic shears.
All hydrodynamic regimes display increasing creep compliance as a function of height (indicating biofilms are softer at greater heights), with stationary biofilms exhibiting significantly larger compliances than either of the two flow regimes. These results suggest that for S. aureus, there is a response to higher shear stresses to produce more rigid early-stage biofilms. This is in agreement with established results for other bacteria species in the literature. Galy et al. showed using magnetic microparticle actuation that the spatial distribution of creep compliance for an F pilus-producing E. coli biofilm grown after 24 h is dependent on height and inversely dependent on shear stress, corroborating the pattern and magnitudes of results presented in this study.26 Fluorescent beads have also been used to examine biofilm viscoelasticity through microrheology. A 2016 study by Cao et al. found characteristic creep compliances an order of magnitude larger than stated in this study but were limited to larger lag times due to the slow acquisition speed of the confocal scanning microscope used in their experiment. Using their technique, they found no significant difference in creep compliance between increasing height layers, contrary to our results. However, it should be noted that they were observing more mature biofilms (24 and 48 h) that may be denser due to extended proliferation.27 An advantage of our particle tracking technique is the absence of physical perturbation caused by the addition of magnetic or fluorescent beads to the biofilm during growth, which could act as abiotic surfaces for the bacteria to attach to other than the surfaces of the flow cell.
Figure 4a shows
the ratio of the Ripley-K-function, K(r), to the expected Ripley-K-function
for complete spatial randomness, E[K(r)], when the clustering radius, r, is equal to 10 μm for all three hydrodynamic growth regimes
at incremental heights after 5 h. A biological interpretation of the
rescaled Ripley’s K-function, 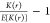 , is
the fractional difference in the number
of bacteria that would be expected within a circle centered on a random
bacterium defined by the clustering radius compared to the same total
number of bacteria but randomly distributed. For example, the data
suggests that for any bacteria at a height of 10 μm above the
surface of the flow cell after 5 h of growth when there is no flow
present, one would expect to find 32 ± 13% more bacteria present
within a circle of radius of 10 μm when compared to a randomly
distributed arrangement of the same total number of bacteria. As the
biofilm grows vertically, the degree of clustering becomes more significant,
indicating biofilm growth away from the surface occurs in narrowing
columns, rather than homogeneously. As an example, after 5 h in a
biofilm subject to 10 mPa hydrodynamic shear in a 10 μm radius
circle, our data suggests at the surface there would be 3 ± 1%
more bacteria present than a completely random arrangement, whereas
at 20 μm away from the surface, there would be 46 ± 19%
more bacteria than an equivalent number arranged randomly. This trend
occurs regardless of the hydrodynamic shear experienced by the biofilm. Figure 4b,c shows the rescaled
Ripley-K-function at the flow cell surface and first
height increment 5 μm above the surface for all shear regimes.
For stationary environment biofilms, the initial distribution of bacteria
remains approximately constant for the duration of the experiment,
whereas the two flow regimes show a decrease in the rescaled Ripley-K-function value which is indicative of the bacteria becoming
more homogeneously organized within the biofilm. As S. aureus are nonmotile, this result suggests that
bacteria are preferentially growing horizontally rather than vertically
when under flow. Furthermore, the homogeneous distribution of bacteria
may be aiding the structural stability of the biofilm, as extracellular
matrix is being produced evenly at the base, rather than in localized
clusters. The full dataset of the rescaled Ripley-K-function values over the course of the experiment for all three
hydrodynamic shear regimes is shown in Figure S4.
