Abstract
Background:
The use of platelet-rich plasma (PRP) in the Medicare population is not well described.
Purpose:
To investigate the national use of PRP among Medicare beneficiaries, including the incidence and conditions for which it was used in both operative and nonoperative settings, and determine charges to Medicare.
Study Design:
Descriptive epidemiology study.
Methods:
The Medicare Standard Analytical Files within the PearlDiver database were queried for PRP injections by use of Current Procedural Terminology (CPT) code 0232T from 2010 to 2014. A search of every associated International Classification of Diseases, 9th Revision, code and CPT code on the day of the injection was performed, and codes were broadly categorized as shoulder, knee, elbow, hip, and foot/ankle. These categories were then subdivided into 2 groups based on whether the injection was performed at the time of surgery or for a nonoperative condition. The patient data were analyzed by demographics and geographic region. In further analysis, the charges sent to Medicare for PRP injections were stratified by year and musculoskeletal site.
Results:
A total of 3654 PRP injections were coded for and administered during the study period; 57% of recipients were men and 33% were 65 to 69 years of age. We found that 42% of all PRP injections were administered in the southern geographic region. PRP injections were most commonly associated with shoulder diagnoses, followed closely by the foot and ankle and by the knee. The majority of injections given for shoulder conditions were performed at the time of surgery, whereas the majority of knee conditions treated with PRP were associated with nonoperative treatments. Annual charges to Medicare for PRP injections increased 400%, from $500,000 in 2010 to more than $2 million in 2014.
Conclusion:
The use and breadth of PRP therapy have increased substantially in Medicare beneficiaries. Further research is required to obtain a consensus on treatment recommendations for PRP use in this population in addition to strategies to obtain insurance reimbursement.
Keywords: platelet-rich plasma, Medicare, charges
Platelet-rich plasma (PRP) continues to gain interest as a popular and promising biologic treatment for various musculoskeletal injuries and conditions.17,22,26,30 PRP is defined in the literature as a sample of autologous blood with platelet concentrations approximately 4 to 5 times above baseline that is produced by the centrifugation of whole blood.22,26,27,29 In addition to containing platelets, PRP contains multiple factors that are thought to enhance the local delivery of growth factors, modify inflammatory responses, and promote cell proliferation and differentiation.26 This biologic rationale has prompted numerous studies to determine the therapeutic efficacy of PRP as both a surgical adjunct and a nonoperative therapeutic treatment.22,27 Such clinical studies have evaluated the use of PRP in the treatment of tendon injuries and osteoarthritis, management of nonunion, and surgical augmentation of rotator cuff repair and anterior cruciate ligament reconstruction.22,27 Although reports of treatment with PRP for such musculoskeletal conditions have increased nearly exponentially in the past 10 years, few studies have provided epidemiological data on the use on PRP on a nationwide scale, specifically in Medicare beneficiaries.22,27,42 The absence of formal indications and labeling for PRP has likely led insurance and Medicare programs to offer limited reimbursement for PRP therapy. Currently, the Centers for Medicare & Medicaid Services (CMS) reimburses autologous PRP only for patients who have chronic nonhealing diabetic, pressure, and/or venous wounds when the patient is enrolled in an approved clinical research study.3 Increasing evidence on the benefits of PRP injections for the treatment of various orthopaedic conditions has encouraged widespread enthusiasm about the therapeutic potential of PRP within the orthopaedic community.1,32 Given the current health care focus on cost-effective and value-based care, it is important to gain a national perspective on the use of new therapies such as PRP. Therefore, the goal of the current study was to use a national insurance database to investigate trends in PRP use in the Medicare beneficiaries billed for its use. Specifically, we sought to determine and characterize the demographics of Medicare patients receiving PRP as well as describe the musculoskeletal anatomic sites treated over time, operative and nonoperative uses, and Medicare charges by year for PRP injections. Although CMS coverage for PRP is limited to specific indications, we hypothesized that the overall number of PRP charges would substantially increase over the time period studied for several conditions not reimbursed by CMS.
