Abstract
Background:
Influenza causes significant morbidity and mortality in adults, and numerous patients require intensive care unit (ICU) admission. Acute respiratory distress syndrome (ARDS) is clearly described in this context, but other clinical presentations exist that need to be assessed for incidence and outcome. The primary goal of this study was to describe the characteristics of patients admitted in ICU for influenza, their clinical presentation, and the 3-month mortality rate. The second objective was to search for 3-month mortality risk factors.
Methods:
This is a retrospective study including all patients admitted to 3 ICUs due to influenza-related disease between October 2013 and June 2016, which assesses the 3-month mortality rate. We compared clinical presentation, biological data, and outcome at 3 months between survivors and non-survivors. We created a predicting 3-month mortality model with Classification and Regression Tree analysis.
Results:
Sixty-nine patients were included, 50 patients (72.5%) for ARDS, 5 (7.2%) for myocarditis, and 14 (20.3%) for acute respiratory failure without ARDS criteria. Non-typed influenza A was found in 30 cases (43.5%), influenza A H1N1 in 18 (26.1%), H3N2 in 3 (4.3%), and influenza B in 18 cases (27.5%). The 3-month mortality rate was 29% (n = 20). Extracorporeal membrane oxygenation (ECMO) was implanted in 23 patients, without any significant increase in mortality (39% vs 24% without ECMO, P = .19). A creatinine serum superior to 96 μmol/L, an aspartate aminotransferase level superior to 68 UI/L, and a Pao2/Fio2 ration below 110 were associated with 3-month mortality in our predictive mortality model.
Conclusion:
Influenza in ICUs may have several clinical presentations. The mortality rate is high, but ECMO may be an effective rescue therapy.
Keywords: Influenza, ARDS, ICU, ECMO, acute respiratory failure, myocarditis
Introduction
Influenza is the second most deadly pandemic in human history – the so-called Spanish Flu.1 Influenza is a disease caused by a single strain RNA genome virus belonging to the Orthomyxoviridae family. Four types have been identified (A, B, C, and D), with only types A and B causing significant infections in humans. This virus is classified according to hemagglutinin and neuraminidase protein characteristics.2 In 2009, an antigenic shift of influenza A H1N1 led to a global influenza pandemic.3,4 Influenza virus strain H1N1pdm09 is responsible for 20% to 40% of the mortality rate and poses a worldwide challenge for intensive care units (ICUs).5-7 However, vaccination coverage remains low despite recommendations.8,9 Furthermore, new virus sub-types cause outbreaks that pose different public health challenges.10
Acute respiratory failure progressing into acute respiratory distress syndrome (ARDS) is the most common presentation in ICUs.11,12 In some cases, this is associated with myocarditis, which can lead to heart failure.13 Treatment is based on neuraminidase inhibitor administration as soon as influenza is suspected, protective lung ventilation, and general organ support.14,15 In the most severe cases, veno-venous extracorporeal membrane oxygenation (VV-ECMO) can be implanted.16,17
Herein, we did a retrospective study including adult patients admitted to 3 referral ICUs of a tertiary care teaching hospital for severe influenza. The primary goal was to describe the characteristics of these patients, their clinical presentation, and the 3-month mortality rate. The second objective was to investigate the 3-month mortality risk factors.
Materials and Methods
Study setting
This was a retrospective observational study including all adult patients admitted with severe influenza to one of the 3 ICUs at Toulouse University Hospital, France, between October 2013 and June 2016. This study was approved by the ‘Commission nationale d’informatique et des libertés’ (French Data Protection Authority) (No. 2173146v0). According to French legislation, the need for consent was waived.
Definitions and management
Influenza cases were defined as a clinical influenza-like illness with an influenza-positive laboratory test (nasal swab, tracheal suction, or bronchoalveolar lavage, with reverse transcription polymerase chain reaction testing [RT-PCR]).
Acute respiratory distress syndrome was defined according to the Berlin consensus, and patients were treated as per the experts’ recommendations.18
The implementation of VV-ECMO was discussed on the basis of regional protocol and Extracorporeal Life Support Organization (ELSO) guidelines, in the case of severe ARDS with refractory hypoxaemia or uncontrolled hypercarbia despite conventional management including prone positioning.16,17
Myocarditis was defined as a change in the ST segment associated with elevated serum troponin levels and normal coronary angiography (or no compatible lesion). In the case of refractory cardiogenic shock, veno-arterial extracorporeal membrane oxygenation (VA-ECMO) implementation was discussed.
All patients with VV-ECMO or VA-ECMO located in our region were transferred to and managed in our ICU.
In our unit, neuraminidase inhibitor (oseltamivir) was given as soon as influenza was suspected. Treatment was continued until the RT-PCR tested negative, with a minimum of 5 days. The test was carried out twice a week once diagnosis was confirmed.
