Abstract
Objective
To assess the usefulness of diffusion tensor imaging and its fractional anisotropy map along with conventional T2-weighted imaging in evaluating the anisotropic water diffusion variations of annulus fibres involved in herniation disc pathology.
Materials and methods
Seventy-five patients with previous medical ethics committee approval and informed consent experiencing low back pain were selected for this prospective randomised blinded trial. Lumbar disc fractional anisotropy maps were obtained acquiring diffusion tensor sequences on a 3T machine. The matrix of nucleus pulposus and structures of annulus fibres were analysed using fractional anisotropy textural features to highlight any presence of lumbar disc herniation. Observer variability and reliability between two neuroradiologists were evaluated. The χ2 test, two-tailed t test and linear regression analysis were used to focus differences in patients’ demographic data and magnetic resonance imaging findings.
Results
Annular fissures with extrusions were identified using diffusion tensor imaging in 10 out of 17 discs (study group) previously assessed as bulging discs using conventional magnetic resonance imaging. Eighteen extrusions out of 39 (study group) disc levels were identified on diffusion tensor imaging compared to eight extrusions highlighted on T2-weighted imaging (P < 0.01). All eight (study group) disc extrusions evaluated on T2-weighted imaging showed annular fissures on diffusion tensor imaging. Seven out of 14 (study group) protrusions highlighted on T2-weighted imaging had no annular fissures on diffusion tensor imaging; thirty-six disc levels in the control group had no evidence of annular fissures on diffusion tensor imaging (P > 0.01).
Conclusions
The addition of diffusion tensor imaging sequences and fractional anisotropy mapping to a conventional magnetic resonance imaging protocol could be useful in detecting annular fissures and lumbar disc herniation.
Keywords: Lumbar disc herniation, fractional anisotropy, diffusion tensor imaging, annular fissures
Introduction
Low back pain affects about 80% of adults1–3 and 4% of these patients have a lumbar disc herniation4 with involvement of the L5–S15–7 level and referred pain to the sciatic nerve dermatomes. Magnetic resonance imaging (MRI) examination is the most used diagnostic test for the assessment of the pathology of lumbar intervertebral disc. The characteristics of the T2 signal reflect the modifications and degenerative aspects of the intervertebral disc due to aging or degenerative lumbar disc pathology.8–10 The fast spin-echo (FSE) sequence was introduced in the early 1990s11 in MRI of the spinal column studies; this technique, currently widely used, offers an excellent contrast between the different structural components of the intervertebral disc.12 According to some authors,13 diffusion weighted imaging (DWI) is more useful to detect microstructural changes related to lumbar disc degeneration especially in the early stages of the disease; in fact, this sequence led to evaluate annulus fibers and nucleus pulposus water diffusion in response to the biochemical changes.13 Furthermore, these signal alterations are mainly seen at the L5–S1 level because of high load stress and mechanical factors at this level space.14 Up to now the diffusion tensor imaging (DTI) sequence has been used only for the evaluation of degenerative disc changes using fractional anisotropy (FA) of the nucleus pulposus, especially in the early stages of the disease.14 The use of echoplanar DTI sequences in evaluating lumbar spine discs and vertebral bone structures is well established, although the echoplanar DWI signal is associated with susceptibility and ghosting artifacts in the lumbar spine.14 However, the FSE T2 sequence with and without fat signal saturation, used in the standard MRI protocol, still remains the reference method for the evaluation of lumbar disc herniation.10 In our study, we investigated the usefulness of DTI in lumbar disc herniation assessing all disc FA and in particular the anisotropic water diffusion variations of annulus fibres involved in herniation disc pathology. Finally, we compared this technique to the standard MRI protocol suggested by the American task force constituted by the North American Spine Society (NASS), the American Society of Spine Radiology (ASSR) and the American Society of Neuroradiology (ASNR).15
Materials and methods
Between May 2016 and March 2018 130 subjects with low back and leg pain were identified. They underwent recent (less than 6 months) lumbar spine MRI examination and many of these exams were performed outside our department. All the exams were reviewed by a neuroradiologist with 30 years of experience. A total of 75 patients (38 men and 37 women) were selected from this cohort of 130 subjects for inclusion in a randomised blind trial approved by the medical ethics committee of our institution, and all participants gave written informed consent prior to enrollment. We considered as exclusion criteria bone and spinal cord tumours, spinal surgery and intradiscal injections of cortisone or O2–O3 mixture; only low back and leg pain for at least one year’s duration proved to have lumbar discal pathology or only facet joint inflammation on MRI were considered as inclusion criteria. Furthermore, the same neuroradiologist administered the Oswestry low back pain disability index (ODI) questionnaire.16 An Oswestry score over 20% was considered as a cut-off for selecting study group patients; we used this study method in order to select control group patients with a disability score under 20% who have low back and/or leg pain due to spondyloarthrosis or facet joint syndrome without discal pathology documented on previous MRI exams. Finally, 36 out of 75 selected patients were classified as a control group having low back and leg pain due to spondyloarthrosis or facet joint syndrome without discal pathology documented on previous MRI exams, and therefore negative according to the guidelines of the American task force16 and positive for classification of Pfirrmann et al.12 The remaining 39 out of 75 patients were classified as the study group and included in the study based on the disc pathology assessed in the previous MRI examination.
