Abstract
Background
Cerebrovascular complications of tuberculous meningitis (TBM) are associated with increased morbidity and mortality. We retrospectively reviewed clinicoradiological findings of 90 TBM patients who presented to a tertiary care hospital, with emphasis on frequency and distribution of infarcts on diffusion imaging and pattern of vascular involvement on magnetic resonance (MR) angiography (MRA).
Materials and methods
MR images of 90 TBM patients at presentation (2012–2018) were coanalyzed by two radiologists for tuberculomas, leptomeningeal enhancement (LM), hydrocephalus, infarct and vascular abnormalities. Infarcts were categorized based on location (“tubercular” (TB) or “ischemic” zones) and arterial supply (perforators and cortical branches). Clinical and laboratory findings were correlated with imaging data.
Results
Ninety TBM patients (age 10–82 years) were enlisted after application of inclusion criteria. Tuberculomas were most common (100%) followed by LM (84.4%), cerebral infarcts (57.7%) and hydrocephalus (29%). Location-wise, 35% infarcts were in ischemic, 13% in TB and 15% in both zones. According to arterial supply, infarcts equally (50%) involved perforators from the lateral lenticulostriate and posterior cerebral (PCA)/basilar artery (BA) followed by medial lenticulostriate arteries (23%). MRA was available in 74.4% and abnormal in 43.2%. The middle cerebral artery was frequently involved (76%) followed by the anterior cerebral artery (38%), internal carotid artery (31%), PCA and BA. Six had diffuse narrowing with a paucity of distal vessels. Cerebral infarction was associated with hydrocephalus (p = .0019) and vasculitis (p < .001).
Conclusion
In TBM, strokes are common and mainly involve the perforators and cortical branches. MR is the imaging modality of choice for early diagnosis and timely management.
Keywords: Cerebral infarcts, cerebrovascular complications, ischemic zone, perforators, tubercular meningitis, tubercular zone
Introduction
Tuberculous meningitis (TBM) is the most severe form of extrapulmonary tuberculosis, with mortality of 25% and long-term neurological disability of 50%.1 Cerebrovascular complications (CVCs) of TBM have been reported as a poor prognostic predictor with adverse outcomes. There is predominantly arterial involvement with occasionally venous involvement. Cerebral infarcts have been described as involving mostly the “tubercular” (TB) zone compared with the “ischemic” zone.2–13
Hsieh et al. described 75% of infarcts in the TB zone, supplied by the medial lenticulostriate artery (MLA) and thalamo-perforating arteries, and only 11% infarcts in the ischemic zone, supplied by the lateral lenticulostriate (LLA), anterior choroidal and thalamogeniculate arteries.14 This pattern of involvement has also been supported by other studies.3,13,15,16 Tai and colleagues reexamined the concept of TB and ischemic zones and discovered that stroke in TBM involves mainly perforators and terminal cortical branches (CBs), rather than TB vs ischemic zone and suggested that the vascular classification may be an accurate method of classifying cerebral infarcts.5 The MLA includes the anterior cerebral artery (ACA)-A1 and middle cerebral artery (MCA)-M1 segments. The MLA supplies the caudate head, the anterior part of the globus pallidus, genu and anterior limb of the internal capsule. The LLA includes the M1 and M2 segments of the MCA and supplies the head and body of the caudate nucleus, most of the globus pallidus, putamen, posterior limb of the internal capsule, corona radiata and the external capsule. Whereas the posterior thalamo-perforating arteries are branches from P1, the P2 segments of the posterior cerebral artery (PCA) supply the midbrain and thalamus.5
Extensive literature exists on the cerebrovascular manifestations of TBM, digital subtraction angiography (DSA), computed tomographic angiography (CTA), diffusion-weighted imaging (DWI) and magnetic resonance angiography (MRA). Contrast-enhanced magnetic resonance imaging (MRI) of the brain is currently considered the standard of care for initial workup and in follow-up of TBM patients. A few recent studies have stressed the distribution of infarcts (TB vs ischemic zone) and pattern of vascular involvement.3,5 The objective of this retrospective study was to recognize the frequency and distribution of infarcts and evaluate the pattern of vascular involvement in a large sample of TBM patients pooled from a tertiary care hospital at their initial presentation. In addition, we correlated clinicobiochemical findings of these patients with imaging findings.
Material and methods
Participants
We retrospectively reviewed MRIs of TBM patients who presented to a tertiary care center (June 2012–May 2018) with an emphasis on CVCs. Ethical approval was obtained from the ethics committee, SGPGIMS, for retrospective analysis. A search was conducted in the radiology imaging archives using the specific keywords “tubercular”, “tubercular meningitis”, “tubercular stroke” and “infarct” either in the provided clinical history or within the body or impression of the MRI report, yielding 100 cases. The clinical medical records of these patients were examined to exclude patients who were either clinically not suspected to be TBM or had a readily apparent alternate diagnosis based on laboratory tests or biopsy for clinical and imaging manifestations (e.g. metastases, fungal or parasitic infections, lymphoma).
Inclusion and exclusion criteria
Inclusion criteria were TBM patients having brain MRI with or without MRA. Exclusion criteria were 1) brain MRI without DWI, 2) confirmation of another diagnosis on microbiological or histopathological evaluation, and 3) patient was reclassified as not having TBM on discharge.
All patients diagnosed with TBM as per the consensus case definition given by Marais and colleagues were enrolled in the study.17,18 This yielded 90 patients who potentially met the diagnostic criteria for TBM and excluded 10 patients (six metastases, two cryptococcal and two toxoplasmosis). The clinical, biochemical and histopathological data were evaluated to see whether they met the diagnostic criteria for definite, probable or possible diagnosis (Table 1). Images were co-reviewed, and discrepancy was resolved by consensus.
Table 1.
Clinical and laboratory variables in TBM patients with cerebral infarcts (n = 52); and with and without cerebral infarcts (n = 90).
| Variable | TBM patients with cerebral infarcts (n = 52) | TBM patients with and without cerebral infarcts (n = 90) |
|---|---|---|
| Age range; mean, y | 10–82; 32.3 | 10–82; 32 |
| <40 years | 39 (75%) | 66 (73.3%) |
| ≥40 years | 13 (25%) | 24 (27.7%) |
| Sex, Male:Female | 26:26 | 42:48 |
| Duration of symptoms mean (range), d | 66 (3–240) | 63.7 (2–240) |
| Clinical symptoms, n (%) | ||
| Fever | 52 (100%) | 90 (100%) |
| Headache | 46 (88.4%) | 76 (84.4%) |
| Vomiting | 33 (63.4%) | 46 (51.1%) |
| Seizures | 10 (19.2%) | 15 (16.6%) |
| Altered sensorium | 33 (63.4%) | 48 (53.3%) |
| Hemi/paraparesis/focal weakness | 12 (23%) | 20 (22.2%) |
| CSF parameters | (n = 48) | (n = 75) |
| Cell count mm3, mean (range) | 120 (20–635) | 138 (6–733) |
| Protein mg/dl | 133 (44–495) | 133 (44–495) |
| Glucose mmol/l | 1.54 (0.1–7.0) | 1.54 (0.1–7.0) |
| Mycobacterial culture positive, n (%), | 14/41 (34%) | 18/62 (29%) |
| Tuberculous polymerase chain reaction, n (%) | 5/41 (12%) | 9/62 (14.5%) |
| Other tests for fungal and cryptococcal antigen | 0/28 | 0/40 |
| Diagnostic category | ||
| Definite | 12 (23%) | 21 (23.3%) |
| Probable | 17 (32.7%) | 33 (36.7%) |
| Possible | 23 (44.2%) | 36 (40%) |
| TBM grade | ||
| I | 12 (23%) | 26 (28.8%) |
| II | 27 (52%) | 48 (53.3%) |
| III | 13 (25%) | 16 (17.7%) |
| Comorbidities DM and HT/ALD/SLE/HIV | 4/1/1/0 | 6/1/1/1 |
| Extraneural involvement tuberculosis | 12 (23%) (lung = 4, abdomen = 2, disseminated TB = 5, spine = 2) | 19 (21%) (lung = 9, abdomen = 2, disseminated TB = 5, spine = 3) |
| Outcome Good outcome (mRS 0–2) | 41/52 (78.8%) | 73/90 (81.1%) |
| Poor outcome (mRS 3–6) | 11/52 (21.1%) 4 died | 17/90 (18.8%) 4 died |
| Neural biopsy | 1 | 1 |
ALD: alcoholic liver disease; DM: diabetes mellitus; HIV: human immunodeficiency virus; HT: hypertension; mRS: modified Rankin scale; SLE: systemic lupus erythematosus; TBM: tuberculous meningitis; MRV-N: Magnetic Resonance Venography-Normal; MB= Midbrain.