Data collection
Demographic data, the length of time from onset of clinical signs to ICU admission or initiation of anti-neuraminidase treatment, invasive ventilation and vasopressor infusion, concomitant bacterial infection, strain lineage, and the administration of ARDS adjunct therapy were recorded. Thirty-day and 3-month mortality were collected from medical records if available or by calling patients or their relative or medical referent when patients were not available.
Statistical analysis
Following initial descriptive statistics comprising variable distribution analysis (Shapiro-Wilk test), the study population was divided into 2 groups: 3-month survivors and non-survivors. The characteristics of both groups were compared using the Mann-Whitney test for quantitative variables and the Fisher test and χ2 test for qualitative variables. Results are expressed as median values with interquartile range or as percentages, where appropriate. Significant quantitative explanatory variables were assessed with receiver operating characteristic curves and associated area under the curve (AUC) to determine the optimal cut-off value associated with 3-month mortality prior to multivariate analysis. Survival probability based on the significant explanatory variable was assessed using the Kaplan-Meier method.
Covariate selection for the multivariate analysis was based on a value of P < .2 with univariate analysis. The prognostic value of the covariates of interest was assessed using the Cox proportional hazards model. The results are presented as hazard ratios (HR) with a 95% confidence interval (CI).
Patients with the best chances of survival were highlighted by separating the population according to Classification and Regression Tree (CART) analysis.19 The purpose of this approach was to describe the method for distributing the population between homogeneous groups based on 3-month survival and the covariates previously selected for the multidimensional analysis.
Statistical analyses were conducted using SPSS for Windows version 23.0 (IBM Corporation, Armonk, NY, USA). A value of P < .05 was considered statistically significant.
Results
Baseline characteristics of the study population
Sixty-nine patients satisfied the inclusion criteria between October 2013 and June 2016. They were mostly men (n = 42, 60.9%), middle-aged (60 [48-68] years), and non-institutionalised (n = 64, 92.8%) (Table 1). Fourteen patients (20.3%) were hospitalised in the month prior to the studied hospital admission.
Table 1.
Summary of demographic, clinical, and biological data of 3-month survivors and non-survivors.
| Survivors | Non-survivors | P value | |
|---|---|---|---|
| n = 49 | n = 20 | ||
| Age, y, median (IQR)a | 56 (43 to 66) | 67 (58 to 76) | .01 |
| BMI, kg/m2, median (IQR)a | 24 (22 to 31) | 25.5 (22 to 29.5) | .93 |
| SAPS II, median (IQR)a | 36 (31 to 53) | 71 (51 to 79) | <.01 |
| Male, No. (%)b | 22 (44.9) | 5 (25) | .17 |
| Medical history | |||
| Hospitalisation in the month prior to admission No. (%)b | 10 (20.4) | 4 (20) | .99 |
| COPD, No. (%)b | 7 (14.3) | 6 (30) | .17 |
| Heart failure, No. (%)b | 7 (14.3) | 7 (35) | .09 |
| Arterial hypertension, No. (%)b | 13 (26.5) | 12 (60) | .01 |
| Vaccination, No. (%)b | 4 (8.6) | 1 (5.8) | 1 |
| Clinical presentation at ICU admissionc | .33 | ||
| Acute respiratory failure, No. (%) | 12 (17.4) | 2 (2.9) | |
| ARDS, No. (%) | 34 (49.3) | 16 (23.2) | |
| Myocarditis, No. (%) | 3 (4.3) | 2 (2.9) | |
| Days between onset and ICU admission, median (IQR)a | 4 (3 to 7) | 6.5 (2 to 8) | .17 |
| Virusc | .68 | ||
| Non-typed influenza A, No. (%) | 21 (42.9) | 9 (45) | |
| H1N1, No. (%) | 15 (30.6) | 3 (15) | |
| H3N2, No. (%) | 1 (2) | 2 (10) | |
| Influenza B, No. (%) | 12 (24.5) | 6 (30) | |
| Presentation at admission | |||
| Body temperature, °C, median (IQR)a | 38.5 (38.1 to 39) | 38.8 (37.9 to 39.2) | .82 |