MRI protocols
Each selected patient underwent a new MRI examination in our department using a magnetic resonance 3T scanner (Ingenia, Philips Healthcare, Best, The Netherlands) with a SENSE (sensitivity encoding) spine coil. Two study protocols were considered for each patient: the standard and DTI protocols. The first one was performed using sequences characterised by these parameters and scanning planes: axial and sagittal T2 Dixon sequences (TR/TE 2208 ms/10 ms; echo train length 22; field of view (FOV) 283 mm; matrix 380 × 262; NEX 1; acquisition time 2:20 minutes; slice thickness/gap 1 mm/1 mm). For the T2 Dixon technique, a modified FSE sequence was used to acquire two images (one in-phase and the other out-of-phase). The DTI protocol was obtained by acquiring images on the axial plane with these parameters: TR 2000 ms, TE 68.3 ms, layer thickness 2 mm, matrix 288 × 160, FOV 30 × 30 cm, NEX 16, b value 800 s/mm2 and 30 directions of diffusion of water molecules. The acquisition time was about 6 minutes. The DTI sequence was acquired with a b value of 800 s/mm2 for minimising diffusion-related loss of signal intensity in disc tissue and particular nucleus pulposus and subsequent inaccuracy in evaluating the presence of annular fissures in annulus fibrosus or partial herniation of disc material.17–19 Fiber reconstructions were achieved using a dedicated workstation and Fibertrak software (release V2.1.3 Philips Healthcare, Cleveland, OH, USA); subsequently the values of anisotropy (FA) were calculated.
Qualitative and quantitative analysis of images
The MRI exams were acquired for every patient of both groups using standard protocol sequences and subsequent DTI. Every exam was independently analysed by two neuroradiologists with 25 and 6 years of experience, respectively. Lumbar disc pathologies were classified on T2 images according to the guidelines of the American task force,15 while the degree of disc degeneration was classified according to the classification of Pfirrmann et al.12 On the DTI images the disc annulus fiber integrity was evaluated visualising the FA corresponding map. So DTI axial images were elaborated with the FiberTrak tool and the values of FA were calculated in the presence of integrity or interruption of the annulus fibers by placing a region of interest (ROI) on the intermediate axial layer of every studied intervertebral disc.
Statistical analysis
The intra and interobserver agreement of the MRI evaluations for the qualitative analysis was estimated using percentages of concordance and kappa statistics on the basis of the Landis and Koch method.20 A final consensus was reached among neuroradiologists after all the data were collected. A final agreement in FA measurements was obtained using the intraclass correlation coefficient and calculating the mean difference between the evaluators and the 95% confidence interval (CI). For the comparison of the categorical variables the χ2 test was used, while for the quantitative variables a two-tailed t test was used. Linear regression analysis was performed to analyse whether there was a correlation between additional fissures or extrusions identified by the DTI protocol and Oswestry score; the same statistical test was used in order to evaluate if FA of the disc was influenced by body mass index (BMI), spinal level or degree of disc degeneration.