Methods
An insurance-based, for-fee database of patient records was used for the present study (PearlDiver Patient Records Database; www.pearldiverinc.com). The PearlDiver database contains data from both Medicare and private insurers including United Healthcare and Humana. The patients in the present study were taken from the Medicare data set because it contains 100% of beneficiaries, which allows a true study of national trends, and because limited data have been published regarding PRP use in this population.
The Medicare data set contains procedural volumes, basic patient demographics, and laterality modifiers for patients with International Classification of Diseases, 9th Revision (ICD-9), diagnoses and procedures or Current Procedural Terminology (CPT) codes. The Medicare database is from the 100% Standard Analytical Files and includes patients insured from 2005 to 2014. These patients can be tracked across all locations (inpatient, outpatient, etc) of patient care throughout the years covered by the database. In total, the Medicare database contains approximately 55 million patients. Data within the system are deidentified and anonymous, and thus, the current study was exempt from institutional review board approval.
The database was queried for patients who underwent a PRP injection through use of a category III tracking CPT code, CPT-0232T, from 2010 to 2014 that was billed to and reimbursed by Medicare. Patients who received an injection but did not bill Medicare cannot be captured by this database. The CPT code for PRP injections was introduced in 2010, and data on injections administered before 2010 are therefore not readily identifiable within the database. An exhaustive search of every ICD-9 and CPT code was then performed on the day of the PRP injection to determine which musculoskeletal condition was being treated. These sites were categorized broadly as shoulder, knee, elbow, hip, and foot and ankle. These were then subdivided into 2 groups based on whether the injection was performed at the time of surgery (using the CPT codes obtained from the same-day search) or was administered for a nonoperative condition (using ICD-9 codes obtained from the same-day search and confirming the absence of a concomitant CPT code). For all PRP injections, patient demographics were recorded and analyzed, including age, sex, and US geographic region. Trends in PRP use over time were then described as the number of PRP injections administered annually for each musculoskeletal site. Use of PRP as a surgical adjunct versus treatment for a nonoperative condition was then compared for each musculoskeletal site and described. Finally, the charges sent to Medicare for PRP injections were stratified by year and musculoskeletal site.
Descriptive statistics were used to report data. Comparative analyses over time were performed with linear regression analyses. Charge data were compared by use of t tests. An alpha value less than .05 was considered statistically significant. All statistical analyses were performed in SPSS (Version 24; IBM Corp).
Results
In total, 3654 PRP injections had codes submitted to Medicare for patients within the database from 2010 to 2014. Characteristics of Medicare patients receiving PRP injections during the study period can be seen in Table 1. Males (57%) received more PRP injections than females (43%) during the study period. Younger Medicare patients received substantially more PRP injections than the older cohorts, as nearly 60% of PRP injections were received by patients 69 years and younger. Geographic distribution showed that the majority of patients receiving PRP injections were in the South (1537; 42%), whereas the fewest PRP injections were administered in the Northeast (558; 15%).
Table 1.
Characteristics of the Patient Population
| Characteristic | n | % of Total |
|---|---|---|
| Sex | ||
| Male | 2088 | 57 |
| Female | 1566 | 43 |
| Age group | ||
| <65 y | 919 | 25 |
| 65-69 y | 1212 | 33 |
| 70-74 y | 778 | 21 |
| 75-79 y | 471 | 13 |
| 80-84 y | 187 | 5 |
| ≥85 y | 87 | 2 |
| Geographic region | ||
| Midwest | 730 | 20 |
| Northeast | 558 | 15 |
| South | 1537 | 42 |
| West | 829 | 23 |
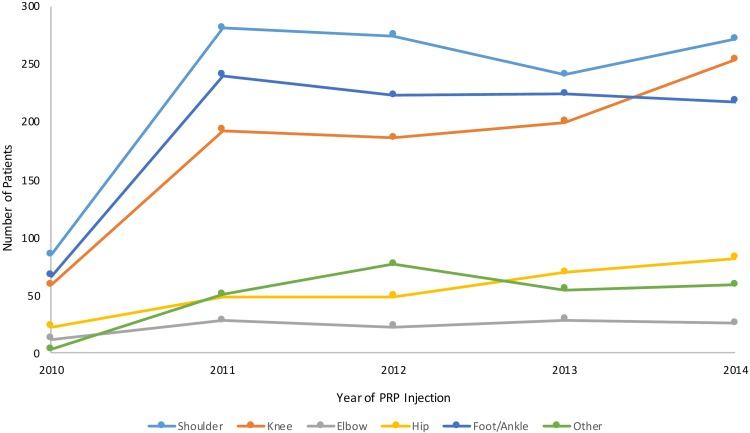
The overall number of PRP injections billed to CMS increased from 249 in 2010 to 911 in 2014 (P < .0001). PRP injections were used for a variety of musculoskeletal sites. The annual incidence of PRP use stratified by condition location over time can be seen in Figure 1.