| Leukocyte, cell/mm3, median (IQR)a | 8890 (5170 to 13 720) | 9670 (5512 to 14 212) | .72 |
| pH, median (IQR)a | 7.42 (7.31 to 7.48) | 7.4 (7.24 to 7.45) | .37 |
| Pao2, mm Hg, median (IQR)a | 58 (50 to 71) | 57 (44 to 66) | .54 |
| Pao2/Fio2, median (IQR)a | 120 (100 to 130) | 90 (50 to 100) | .03 |
| Paco2, mm Hg, median (IQR)a | 37 (31 to 49) | 36 (29 to 43) | .49 |
| Alkaline reserve, mmol/L, median (IQR)a | 22 (20 to 29) | 20 (17 to 24) | .04 |
| Base excess, mmol/L, median (IQR)a | −1.3 (−4 to 3.1) | −4.2 (−8.9 to −1.4) | .01 |
| Lactates, mmol/L, median (IQR)a | 1.5 (1 to 2.2) | 2.4 (1.8 to 4) | <.01 |
| Creatinine, µmol/L, median (IQR)a | 76 (60 to 91) | 145 (96 to 265) | <.01 |
| Troponin T, ng/L, median (IQR)a | 12 (7 to 49) | 65 (19 to 279) | .02 |
| AST, IU/L, median (IQR)a | 50 (34 to 93) | 130 (82 to 275) | <.01 |
| ALT, IU/L, median (IQR)a | 34 (22 to 73) | 77 (56 to 116) | <.01 |
| Bilirubin, µmol/L, median (IQR)a | 8 (5 to 13) | 13 (9 to 17) | .01 |
| No. of days with norepinephrine, median (IQR)a | 3 (1 to 5) | 5 (3 to 9) | .02 |
| Days under mechanical ventilation, median (IQR)a | 10 (4 to 25) | 9 (8 to 17) | .87 |
| Days with neuromuscular blocking agent, median (IQR)a | 5.5 (2 to 10) | 6 (2.25 to 10) | .82 |
| Nitric oxide, No. (%)b | 10 (20.4) | 5 (25) | .67 |
| Prone positioning sequence, median (IQR)a | 2 (2 to 2) | 1 (1 to 2) | .06 |
| Patient receiving NI, No. (%)b | 43 (87.7) | 16 (80) | .46 |
| Days between onset and NI debut, median (IQR)a | 5 (3 to 7) | 7 (2 to 8) | .85 |
| NI duration, median (IQR)a | 10 (7 to 14) | 8 (5 to 12) | .31 |
| ECMOc | 14 (28.6) | 9 (45) | .26 |
| Veno-venous, No. (%) | 7 (14.3) | 7 (35) | |
| Veno-arterial, No. (%) | 2 (4.1) | 2 (10) | |
| Prone positioning and ECMO, No. (%)b | 10 (20.4) | 7 (35) | .23 |
| No. of days with ECMO, median (IQR)a | 9 (6 to 13) | 7 (3 to 9) | .16 |
Abbreviations: ALT, alanine aminotransferase; ARDS, acute respiratory distress syndrome; AST, aspartate aminotransferase; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; Fio2, O2 inspired fraction; ICU, intensive care unit; IQR, interquartile range; NI, neuraminidase inhibitor; Paco2, CO2 partial pressure; ao2, O2 partial pressure; SAPS II, Simplified Acute Physiology Score II.
Mann-Whitney test.
Fisher test.
χ2 test
Bold values are statistically significant (p < 0.05).
A history of arterial hypertension (n = 25, 36.5%), heart failure (n = 14, 20.3%), and chronic obstructive pulmonary disease (n = 13, 18.8%) was documented in most cases.
Presentation and management of influenza
Five patients (7.2%) were vaccinated in the year of admission, although 54 patients (78.3%) should have been vaccinated according to French guidelines.
In terms of clinical presentation, 50 patients (72.5%) were admitted for ARDS, 5 (7.2%) for myocarditis, and 14 (20.3%) for acute respiratory failure without ARDS criteria.
The median length of time between clinical onset and ICU admission was 5 (2-7) days. Twenty-eight patients (40.6%) were initially admitted to another facility. The median Simplified Acute Physiology Score II (SAPS II) on admission was 44 (33-66).
Non-typed influenza A was found in 30 cases (43.5%), influenza A H1N1 in 18 patients (26.1%), H3N2 in 3 patients (4.3%), and influenza B in 18 patients (27.5%).
Nearly 70% of patients required invasive ventilation for a median duration of 10 (4-25) days. Prone position was required for 26 patients (37.7%) and nitric oxide was administered to 15 patients (21.7%). Extracorporeal membrane oxygenation was implanted in 23 patients (33.3%), namely 19 VV-ECMO and 4 VA-ECMO.
Neuraminidase inhibitor was given to 58 patients (84.1%), for a median duration of 9 (6-14) days. Five patients did not receive treatment because of a late diagnosis, and no specific reason was retrieved for the remaining 6 patients. The 30-day and 3-month mortality rates were 24.6% (n = 17) and 29% (n = 20), respectively.