Methods
MRI was performed on a 3Tesla (GE Healthcare Signa HDxt; General Electric, Milwaukee, WI, USA) system using a 16-channel head, neck and spine coil. Axial-T1 weighted image (T1WI) with and without gadolinium contrast enhancement (0.1 mmol/kg), T2WI, fluid-attenuated inversion recovery and DW-MR sequences were evaluated. Three-dimensional time of flight (3D-TOF) MRA was performed with the following parameters: –3D overlapping slabs with slice thickness of 1.6 mm with an overlap of –0.6 mm, repetition time (TR) = 18 msec, echo time (TE) = 2.4 msec, flip angle = 15 degrees, bandwidth = 31.2 kHz, field of view = 220 × 160 mm and matrix = 320 × 224. All MRA source images were postprocessed using the maximum intensity projection method and reviewed on a GE workstation. In a few cases, an available MR venography (MRV) sequence was also evaluated for venographic abnormalities such as thrombus and stenosis. Imaging findings for these 90 TBM patients were coanalyzed by two readers (SK and NS) with 25 and 10 years’ experience, respectively, in neuroimaging. The reviewers were blinded to each patient’s condition and disease stage. The final assessments and all abnormal imaging findings were recorded in consensus. Imaging findings were evaluated for the presence and number of tuberculomas (<5 and ≥5), leptomeningeal enhancement (LM), hydrocephalus (mild, moderate, severe and ventriculitis), infarct frequency and distribution (TB vs ischemic zone and vascular distribution) and angiographic abnormalities (frequency and pattern of vascular involvement). Demographic, clinical and laboratory findings are summarized in Table 1. TBM was categorized as “definite,” “probable” and “possible” based on the Thwaites criteria.18
Criteria for characterizing infarcts
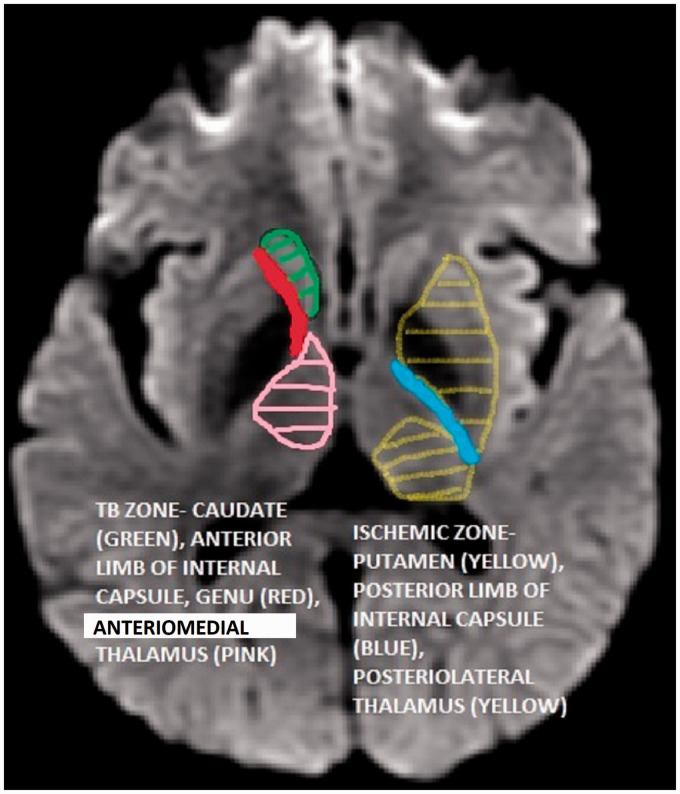
Cerebral infarcts were assessed by two methods as described by Hsieh and colleagues14 and Tai et al.5 Hsieh et al.14 based their classification on TB (caudate head, genu, anterior limb of the internal capsule and anteromedial thalamus) and ischemic (lentiform nucleus, posterolateral thalamus and posterior limb of internal capsule) zones (Figure 1). Tai and colleagues5 based their classification of vascular supply: MLAs, LLAs, CBs, basilar artery (BA) terminal penetrating arteries and PCA perforators.
Figure 1.
Tubercular (TB) zone and ischemic zone.
Criteria for characterizing MRA abnormalities
Cerebral vasculitis was diagnosed based on different patterns of MRA abnormality such as vasculitis single- or multiple-vessel involvement, short- or long-segment luminal stenosis, occlusion of a major vessel, and vasospasm (diffuse irregular calibers of intracranial arteries with or without reduction in distant branches). Stenosis was graded as mild: 50% or less; moderate: 50% or more to 69%; severe: 70% or more to 99%; and occlusion: greater than 99% narrowing of the vessel lumen.19
Criteria for other brain MRI findings
Hydrocephalus was categorized as mild, moderate and severe generalized or focal dilation of the ventricles. Ventriculitis included ependymal enhancement. The number ( < 5 and ≥ 5) and location of the tuberculomas were noted. Severity of meningitis was graded according to British Medical Research Council criteria: stage 1 for Glasgow Coma Scale (GCS) 15 with meningeal signs only, stage 2 for GCS 11–14 or 15 with focal neurological signs or altered sensorium, and stage 3 for either stupor, delirium, hemiplegia or paraplegia (GCS ≤ 10). 18 Neurological disabilities were measured and an evaluation of clinical outcome on discharge was conducted using the modified Rankin Scale (mRS) and classified as good (mRS score 0–2) or poor (mRS score 3–6).20 Cerebrospinal fluid (CSF) was analyzed for cell count, protein and sugar, and underwent smear and culture for acid-fast bacilli, other bacteria and fungus. Enzyme-linked immunosorbent assay for immunoglobulin M antibody and polymerase chain reaction (PCR) for Mycobacterium tuberculosis DNA were also performed.
Statistical analysis
Descriptive statistics were compiled. Continuous variables were expressed as means, and categorical variables were expressed as frequencies and percentages. Chi-square test (or Fisher exact test) was performed to analyze the association of cerebral infarcts with clinical parameters (age, comorbidities and functional outcomes) and imaging parameters (LM, hydrocephalus and vasculitis). The Statistical Package for Social Sciences, SPSS (SPSS Inc, Chicago, IL, USA) was used, and p less than .05 was considered significant.