Figure 1.
Trends in platelet-rich plasma (PRP) billing for various musculoskeletal sites in the Medicare database from 2010 to 2014.
The number of PRP injections increased significantly over the study period for each injection body site: shoulder (220%; P < .0001), knee (331%; P < .0001), elbow (117%; P = .013), hip (257%; P < .0001), and foot/ankle (225%; P < .0001). Overall, injections were most commonly billed for conditions involving the shoulder, followed closely by the foot/ankle and then the knee. Elbow conditions represented the least frequent location for PRP injections within the database.
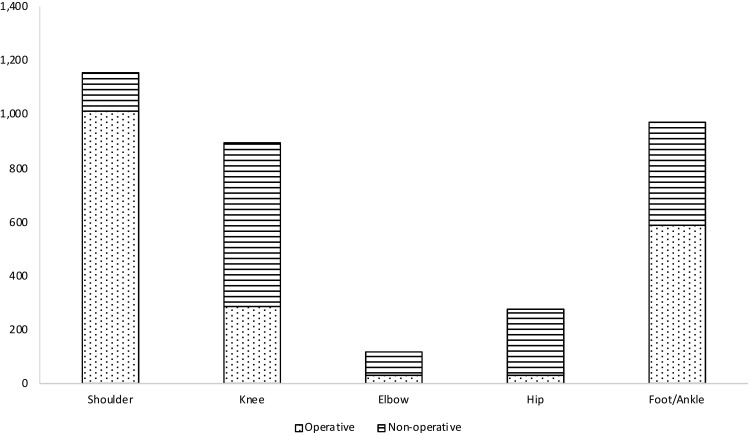
PRP injections were used as both a surgical adjunct and in the nonoperative management of each condition studied. Comparisons of total procedural volumes for each joint between surgical and nonoperative uses can be seen in Figure 2.
Figure 2.
Total platelet-rich plasma charges for each condition for operative and nonoperative uses in the Medicare database.
The majority of PRP injections reported in the treatment of shoulder conditions were injected at the time of surgery (88%; 1012/1154). In contrast, the majority of PRP injections used in the treatment of knee (68%; 606/892), elbow (73%; 86/118), and hip conditions (96%; 244/253) were administered in a nonoperative setting. Foot and ankle conditions treated with PRP showed the most balance between injections at the time of surgery (60%) and those performed in a nonoperative setting (40%).
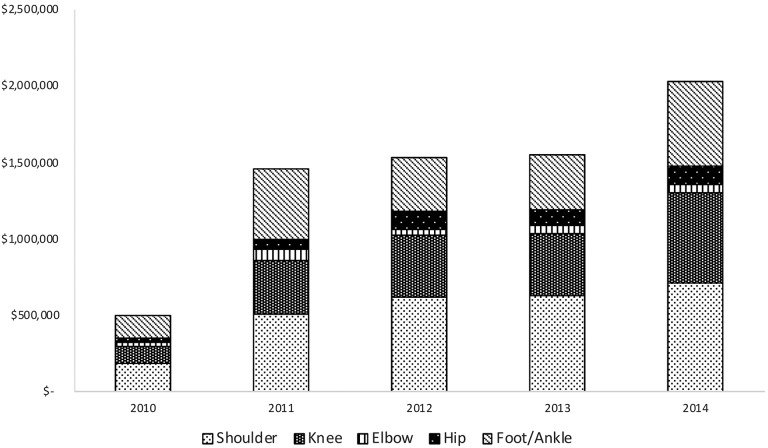
The overall annual Medicare charges for PRP injections increased from 2010 to 2014 (Figure 3). Over the time period studied, the mean charge per injection for shoulder conditions was $2301, knee conditions $2032, elbow conditions $2058, hip conditions $1585, and foot and ankle conditions $1984. Notably, no information on the procedural cost borne by the provider or the reimbursement provided by CMS was available for analysis.