Predictors for 3-month mortality
Univariate analysis revealed a significant correlation between 3-month mortality and higher patient’s age (odds ratio [OR]: 1.05, 95% confidence interval [CI], 1.02-1.1, P = .008), higher SAPS II (OR: 1.07, 95% CI: 1.04-1.11, P < .001), a medical history of arterial hypertension and/or cardiac medication (OR: 4.15, 95% CI: 1.41-12.92, P = .011), lower Pao2/Fio2 ratio (OR: 0.98, 95% CI: 0.95-0.99, P = .026), higher serum lactate level (OR: 1.49, 95% CI: 1.11-2.15, P = .017), higher serum creatinine level (OR: 1.009, 95% CI: 1.003-1.012, P = .009), lower glomerular filtration rate (estimated by CKD EPI [Chronic Kidney Disease Epidemiology Collaboration] formula) (OR: 0.96, CI 95%: 0.94-0.98, P < .001), higher troponin level (OR: 1.009, 95% CI: 1.003-1.012, P = .009), higher aspartate aminotransferase (AST) (OR: 1.01, 95% CI: 1.005-1.016, P = .012) and alanine aminotransferase (ALT) (OR: 1.008, 95% CI: 1.002-1.01, P = .026), bilirubin levels on admission (OR: 1.003, 95% CI = 1.001-1.005, P = .033), and the duration of norepinephrine infusion (in days) (OR: 1.84; 95% CI: 1.26-2.09, P = .039). Virus type and lineage was not linked to worst outcome.
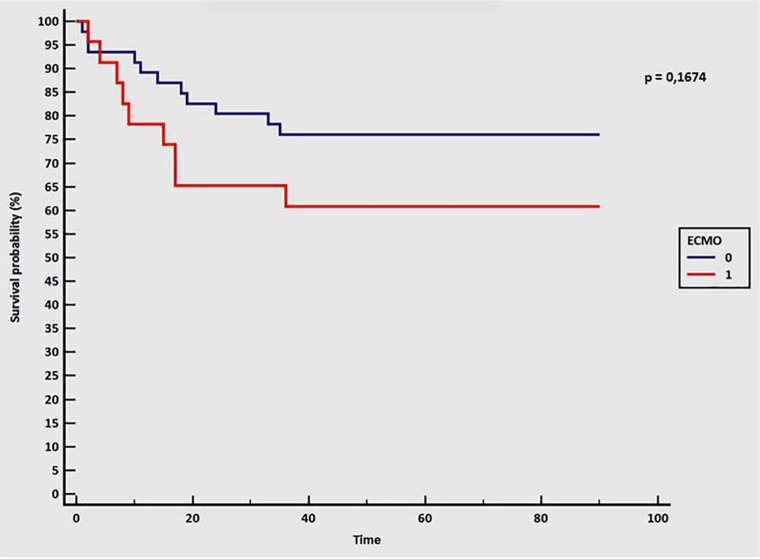
A sub-group analysis of patients treated with ECMO did not highlight any increase in mortality compared with patients without ECMO (39% vs 24%, respectively; P = .19), even with a higher SAPS II value (predicted in-hospital mortality of 70% for patients with ECMO versus 19.6% for those without) (Figure 1 and Table 2). Patients treated with ECMO had higher BMI, higher creatinine serum level, lower Pao2/Fio2 ratio, and higher AST, ALT, and bilirubin level at ICU admission than patients without ECMO, without significant difference in hemodynamic parameters, lactate level, or pH.
Figure 1.
Kaplan-Meier survival curve at 3 months. Blue line indicates patient without ECMO; red line indicates patients with ECMO. Time in days. A value of P < .05 was considered statistically significant. ECMO indicates extracorporeal membrane oxygenation.
Table 2.
Demographic data of ECMO and non-ECMO patients.
| Non-ECMO (n = 46) | ECMO (n = 23) | P value | |||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Age | 62 | 50-75 | 53 | 47-64 | .09 |
| BMI | 24 | 22-29.5 | 29 | 24-32 | .039 |
| SAPS II | 37 | 31.5-51 | 61 | 45.5-72.5 | .002 |
| Heart rate | 101 | 85-114 | 97 | 90-113 | .86 |
| SAP | 115 | 90-129 | 120 | 100-125 | .96 |
| DAP | 70 | 60-80 | 70 | 60-80 | .59 |
| Creatinine | 78.5 | 58.5-97 | 93 | 76-165 | .049 |
| GFR | 82.5 | 49.5-105 | 61 | 38-90.5 | .12 |
| Troponin | 28 | 9-92 | 18.8 | 7.5-86.5 | .73 |
| Lactates | 1.5 | 1-2.5 | 2 | 1.5-3.5 | .09 |
| Pao2 | 60.9 | 58-73.5 | 53 | 38.5-63.5 | .06 |
| Pao2/Fio2 | 125 | 100-134 | 68 | 50-71 | <.001 |
| Paco2 | 37 | 31-49 | 33.4 | 30-42 | .09 |
| pH | 7.40 | 7.29-7.47 | 7.44 | 7.39-7.5 | .12 |
| AST | 50.5 | 36-96 | 93 | 66-233 | .01 |
| ALT | 34 | 22.5-69.5 | 77 | 53.5-115 | .002 |
| Bilirubin | 7.85 | 5-13 | 13 | 11-18 | .001 |
| ICU length of stay | 10 | 6-20 | 20 | 11-32 | .02 |
| Number of days of MV | 6 | 2-10 | 24 | 11-32 | <.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DAP, diastolic arterial pressure; ECMO, extracorporeal membrane oxygenation; GFR, glomerular filtration rate; ICU, intensive care unit; IQR, interquartile range; MV, mechanical ventilation; SAP, systolic arterial pressure; SAPS II, Simplified Acute Physiology Score II.