, is
the fractional difference in the number
of bacteria that would be expected within a circle centered on a random
bacterium defined by the clustering radius compared to the same total
number of bacteria but randomly distributed. For example, the data
suggests that for any bacteria at a height of 10 μm above the
surface of the flow cell after 5 h of growth when there is no flow
present, one would expect to find 32 ± 13% more bacteria present
within a circle of radius of 10 μm when compared to a randomly
distributed arrangement of the same total number of bacteria. As the
biofilm grows vertically, the degree of clustering becomes more significant,
indicating biofilm growth away from the surface occurs in narrowing
columns, rather than homogeneously. As an example, after 5 h in a
biofilm subject to 10 mPa hydrodynamic shear in a 10 μm radius
circle, our data suggests at the surface there would be 3 ± 1%
more bacteria present than a completely random arrangement, whereas
at 20 μm away from the surface, there would be 46 ± 19%
more bacteria than an equivalent number arranged randomly. This trend
occurs regardless of the hydrodynamic shear experienced by the biofilm. Figure 4b,c shows the rescaled
Ripley-K-function at the flow cell surface and first
height increment 5 μm above the surface for all shear regimes.
For stationary environment biofilms, the initial distribution of bacteria
remains approximately constant for the duration of the experiment,
whereas the two flow regimes show a decrease in the rescaled Ripley-K-function value which is indicative of the bacteria becoming
more homogeneously organized within the biofilm. As S. aureus are nonmotile, this result suggests that
bacteria are preferentially growing horizontally rather than vertically
when under flow. Furthermore, the homogeneous distribution of bacteria
may be aiding the structural stability of the biofilm, as extracellular
matrix is being produced evenly at the base, rather than in localized
clusters. The full dataset of the rescaled Ripley-K-function values over the course of the experiment for all three
hydrodynamic shear regimes is shown in Figure S4.
Figure 4.
(a) Rescaled Ripley-K analysis as a function of height for biofilm grown after 5 h at 37 °C. Rescaled Ripley-K-function analysis for biofilm at the bottom layer (b) and 5 μm above surface of flow cell (c) over all time points and for all hydrodynamic regimes.
To elaborate on this further, Figure 5a,b shows the locations of bacteria after 5 h in 10 mPa flow conditions at heights of 0 and 15 μm, respectively, where the bacteria have been color coded based on their individual creep compliance value at a reference time of 10 ms. Despite there being fewer bacteria at 15 μm height, it is apparent visually that the distribution is not evenly spatially distributed, especially in comparison to the bacteria at the surface. Figure 5c,d displays the corresponding L(r)–r functions with 97.5 and 2.5% quantiles from 50 Monte Carlo simulations of randomly distributed hard-shell particles. Any positive value greater than the error quantiles indicates clustering at that value of d (given in units of pixels in these figures). A circle of r = 140 pixels originating from a randomly chosen bacterium is shown on Figure 5b, where the corresponding L(r)–r function peaks, to illustrate the most statistically likely clustering size.
Figure 5.
Comparative examples of bacteria distributions color coded by creep compliance of each bacteria for biofilms grown under 10 mPa hydrodynamic shear stress for 5 h at 37 °C at the surface of the flow cell (a) and 15 μm above the surface (b). In (b), a circle is shown with an arbitrary radius, r originating from a randomly selected bacterium to convey the bacteria selected within a certain radius. (c) and (d) show the normalized Ripley-K-function (L(r)−r) corresponding to (a) and (b) respectively, to demonstrate the extent of clustering at a radius, r = 10 μm.
To combine the ideas of spatial heterogeneity and characteristic creep compliance, Figure 6 shows the all mean creeps at all time points and heights as a function of bacteria density, with an inset showing the mean creeps as a function of rescaled Ripley-K-function. Linear fits are shown as black lines, revealing an inverse relationship between creep and cell density, but a positive correlation between creep and rescaled Ripley-K-function. Intuitively, a greater number of adjacent bacteria would result in a stiffer biofilm due to more overall cell–cell adhesion and shared extracellular material. Moreover, the increase in creep associated with larger spatial heterogeneity could be due to isolated columns of bacteria with no surrounding structural support.
Figure 6.

Characteristic creep compliance as a function of bacteria density for all time points, heights, and hydrodynamic regimes, with a black fit line showing an inverse linear correlation. The inset shows the same mean creep compliances plotted as a function of the rescaled Ripley-K-function, with a black fit line displaying a positive linear correlation.