Results
Demographic data
At presentation both groups did not show any significant difference (P > 0.01) in terms of the male to female ratio, median age and BMI (Table 1), while the study group Oswestry disability index (ODI) score was higher in respect to the control group (P < 0.01). In accordance with the guidelines of the American task force15 all patients in the control group did not have significant disc alterations in respect to the study group patients who showed a wide range of different herniation types (Table 1). Beside that the study group had a tendency to have lower disc levels involved, especially L5–S1 (Table 1). Most of the patients in the control group showed a low grade of disc degeneration according to classification of Pfirrmann et al.16 (Table 1).
Table 1.
Demographic data.
| Study group (39) | Control group (36) | P value | |
|---|---|---|---|
| Male to female ratioa | 18:21 | 20:16 | 0.66d |
| Median ageb | 46 | 45 | 0.98c |
| Median BMI (kg/m2)b | 25.6 ± 3.3 SD | 25.9 ± 3.3 SD | 0.89c |
| ODI score (%)b | 72 ± 0.23 SD | 19 ± 0.32 SD | 0.01c |
| Herniation typea | |||
| Protrusion | 14 | 0 | 0.009d |
| Extrusion | 8 | 0 | 0.01d |
| Bulging | 17 | 0 | 0.001d |
| Annular fissures | 8 | 0 | 0.01d |
| No alteration | 0 | 36 | 0.0005d |
| Spinal levela | |||
| L2–L3 | 2 | 9 | 0.01d |
| L3–L4 | 5 | 8 | 0.42d |
| L4–L5 | 7 | 10 | 0.42d |
| L5–S1 | 25 | 9 | 0.01d |
| Grade of disc degeneration (I–V)a | |||
| I | 0 | 12 | 0.005d |
| II | 12 | 9 | 0.45d |
| III | 10 | 8 | 0.65d |
| IV | 9 | 5 | 0.01d |
| V | 8 | 2 | 0.01d |
Data are expressed as numbers of patients.
Data are expressed as mean values ± standard deviation.
Numbers are calculated with the two-tailed t test for equality of means.
Numbers are calculated with the χ2 test for equality of means.
BMI: body mass index; ODI: Oswestry Disability Index.
Qualitative assessment using DTI along with standard MRI protocol
Intraobserver agreement was ‘excellent’ for both readers, with kappa values ranging from 0.83 to 0.90 and 0.81 to 0.93, while interobserver agreement ranged from substantial to excellent, with kappa values ranging from 0.68 to 0.82 and 0.63 to 0.83, for herniation type analysis on standard MRI and DTI protocol, respectively. The consensus reading results for these two protocols among study group patients are shown in Table 2. The DTI protocol showed that 10 out of 17 study group patients with 17 intervertebral disc levels classified as bulging discs assessed in standard MRI protocol, had annular fissures together with extrusions (Figure 1(a) and (b)). Overall, using the DTI protocol we identified 18 extrusions out of 39 disc levels in 39 study group patients compared to eight disc levels involved as extrusions highlighted in the standard MRI protocol (P < 0.01) (Figure 2(a), (b) and (c)) (Table 2). All eight study group patients with eight different involved herniated disc levels evaluated in the standard MRI protocol showed annular fissures also in DTI. Seven out of 14 study group patients, with a total of 14 intervertebral disc level protrusions highlighted on standard MRI sequences did not show annular fissures in DTI; then they were better framed as bulging discs using DTI (Figure 3(a), (b) and (c)). Therefore, the DTI protocol identified in a total of 39 study group patients, seven protrusion discs instead of 14 evaluated in the standard protocol (P < 0.01) (Table 2). Taking into account the results of both MRI standard and DTI protocols, the discal annulus fibrosus of 36 healthy volunteers had no evidence of annular fissures (P > 0.01) (Figure 4(a) and (b)). There was major correlation between the ODI score and herniation types assessed using DTI with respect to the observations obtained by the MRI standard protocol (P = 0.001 to P = 0.0001) (Table 2).
Table 2.
Comparison of qualitative results between DTI and standard MRI protocol in study group patients.
| DTI protocol (39 patients) | Standard MRI protocol (39 patients) | P valueb,c | |
|---|---|---|---|
| Herniation typea | |||
| Protrusion | 7 | 14 | 0.01 0.001c |
| Extrusion | 18 | 8 | 0.001 0.0001c |
| Bulging | 14 | 17 | 0.25 0.003c |
| Annular fissures | 25 | 8 | 0.0001 0.00001c |
| No alteration | 0 | 0 | 0.99 0.99c |
Data are numbers of patients.