Results
Clinical parameters
Ninety TBM patients (mean age 32 years; range, 10–82 years) were included. The baseline clinical, demographic and laboratory characteristics of the 90 TBM patients are summarized in Table 1. CSF analysis results were available in 75 patients with predominantly lymphocytic pleocytosis, increased protein and decreased glucose. Out of 62 tested for mycobacterial culture and CSF-PCR, only 18 had a positive culture. CSF test results were negative for fungal and cryptococcal antigen in 40 patients. One patient had a surgical neural tissue biopsy.
Radiological features
MRIs were abnormal in all patients, including most common tuberculomas (100%), LMs (84.4%), cerebral infarcts (57.7%) and hydrocephalus (29%) (Table 2). With regard to CVCs, infarcts were seen in 52 (acute 47, subacute 3, chronic 2), and TBM patients had equal sex distribution and were mostly younger than 40 years. The baseline clinical, demographic and laboratory characteristics of the 52 TBM patients with infarct are summarized in Table 1. Forty patients (44.4% of all TBM patients, 77% of patients with infarcts) had multiple infarcts, and the majority were in the supratentorial region (basal ganglia and thalamus (63%), brainstem (28.4%) and cerebellum (7.7%)). Radiological findings of all TBM patients with infarcts are summarized in Tables 2–4. According to the Hsieh classification,14 35% had infarcts in the ischemic zone (Figures 2 and 3), 13% in the TB zone (Figure 4) and 15% in both zones (Figure 5). According to vascular supply, 50% of the infarcts were in the area supplied by perforators of the PCA and BA, 50% of the infarcts were in territory supplied by the LLAs, and 23% of the infarcts were in the territory supplied by MLAs. Brainstem and cerebellar infracts were seen in 15 and four patients, respectively (Figure 7). Infarction of cortical areas was less common (15.3%), and all presented in the MCA or PCA territories (Figures 2, 3 and 7). One patient had an infarct in the internal border zone of the MCA, and two patients had infarcts due to total occlusion of major intracranial arteries (Figure 6).
Table 3.
Radiological findings in TBM patients with cerebral infarcts (n = 52).
| Features | n (%) |
|---|---|
| Abnormal MRI | 52 (57.7%) |
| Single | 12 (23%) |
| Multiple | 40 (77%) |
| Bilateral infarcts | 5 (9.6%) |
| Acute | 47 |
| Subacute | 3 |
| Chronic | 2 |
| Location of infarct | |
| Cortical | 8 (15.4%) |
| Basal ganglia and thalamus | 33 (63%) |
| Brainstem | 15 (28.4%) |
| Cerebellum | 4 (7.7%) |
MRI: magnetic resonance imaging; TBM: tuberculous meningitis.
Table 2.
Radiological findings in TBM patients with cerebral infarcts (n = 52) and with and without cerebral infarcts (n = 90).
| Features | With cerebral infarcts (n = 52) | With and without cerebral infarcts (n = 90) |
|---|---|---|
| Tuberculomas | 52 (100%) | 90 (100%) |
| Numbers ≤5 and ≥5 | 15 (29%); 37 (71%) | 22 (24.5%); 68 (75.5%) |
| Leptomeningeal enhancement | 46 (88%) | 76 (84.4%) |
| Hydrocephalus Severity | 20 (38%) | 26 (29%) |
| Mild | 4 (20%) | 5 (19.2) |
| Moderate | 12 (60%) | 14 (53.8%) |
| Severe | 4 (20%) | 7 (27%) |
| Ventriculitis | 4 (20%) | 5 (19.2%) |
| MRA available | 39/52 (75%) | 67/90 (74.4%) |
| Abnormal MRA | 26/39 (66.6%) | 29/67 (43.2%) |
| MRV | 7/52 | 10/90 |
MRA: magnetic resonance angiography; MRV: magnetic resonance venography; TBM: tuberculous meningitis.
Table 4.
Radiological findings in TBM patients with cerebral infarcts (n = 52).
| Patients, n = 52 | Left (n, %) | Right (n, %) | Both (n, %) | |
|---|---|---|---|---|
| Cerebral infarcts (n, % out of all TBM patients) | (52, 57.7%) | |||
| Thalamus | 17 | 5 | 8 | 4 |
| Anteromedial thalamus | 5 | 2 | 2 | 1 |
| Posterolateral thalamus | 4 | 2 | 1 | 1 |
| Whole thalamus | 8 | 1 | 5 | 2 |
| Basal ganglia | 20 | |||
| Basal ganglia | 9 | 2 | 7 | |
| Globus pallidus | 6 | 2 | 3 | 1 |
| Putamen | 5 | 5 | ||
| Caudate | 12 | 4 | 8 | 0 |
| Head | 5 | 2 | 3 | |
| Head and body of caudate | 7 | 2 | 5 | |
| Temporal | 4 | 3 | 1 | |
| Parietal | 2 | 1 | 1 | |
| Frontal | 11 | 4 | 6 | 1 |
| Occipital | 3 | 2 | 1 | |
| Opercular region | 7 | 2 | 5 | |
| Corona radiata | 10 | 4 | 6 | |
| Corpus callosum (body/genu/splenium) | 5 (1/1/3) | |||
| Internal capsule | 21 | 8 | 13 | |
| Anterior limb | 6 | 3 | 3 | |
| Genu | 3 | 2 | 1 | |
| Posterior limb | 12 | 3 | 9 | |
| Midbrain | 6 | |||
| Pons | 8 | |||
| Medulla | 1 | |||
| Cerebellar vermis | 1 | |||
| Cerebellar hemisphere | 3 | 2 | 1 | |
| Multiple infarcts | 40 (44.4% of all TBM patients, 77% of patients with infarcts) | |||
| Classification of cerebral infarction according to vascular supply (n, % out of all 52 patients with infarcts) | ||||
| Medial lenticulostriate arteries | 12 (23%) | |||
| Lateral lenticulostriate arteries | 26 (50%) | |||
| Perforators from posterior cerebral artery and basilar artery | 26 (50%) | |||
| Combined cortical and perforators branches | 22 (42.3%) | |||
| Only cortical branches | 8 (15.4%) | |||
| Superior cerebellar artery | 1 (2%) | |||
| Posterior inferior cerebellar artery | 3 (6%) | |||
| Classification of cerebral infarction according to Hsieh classification (n, % out of all 52 patients with infarcts) | ||||
| TB zone | 7 (13%) | |||
| Ischemic zone | 18 (35%) | |||
| Combined TB zone and ischemic zone | 8 (15%) | |||
| Vasculitis (n, % out of all 29 patients with abnormal MRA) | ||||
| Terminal internal carotid artery | 9 (31.0%) | 2 mild, 2 moderate, 1 severe | 1 moderate | 3 moderate |
| Middle cerebral artery | 22 (75.8%) | 3 mild, 2 moderate, 1 severe, 1 occlusion | 4 moderate 3 severe | 5 mild, 2 moderate, 1 occlusion |
| Anterior cerebral artery | 11 (37.9%) | 2 moderate, 1 severe | 4 moderate, 2 severe | 1 mild, 1 moderate |
| Posterior cerebral artery | 6 (20.7%) | 1 severe | 1 moderate | 3 moderate, 1 severe |
| Basilar artery | 3 (10.3%) (1 mild, 2 severe) | |||
| Diffuse vasospasm with generalized involvement, distal paucity of vessels and beaded appearance | 6 (20.7%) |
MRA: magnetic resonance angiography; TB: tubercular; TBM: tuberculous meningitis.