The early-stage biofilm structure investigated in this study may be the precursor to the macroscale features of mature biofilms that have been studied in the literature. For example, Stoodley et al. showed that mature biofilms in laminar flow elongate into long streamers, as opposed to circular clusters in turbulent flow conditions.28 Three-dimensional streamers are also present in motile bacteria (such as Pseudomonas aeruginosa) biofilms, as demonstrated by Drescher et al., as a response to unusual geometries, such as flow obstacles, gaps, or corners.29 To visualize the degree of clustering as a function of depth in the biofilm, Figure 7 shows a rendering of the segmented bacteria calculated at 5 μm increments within the same sample for a static biofilm at the end of the observation period. Colored bacteria correspond to those resolved for the calculation of the creep compliances and spatial statistical analysis (with out of focus bacteria shown as gray outlines). Tapered columns can be seen at greater heights with broader bases attached to the glass surface, reflecting the increase in spatial heterogeneity at the higher elevations furthest from the surface.
Figure 7.

Rendering of the bacteria distributed within a biofilm subject to no hydrodynamic shear after 6 h of growth at 37 °C. Bacteria positions are extracted from tracking data and color coded based on height above surface in 5 μm height intervals. Gray outlines represent bacteria in the spaces between focal planes. The arrows indicate the direction of shear flow. Tapered columns can be seen with their bases on the surface of attachment.
Figure 8 shows the characteristic creep compliance value at a reference time of 10 ms for biofilm grown at 37 °C subject to a hydrodynamic shear of 1 mPa after 5 h in the presence of proteinase K and DNase-1. Biofilms formed in the presence of proteinase K were unable to grow past 10 μm, suggesting that the shear forces from the surrounding flow overcame the intracellular attachment when a protein biofilm component is removed. Addition of proteinase K results in a significant increase in the compliance of the biofilm compared with the control (TSB alone). Bacterial biofilm on the surface of the flow cell had a mean creep compliance of 0.369 ± 0.005 Pa–1 compared to the samples exposed to proteinase K, which had a mean creep compliance at the surface of 1.394 ± 0.017 Pa–1, indicating much softer viscoelastic structures form. In contrast, the addition of DNase-1 at the same time point and height reduced the mean compliance to 0.321 ± 0.006 Pa–1. This pattern was observed for all heights and all time points. In their study on DNase-1 dependence on biofilm coverage, Moormeier et al. found that there was no statistically significant discrepancy between UAMS-1 S. aureus biofilms grown with and without DNase-1 until after 6 h, possibly because cell lysis is not induced until after this time point.30,31 Characteristic creep compliances across all time points and all heights in the presence proteinase K and DNase-1 are shown in Figure S5.
Figure 8.

Mean compliances calculated at a characteristic time (10 ms) plotted after 5 h for two different enzymes (proteinase K and DNase-1) and the control (just TSB). Biofilms grown in the presence of proteinase K exhibited much larger creep compliances, characteristic of softer biofilms. Biofilms, grown in the presence of DNase-1, showed a slight decrease in creep compliance compared to no enzyme present.
The elasticity gradient of the bacterial columns means that there is not a single threshold value of shear stress associated with S. aureus biofilm detachment. Instead, a broad range of elasticities and thus detachment stresses are presented by the biofilm, which may help to insure both persistent colonization of the chosen surface and subsequent detachment events to colonize other surfaces or interfaces.
In the future, we intend to combine this passive-microrheology technique with super-resolution fluorescence microscopy to observe the structure of intact biofilms during development with ∼20 nm resolution. Under restricted nutrient availability, Staphylococcal strains have been shown to produce amyloid fibers that are phenol-soluble modulins attached to biofilm macromolecules such as eDNA.32,33 Such amyloid fibers are known to be pathogenic causing lysis in human neutrophils.34 It would be interesting to explore their role in the micromechanics of biofilms.