Bold values were statistically significant (p < 0.05).
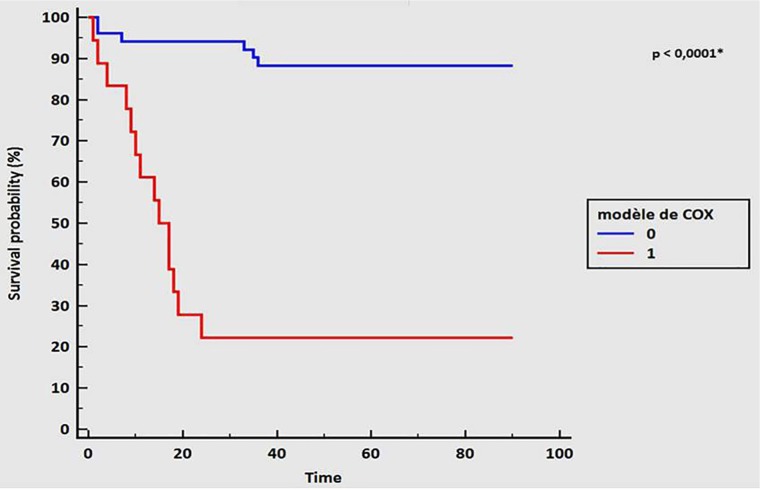
Multivariate Cox proportional hazard regression analysis using backward elimination was performed to identify the risk factors predicting 3-month mortality. This model confirmed that an AST level superior to 68 IU/L and a creatinine level superior to 96 μmol/L on admission are associated with 3-month mortality (HR: 7.68 [1.68-35.1] and 4.73 [1.61-13.92], respectively, with P < .01 for each). The AUC for this model was 0.89 (95% CI: 0.79-0.95), with a sensitivity of 70%, a specificity of 92%, a positive predictive value of 78%, and a negative predictive value of 88% (Figure 2 and Table 3).
Figure 2.
Survival curve according to Cox model variable, 1 (red line): creatinine >96 μmol/L, AST >68 IU/L, and P/F ⩽110; 0 (blue line): creatinine <96 μmol/L, AST <68 IU/L, and P/F >110. Time in days. A value of P < .05 was considered statistically significant. AST indicates aspartate aminotransferase; P/F, O2 partial pressure/O2 inspired fraction ratio.
Table 3.
Multivariate analysis with Cox model for 3-month mortality-associated factor.
| Hazard ratio | 95% CI | P value | |
|---|---|---|---|
| Significant covariables | |||
| AST >68 IU/L | 7.68 | 1.68-35.1 | <.01 |
| Creatinine >96 µmol/L | 4.73 | 1.61-13.92 | <.01 |
| Non-significant variable included in segmentation tree | |||
| P/F ⩽110 | 1.36 | 0.47-3.92 | .57 |
Abbreviations: AST, alanine aminotransferase; CI, confidence interval; P/F, O2 partial pressure/O2 inspired fraction ratio.
A value of P < .05 was considered statistically significant.
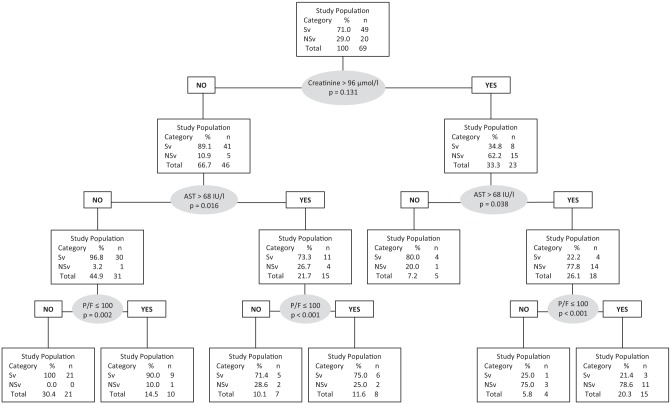
With the segmentation tree and using the CART method, we increased the positive predictive value to 85.5% by including a new variable: Pao2/Fio2 ratio <110 (Figure 3). We defined 7 sub-groups by dividing the study population step by step according to the variables included in the model. Those sub-groups differed considerably for outcome: the sub-group with no risk factor (n = 21), as defined by our model, had a 100% survival rate compared with 21.3% for the sub-group presenting all the risk factors (n = 15). The other sub-groups had intermediate outcomes, depending on how many risk factors they presented.