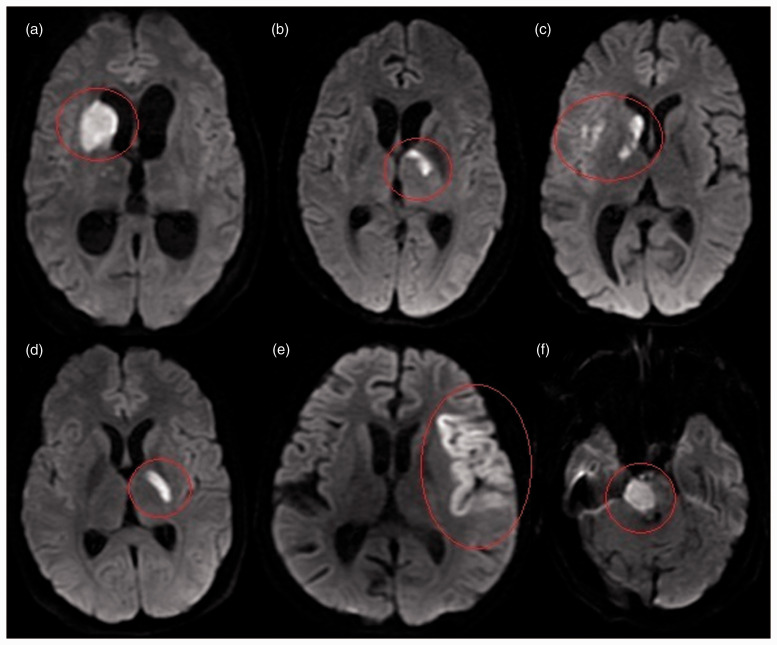
Figure 2.
Diffusion-weighted images of different patients showing distribution of cerebral infarcts. (a–c) Tubercular zone: (a) right caudate, anterior globus pallidus and anterior limb of internal capsule; (b) left anteromedial thalamus; (c) caudate, genu and right middle cerebral artery (MCA) cortical branches. (d) Ischemic zone: posterior part of globus pallidus and posterior limb of internal capsule. (e) Cortical: left MCA cortical branches. (f) Posterior circulation: right hemipons.
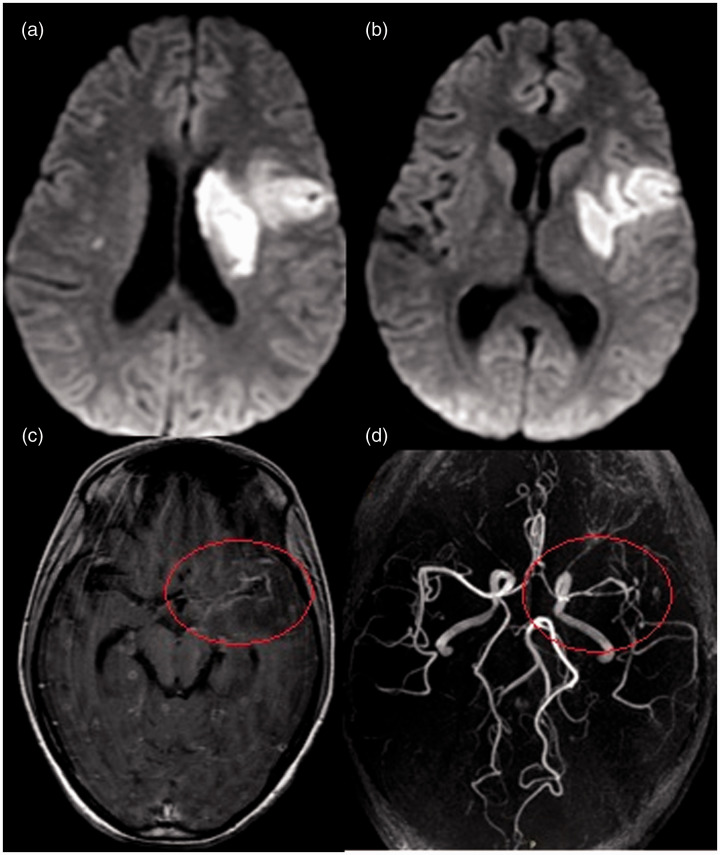
Figure 3.
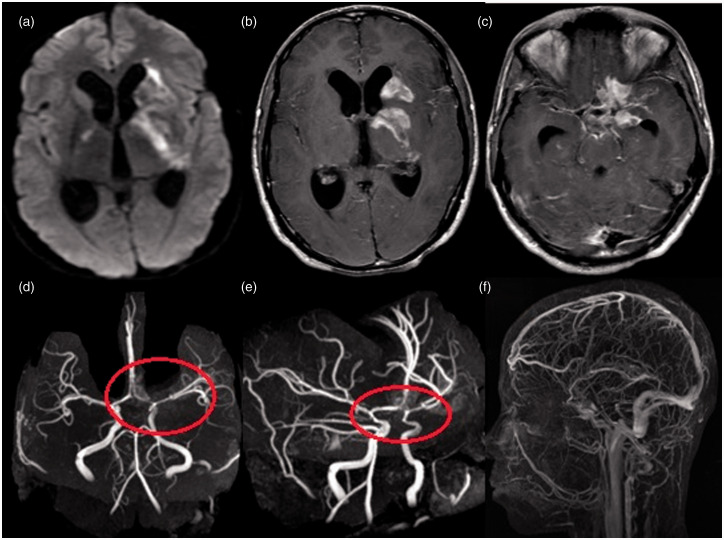
Brain magnetic resonance imaging of a 28-year-old woman with stage 2 tubercular meningitis and vasculitis. (a and b) Diffusion-weighted images show acute infarct (both perforators and cortical branches) in the left middle cerebral artery (MCA) territory involving the centrum semiovale, putamen (ischemic zone) and frontotemporal region. (c) Axial T1 postcontrast image shows basal leptomeningeal enhancement predominantly in the left MCA region (circle) and multiple enhancing cerebral tuberculomas. (d) Three-dimensional time-of-flight noncontrast angiogram shows luminal narrowing of the left MCA with attenuated distal cortical branches and luminal narrowing of the anterior cerebral artery-A1 segment (circle).
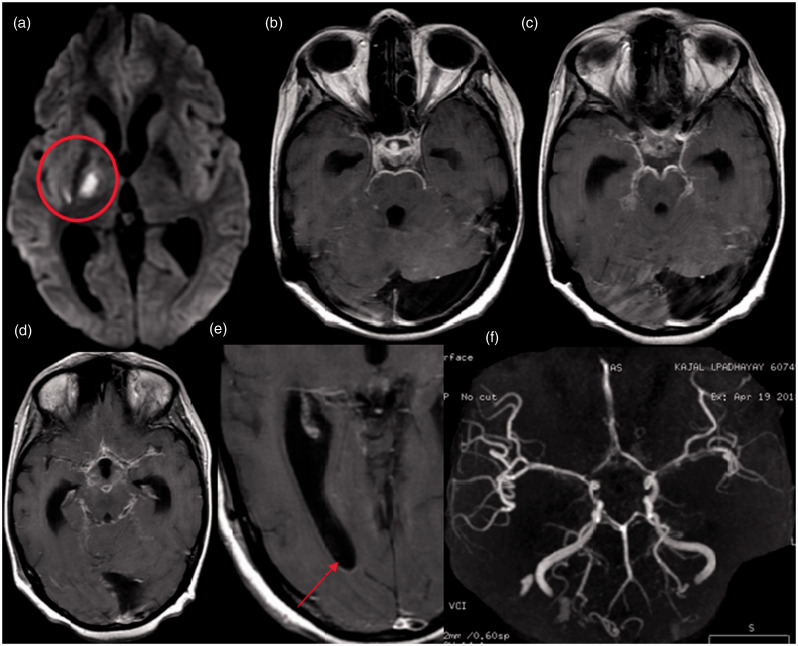
Figure 4.
