Abstract
Objective
Evaluate the physiological and autonomic nervous system responses of premature newborns to body position and noise in the neonatal intensive care unit.
Methods
A quasi-experimental study. The autonomic nervous system of newborns was evaluated based on heart rate variability when the newborns were exposed to environmental noise and placed in different positions: supine without support, supine with manual restraint and prone.
Results
Fifty premature newborns were evaluated (gestational age: 32.6 ± 2.3 weeks; weight: 1816 ± 493g; and Brazelton sleep/awake level: 3 to 4). A positive correlation was found between environmental noise and sympathetic activity (R = 0.27, p = 0.04). The mean environmental noise was 53 ± 14dB. The heart rate was higher in the supine position than in the manual restraint and prone positions (148.7 ± 21.6, 141.9 ± 16 and 144 ± 13, respectively) (p = 0.001). Sympathetic activity, represented by a low frequency index, was higher in the supine position (p < 0.05) than in the other positions, and parasympathetic activity (high frequency, root mean square of the sum of differences between normal adjacent mean R-R interval and percentage of adjacent iRR that differed by more than 50ms) was higher in the prone position (p < 0.05) than in the other positions. The complexity of the autonomic adjustments (approximate entropy and sample entropy) was lower in the supine position than in the other positions.
Conclusion
The prone position and manual restraint position increased both parasympathetic activity and the complexity of autonomic adjustments in comparison to the supine position, even in the presence of higher environmental noise than the recommended level, which tends to increase sympathetic activity.
Keywords: Heart rate/physiology; Prone position; Infant, newborn; Autonomic nervous system; Noise
INTRODUCTION
The autonomic nervous system (ANS) is part of the peripheral nervous system and is responsible for the automatic homeostasis of an organism. The ANS is subdivided into two systems that work in synergy: the sympathetic and parasympathetic nervous systems.(1) In the first months of life, the ANS develops rapidly. The sympathetic nervous system starts developing at the beginning of gestation, and the parasympathetic nervous system develops more in the perinatal period. Thus, the development and control of the parasympathetic nervous system are susceptible to stimuli in the surrounding environment, such as excessive noise and body position.(2,3) The first six months of the postnatal period are fundamental for the maturation of the parasympathetic nervous system.(4)
Noise, hypoxia and premature birth are common in neonatal intensive care units (ICUs) and are associated with increases in catecholamines and sympathetic activity in newborns.(5) Premature newborns have greater difficulty decelerating their heartbeat in the occurrence of external noise than full-term infants.(6) The external stimuli to which premature newborns are often subjected include pain, light and noise, which are stressors that cause instability with regard to physiological control. Body positioning is a common practice and is necessary for the development of premature newborns who are not able to resist the force of gravity, maintain body alignment on the midline or maintain a physiologic flexion position on their own, all of which are fundamental to adequate neurological development. Inadequate positioning is another source of stress for this population, affecting behavior and probably autonomic control. Candia et al.(7) evaluated the salivary cortisol levels of premature newborns and found lower levels when the newborns were in the prone position than in other positions, thus associating this position with the reduction of stress.
Body positioning, autonomic control and environmental stress in the neonatal ICU occur simultaneously. According to Peng et al.,(8) premature newborns exhibit more stress in the supine position than in the prone position, which is associated with better respiratory mechanics and lower stress. However, studies show that this position is associated with a greater incidence of sudden infant death, less heart rate variability (HRV) and greater sympathetic activity during sleep. The hypothesis is that with regard to clinical variables and ANS responses, awake premature newborns, such as those in the study by Peng et al.,(8) who are subjected to habitual environmental stimuli in the neonatal ICU may benefit from the prone position and the supine position with manual restraint for flexion, thus maintaining the body on the midline in comparison to the supine position without support.
The aim of the present study was to evaluate the physiological and ANS responses of premature newborns to body position and noise in the neonatal ICU.
METHODS
A quasi-experimental prospective study was conducted with a single group of newborns in which each child served as his/her own control for each position.
This study was conducted in accordance with the precepts stipulated in the Declaration of Helsinki and the Nuremberg Code as well as the norms governing research involving human subjects (Resolution 466/2012 of the Brazilian National Board of Health). The study received approval from the Human Research Ethics Committee of the Hospital do Mandaqui and Universidade Nove de Julho (certificate of approval: 1.613.732/2016).