Figure 3.
Prediction model of 3-month mortality by segmentation tree (Classification and Regression Tree analysis) according to creatinine level, AST level, and P/F ratio. A value of P > .05 is significant and means predictive value improvement of the step. AST indicates alanine aminostransferase; NSv, 3-month non-survivors; P/F, O2 partial pressure/O2 inspired fraction ratio; Sv, 3-month survivors.
Discussion
This article reports on a retrospective cohort of 69 patients admitted to ICU for severe influenza, with 3 main clinical presentations: ARDS (72.5%), acute respiratory failure without ARDS criteria (21.7%), and myocarditis (7.2%). Influenza ARDS and respiratory failure are clearly described, especially during the 2009 pandemic, whereas viral myocarditis is less well known.4,11,14,15,20
In their review of extrapulmonary influenza complications, Sellers et al13 highlight 44 reported cases of influenza-related myocarditis, with VA-ECMO implemented in 16 of them. In our study, 4 patients benefited from VA-ECMO, of whom 2 died. Pathophysiology is not well documented, but direct viral invasion seems to be the primum movens.13
The mortality rate in our study was 29% (32% for patients admitted for ARDS, 40% for those admitted for myocarditis, and 11.8% for those admitted for non-ARDS acute respiratory failure). Studies on influenza in ICUs report various mortality rates, but the rate remains high, between 8% and 50%, depending on the population studied, resources, the chosen time for end point, and the type of virus.3,4,6,21-23 Most of these studies focused on influenza A H1N1, and more specifically on the 2009 pandemic. We report on cases of all influenza virus clinical presentations encountered over a 4-year period in a large regional tertiary hospital ICU.
In our study, we found no difference in mortality rate between patients with and without ECMO, despite the fact that patients with ECMO were more severely ill. The conventional ventilation or ECMO for severe adult respiratory failure (CESAR) trial showed improved survival rates with VV-ECMO in ARDS, but did not focus on influenza.24 The main limit of this study is that all patients in the interventional group were transferred to the same unit for ECMO, making it difficult to extrapolate the results. Furthermore, in some cases, ECMO was not implemented because conventional management was sufficient when applied according to the guidelines. Conversely, the recent ECMO to rescue lung injury in severe ARDS (EOLIA) trial did not find an improved survival rate and was stopped early for futility.25 However, cross-over between the control group and the interventional group (ie, conventional ventilation and ECMO) was permitted in EOLIA study, making it difficult to interpret those results. In their study, Noah et al26 highlighted improvement in mortality rates in influenza ARDS with ECMO in a retrospective cohort with a propensity score matching. Pham et al27 found no difference in mortality rate using a similar method, but some patients treated with ECMO were not included in their propensity score analysis because there was no match for comparison, and those patients had better outcomes, with more severe respiratory criteria, than those included.
Several predictive mortality scores exist for ARDS patients treated with VV-ECMO,28 and one was specifically designed for influenza.29 This score includes hospital length of stay before VV-ECMO, haematocrit, and mean arterial pressure as well as creatinine and bilirubin. In our 3-month mortality-predicting model, we included serum creatinine and AST on admission, which were significantly associated with death when superior to 96 µmol/L and 68 IU/L, respectively. Extracorporeal membrane oxygenation may be effective in this indication because ECMO implementation was not significantly associated with mortality and was not included in our model. However, the patients who would most benefit from this should be better defined.
Acute kidney injury (AKI) is a well-known associated mortality factor in ICU.30 Several studies have described the same association with influenza, especially ARDS-related influenza A H1N1 during the 2009 pandemic.31-33 Acute tubular necrosis seems to be the main pathological finding in an autopsy series of patients with AKI who died in the 2009 pandemic.34 Pathophysiology is poorly understood, but AKI is probably multifactorial involving renal hypoperfusion, hypoxia, rhabdomyolysis, vasoconstriction, and SIRS (systemic inflammatory response syndrome). Liver injury is described to a less extent, but seems to be associated with worse outcomes.35,36 In their study, Gao et al35 found that an AST rate superior to 40 IU/L was associated with a worse outcome for patients with ARDS and influenza A H7N9. In their retrospective study including 97 patients with seasonal and 2009 pandemic influenza, Papic et al36 found a correlation between serum liver enzyme and hypoxia. Interestingly, they found that serum liver enzyme elevation was significantly higher in the 2009 pandemic influenza than in seasonal flu. Once again, the mechanisms involved have yet to be defined, but appear to include hypoxia and SIRS. Cor pulmonale is already known to induce biological abnormalities, including AST and creatinine elevation, and is frequently associated with ARDS and poor outcome.37 Here we assume that this provides one explanation for our findings, but our study was not designed for this purpose, and further studies are needed.