Brain magnetic resonance imaging of a 36-year-old man with stage 3 tubercular meningitis, ventriculitis and diffuse vasospasm. (a) Diffusion-weighted image shows acute infarct in the right anterior thalamus (tubercular zone). (b–d) Postcontrast images show typical thick leptomeningeal enhancement involving all the basal cisterns and along the bilateral sylvian fissures. (e) Postcontrast image shows hydrocephalus with ventriculitis (ependymal enhancement) and multiple tuberculomas. (f and g) Three-dimensional time-of-flight noncontrast angiogram shows diffuse vasospasms involving the basilar artery, bilateral P1 segments, bilateral middle cerebral artery, bilateral anterior cerebral artery and bilateral distal internal carotid artery.
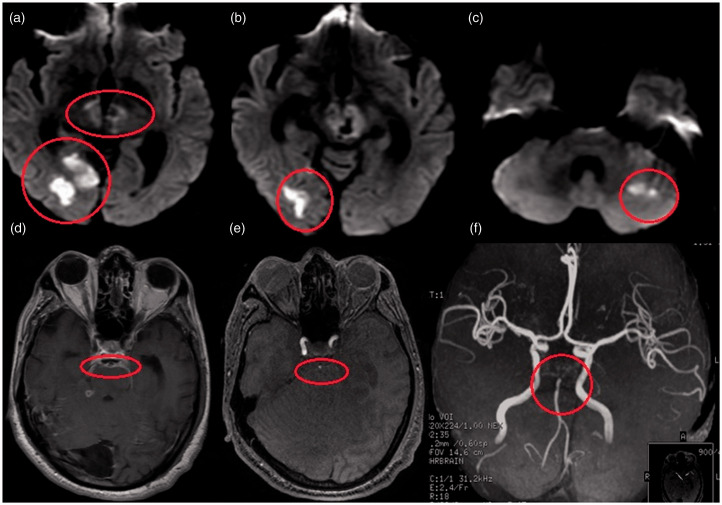
Figure 5.
A 40-year-old man with tubercular meningitis, hydrocephalus and vasculitis. (a) Diffusion-weighted image shows subacute infarct involving both tubercular zone (left caudate) and ischemic zone (left lentiform, posterior limb of internal capsule). (b and c) Axial T1 postcontrast images show enhancement in the areas of infarct (subacute infarct) and basal leptomeningeal enhancement with hydrocephalus. (d and e) Three-dimensional time-of-flight (TOF) noncontrast angiogram maximum intensity projection (MIP) images reveal narrowing of the supraclinoid bilateral internal carotid artery, bilateral middle cerebral artery-M1 segment (left > right), and left anterior cerebral artery-A1 vessels. (e) Three-dimensional TOF contrast venogram MIP image reveals normal major venous sinuses, superficial as well as deep cortical veins, with no evidence of venous thrombosis.
Figure 7.
Brain magnetic resonance imaging of a 31-year-old man with stage 2 tubercular meningitis and vasculitis. (a–c) Diffusion-weighted images show acute infarct in the posterior circulation involving the right occipital, bilateral thalami, midbrain and left cerebellar hemisphere. (d) T1 postcontrast image shows thick basal leptomeningeal enhancement in the pre- and peripontine cisterns encasing the basilar artery (BA) (circle). (e and f) Three-dimensional time-of-flight noncontrast angiogram shows luminal narrowing of the BA and nonvisualization of the bilateral P1 and P2 segments on (f) maximum intensity projection image.
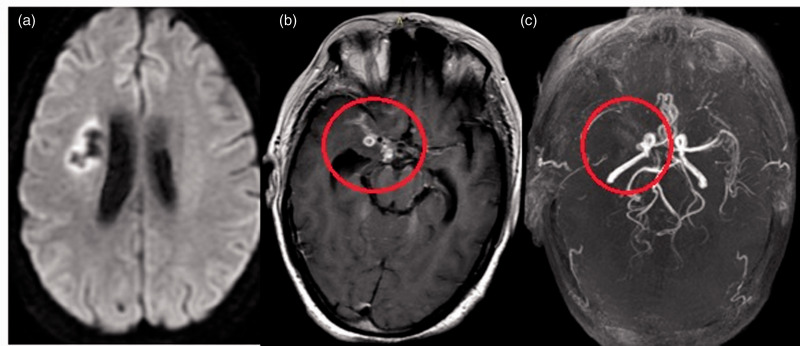
Figure 6.
Brain magnetic resonance imaging of a 35-year-old woman with a history of tubercular meningitis and vasculitis. (a) Diffusion-weighted image shows chronic infarct in the right centrum semiovale. (b) Axial T1 postcontrast image shows basal leptomeningeal enhancement and enhancing tuberculomas concentrated around the left middle cerebral artery (MCA) (circle). (c) Three-dimensional time-of-flight magnetic resonance angiogram shows typical narrowing involving the right supraclinoid internal carotid artery and total occlusion of the right MCA with nonvisualization of distal cortical branches (circle); normal-caliber posterior fossa vessels.
MRA was available for 74.4% patients and were found to be abnormal in 43.2% (29/67) patients. In 52 patients with infarct, MRA was available in 39 (75%) and was abnormal in 26 (66.6%) patients. The most common vascular involvement was the MCA (75.8%) followed by the ACA (37.9%), ICA (31%), PCA (20.7%) and BA (11.5%). Diffuse vasospasm of the circle of Willis with a distal paucity of vessels was observed in 20.7% patients who had thick basal meningeal enhancement and exudates encircling the major arteries. Thirteen patients with infarctions had no MRA abnormalities. Three patients had abnormal MRA without infarcts. All 10 MRVs were normal. Overall, clinical outcome was good (78.8%) in TBM patients with infarcts. We compared the clinical and imaging characteristics of 52 patients with infarcts with 38 TBM patients without infarcts. Table 5 shows an association of cerebral infarcts with age, hydrocephalus, vasculitis, LM and outcome. Cerebral infarction showed a statistically significant association with hydrocephalus (p = .019) and vasculitis (p = .001).
Table 5.
Association of cerebral infarcts with clinical and imaging parameters.
| TBM patients |
|||
|---|---|---|---|
| Variable | With cerebral infarcts (n = 52) | Without cerebral infarct s(n = 38) | p value |
| Age, y mean ± SD (range) | 32.28 ± 16.30 (10–82) | 30.62 ± 14.70 (15–56) | .620 |
| Comorbidities | 6 | 3 | .728 |
| Leptomeningeal enhancement | 46 (88%) | 30 (79%) | .219 |
| Hydrocephalus | 20 (38%) | 6 (15.7%) | .019 |
| Vasculitis | 26/39 (66.6%) | 3 (7.8%) | <.001 |
| Functional outcome | |||
| Good outcome (mRS 0–2) | 41 (78.8%) | 32 (84.8%) | .521 |
| Poor outcome (mRS 3–6) | 11 (21.2%) | 6 (15.2%) | |
RS: modified Rankin Scale; TBM: tuberculous meningitis.