Numbers are calculated with the χ2 test for equality of means.
As calculated at logistic regression analysis with disability using the Oswestry score as covariate.
DTI: diffusion tensor imaging; MRI: magnetic resonance imaging.
Figure 1.
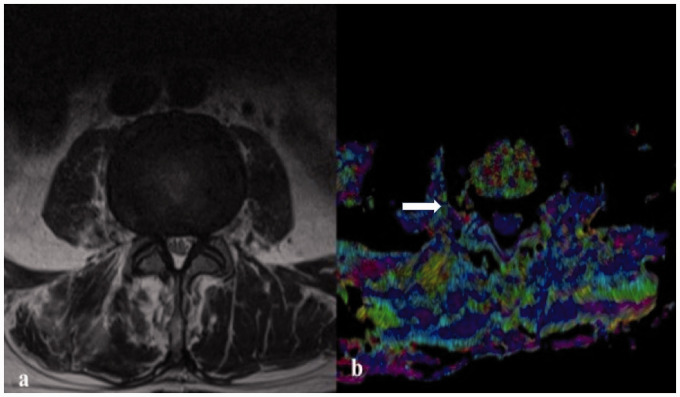
(a) Axial T2-weighted image that shows a bulging disc at the L3–L4 level in a 48-year-old female patient; (b) fractional anisotropy in diffusion tensor imaging at the same level shows the presence of a radial annular fissure with partial herniation of the nucleus pulposus near the edge of the disc at the level of the right far lateral zone (white arrow).
Figure 2.
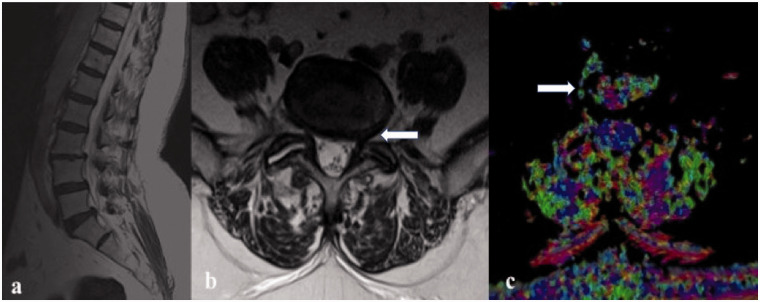
(a) Left paramidline sagittal T2-weighted image that shows different degenerative disc grades at different levels of a 56-year-old male patient; in particular at L5–S1; (b) in axial T2-weighted image there is a protrusion disc narrowing the left neural exit foramina (white arrow); on fractional anisotropy (c) it is possible to note annular fissures with partial extrusion at the same disc level near the right far lateral zone (white arrow).
Figure 3.
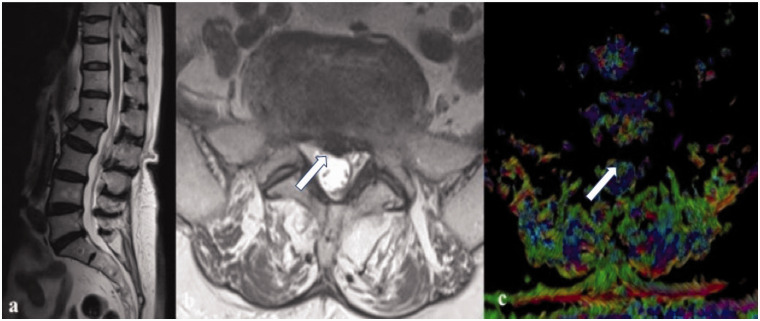
(a) Midline sagittal T2-weighted image that shows different degenerative disc grades at different levels of a 55-year-old female patient; in particular at L5–S1; (b) in axial T2-weighted image there is a central disc protrusion with an imprint on the dural sac (white arrow) in the context of a bulging disc; on fractional anisotropy (c) at the same disc level it is possible to note the homogeneous signal of the annulus fibrosus (white arrow), therefore these aspects indicate only a symmetrical bulging disc.