Figure 3.
Evaluation of annual charges sent to Medicare for cumulative platelet-rich plasma injections subdivided by anatomic site.
Discussion
Alongside basic science and animal studies that have demonstrated the promising effects of PRP in the healing process of multiple musculoskeletal conditions or in association with symptom modification, an increasing body of clinical research on the therapeutic efficacy of PRP is emerging for some indications.22,27,38 Given these early results, PRP has garnered a substantial amount of interest by both the medical and the lay press for its use in surgical and nonoperative settings. Although the number of clinical studies and case series investigating the role of PRP in treating various orthopaedic conditions has dramatically increased, few studies have offered a national perspective on the trends of PRP use and associated charges in a Medicare population. The current study shows a significant increase in the charges submitted per year for PRP among Medicare beneficiaries over the time period studied. Notably, charge data are not reflective of the cost of goods and services required to provide PRP in various settings, and actual CMS payments for the use of PRP are not publicly available.
Increased interest for the use of PRP as a biologic adjuvant to enhance healing at the bone-tendon interface has prompted a number of clinical studies to examine both the clinical efficacy and cost-effectiveness of PRP use at the time of rotator cuff repair.§ Several studies have demonstrated lower retear rates, increased vascularity of repair sites, and improved patient-reported outcome scores with PRP use.9,19,24,31,36,41 The increase in number of clinical trials has led to recent meta-analyses of data that provide a more complete perspective. Hurley et al21 recently examined 18 randomized clinical trials with 1147 patients and reported that the use of PRP in rotator cuff repair resulted in improved healing rates, pain levels, and functional outcomes. Vavken et al39 also performed a meta-analysis with a focus on cost-effectiveness and found a decreased relative risk of retear after repair of small to medium tears. However, those investigators noted that this decreased risk was cost-effective only when the cost of PRP was less than $652.11, and thus, they concluded that PRP in the context of decreasing risk of rotator cuff retears was not cost-effective. However, with increasing use of PRP and efficiency of administration, one may anticipate the price per injection to decrease with time. Even with an increasing body of encouraging human research on PRP therapy and rotator cuff repair outcomes, a clinical consensus for the standardization of PRP use in both operative and nonoperative conditions for the shoulder has not been achieved. The current study shows that PRP use at the time of shoulder surgery is the most common use for PRP billed to Medicare, which does not capture patients who pay cash or use other means to pay for PRP.
Knee conditions experienced the most significant increase in PRP use billed to Medicare, which secondarily led to charges for PRP injections increasing from approximately $500,000 in 2010 to more than $2 million in 2014. Similar to evidence seen in the shoulder literature, evidence for PRP therapy for symptomatic knee arthritis has continued to show encouraging results when compared with more established treatments such as corticosteroid and hyaluronic acid (HA) preparations.11,13,15,25,37,38 Many of the trials evaluating the efficacy of PRP for knee arthritis have reported positive short-term results with follow-up of 6 to 12 months.4,12,13,20 Di Martino et al13 recently randomized 192 patients with symptomatic Kellgren-Lawrence grade 0 to 3 knee osteoarthritis to undergo 3 blinded weekly intra-articular injections of either PRP or HA. Prospective evaluation at a mean follow-up of 64 months demonstrated that both treatments were effective in improving knee functional status and symptoms over time. The authors suggested that further research is needed to demonstrate whether PRP can definitively yield more durable and cost-effective results than traditional treatments. We believe that given the increasing number of level 1 studies that demonstrate significant clinical improvement with the use of PRP for the treatment of knee osteoarthritis, the current use of PRP to treat symptomatic osteoarthritis as an alternative to HA and corticosteroid preparations, which have had their own reimbursement and efficacy challenges, will continue to increase.