P less than .05 is significant, as indicated in bold.
Discussion
We retrospectively analyzed the clinical and imaging manifestations of 90 TBM patients with emphasis on CVCs. Cerebral infarction was present in 57.7% and abnormal MRA in 43.2% of all TBM patients, consistent with earlier reports (Table 6). The clinical presentations were in concordance with the available literature.2–5,8,11–13,16,21,22 The majority of the patients (53.3%) in the current study experienced altered sensorium, and 22% presented with motor weakness. Even though several studies have assessed the imaging findings in TBM patients, only a few studies have addressed the CVCs with a dedicated imaging review. Table 6 lists the various imaging manifestations reported in TBM in prior studies as well as our current cohort experience. With this large retrospective review, the authors attempt to further refine and expand on the frequency and distribution of cerebral stroke and the pattern of vascular involvement. Tuberculomas were the most common imaging abnormality (100%) identified and the majority (75.5%) were multiple, located at the gray-white matter junction in the supratentorial region. In previously reported studies, these have been variably reported in 15% to 70% of TBM patients.2,3,8,11,13,15,16
Table 6.
Prevalence of imaging findings in TBM patients with infarcts, as reported in the available literature, compared with the current study. Numbers in parentheses indicate percentage (%).
| First author, yearref | N | MA, y (range) | MRA (%) | Abnormal MRA (%) | T % | LM% | HC% | IN (%) | Multiple infarcts % | Infarcts distribution (%) | MCA/ACA/ PCA/ BA (%) | TBM stage 1/2/3 (%) | Outcome good/ poor (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wasay, 20182 | 559 (CT + MRI) | 41.9 | 67 | NA | 57.6 | 42.4 | 48.6 | 144 (26) | 52.8 | BG and TH 21% BS and CB 20% CO 13% | NA | 35/60/49 | |
| Kalita, 20183 | 26 | 23 (11–75) | 25 | 11 (42.3) | 69.2 | 61.5 | 57.7 | 13 (50) | TBZ 25; ISZ 1; CB 2; MB 1; C 1 | MCA 11; PCA 3; BA 2; ACA 2; VA 1 MRV normal | 7/12/7 | 18 (69)/11(31) | |
| Tai, 20174 | 54 | 35 | 43 (CT + MR) | 21 (49) Vasculitis 37% Vasospasm 11% | 53.7 | 72.2 | 70.4 | 34 (63) | GP 26%, TH 24, C 25.5%, P 22.2% | 18 (39)/33 (61) | |||
| Tai, 20165 | 51 | 35 | 51 | Vasculitis 15 (37) Vasospasm 6 (15) | 71 | 34 (67) | 41 | TBZ 2 (6); ISZ (35); both 20 (59); TH 13 (26); BG 25 (49); CB 2 | 20 (39)/4 (8)/9 (18) | 15 (30)/36 (70) | |||
| Rohlwink, 20166 | CT 44 (MRI in 39) | 3.3 median (0.3–13) | 29 | 16 (55) | 59 | 100 | 61.5 | 25 (66) | MCA 23 (42); BG 20 (80) | MCA 94 | 32 (73)/12 (27) | ||
| Chatterjee, 20157 | 51 autopsies | 3 mo–72 y | Autopsy study | 25.5 | 99% | 37 (72.5) | 50% bilateral | BG 13 (25.5); TH 1 (2); CB 1 (2); MB 1 (2); pons 4 (8)/C 13 (25.5) | BA 43%; MCA 47% Smaller arteries of BA (100%) and MCA (96%) | ||||
| Wasay, 20148 | 404 (CT 153,MRI 313) | 43 | ND | ND | 50 | 32 | 26 | 77 (25) | 58 | – | ND | ||
| Pasticci, 20139 | 30 | 26 | 6 (23) | BG 4 (67) | MCA 2; PCA 1; ICA 1 | 20/1/9 | |||||||
| Singh, 201210 | CT 47 | Median 28 (12–65) | CTA 47 | CTA 33 (70.2) | 17 | 96 | 76.6 | 19 (40.5) | 8.5 | BG + IC 25.5 | MCA 13; ACA 23; ICA 17; PCA 8; BA 2 | 12 (25)/13 (27)/22 (47) | 35 (74)/12 (26) |
| Sheu, 201211 | 91 (CT + MRI) | 53.2 | 15 | 62 | 46 | 30 (33) | 65 | ||||||
| Kalita, 201213 | 67 | Median 34 | 67 | 50.7 | 49.2 | 36 | 34 | 59.7 | 52.5 | BG 21 (52); TH 15 (37.5); BS 12 (30); CB 8 (20); C 18 (45) | MCA 17/ACA 5/PCA 14/ICA 8/BA 5/VA 1 | ||
| Soni, 2019 (present study) | 90 | 32 (10–82) | 67 (74.4) | 29 (43.2) | 100 | 84.4 | 29 | 52 (57.7) | 77 | ISZ 18 (35); TBZ 7 (13); both 8 (15); BG and TH 33 (63); BS 15 (28.4); C 8 (15.4); CB 4 (7.7) | MCA 22 (76); ACA 11 (38); ICA 9 (31); PCA 6 (20.7); BA 3 (10.3) MRV-N | 12 (23)/27 (52)/13 (25) |
ACA: anterior cerebral artery; BA: basilar artery; BG: basal ganglia; BS: brainstem; C: caudate; CB: cerebellum; CO: cortical; CT: computed tomography; CTA: computed tomography angiography; GP: globus pallidus; HC: hydrocephalus; ICA: internal carotid artery; IN: frequency of infarct – total No. of infarct (%); ISZ: ischemic zone; LM: leptomeningeal enhancement; N: number of participants; NS: not specified; MA: mean age; MCA: middle cerebral artery; MRA: magnetic resonance angiography; MRI: magnetic resonance imaging; MRV: magnetic resonance venography; ND: not determined; P: putamen; PCA: posterior cerebral artery; T: tuberculomas; TBZ: tubercular zone; TH: thalamus; VA: vertebral artery.
Various studies have reported a wide range of leptomeningeal involvement ranging between 32% and 100% (Table 5).6,7,23 In our study population, it was the second most common imaging manifestation and was noted predominantly at the interpeduncular fossa, sylvian fissure, prepontine and quadrigeminal cisterns. Prior studies have reported variable correlations between leptomeningeal involvement and increased CVCs. However, we found no statistically significant associations between the two. Hydrocephalus (29%) was communicating in all patients and moderate in severity (54%). Five patients had ventriculitis, likely secondary to ependymal inflammation. No differences were found regarding CSF cell count, sugar, protein, microscopy or growth of M. tuberculosis among patients with or without stroke. Clinical outcome at discharge was good (78.8%) in patients with stroke, similar to previously reported studies (Table 6).