The study was conducted at the neonatal ICU of the Hospital do Mandaqui. The sample was composed of 50 newborns with 32 to 40 weeks of corrected age who were born between gestational ages of 30 and 36 weeks. The sample calculation was performed from a pilot study with 15 newborns in which an analysis of the correlation between sympathetic activity and noise was conducted (r = 0.40). Using a two-tailed significance of p = 0.05, beta error of 0.20, and power of 80%, the study sample should have at least 47 newborns. Fifty were included to guarantee the calculated sample size.
Inclusion criteria
Were included newborns with 32 to 40 weeks of corrected age; weight less than 2500g and more than 1100g (only low-weight and very-low-birth-weight infants were included to avoid excessive handling of extremely low-birth-weight infants and to guarantee sample homogeneity); clinical stability with weight gain in the previous 72 hours; more than 72 hours of life; and stage 3 or 4 on the Brazelton sleep/awake scale.(9)
Exclusion criteria
Were not included in the sample neonates using invasive or noninvasive mechanical ventilation; in use of oxygen therapy; with clinical instability; with active infection; and refusal to sign informed consent by a legal guardian.
Experimental procedure
Measurement of environmental noise in the neonatal intensive care unit
Noise was measured near each child's crib/incubator with the aid of a Sound Datalogger(tm) for 30 minutes during the normal care routine in the neonatal ICU.(10) The data were subsequently analyzed using a specific software program and expressed in decibels (dB). When the infant was placed in the supine position with no support, in the dorsal decubitus position with restraint (Figure 1) or in the prone position, noise was measured minute by minute for 10 minutes in each position along with vital signs and HRV.
Figure 1.
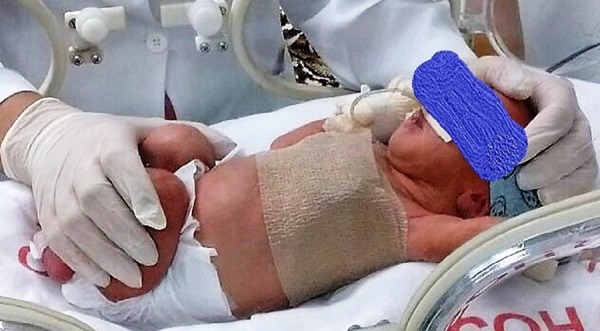
Manual restraint.
Heart rate variability
Sympathetic and parasympathetic activity was measured based on the analysis of HRV,(10) which was determined using a Polar(tm) V800 portable monitor. The HRV indices analyzed in the time domain were the mean R-R interval (iRR), the standard deviation of all iRR (SDNN), the root mean square of the sum of differences between normal adjacent iRR (RMSSD), the percentage of adjacent iRR that differed by more than 50ms (pNN50) and the triangular index (total number of iRRs divided by the maximum height of the histogram of all iRRs). The HRV indices analyzed in the frequency domain were low frequency (LF: 0.04 to 0.15Hz), high frequency (HF: 0.15 to 0.4Hz) and the LF/HF ratio.(11) Moreover, the following nonlinear indices were evaluated: standard deviation 1 (SD1), standard deviation 2 (SD2), approximate entropy (ApEn) and sample entropy (SampEn).(12,13) The readings lasted 30 minutes: 10 minutes in the supine position without support, 10 minutes in the prone position and 10 minutes during manual restraint.
Statistical analysis
The statistical analysis was performed using Minitab 14. The normality of the data was determined through a visual inspection of the curve and using the Shapiro-Wilk test. One-way analysis of variance (ANOVA) and Tukey's post hoc test were used for comparisons of the clinical variables and HRV for the different body positions. The level of significance was set to 5% (p < 0.05).
RESULTS
The sample was composed of premature newborns with very-low birth weight, the majority of whom had APGAR scores of 9 in the fifth minute following birth. The newborns were hemodynamically stable with spontaneous breathing. The majority (56%) were born via a cesarean section procedure, and 44% were born via natural childbirth. Table 1 displays the characteristics of the sample.
Table 1.