Graphene oxide coatings show promise for loading antimicrobial peptides.35 Particle tracking microrheology is seen to be a sensitive method to probe antibiofilm treatments and could be used to investigate the effectiveness of graphene coatings. It could also be used for large-scale screening of candidate antibiofilm drug molecules and used in combination with bactericidal assays to quantify the combined effects of mixed formulations that both disrupt biofilms and kill bacteria e.g., proteinases mixed with penicillin. A consensus is currently developing that effective antimicrobial treatments need a combination of antibiofilm and antibacterial drugs, since both properties are not demonstrated by the same molecules.
4. Conclusions
Passive-microrheology experiments on S. aureus biofilms show clear evidence that the characteristic creep compliances become smaller under flow. S. aureus and thus reacts to an increase in shear force by producing a more rigid biofilm in which it is embedded, by up to a factor of 3 in relative compliance. A statistical spatial analysis reveals early-stage biofilms grow vertically in column-like arrangements. However, biofilms under flow grow more homogeneously at layers closer to the surface over time, compared to biofilms grown in static conditions. Coupled with the viscoelastic response of biofilms, this suggests a reinforcement of the lower layers close to the attachment surface to prevent complete detachment of the biofilm under shear stress. A vertical gradient of viscoelasticity and spatial heterogeneity also facilitates biofilm dispersal, allowing loosely bound bacteria to be removed from the biofilm structure while retaining an entrenched layer close to the attachment surface. Treatment with proteinase K had a large effect on softening the biofilms, whereas DNase-1 in contrast has the opposite effect, slightly hardening the biofilms.
This methodology could be extensively used to screen different antibiofilm compounds (as opposed to antibacterial compounds that directly kill bacteria). It is thought that effective treatment of microbial infections associated with biofilms will require mixed formulations targeted at both the bacteria (concentrated antibiotics) and the biofilms (antibiofilm molecules). Both DNases and proteinases are primary candidates for antibiofilm molecules in such formulations.
Acknowledgments
The CDT in Regenerative Medicine (EPSRC/MRC) supported the PhD of Jack Hart.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.langmuir.8b04252.
The Supplementary Information contains five figures with additional details to convey the complete dataset for all biofilm studies. First, Figure S1 shows the bacteria counts corresponding to the same experiments. No significant differences in bacteria population are observed for biofilms grown under flow, but fewer bacteria were observed in the stationary biofilm at greater heights. Figure S2 shows the identified bacteria radii across all experimental time points, heights (indicated by color), and hydrodynamic regimes. As the maximum variation is on the order of 6%, discrepancies in the bacteria size cannot alone explain the changes in creep compliance observed. Figure S3 shows the characteristic creep compliance at a reference time of 10 ms over the complete experiment time course of 6 h for the hydrodynamic shear stresses (no flow, 1 and 10 mPa). Figure S4 shows the rescaled Ripley-K-function for a radius of 10 μm for all time points and flow regimes, indicating the increase in spatial heterogeneity as a function of height. Figure S5 conveys the biofilm characteristic creep compliances at a shear rate of 1 mPa when subject to 60 μg/mL of proteinase K and 100 μg/mL of DNase-1. A large increase in creep is seen for all time points in the biofilm treated with proteinase K, however DNase-1 made little impact (compare with Figure S3b) (PDF)
The authors declare no competing financial interest.
This paper was published ASAP on February 15, 2019, with an error in an author middle initial. The corrected version was reposted on February 20, 2019.