TBM is one of the most severe forms of extrapulmonary tuberculosis, and cerebral infarctions complicate a variable proportion of TBM cases, especially in the advanced stage. Stroke has been reported in 15% to 67% of TBM patients and is associated with poor outcome.5,15 In our study, we noted cerebral infarcts in 57.7% of TBM patients. There were a greater number of reported infarct cases compared with previous studies; however, less than reported by Tai et al.5 The most common sites of infarcts were in the basal ganglia and thalamus (63%), slightly higher compared with prior studies.5,5,15,16,24,25 We noted infarcts mostly in the ischemic zone; however, previous studies have noted a predilection for the TB zone.3,13,14,16 The higher number of infarcts in our cohort may be due to the use of DWI with MRA and our dedicated imaging review, especially evaluating for CVCs. Most of the infarcts were multiple (77%), and a few showed bilateral basal ganglia and thalamic involvement, representing imaging characteristics of TBM stroke compared with atherosclerosis.5,15 All the patients showed involvement of perforators and the terminal CBs, which equally involved the perforators from the LLA and PCA/BA, followed by the MLAs.
The high prevalence of strokes in our study is concordant with the reported literature, including one autopsy study.3–5,7,16 Chatterjee et al.7 reported infarcts in 72.5% of TBM cases in their autopsy study. The majority (53%) of infarcts were macroscopic in the MCA territory and microscopic infarcts in the BA distribution, with an overall equal number of infarctions in both territories.7 Microscopic infarction is thought to be responsible for increased mortality and morbidity. The imaging methods used for evaluation showed a variable sensitivity for the detection of small brainstem infarctions, explaining the different frequency in the literature. MRI showed high sensitivity in detecting small brainstem infarcts and revealed that 40% to 46% of patients with TBM may have brainstem infarcts.26,27 Incidence of brainstem infarctions in our study is concordant with Tai and colleagues.5
Vasculitis is an important feature of TBM and can involve large, medium and small arteries as well as veins. Most of the earlier TBM studies have reported vascular abnormalities on conventional angiography.15 Lehrer demonstrated the characteristic “angiographic triad” of narrowing of the supraclinoid ICA, a widely sweeping pericallosal artery, and delayed circulation in the MCA with early draining veins.28 A moyamoya-like pattern of angiographic changes has also been described in the literature.29,30 Chatterjee et al.7 reported that the type of vascular lesions varies according to vessel size and course of disease. Proliferative lesions follow a chronic course and involve larger arteries, which results in hemiplegia or quadriplegia. Necrotizing lesions occur in the early stage and affect smaller, mostly medial arteries in the circle of Willis, resulting in monoplegia. Infiltrative lesions are the most common and affect arteries of all sizes.7,10 Even though the exact etiology remains unclear, there have been a few hypothesized mechanisms that increase the risk of stroke in TBM. The predominant cause is direct vessel wall invasion, including other contributory factors such as an immunologic reaction against vessel walls and an inflammatory response promoting atherosclerosis. Vasculitis is believed to be the result of the spread of inflammation from the meninges to the arteries. Thick inflammatory basal exudates encircle the circle of Willis and result in infiltrative, proliferative and necrotizing vascular pathologies. Vasospasm may cause strokes early in the course of the disease, whereas proliferative intimal disease contributes to late strokes. Systemic TB can mount an immunological reaction against the vessel wall due to the molecular mimicry between TB bacilli and vessel-wall antigens. It is also presumed that TB may act as a risk factor promoting atherogenesis. Studies have both argued and refuted this hypothesis, and therefore the level of evidence is low.12,31 Very little is known about venous involvement; a few factors such as stasis, endothelial inflammation and hypercoagulable state are considered to contribute to venous sinus thrombosis.32–34
MRA abnormality presented in 43.2% of all TBM cases, which differs from prior studies, probably related to the imaging techniques used: DSA, CTA and MRA.3,5,6,13,15 The MCA (84.4%) was the most common artery involved, followed by the ACA (42.3%) and ICA (34.6%), consistent with previous studies (Table 6). The most common pattern of involvement was short-segmental stenosis of the proximal vessel at the base of the brain. Six patients showed diffuse, irregular-caliber intracranial vessels with a paucity of distal vessels. The pattern of involvement was consistent with prior studies and may be explained by the common location of the LM and basal exudates encircling the vessels at the base of the brain (Table 6). Lu et al.1 described two patterns of intracranial vascular involvement on MRA. The first is disseminated, irregular-caliber intracranial arteries with or without a paucity of distant branches, and the second is localized stenosis at the base of the brain. The first pattern relates to hydrocephalus, resulting in decreased blood circulation due to raised intracranial pressure or stretching of the intracranial vessels by enlarging ventricles. The second pattern shows a statistically significant correlation with TBM stage, duration of disease, basal exudates and cerebral infarcts.1
We reported poor outcome in only 21% of TBM patients with infarcts, which is less compared with other studies (Table 5). We found no correlation between TBM-related strokes and LM, age or outcomes, which is consistent with another recent study.2 TBM patients with infarcts showed worse outcome independent of TBM grade. Many studies have found various infarct predictors (age, diabetes mellitus, hypertension and dyslipidemia) in TBM patients and have shown a more complex mechanism for development of cerebral infarcts.2,35–37 Antituberculous treatment appears to be relatively ineffective in preventing CVCs. The role of corticosteroids in reducing mortality is well established now; however, its role as a preventive therapy is still being investigated. It is important to know the exact etiology of an infarction in TBM because antiplatelets may be effective for an arterial infarct, whereas an anticoagulant may be effective for a venous infarct.3,15 Our study results show an increased prevalence of stroke in TBM patients with predominant involvement of the perforators. DWI and MRA play a crucial role in early detection and timely management of CVCs.
Limitations
There are a few limitations of this study such as its retrospective design, observations or selection bias because the data were gathered from a tertiary care hospital, the absence of any control group, no matching CT evaluation and inclusion of descriptive statistics. The number of definite TBM cases was less than in a few of the previous studies. Clinical and laboratory details were not available for all patients, which may limit the extrapolation of our results to other populations. Similarly, MRA was not available for all patients, which might have introduced referral bias. Follow-up imaging studies were not available for many patients. Despite these limitations, our study results support recently published study findings and strengthen the fact that cerebral infarcts in TBM patients involve the perforators and CBs rather than the TB vs ischemic zone.5 Diffusion with MRA has proven to be beneficial for early diagnosis and timely management of TBM-related CVCs. High-resolution vessel-wall imaging (HR-VWI) can identify early vessel-wall changes that have not yet caused changes in the vessel lumen and that might be underestimated by routine CTA and MRA. Therefore, early recognition of vessel-wall changes by HR-VWI will greatly improve management in TBM patients compared with routine MRA. A recent study by Lu and colleagues38 retrospectively evaluated MRA and HR-VWI in 27 TBM patients and concluded that cerebral artery involvement in TBM patients is much more common and extensive than reported in the literature. Future prospective studies may be conducted using DWI, MRA and HR-VWI in all patients suspected to have TBM. This may be extremely useful in understanding the pathophysiology and consequences of TBM patients.