The strength of our study is its description of severe H1N1 and non-H1N1 influenza-related ICU patients, with several clinical presentations. Myocarditis was not rare, emphasising the need to take into consideration influenza in epidemic season for patient with this clinical presentation. We have developed a simple and efficient predictive mortality model, including clinical and biological data availed in daily practice. The association of a high AST level and 3-month mortality raises questions about pathophysiologic mechanisms in influenza infection that require specific studies on this subject, especially the hypothesis of a right heart–specific injury.
However, our study presents several limitations. Data are missing because of its retrospective design. Only 69 patients with heterogeneous presentations and characteristics were enrolled. It is a single-centre study, and as we are a tertiary centre, our patients may not represent patients admitted to other facilities. This may reduce the accuracy of our model. Furthermore, the low rate of events (ie, death at 3 months) due to the small number of patients meant that we could not include more than 2 variables in the model.
Conclusions
Influenza is still a life-threatening disease. Respiratory failure is the main cause of ICU admission, although myocarditis is not rare. While previous scientific reports and media attention focus on H1N1 influenza, seasonal and new emergent variant-related influenza should be borne in mind when it comes to causes of severe infection. Three simple, practical, and available in daily practice variables were found to be significant predictors of 3-month mortality. These could prove useful in providing a more accurate evaluation of severity to tailor additional therapies. Extracorporeal membrane oxygenation may be beneficial in the most severe cases.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interest:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: TA – writting, methodology and statistics. CD – conceptualisation, data collection, supervision, writting, and reviewing of the initial and final draft; FVB – reviewing of final draft. FB – data collection and reviewing of the initial and final draft. LC, TS, BRP, SR, AR, PC and BG – data collection and reviewing of the final draft. JMC – conceptualisation, data collection, methodology and statistics and reviewing of the initial and final draft. VM – reviewing of the final draft.
ORCID iDs: Timothée Abaziou  https://orcid.org/0000-0003-0493-3441
https://orcid.org/0000-0003-0493-3441
Clément Delmas  https://orcid.org/0000-0001-9180-9128
https://orcid.org/0000-0001-9180-9128
References
- 1. Wever PC, van Bergen L. Death from 1918 pandemic influenza during the First World War: a perspective from personal and anecdotal evidence. Influenza Other Respir Viruses. 2014;8:538-546. doi: 10.1111/irv.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen X, Liu S, Goraya MU, Maarouf M, Huang S, Chen J-L. Host immune response to influenza a virus infection. Front Immunol. 2018;9. doi: 10.3389/fimmu.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar A. Critically ill patients with 2009 influenza a (H1N1) infection in Canada. JAMA. 2009;302:1872. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 4. Critical care services and 2009 h1n1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925-1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 5. Dwyer DE, Lynfield R, Losso MH, et al. Comparison of the outcomes of individuals with medically attended influenza A and B virus infections enrolled in 2 international cohort studies over a 6-year period: 2009–2015. Open Forum Infect Dis. 2017;4:ofx212. doi: 10.1093/ofid/ofx212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mytton OT, Rutter PD, Donaldson LJ. Influenza A(H1N1)pdm09 in England, 2009 to 2011: a greater burden of severe illness in the year after the pandemic than in the pandemic year. Euro Surveill. 2012;17:20139. [PubMed] [Google Scholar]
- 7. Ortiz JR, Neuzil KM, Shay DK, et al. The burden of influenza-associated critical illness hospitalizations. Crit Care Med. 2014;42:2325-2332. doi: 10.1097/CCM.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catania J, Que LG, Govert JA, Hollingsworth JW, Wolfe CR. High intensive care unit admission rate for 2013–2014 influenza is associated with a low rate of vaccination. Am J Respir Crit Care Med. 2014;189:485-487. doi: 10.1164/rccm.201401-0066LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minchole E, Figueredo AL, Omeñaca M, et al. Seasonal influenza A H1N1pdm09 virus and severe outcomes: a reason for broader vaccination in non-elderly, at-risk people. PLoS ONE. 2016;11:e0165711. doi: 10.1371/journal.pone.0165711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Human infection with influenza A(H7N9) virus in China. http://www.who.int/csr/don/2013_04_01/en/index.html. Accessed September 5, 2013.