Characteristics of the study sample
| Variables | N (%) | Minimum | Maximum | Mean | Standard deviation |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 26 (52) | ||||
| Female | 24 (48) | ||||
| Gradational age (weeks) | 30 | 36 | 32.6 | 2.3 | |
| 30 | 11 (22) | ||||
| 31 | 2 (4) | ||||
| 32 | 7 (14) | ||||
| 33 | 7 (14) | ||||
| 34 | 11 (22) | ||||
| 35 | 10 (20) | ||||
| 36 | 2 (4) | ||||
| Chronological age (days) | 3 | 28 | 9.23 | 9.5 | |
| APGAR (1 minute) | 5 | 9 | 6.65 | 2.26 | |
| APGAR (5 minutes) | 5 | 10 | 8.42 | 1.3 | |
| Birth body weight (g) | 1,100 | 2,500 | 1,816.3 | 493.7 | |
| Study body weight (g) | 1,260 | 3,300 | 2,123.4 | 671.5 | |
| Type of birth | |||||
| Cesarean | 28 (56) | ||||
| Natural | 22 (44) |
With regard to HRV variables, greater parasympathetic activity was found in the prone position than in the supine position in all domains, with a significant difference between the two positions in the frequency domain. In the analysis of nonlinear variables, manual restraint and the prone position promoted greater complexity in the autonomic adjustments than the supine position, which is considered positive from a physiological standpoint. Table 2 displays the HRV variables.
Table 2.
Heart rate variability in the three positions
| Variables | Supine | Restraint | Prone |
|---|---|---|---|
| Time domain | |||
| Mean RR | 466.1 ± 167.4 | 426.8 ± 52.7 | 433.9 ± 113.8 |
| SDNN | 27.02 ± 20.56 | 24.07 ± 13.52 | 29.52 ± 28.43 |
| Mean HR | 147.2 ± 25.1 | 142.20 ± 17.9 | 140.45 ± 20.7 |
| RMSSD | 9.87 ± 9.4 | 9.81 ± 7.3 | 11.19 ± 7.7 |
| pNN50 | 0.62 ± 0.20 | 1.12 ± 4.4 | 1.45 ± 4.2 |
| Frequency domain | |||
| LF | 82.24 ± 6.7* | 80.49 ± 7.7 | 77.79 ± 9.4 |
| HF | 17.73 ± 6.7* | 19.44 ± 7.6 | 21.57 ± 9.6 |
| LF/HF | 5.68 ± 32 | 5.08 ± 2.7 | 4.49 ± 2.9 |
| Nonlinear | |||
| SD1 | 6.08 ± 3.8 | 6.98 ± 5 | 7.54 ± 5.1 |
| SD2 | 38.21 ± 28.9 | 33.41 ± 17.6 | 45.79 ± 52.9 |
| ApEn | 0.77 ± 0.28†* | 0.96 ± 0.22 | 0.86 ± 0.29 |
| SampEn | 0.63 ± 0.36†* | 0.88 ± 0.37 | 0.74 ± 0.36 |
RR - R-R interval; SDNN - mean standard deviation of all iRRs; HR - heart rate; RMSSD - root mean square of the sum of squared differences between iRRs; pNN50 - percentage of difference between adjacent normal R-R intervals greater than 50ms computed over the entire height of the histogram created by charting all R-R intervals; LF - low frequency; HF - high frequency; SD - standard deviation; ApEn - approximate entropy; SampEn: sample entropy;
p < 0.05 prone versus supine,
p < 0.05 supine versus restraint. Data are expressed as the mean and standard deviation.