Supplementary Material
References
- Branda S. S.; Chu F.; Kearns D. B.; Losick R.; Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 2006, 59, 1229–1238. 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- Yarwood J. M.; Bartels D. J.; Volper E. M.; Greenberg E. P. Quorum sensing in Staphylococcus aureus biofilms. J. Bacteriol. 2004, 186, 1838–1850. 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T.; Kirketerp-Møller K.; Kristiansen S.; Phipps R.; Nielsen A. K.; Jensen PØ.; Høiby N.; Givskov M. Silver against Pseudomonas aeruginosa biofilms. Apmis 2007, 115, 921–928. 10.1111/j.1600-0463.2007.apm_646.x. [DOI] [PubMed] [Google Scholar]
- Flemming H.-C.; Wingender J.; Szewzyk U.; Steinberg P.; Rice S. A.; Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563. 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- Arnaouteli S.; MacPhee C. E.; Stanley-Wall N. R. Just in case it rains: building a hydrophobic biofilm the Bacillus subtilis way. Curr. Opin. Microbiol. 2016, 34, 7–12. 10.1016/j.mib.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Stalder T.; Top E. Plasmid transfer in biofilms: a perspective on limitations and opportunities. NPJ Biofilms Microbiomes 2016, 2, 16022 10.1038/npjbiofilms.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westall F.; de Wit M. J.; Dann J.; van der Gaast S.; de Ronde C. E.; Gerneke D. Early Archean fossil bacteria and biofilms in hydrothermally-influenced sediments from the Barberton greenstone belt, South Africa. Precambrian Res. 2001, 106, 93–116. 10.1016/S0301-9268(00)00127-3. [DOI] [Google Scholar]
- Janssens J. C.; Steenackers H.; Robijns S.; Gellens E.; Levin J.; Zhao H.; Hermans K.; De Coster D.; Verhoeven T. L.; Marchal K.; et al. Brominated furanones inhibit biofilm formation by Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2008, 74, 6639–6648. 10.1128/AEM.01262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R. A.; Graeme A.; Leid J. G.; Costerton J. W.; Shirtliff M. E.; et al. Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment. Infect. Immun. 2011, 79, 1797–1803. 10.1128/IAI.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter B. Slimy business—the biotechnology of biofilms. Nat. Biotechnol. 2003, 21, 361. 10.1038/nbt0403-361. [DOI] [PubMed] [Google Scholar]
- Gan T.; Gong X.; Schönherr H.; Zhang G. Microrheology of growing Escherichia coli biofilms investigated by using magnetic force modulation atomic force microscopy. Biointerphases 2016, 11, 041005 10.1116/1.4968809. [DOI] [PubMed] [Google Scholar]
- Otto M.Staphylococcal Biofilms. In Bacterial Biofilms; Springer, 2008; pp 207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal D.; Foreman A.; Bardy J. J.; Wormald P. J. Staphylococcus aureus biofilms: Nemesis of endoscopic sinus surgery. Laryngoscope 2011, 121, 1578–1583. 10.1002/lary.21805. [DOI] [PubMed] [Google Scholar]
- Shukla S. K.; Rao T. S. Dispersal of Bap-mediated Staphylococcus aureus biofilm by proteinase K. J. Antibiot. 2013, 66, 55. 10.1038/ja.2012.98. [DOI] [PubMed] [Google Scholar]
- Izano E. A.; Amarante M. A.; Kher W. B.; Kaplan J. B. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 2008, 74, 470–476. 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz G. V.; Artemenko N. K.; Tetz V. V. Effect of DNase and antibiotics on biofilm characteristics. Antimicrob. Agents Chemother. 2009, 53, 1204–1209. 10.1128/AAC.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waigh T. A. Microrheology of complex fluids. Rep. Prog. Phys. 2005, 68, 685–742. 10.1088/0034-4885/68/3/R04. [DOI] [PubMed] [Google Scholar]
- Rogers S. S.; Van Der Walle C.; Waigh T. A. Microrheology of bacterial biofilms in vitro: Staphylococcus aureus and Pseudomonas aeruginosa. Langmuir 2008, 24, 13549–13555. 10.1021/la802442d. [DOI] [PubMed] [Google Scholar]