Figure 4.
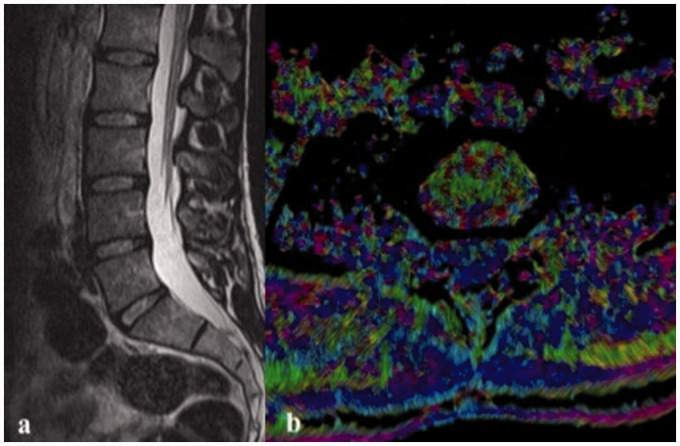
Both on midline sagittal T2-weighted image (a) of the lumbar spine and axial fractional anisotropy image (b) at the disc level of L4–L5 of a 30-year-old male control group patient, there is no evidence of lumbar disc herniation or annular fissures.
Quantitative analysis of FA in the study and control groups
The intraclass correlation coefficient between the two readers for the FA measurements was 0.95 (95% CI 0.95, 0.99) for both groups. The mean interobserver differences for the FA measurements were less than 0.05. The FA values were different between the study and control groups at L3–L4, L4–L5 and L5–S1 levels (P < 0.01) that showed an increase from higher to lower levels (Table 3). Linear regression analysis revealed a significant influence (P < 0.01) of age, sex, BMI, spinal level and in particular the degree of disc degeneration on FA values for either group (Table 4).
Table 3.
Results of quantitative analysis of fractional anisotrophy in study and control groups.
| Study group (39) | Control group (36) | P valueb | |
|---|---|---|---|
| Fractional anisotrophya | |||
| L2–L3 | 0.23 ± 0.09 | 0.20 ± 0.03 | 0.19 |
| L3–L4 | 0.27 ± 0.11 | 0.22 ± 0.06 | 0.01 |
| L4–L5 | 0.32 ± 0.08 | 0.25 ± 0.07 | 0.01 |
| L5–S1 | 0.36 ± 0.08 | 0.30 ± 0.05 | 0.005 |
Data are expressed as mean values ± standard deviation.
Numbers are calculated with the two-tailed t test for equality of means.
Table 4.
Results of linear regression analysis for fractional anisotrophy and different independent variables in study and control groups.
| Variables | Regression coefficient | 95% CI | Standardised regression coefficient | P value | |
|---|---|---|---|---|---|
| Study group (39) | Age | 0.64 | 0.58 to 0.70 | 0.66 | 0.01 |
| Sex | 0.42 | 0.36 to 0.49 | 0.46 | 0.001 | |
| BMI | 0.72 | 0.61 to 0.85 | 0.73 | 0.002 | |
| Spinal level | 0.78 | 0.49 to 0.91 | 0.77 | 0.01 | |
| GODD | −0.83 | −0.63 to −0.96 | −0.84 | 0.004 | |
| Control group (36) | Age | 0.67 | 0.53 to 0.73 | 0.64 | 0.01 |
| Sex | 0.47 | 0.33 to 0.51 | 0.49 | 0.002 | |
| BMI | 0.76 | 0.59 to 0.87 | 0.79 | 0.004 | |
| Spinal level | 0.74 | 0.46 to 0.95 | 0.76 | 0.01 | |
| GODD | −0.85 | −0.64 to −0.94 | −0.88 | 0.006 |
GODD: grade of disc degeneration; CI: confidence interval.