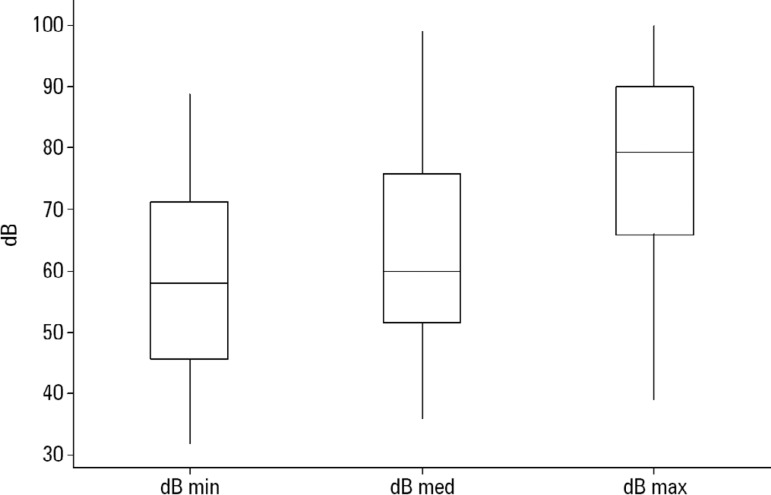
Figure 2 illustrates the levels of noise in the neonatal ICU during the evaluation period, all of which surpassed the recommended level of 45dB.
Figure 2.
Decibels measured in the neonatal intensive care unit.
min - minimum; med - medium; max - maximum.
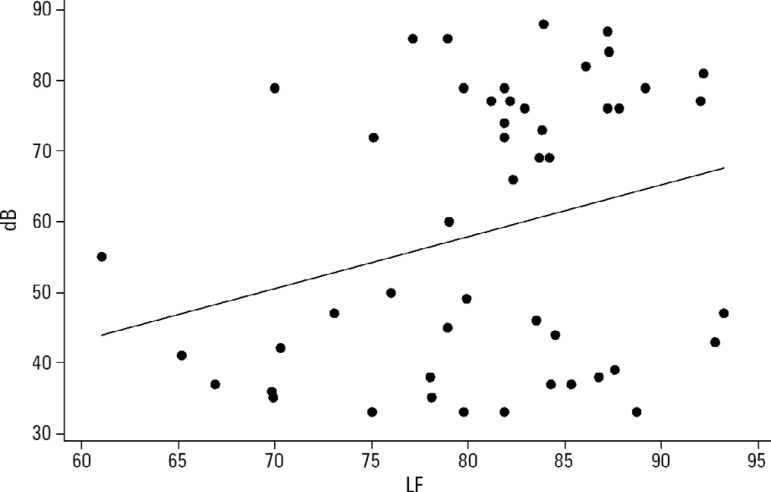
A positive correlation was found between environmental noise and sympathetic activity in the newborns (Figure 3).
Figure 3.
Correlation between noise and the sympathetic activity of the autonomic nervous system.
HRV - heart rate variability; LF - low frequency.
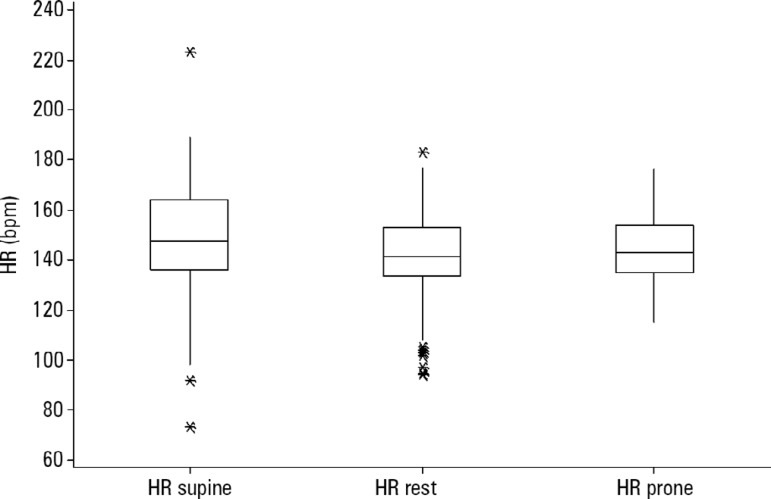
Figure 4 displays the mean heart rates (bpm) in the supine, prone and restrained positions. Higher means were found in the supine position than in the other two positions (*) (p < 0.05).
Figure 4.
Mean heart rate (bpm) in different positions.
HR - heart rate.
DISCUSSION
The main finding of the present study was that the prone position promoted a reduction in stress behavior(8,14,15) due to low visual stimulation as well as the known effects regarding improvements in respiratory mechanics.(16) The reduction in visual stressors due to positioning promotes better self-regulation and homeostasis, with a consequent improvement in autonomic adjustments.(8) Indeed, an increase in parasympathetic activity and good adjustment complexity demonstrated by the nonlinear HRV variables were found in the present study.