Given the CMS guidelines previously mentioned, we expected foot and ankle conditions to produce the highest reported procedural volumes and charges per year for PRP use. Similar to the shoulder and knee literature, evidence for PRP therapy for foot and ankle conditions is less robust.18 In the present study, foot and ankle conditions treated with PRP trailed shoulder conditions throughout the study period, and knee conditions surpassed those of foot and ankle in 2014. Interestingly, more than half of PRP injections billed for foot and ankle conditions were performed at the time of surgery. When we exclude PRP use at the time of surgery and consider only nonoperative injections that would satisfy CMS guidelines for lower extremity use, the overall number of approved PRP injections represented an exceedingly small portion of the nearly 4000 injections coded for in Medicare recipients over this 4-year period.
The current study has several shortcomings, many of which are consistent with other database studies and those focusing on newly developed therapies that are not consistently coded.5,9 First, this cohort is not meant to represent all Medicare patients who received PRP injections during the time period studied. It is more likely that this is a small subset of such patients given that many PRP injections are paid for with cash and not submitted to Medicare for reimbursement; thus, the results should be viewed within this context. Second, the goals of the present study were to report on the relative use of PRP within this population, not infer or evaluate the efficacy of PRP for various musculoskeletal injuries. Third, the quality of the data relies on the accuracy of coding and noncoding within the Medicare data set. However, a recent CMS improper payments report estimates an overall coding error rate of approximately 1.3%.8 Therefore, although this is a significant limitation when administrative databases are used, the overall coding error rate remains low. Fourth, prior epidemiological data have suggested that patients older than 65 years are not the largest consumers of PRP therapy.42 Thus, the trends data demonstrated in the current study might not apply to a younger, privately insured group of patients receiving PRP. Fifth (and perhaps most challenging as a study limitation), similar to prior epidemiological trends studies using administrative databases, there is the possibility that patients receiving PRP therapy are recorded through use of “unspecified” codes or not coded at all as an alternative to the use of the CPT code 0232T.42 This is especially relevant because reimbursement for this CPT code is rarely provided by any payer, including CMS. These patients would summarily be excluded from our search and would not be represented in our analysis.6,7
Although the efficacy of PRP injections for musculoskeletal conditions continues to be debated, an increasing body of positive evidence supports the widespread enthusiasm for the use of PRP. The current study reflects such use in a subset of the Medicare population. Future direction in biologics research should focus on treatment algorithms, indications, and the standardization of protocols for processing, delivery, and outcome reporting. Once such objectives have been validated and a clinical consensus has been achieved, regulatory processes similar to drug approval processes will be helpful to encourage reimbursement from CMS or third-party payors. Until this time, the present study suggests that the use and breadth of indications for PRP therapy will continue to increase in the Medicare population.
Conclusion
The use and breadth of PRP therapy have increased substantially in the Medicare database. Further research is needed to obtain a consensus on treatment recommendations for PRP use.
Footnotes
Final revision submitted October 18, 2019; accepted October 23, 2019.
One or more of the authors has declared the following potential conflict of interest or source of funding: B.C.W. has received educational support from Arthrex and Supreme Orthopaedic Systems and hospitality payments from Arthrex, Integra Lifesciences, and Supreme Orthopaedic Systems. J.M.C. has received educational support from Medacta, Smith & Nephew, and Supreme Orthopaedic Systems. N.N.V. has received consulting fees from Arthrex, Medacta, and Smith & Nephew; speaking fees from Arthrex and Pacira; and royalties from Smith & Nephew. B.J.C. has received royalties from Arthrex and DJO; consulting fees from Acumed, Anika Therapeutics, Arthrex, Bioventus, Flexion Therapeutics, Geistlich Pharma, Genzyme, Pacira, Smith & Nephew, Verical, and Zimmer Biomet; speaking fees from Arthrex, Carticept Medical, Lifenet Health, and Pacira; educational support from Medwest; honoraria from Vericel; and hospitality payments from Geistlich Pharma and GE Healthcare. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Alcerro JC, Lavernia CJ. Stem cells and platelet-rich plasma for knee osteoarthritis. J Am Acad Orthop Surg. 2019;27(20):779–783. [DOI] [PubMed] [Google Scholar]
- 2. Antuña S, Barco R, Martínez Diez JM, Sánchez Márquez JM. Platelet-rich fibrin in arthroscopic repair of massive rotator cuff tears: a prospective randomized pilot clinical trial. Acta Orthop Belg. 2013;79(1):25–30. [PubMed] [Google Scholar]
- 3. Autologous platelet-rich plasma. https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/Autologous-Platelet-rich-Plasma.html. Accessed December 8, 2018.