Discussion
Most previous studies on lumbar discs focused on anatomical aspects and degeneration assessed on MRI.21,22 One of these previous works, written to reorganise and standardise the terminologies used by providing guidelines, was realised in 2001 by the collaborative efforts of the NASS, the ASSR and the ASNR.23 Many advances have been reached in understanding lumbar disc herniation during the past 15 years that led to a revision and modification of the above-mentioned study in 2014, preserving the format and most of the terminology, however, consistent with current concepts in radiological and clinical care. One of the most important grading systems and algorithms based on MRI signal intensity of disc structure and disc height is Pfirrmann’s classification system.12 This grading system is performed on T2-weighted FSE images and it assigns five grades of disc degeneration, ranging from grade I which is characterised by homogeneous disc structure, bright hyperintense white signal intensity and a normal disc height, while the highest grade (V) is characterised by inhomogeneous structure and black hypointense disc signal intensity. The signal alteration of the disc on T2-weighted MRI correlates with the progressive degenerative changes of the intervertebral disc12,25 due to a reduction in nucleus pulposus proteoglycan concentration and not the water or collagen contents.24 The classification of Modic et al.25 (types 1–3) identifies changes in the vertebral body marrow adjacent to the intervertebral space for further specification of a degenerated intervertebral disc. Although conventional MRI is very useful in the diagnosis of lumbar disc degeneration, ultrastructural alterations are difficult to identify in T2-weighted images.26 Previous studies, such as that by Boos et al.27 demonstrated that variations in disc hydration or composition can be detected non-invasively by using cohort studies with sufficient accuracy using T1 and T2 relaxation times. Low back pain is closely related to lumbar disc degeneration, thus the early diagnosis and control of lumbar disc degeneration is the most important step for the treatment of the low back pain.28,29 The disc degeneration process begins at approximately 16–20 years of age, and is caused by physiological degeneration, high load stress28 and other factors such as obesity.30 It is recognised in the literature that DWI and DTI are sensitive and non-invasive methods for detecting microstructural characteristics of early lumbar disc degeneration.13,14 The initiating factors of early lumbar disc degeneration are the regression of proteoglycans and the destruction of collagen cross-linking in the nucleus pulposus.31,32 These observations are supported by a study on early degeneration of articular cartilage showing that the reduction of proteoglycans might cause an increase in T2 values initially; these results were supported by histological evidence.33 DWI and its corresponding ADC map identify the capacity of water diffusion while DTI and FA describe the preferential direction of the water molecules inside the intervertebral disc. The diffusion of water molecules is divided into isotropic and anisotropic types. Isotropic diffusion occurs when no cell membranes limit diffusion and when the water molecules have a degree of diffusion equal in all directions in space; biological tissues are highly anisotropic, meaning that their water diffusion rates are not the same in every direction. Sampling of the diffusion tensor gives information about the principal direction of diffusion. FA is defined as the ratio of the anisotropic component of the diffusion tensor to the whole diffusion tensor and serves as a rotationally invariant scalar that quantifies the shape of the diffusion tensor. As described in the previous experience of Shen et al.14 higher contents of proteoglycans and water molecules appear in the centre of the disc nucleus pulposus confirmed by high ADC and low FA values while the water content gradually declined and collagen fibres increased in the intervertebral disc periphery reducing ADC values with high FA values. In DTI, disc annulus fibres have a lower signal than the nucleus pulposus due to the increase of FA. In our experience this peculiarity allowed us to visualise more annular fissures and extrusions in the study group with respect to the standard MRI protocol (P < 0.01). In order to minimise diffusion-related loss of signal intensity in disc tissue, particularly annulus fibrosus and subsequent inaccuracy in evaluating the presence of annular fissures or extrusions, we