- Nauman E. A.; Risic K. J.; Keaveny T. M.; Satcher R. L. Quantitative assessment of steady and pulsatile flow fields in a parallel plate flow chamber. Ann. Biomed. Eng. 1999, 27, 194–199. 10.1114/1.173. [DOI] [PubMed] [Google Scholar]
- Rogers S. S.; Waigh T. A.; Zhao X.; Lu J. R. Precise particle tracking against a complicated background: polynomial fitting with Gaussian weight. Phys. Biol. 2007, 4, 220. 10.1088/1478-3975/4/3/008. [DOI] [PubMed] [Google Scholar]
- Waigh T. A. Advances in the microrheology of complex fluids. Rep. Prog. Phys. 2016, 79, 074601 10.1088/0034-4885/79/7/074601. [DOI] [PubMed] [Google Scholar]
- Mason T. G.; Weitz D. A. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys. Rev. Lett. 1995, 74, 1250–1253. 10.1103/PhysRevLett.74.1250. [DOI] [PubMed] [Google Scholar]
- Xu J.; Viasnoff V.; Wirtz D. Compliance of actin filament networks measured by particle-tracking microrheology and diffusing wave spectroscopy. Rheol. Acta 1998, 37, 387–398. 10.1007/s003970050125. [DOI] [Google Scholar]
- Ripley B. D. Modelling spatial patterns. J. R. Stat. Soc. Ser. B 1977, 172–212. 10.1111/j.2517-6161.1977.tb01615.x. [DOI] [Google Scholar]
- Besag J. Comments on Ripley’s paper: Royal Statistical Society. Journal 1977, 39, 193–195. [Google Scholar]
- Galy O.; Latour-Lambert P.; Zrelli K.; Ghigo J. M.; Beloin C.; Henry N. Mapping of bacterial biofilm local mechanics by magnetic microparticle actuation. Biophys. J. 2012, 103, 1400–8. 10.1016/j.bpj.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H.; Habimana O.; Safari A.; Heffernan R.; Dai Y.; Casey E. Revealing region-specific biofilm viscoelastic properties by means of a micro-rheological approach. npj Biofilms Microbiomes 2016, 2, 5 10.1038/s41522-016-0005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P.; Dodds I.; Boyle J. D.; et al. Influence of hydrodynamics and nutrients on biofilm structure. J. Appl. Microbiol. 1998, 85, 19S–28S. 10.1111/j.1365-2672.1998.tb05279.x. [DOI] [PubMed] [Google Scholar]
- Drescher K.; Shen Y.; Bassler B. L.; Stone H. A. Biofilm streamers cause catastrophic disruption of flow with consequences for environmental and medical systems. Proc. Natl. Acad. Sci. USA 2013, 110, 4345–4350. 10.1073/pnas.1300321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moormeier D. E.; Bose J. L.; Horswill A. R.; Bayles K. W. Temporal and stochastic control of Staphylococcus aureus biofilm development. mBio 2014, 5, e01341 10.1128/mBio.01341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann E. E.; Rice K. C.; Boles B. R.; Endres J. L.; Ranjit D.; Chandramohan L.; Tsang L. H.; Smeltzer M. S.; Horswill A. R.; Bayles K. W. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 2009, 4, e5822 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K.; Ganesan M.; Payne D. E.; Solomon M. J.; Boles B. R. Extracellular DNA facilitates the formation of functional amyloids in S taphylococcus aureus biofilms. Mol. Microbiol. 2016, 99, 123–134. 10.1111/mmi.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.; Joo H.-S.; Nair V.; Le K. Y.; Otto M. Do amyloid structures formed by Staphylococcus aureus phenol-soluble modulins have a biological function?. Int. J. Med. Microbiol. 2018, 308, 675–682. 10.1016/j.ijmm.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.; Braughton K. R.; Kretschmer D.; Bach T.-H. L.; Queck S. Y.; Li M.; Kennedy A. D.; Dorward D. W.; Klebanoff S. J.; Peschel A. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007, 13, 1510 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Li R.Fluorescent Quenching from Functionalised Graphene Oxide Films. Ph.D. Dissertation, The University of Manchester: Manchester, UK, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