- 4. Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthrosc J Arthrosc Relat Surg. 2015;31(11):2213–2221. [DOI] [PubMed] [Google Scholar]
- 5. Cancienne JM, Brockmeier SF, Gulotta LV, Dines DM, Werner BC. Ambulatory total shoulder arthroplasty: a comprehensive analysis of current trends, complications, readmissions, and costs. J Bone Joint Surg Am. 2017;99(8):629–637. [DOI] [PubMed] [Google Scholar]
- 6. Cancienne JM, Gwathmey FW, Miller MD, Werner BC. Tobacco use is associated with increased complications after anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(1):99–104. [DOI] [PubMed] [Google Scholar]
- 7. Cancienne JM, Werner BC, Browne JA. Is there an association between hemoglobin A1C and deep postoperative infection after TKA? Clin Orthop Relat Res. 2017;475(6):1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Medicare & Medicaid Services. Medicare Fee-for-Service 2012 Improper Payments Report. Baltimore, MD: Centers for Medicare & Medicaid Services; 2013. [Google Scholar]
- 9. Chahla J, Cinque ME, Piuzzi NS, et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting. J Bone Joint Surg Am. 2017;99(20):1769–1779. [DOI] [PubMed] [Google Scholar]
- 10. Charousset C, Zaoui A, Bellaïche L, Piterman M. Does autologous leukocyte-platelet-rich plasma improve tendon healing in arthroscopic repair of large or massive rotator cuff tears? Arthroscopy. 2014;30(4):428–435. [DOI] [PubMed] [Google Scholar]
- 11. Cook CS, Smith PA. Clinical update: why PRP should be your first choice for injection therapy in treating osteoarthritis of the knee. Curr Rev Musculoskelet Med. 2018;11(4):583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthrosc J Arthrosc Relat Surg. 2017;33(3):659–670.e1. [DOI] [PubMed] [Google Scholar]
- 13. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47(2):347–354. [DOI] [PubMed] [Google Scholar]
- 14. Flury M, Rickenbacher D, Schwyzer H-K, et al. Does pure platelet-rich plasma affect postoperative clinical outcomes after arthroscopic rotator cuff repair? A randomized controlled trial. Am J Sports Med. 2016;44(8):2136–2146. [DOI] [PubMed] [Google Scholar]
- 15. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958–965. [DOI] [PubMed] [Google Scholar]
- 16. Gwinner C, Gerhardt C, Haneveld H, Scheibel M. Two-staged application of PRP in arthroscopic rotator cuff repair: a matched-pair analysis. Arch Orthop Trauma Surg. 2016;136(8):1165–1171. [DOI] [PubMed] [Google Scholar]
- 17. Halpern B, Chaudhury S, Rodeo SA, et al. Clinical and MRI outcomes after platelet-rich plasma treatment for knee osteoarthritis. Clin J Sport Med. 2013;23(3):238–239. [DOI] [PubMed] [Google Scholar]
- 18. Henning PR, Grear BJ. Platelet-rich plasma in the foot and ankle. Curr Rev Musculoskelet Med. 2018;11(4):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holtby R, Christakis M, Maman E, et al. Impact of platelet-rich plasma on arthroscopic repair of small- to medium-sized rotator cuff tears: a randomized controlled trial. Orthop J Sports Med. 2016;4(9):2325967116665595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang G, Hua S, Yang T, Ma J, Yu W, Chen X. Platelet-rich plasma shows beneficial effects for patients with knee osteoarthritis by suppressing inflammatory factors. Exp Ther Med. 2018;15(3):3096–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hurley ET, Lim Fat D, Moran CJ, Mullett H. The efficacy of platelet-rich plasma and platelet-rich fibrin in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Am J Sports Med. 2019;47(3):753–761. [DOI] [PubMed] [Google Scholar]
- 22. Hussain N, Johal H, Bhandari M. An evidence-based evaluation on the use of platelet rich plasma in orthopedics—a review of the literature. SICOT J. 2017;3:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035–1045. [DOI] [PubMed] [Google Scholar]