used a b value of 800 s/mm2 as described in the literature.17–19 The absence of histological data and the inability to perform MRI lumbar discography are considered limitations of our study to confirm the presence or absence of annular fissures. Up to now there have been no published studies that test this method to evaluate herniation disc pathology; however Shen et al.14 used this technique in order to observe a gradual increase of ADC and FA values in the lumbar discs with the lowering of anatomical location compared with those of upper discs as an expression of the early disc degeneration process. We found similar observations in our study samples and especially in the study group despite, in our experience, using a study method positioning the ROI on the whole lumbar intervertebral disc and our patients’ age being older in comparison with those of Shen et al.14 Due to these factors we believe that our similar results could be caused by the presence of herniated disc pathology in our study group patients. We also observed only a slight increase of FA value in our control group maybe partially related to mild lumbar disc degeneration. In our experience these reports and especially the effect of the disc degeneration process on FA are in accordance with the results of Kealey et al.34 and Wu et al.35 They observed reduced ADC values in degenerated lower lumbar discs with respect to upper levels as we observed in our control group patients who showed an increase of FA values especially in the lower intervertebral disc levels. In contrast, we observed an important increase of FA values of the lower intervertebral disc levels of the study group patients, confirming that a significant increase in FA disc values is a possible biomarker of early degeneration and also herniation of discs. We believe that the combination of DTI and FA mapping, along with standard MRI imaging, could allow a better assessment of the intervertebral disc state, the presence or absence of intracanal hernia fragments and conditions of lumbar stenosis to distinguish patients who are candidates for conservative treatment, such as discogel or oxygen-ozone discolysis, from those who need spine surgery. In conclusion, although the study results need further histological verification, they demonstrated that DTI and FA mapping could prospectively act as a sensitive tool to detect the microstructural annulus fiber ruptures in lumbar disc herniation.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
ORCID iD
Marco Perri https://orcid.org/0000-0001-6113-262X
References
- 1.Andersson GBJ. The epidemiology of spinal disorders. In: Frymoyer JW. (ed). The adult spine: Principles and practice, 2nd edn Philadelphia: Lippincott-Raven, 1997, pp. 93–141. [Google Scholar]
- 2.Splendiani A, Ferrari F, Barile A, et al. Occult neural foraminal stenosis caused by association between disc degeneration and facet joint osteoarthritis: demonstration with dedicated upright MRI system. Radiol Med 2014; 119: 164–174. [DOI] [PubMed] [Google Scholar]
- 3.Barile A, Limbucci N, Splendiani A, et al. Spinal injury in sport. Eur J Radiol 2007; 62: 68–78. [DOI] [PubMed] [Google Scholar]
- 4.Deyo RA, Weinstein JN. Low back pain. N Engl J Med 2001; 344: 363–370. [DOI] [PubMed] [Google Scholar]
- 5.Deyo RA. Early diagnostic evaluation of low back pain. J Gen Intern Med 1986; 1: 328–338. [DOI] [PubMed] [Google Scholar]
- 6.Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA 1992; 268: 760–765. [PubMed] [Google Scholar]
- 7.Gallucci M, Limbucci N, Paonessa A, et al. Degenerative disease of the spine. Neuroimaging Clin N Am 2007; 17: 87–103. [DOI] [PubMed] [Google Scholar]
- 8.Modic MT, Masaryk TJ, Ross JS, et al. Imaging of degenerative disk disease. Radiology 1988; 168: 177–186. [DOI] [PubMed] [Google Scholar]
- 9.Pearce RH, Thompson JP, Bebault GM, et al. Magnetic resonance imaging reflects the chemical changes of aging degeneration in the human intervertebral disk. J Rheumatol Suppl 1991; 27: 42–43. [PubMed] [Google Scholar]
- 10.Sether LA, Yu S, Haughton VM, et al. Intervertebral disk: normal age-related changes in MR signal intensity. Radiology 1990; 177: 385–388. [DOI] [PubMed] [Google Scholar]
- 11.Listerud J, Einstein S, Outwater E, et al. First principles of fast spin echo. Magn Reson Q 1992; 8: 199–244. [PubMed] [Google Scholar]
- 12.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila, PA 1976) 2001; 26: 1873–1878. [DOI] [PubMed] [Google Scholar]