Stress provoked by the surrounding environment leads to an increase in energy expenditure in premature newborns, which exerts a negative impact on neurological integration as well as growth and development. The factors that contribute to environmental stress in the neonatal ICU are light, noise and handling. Previous studies(10,17) found that the noise associated with the care offered in the neonatal ICU surpassed the limit of 45dB recommended by the American Academy of Pediatrics(9) and that the noise could even surpass the maximum limit of safety (70dB). Team training for noise reduction in neonatal ICUs seems to be effective.
Similar results were found in the present study, in which the maximum noise level reached 90dB.(18,19)
Sahni(3) evaluated the effects of the prone position on RR intervals with an electrocardiogram during sleep in newborns and found a reduction in parasympathetic activity, which is in disagreement with the present findings. However, differences were found in the cardiovascular dynamics between the states of sleep and wakefulness. In the present study, evaluations were made on newborns in stages 3 and 4 of the Brazelton scale.
The supine position is associated with greater arousal and greater energy expenditure than the prone position. Bell et al.(20) measured energy expenditure by direct and indirect calorimetry and found that the energy expenditure was 10% lower in the prone position than in the supine position. In contrast, the prone position is associated with a greater risk of sudden infant death and should therefore not be used without supervision in the home environment. In a supervised environment, such as the neonatal ICU, the prone position can be adopted safely and offers numerous benefits, such as a position of flexion, better respiratory mechanics,(21) better neuromotor organization and better autonomic adjustments.(22,23)
The prone position and lateral decubitus position are not safe because they may favor the rebreathing of expired gases and reduce the rate of body heat loss, thus leading to hypoxemia and hypercapnia and an increase in body temperature, predisposing the child to sudden infant death syndrome; therefore, these two position should not be used during sleep. Most studies evaluated a prone position during sleep; in the present study, newborns were awake (Brazelton 3 - 4), which is a divergent factor since there are alterations in the autonomic responses in different stages of sleep and wakefulness.(24)
Shepherd et al.(25) evaluated cerebral blood flow and oxygen extraction in extreme and moderate newborns, and in the extreme population, there was a reduction in cerebral blood flow and a slight increase in oxygen extraction in the prone position, suggesting a greater need for the evaluation of position in this group than in the moderate group. In the group of moderate newborns and term infants,(26) this change did not occur. We suggest strict evaluation of the clinic and the gestational age of the child when using the prone position. As previously stated, there are differences in adjustments during sleep and wakefulness, and when awake, the prone position seems to have benefits in terms of both autonomic adjustments and the reduction of stress.
The idea of offering postural comfort to premature newborns to alleviate stress is not new, but few studies have demonstrated the response to positioning by the ANS, and the population sizes analyzed have rarely surpassed 30 patients. Cong et al.(27) evaluated the autonomic responses of newborns to pain, comparing infants in an incubator and those in kangaroo care (skin-to-skin contact), demonstrating that stability of the autonomic adjustments after pain was quickly achieved when the infant was in a comfortable position.
All the benefits of positioning in this population and the harmful effects of stress from the environment on the premature brain are widely known. This was one of the first studies to show that the apparently simple practice of positioning a newborn also improves autonomic adjustments despite the occurrence of normal noises in the neonatal ICU environment.
The development of the parasympathetic nervous system occurs in the perinatal period. Adequate stimuli result in optimal development of this system, whereas adverse stimuli can exert a negative effect on its development, with the possibility of future abnormalities in childhood and adulthood.(4) The complexity of autonomic adjustments is a marker of good cardiovascular health, and this complexity is normally reduced in pathological situations, which demonstrates its importance.(23,28-30)
The limitations of this study are related to the evaluation of HRV because it is influenced by many factors; therefore, several inclusion criteria were adopted. In addition, the evaluation of HRV by itself with nonlinear methods, both in the time domain and in the frequency domain, is unable to characterize the dynamic parameters of HRV. The nonstationary character of HRV makes the use of stationary approaches to the study of HRV, as in the case of traditional methods, limited, although it provides us with information about the behavior of the ANS.