- 11. Arabi Y, Gomersall CD, Ahmed QA, Boynton BR, Memish ZA. The critically ill avian influenza A (H5N1) patient. Crit Care Med. 2007;35:1397-1403. doi: 10.1097/01.CCM.0000262940.34596.4B. [DOI] [PubMed] [Google Scholar]
- 12. Garg S, Jain S, Dawood FS, et al. Pneumonia among adults hospitalized with laboratory-confirmed seasonal influenza virus infection – United States, 2005–2008. BMC Infect Dis. 2015;15:369. doi: 10.1186/s12879-015-1004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sellers SA, Hagan RS, Hayden FG, Fischer WA., 2nd The hidden burden of influenza: a review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11:372-393. doi: 10.1111/irv.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hui DS, Lee N, Chan PKS. Clinical management of pandemic 2009 influenza A(H1N1) infection. Chest. 2010;137:916-925. doi: 10.1378/chest.09-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Rosa FG, Montrucchio C, Di Perri G. Management of H1N1 influenza virus respiratory syndrome. Minerva Anestesiol. 2009;75:654-660. [PubMed] [Google Scholar]
- 16. ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support Extracorporeal Life Support Organization, Version 1.4. Ann Arbor, MI: ELSO; 2017. [Google Scholar]
- 17. H1N1 Specific Supplements to the ELSO General Guidelines Extracorporeal Life Support Organization, Version 1.4. Ann Arbor, MI: ELSO; 2017. [Google Scholar]
- 18. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526-2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 19. Breiman L, Freidman JH, Olshen RA, Stone CJ, eds. Classification and Regression Trees. Boca Raton, FL: Chapman & Hall; 1998. [Google Scholar]
- 20. Fuhrman C, Bonmarin I, Paty AC, et al. Severe hospitalised 2009 pandemic influenza A(H1N1) cases in France, 1 July-15 November 2009. Euro Surveill. 2010;15:19463. [DOI] [PubMed] [Google Scholar]
- 21. Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12:687-695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 22. Schaffer A, Muscatello D, Cretikos M, Gilmour R, Tobin S, Ward J. The impact of influenza A(H1N1)pdm09 compared with seasonal influenza on intensive care admissions in New South Wales, Australia, 2007 to 2010: a time series analysis:. BMC Public Health. 2012;12:869. doi: 10.1186/1471-2458-12-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Estenssoro E, Rios FG, Apezteguia C, et al. Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010;182:41-48. doi: 10.1164/201001-0037OC. [DOI] [PubMed] [Google Scholar]
- 24. Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351-1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 25. Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965-1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 26. Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306:1659-1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 27. Pham T, Combes A, Rozé H, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)–induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276-285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 28. Rozencwajg S, Pilcher D, Combes A, Schmidt M. Outcomes and survival prediction models for severe adult acute respiratory distress syndrome treated with extracorporeal membrane oxygenation. Crit Care. 2016;20:392. doi: 10.1186/s13054-016-1568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pappalardo F, Pieri M, Greco T, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med. 2013;39:275-281. doi: 10.1007/s00134-012-2747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190-2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 31. Bagshaw SM, Sood MM, Long J, Fowler RA, Adhikari NK. Acute kidney injury among critically ill patients with pandemic H1N1 influenza A in Canada: cohort study. BMC Nephrol. 2013;14:123. doi: 10.1186/1471-2369-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pettilä V, Webb SAR, Bailey M, Howe B, Seppelt IM, Bellomo R. Acute kidney injury in patients with influenza A (H1N1) 2009. Intensive Care Med. 2011;37:763-767. doi: 10.1007/s00134-011-2166-8. [DOI] [PubMed] [Google Scholar]
- 33. Casas-Aparicio GA, León-Rodríguez I, Hernández-Zenteno RJ, et al. Aggressive fluid accumulation is associated with acute kidney injury and mortality in a cohort of patients with severe pneumonia caused by influenza A H1N1 virus. PLoS ONE. 2018;13:e0192592. doi: 10.1371/journal.pone.0192592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tignanelli CJ, Wiktor AJ, Vatsaas CJ, et al. Outcomes of acute kidney injury in patients with severe ARDS due to influenza A(H1N1) pdm09 virus. Am J Crit Care. 2018;27:67-73. doi: 10.4037/ajcc2018901. [DOI] [PubMed] [Google Scholar]
- 35. Gao H-N, Lu H-Z, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368:2277-2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 36. Papic N, Pangercic A, Vargovic M, Barsic B, Vince A, Kuzman I. Liver involvement during influenza infection: perspective on the 2009 influenza pandemic: liver involvement during influenza infection. Influenza Other Respir Viruses. 2012;6:e2-e5. doi: 10.1111/j.1750-2659.2011.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42:862-870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]