- 13.Perri M, Grattacaso G, di Tunno V, et al. T2 shine-through phenomena in diffusion-weighted MR imaging of lumbar discs after oxygen–ozone discolysis: a randomized, double-blind trial with steroid and O2–O3 discolysis versus steroid only. Radiol Med 2015; 120: 941–950. [DOI] [PubMed] [Google Scholar]
- 14.Shen S, Wang H, Zhang J, et al. Diffusion weighted imaging, diffusion tensor imaging, and T2* mapping of lumbar intervertebral disc in young healthy adults. Iran J Radiol 2016; 13: e30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fardon DF, Williams AL, Dohring EJ, et al. Lumbar disc nomenclature: version 2.0: Recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. Spine J 2014; 14: 2525–2545. [DOI] [PubMed] [Google Scholar]
- 16.Fairbank JC, Pynsent PB. The Oswestry disability index. Spine (Phila, PA 1976) 2000; 25: 2940–2952. [DOI] [PubMed] [Google Scholar]
- 17.Pfirrmann CWA, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001; 26: 1873–1878. [DOI] [PubMed] [Google Scholar]
- 18.Kurunlahti M, Kerttula L, Jauhiainen J, et al. Correlation of diffusion in lumbar intervertebral disks with occlusion of lumbar arteries: a study in adult volunteers. Radiology 2001; 221: 779–786. [DOI] [PubMed] [Google Scholar]
- 19.Kerttula L, Kurunlahti M, Jauhiainen J, et al. Apparent diffusion coefficients and T2 relaxation time measurements to evaluate disc degeneration. A quantitative MR study of young patients with previous vertebral fracture. Acta Radiol 2001; 42: 585–591. [DOI] [PubMed] [Google Scholar]
- 20.Landis RJ, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 21.Friberg S, Hirsch C. Anatomical and clinical studies on lumbar disc degeneration. Acta Orthop Scand 1949; 19: 222–242. [DOI] [PubMed] [Google Scholar]
- 22.Raininko R, Manninen H, Battie MC, et al. Observer variability in the assessment of disc degeneration on magnetic resonance images of the lumbar and thoracic spine. Spine 1995; 20: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 23.Fardon DF, Milette PC. Combined Task Forces of the North American Spine Society, American Society of Spine Radiology and American Society of Neuroradiology. Nomenclature and classification of lumbar disc pathology. Recommendations of the combined task forces of the North American Spine Society, American Society of Spine Radiology and American Society of Neuroradiology. Spine (Phila, PA 1976) 2001; 26: E93–E113. [DOI] [PubMed] [Google Scholar]
- 24.Pearce RH, Thompson JP, Bebault GM, et al. Magnetic resonance imaging reflects the chemical changes of aging degeneration in the human intervertebral disk. J Rheumatol Suppl 1991; 27: 42–43. [PubMed] [Google Scholar]
- 25.Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988; 166: 193–199. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Chan Q, Anthony MP, et al. Age-related diffusion patterns in human lumbar intervertebral discs: a pilot study in asymptomatic subjects. Magn Reson Imaging 2012; 30: 181–188. [DOI] [PubMed] [Google Scholar]
- 27.Boos N, Wallin A, Schmucker T, et al. Quantitative MR imaging of lumbar intervertebral disc and vertebral bodies: methodology, reproducibility, and preliminary results. Magn Reson Imaging 1994; 12: 577–587. [DOI] [PubMed] [Google Scholar]
- 28.Sibell DM, Kirsch JR. The 5-Minute Pain Management Consult, Baltimore: Lippincott Williams & Wilkins, 2006, pp. 98–102. [Google Scholar]
- 29.Heuck A, Glaser C. Basic aspects in MR imaging of degenerative lumbar disk disease. Semin Musculoskelet Radiol 2014; 18: 228–239. [DOI] [PubMed] [Google Scholar]
- 30.Samartzis D, Karppinen J, Chan D, et al. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population-based study. Arthritis Rheum 2012; 64: 1488–1496. [DOI] [PubMed] [Google Scholar]
- 31.Zobel BB, Vadala G, Del Vescovo R, et al. T1rho magnetic resonance imaging quantification of early lumbar intervertebral disc degeneration in healthy young adults. Spine (Phila, PA 1976) 2012; 37: 1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther 2003; 5: 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watrin-Pinzano A, Ruaud JP, Olivier P, et al. Effect of proteoglycan depletion on T2 mapping in rat patellar cartilage. Radiology 2005; 234: 162–170. [DOI] [PubMed] [Google Scholar]
- 34.Kealey SM, Aho T, Delong D, et al. Assessment of apparent diffusion coefficient in normal and degenerated intervertebral lumbar disks: initial experience. Radiology 2005; 235: 569–574. [DOI] [PubMed] [Google Scholar]
- 35.Wu N, Liu H, Chen J, et al. Comparison of apparent diffusion coefficient and T2 relaxation time variation patterns in assessment of age and disc level related intervertebral disc changes. PLoS One 2013; 8: e30069. [DOI] [PMC free article] [PubMed] [Google Scholar]