Another difficulty in studying this specific population is that invasive procedures should be avoided, thereby limiting more accurate evaluations. Finally, no medicines that might have interfered with the autonomic responses were adopted, so more rigorous inclusion criteria were adopted.
CONCLUSION
The prone position and manual restraint for premature newborns increase both parasympathetic activity and the complexity of autonomic adjustments in comparison to the supine position, even in the presence of higher environmental noise than the recommended level, which tends to increase sympathetic activity.
Footnotes
Conflicts of interest: None.
Responsible editor: Werther Brunow de Carvalho
REFERENCES
- 1.Lucchini M, Fifer WP, Sahni R, Signorini MG. Novel heart rate parameters for the assessment of autonomic nervous system function in premature infants. Physiol Meas. 2016;37(9):1436–1446. doi: 10.1088/0967-3334/37/9/1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos J, Pearce SE, Stroustrup A. Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr Opin Pediatr. 2015;27(2):254–260. doi: 10.1097/MOP.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahni R, Schulze KF, Kashyap S, Ohira-Kist K, Fifer WP, Myers MM. Postural differences in cardiac dynamics during quiet and active sleep in low birthweight infants. Acta Paediatr. 1999;88(12):1396–1401. doi: 10.1080/080352599750030158. [DOI] [PubMed] [Google Scholar]
- 4.Yiallourou SR, Witcombe NB, Sands SA, Walker AM, Horne RS. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum Dev. 2013;89(3):145–152. doi: 10.1016/j.earlhumdev.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Salavitabar A, Haidet KK, Adkins CS, Susman EJ, Palmer C, Storm H. Preterm infants' sympathetic arousal and associated behavioral responses to sound stimuli in the neonatal intensive care unit. Adv Neonatal Care. 2010;10(3):158–166. doi: 10.1097/ANC.0b013e3181dd6dea. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd KL, Yiallourou SR, Horne RS, Wong FY. Prone sleeping position in infancy: Implications for cardiovascular and cerebrovascular function. Sleep Med Rev. 2018;39:174–186. doi: 10.1016/j.smrv.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Candia MF, Osaku EF, Leite MA, Toccolini B, Costa NL, Teixeira SN, et al. Influence of prone positioning on premature newborn infant stress assessed by means of salivary cortisol measurement: pilot study. Rev Bras Ter Intensiva. 2014;26(2):169–175. doi: 10.5935/0103-507X.20140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng NH, Chen LL, Li TC, Smith M, Chang YS, Huang LC. The effect of positioning on preterm infants' sleep-wake states and stress behaviours during exposure to environmental stressors. J Child Health Care. 2014;18(4):314–325. doi: 10.1177/1367493513496665. [DOI] [PubMed] [Google Scholar]
- 9.Brazelton TB. 2nd ed. 88. London: Spastics International Medical Publications; 1984. Neonatal behavior assessment scale. [Google Scholar]
- 10.Noise: a hazard for the fetus and newborn. American Academy of Pediatrics Committee on Environmental Health. Pediatrics. 1997;100(4):724–727. [PubMed] [Google Scholar]
- 11.Heart rate variability: standards of measurement, physiological interpretation, and clinical use Task Force of the European Society of Cardiology and The North American Society of Pacing Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 12.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci. 1991;88(6):2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piskorski J, Guzik P. Geometry of the Poincaré plot of RR intervals and its asymmetry in healthy adults. Physiol Meas. 2007;28(3):287–300. doi: 10.1088/0967-3334/28/3/005. [DOI] [PubMed] [Google Scholar]
- 14.Antunes LC, Rugolo LM, Crocci AJ. Effect of preterm infant position on weaning from mechanical ventilation. J Pediatr (Rio J) 2003;79(3):239–244. Portuguese. [PubMed] [Google Scholar]