- 24. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears. Am J Sports Med. 2015;43(9):2102–2110. [DOI] [PubMed] [Google Scholar]
- 25. Lana JFSD, Weglein A, Sampson SE, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12(2):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. LaPrade RF, Geeslin AG, Murray IR, et al. Biologic treatments for sports injuries II think tank—current concepts, future research, and barriers to advancement, part 1: biologics overview, ligament injury, tendinopathy. Am J Sports Med. 2016;44(12):3270–3283. [DOI] [PubMed] [Google Scholar]
- 27. Le ADK, Enweze L, DeBaun MR, Dragoo JL. Platelet-rich plasma. Clin Sports Med. 2019;38(1):17–44. [DOI] [PubMed] [Google Scholar]
- 28. Malavolta EA, Gracitelli MEC, Ferreira Neto AA, Assunção JH, Bordalo-Rodrigues M, de Camargo OP. Platelet-rich plasma in rotator cuff repair: a prospective randomized study. Am J Sports Med. 2014;42(10):2446–2454. [DOI] [PubMed] [Google Scholar]
- 29. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–646. [DOI] [PubMed] [Google Scholar]
- 30. Murray IR, LaPrade RF. Platelet-rich plasma: renewed scientific understanding must guide appropriate use. Bone Joint Res. 2016;5(3):92–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pandey V, Bandi A, Madi S, et al. Does application of moderately concentrated platelet-rich plasma improve clinical and structural outcome after arthroscopic repair of medium-sized to large rotator cuff tear? A randomized controlled trial. J Shoulder Elbow Surg. 2016;25(8):1312–1322. [DOI] [PubMed] [Google Scholar]
- 32. Piuzzi N, Ng M, Kantor A, et al. What is the price and claimed efficacy of platelet-rich plasma injections for the treatment of knee osteoarthritis in the United States? J Knee Surg. 2019;32(9):879–885. [DOI] [PubMed] [Google Scholar]
- 33. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518–528. [DOI] [PubMed] [Google Scholar]
- 34. Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing. Am J Sports Med. 2012;40(6):1234–1241. [DOI] [PubMed] [Google Scholar]
- 35. Ruiz-Moneo P, Molano-Muñoz J, Prieto E, Algorta J. Plasma rich in growth factors in arthroscopic rotator cuff repair: a randomized, double-blind, controlled clinical trial. Arthroscopy. 2013;29(1):2–9. [DOI] [PubMed] [Google Scholar]
- 36. Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32(5):906–918. [DOI] [PubMed] [Google Scholar]
- 37. Sánchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070–1078. [DOI] [PubMed] [Google Scholar]
- 38. Southworth T, Naveen N, Tauro T, Leong N, Cole B. The use of platelet-rich plasma in symptomatic knee osteoarthritis. J Knee Surg. 2019;32(1):37–45. [DOI] [PubMed] [Google Scholar]
- 39. Vavken P, Sadoghi P, Palmer M, et al. Platelet-rich plasma reduces retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med. 2015;43(12):3071–3076. [DOI] [PubMed] [Google Scholar]
- 40. Wang A, McCann P, Colliver J, et al. Do postoperative platelet-rich plasma injections accelerate early tendon healing and functional recovery after arthroscopic supraspinatus repair? A randomized controlled trial. Am J Sports Med. 2015;43(6):1430–1437. [DOI] [PubMed] [Google Scholar]
- 41. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306–320. [DOI] [PubMed] [Google Scholar]
- 42. Zhang JY, Fabricant PD, Ishmael CR, Wang JC, Petrigliano FA, Jones KJ. Utilization of platelet-rich plasma for musculoskeletal injuries: an analysis of current treatment trends in the United States. Orthop J Sports Med. 2016;4(12):2325967116676241. [DOI] [PMC free article] [PubMed] [Google Scholar]