- 15.Jarus T, Bart O, Rabinovich G, Sadeh A, Bloch L, Dolfin T, et al. Effects of prone and supine positions on sleep state and stress responses in preterm infants. Infant Behav Dev. 2011;34(2):257–263. doi: 10.1016/j.infbeh.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Gillies D, Wells D, Bhandari AP. Positioning for acute respiratory distress in hospitalised infants and children. Cochrane Database Syst Rev. 2012;(7):CD003645–CD003645. doi: 10.1002/14651858.CD003645.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkataraman R, Kamaluddeen M, Amin H, Lodha A. Is less noise, light and parental/caregiver stress in the neonatal intensive care unit better for neonates. Indian Pediatr. 2018;55(1):17–21. [PubMed] [Google Scholar]
- 18.Calikusu Incekar M, Balci S. The effect of training on noise reduction in neonatal intensive care units. J Spec Pediatr Nurs. 2017;22(3) doi: 10.1111/jspn.12181. [DOI] [PubMed] [Google Scholar]
- 19.Cardoso SM, Kozlowski LC, Lacerda AB, Marques JM, Ribas A. Newborn physiological responses to noise in the neonatal unit. Braz J Otorhinolaryngol. 2015;81(6):583–588. doi: 10.1016/j.bjorl.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell EF, Johnson KJ, Dove EL. Effect of body position on energy expenditure of preterm infants as determined by simultaneous direct and indirect calorimetry. Am J Perinatol. 2017;34(5):493–498. doi: 10.1055/s-0036-1593476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston C, Zanetti NM, Comaru T, Ribeiro SNDS, Andrade LB, Santos SL. I Brazilian guidelines for respiratory physiotherapy in pediatric and neonatal intensive care units. Rev Bras Ter Intensiva. 2012;24(2):119–129. [PubMed] [Google Scholar]
- 22.Sahni R, Schulze KF, Ohira-Kist K, Kashyap S, Myers MM, Fifer WP. Interactions among peripheral perfusion, cardiac activity, oxygen saturation, thermal profile and body position in growing low birth weight infants. Acta Paediatr. 2010;99(1):135–139. doi: 10.1111/j.1651-2227.2009.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariagno RL, Mirmiran M, Adams MM, Saporito AG, Dubin AM, Baldwin RB. Effect of position on sleep, heart rate variability, and QT interval in preterm infants at 1 and 3 months' corrected age. Pediatrics. 2003;111(3):622–625. doi: 10.1542/peds.111.3.622. [DOI] [PubMed] [Google Scholar]
- 24.Moon RY, Task Force on Sudden Infant Death Syndrome SIDS and Other Sleep-Related Infant Deaths: Evidence Base for 2016 Updated Recommendations for a Safe Infant Sleeping Environment. Pediatrics. 2016;138(5) doi: 10.1542/peds.2016-2940. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd KL, Yiallourou SR, Odoi A, Brew N, Yeomans E, Willis S, et al. Effects of prone sleeping on cerebral oxygenation in preterm infants. J Pediatr. 2019;204:103-110.e1. doi: 10.1016/j.jpeds.2018.08.076. [DOI] [PubMed] [Google Scholar]
- 26.Wu TW, Lien RI, Seri I, Noori S. Changes in cardiac output and cerebral oxygenation during prone and supine sleep positioning in healthy term infants. Arch Dis Child Fetal Neonatal Ed. 2017;102(6):F483–F489. doi: 10.1136/archdischild-2016-311769. [DOI] [PubMed] [Google Scholar]
- 27.Cong X, Cusson RM, Walsh S, Hussain N, Ludington-Hoe SM, Zhang D. Effects of skin-to-skin contact on autonomic pain responses in preterm infants. J Pain. 2012;13(7):636–645. doi: 10.1016/j.jpain.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Goto K, Mirmiran M, Adams MM, Longford RV, Baldwin RB, Boeddiker MA, et al. More awakenings and heart rate variability during supine sleep in preterm infants. Pediatrics. 1999;103(3):603–609. doi: 10.1542/peds.103.3.603. [DOI] [PubMed] [Google Scholar]
- 29.Galland BC, Taylor BJ, Bolton DP, Sayers RM. Heart rate variability and cardiac reflexes in small for gestational age infants. J Appl Physiol. 2006;100(3):933–939. doi: 10.1152/japplphysiol.01275.2005. [DOI] [PubMed] [Google Scholar]
- 30.Fyfe KL, Yiallourou SR, Wong FY, Odoi A, Walker AM, Horne RS. The effect of gestational age at birth on post-term maturation of heart rate variability. Sleep. 2015;38(10):1635–1644. doi: 10.5665/sleep.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]