Conclusions
In conclusion, cerebral infarcts and MRA abnormalities are common in TBM patients. Stroke in TBM patients may be clinically silent and difficult to differentiate clinically from preexisting weakness due to tuberculomas or meningitis; this may delay diagnosis and result in a poorer prognosis. Different imaging modalities can affect the sensitivity of diagnosing infarcts, particularly in the brainstem region, and CT is not the reliable imaging modality of choice for detection and determination of the age of the infarct. MRI with angiography has proven to be beneficial for early diagnosis and timely management of TBM-related CVCs and could help to reduce clinical stroke and yield better outcomes in TBM patients. Patients should be managed aggressively. We noted the cerebral infarcts to be present mostly in the ischemic zone, predominantly in distribution of the small perforator of LLAs and PCAs. In line with previous studies, these findings are helpful in the early diagnosis of CVCs and appropriate management to reduce adverse outcomes.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lu TT, Lin XQ, Zhang L, et al. Magnetic resonance angiography manifestations and prognostic significance in HIV-negative tuberculosis meningitis. Int J Tuberc Lung Dis 2015; 19: 1448–1454. [DOI] [PubMed] [Google Scholar]
- 2.Wasay M, Khan M, Farooq S, et al. Frequency and impact of cerebral infarctions in patients with tuberculous meningitis. Stroke 2018; 49: 2288–2293. [DOI] [PubMed] [Google Scholar]
- 3.Kalita J, Singh RK, Misra UK, et al. Evaluation of cerebral arterial and venous system in tuberculous meningitis. J Neuroradiol 2018; 45: 130–135. [DOI] [PubMed] [Google Scholar]
- 4.Tai MLS, Mohd Nor H, Rahmat K, et al. Neuroimaging findings are sensitive and specific in diagnosis of tuberculous meningitis. Neurology Asia 2017; 22: 15–23. [Google Scholar]
- 5.Tai MS, Viswanathan S, Rahmat K, et al. Cerebral infarction pattern in tuberculous meningitis. Sci Rep 2016; 6: 38802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohlwink UK, Kilborn T, Wieselthaler N, et al. Imaging features of the brain, cerebral vessels and spine in pediatric tuberculous meningitis with associated hydrocephalus. Pediatr Infect Dis J 2016; 35: 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee D, Radotra BD, Vasishta RK, et al. Vascular complications of tuberculous meningitis: An autopsy study. Neurol India 2015; 63: 926–932. [DOI] [PubMed] [Google Scholar]
- 8.Wasay M, Farooq S, Khowaja ZA, et al. Cerebral infarction and tuberculoma in central nervous system tuberculosis: Frequency and prognostic implications. J Neurol Neurosurg Psychiatry 2014; 85: 1260–1264. [DOI] [PubMed] [Google Scholar]
- 9.Pasticci MB, Paciaroni M, Floridi P, et al. Stroke in patients with tuberculous meningitis in a low TB endemic country: An increasing medical emergency? New Microbiol 2013; 36: 193–198. [PubMed] [Google Scholar]
- 10.Singh B, Garg RK, Singh MK, et al. Computed tomography angiography in patients with tuberculous meningitis. J Infect 2012; 64: 565–572. [DOI] [PubMed] [Google Scholar]
- 11.Sheu JJ, Hsu CY, Yuan RY, et al. Clinical characteristics and treatment delay of cerebral infarction in tuberculous meningitis. Intern Med J 2012; 42: 294–300. [DOI] [PubMed] [Google Scholar]
- 12.Sheu JJ, Chiou HY, Kang JH, et al. Tuberculosis and the risk of ischemic stroke: A 3-year follow-up study. Stroke 2010; 41: 244–249. [DOI] [PubMed] [Google Scholar]
- 13.Kalita J, Prasad S, Maurya PK, et al. MR angiography in tuberculous meningitis. Acta Radiol 2012; 53: 324–329. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh FY, Chia LG, Shen WC. Locations of cerebral infarctions in tuberculous meningitis. Neuroradiology 1992; 34: 197–199. [DOI] [PubMed] [Google Scholar]
- 15.Misra UK, Kalita J, Maurya PK. Stroke in tuberculous meningitis. J Neurol Sci 2011; 303: 22–30. [DOI] [PubMed] [Google Scholar]
- 16.Nair PP, Kalita J, Kumar S, et al. MRI pattern of infarcts in basal ganglia region in patients with tuberculous meningitis. Neuroradiology 2009; 51: 221–225. [DOI] [PubMed] [Google Scholar]
- 17.Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect Dis 2010; 10: 803–812. [DOI] [PubMed] [Google Scholar]
- 18.Thwaites GE, Nguyen DB, Nguyen HD, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004; 351: 1741–1751. [DOI] [PubMed] [Google Scholar]
- 19.Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000; 21: 643–646. [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: Use of a structured interview to assign grades on the modified Rankin Scale. Stroke 2002; 33: 2243–2246. [DOI] [PubMed] [Google Scholar]
- 21.Tai ML, Nor HM, Kadir KA, et al. Paradoxical manifestation is common in HIV-negative tuberculous meningitis. Medicine (Baltimore) 2016; 95: e1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modi M, Sharma K, Prabhakar S, et al. Clinical and radiological predictors of outcome in tubercular meningitis: A prospective study of 209 patients. Clin Neurol Neurosurg 2017; 161: 29–34. [DOI] [PubMed] [Google Scholar]
- 23.Singh P, Paliwal VK, Neyaz Z, et al. Clinical and magnetic resonance imaging characteristics of tubercular ventriculitis: An under-recognized complication of tubercular meningitis. J Neurol Sci 2014; 342: 137–140. [DOI] [PubMed] [Google Scholar]
- 24.Lan SH, Chang WN, Lu CH, et al. Cerebral infarction in chronic meningitis: A comparison of tuberculous meningitis and cryptococcal meningitis. QJM 2001; 94: 247–253. [DOI] [PubMed] [Google Scholar]
- 25.Chan KH, Cheung RT, Lee R, et al. Cerebral infarcts complicating tuberculous meningitis. Cerebrovasc Dis 2005; 19: 391–395. [DOI] [PubMed] [Google Scholar]
- 26.van der Merwe DJ, Andronikou S, Van Toorn R, et al. Brainstem ischemic lesions on MRI in children with tuberculous meningitis: With diffusion weighted confirmation. Childs Nerv Syst 2009; 25: 949–954. [DOI] [PubMed] [Google Scholar]
- 27.Pienaar M, Andronikou S, van Toorn R. MRI to demonstrate diagnostic features and complications of TBM not seen with CT. Childs Nerv Syst 2009; 25: 941–947. [DOI] [PubMed] [Google Scholar]
- 28.Lehrer H. The angiographic triad in tuberculous meningitis. A radiographic and clinicopathologic correlation. Radiology 1966; 87: 829–835. [DOI] [PubMed] [Google Scholar]
- 29.Kumar RM, Saini L, Kaushik JS, et al. A combination of moyamoya pattern and cerebral venous sinus thrombosis: A case of tubercular vasculopathy. J Trop Pediatr 2015; 61: 393–396. [DOI] [PubMed] [Google Scholar]
- 30.Mun HY, Nam TK, Choi HH, et al. Rupture of a middle meningeal artery pseudoaneurysm in moyamoya syndrome related with tuberculous meningitis. J Cerebrovasc Endovasc Neurosurg 2018; 20: 187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lammie GA, Hewlett RH, Schoeman JF, et al. Tuberculous cerebrovascular disease: A review. J infect 2009; 59: 156–166. [DOI] [PubMed] [Google Scholar]
- 32.Bansod A, Garg RK, Rizvi I, et al. Magnetic resonance venographic findings in patients with tuberculous meningitis: Predictors and outcome. Magn Reson Imaging 2018; 54: 8–14. [DOI] [PubMed] [Google Scholar]
- 33.Ramdasi R, Mahore A, Kawale J, et al. Cerebral venous thrombosis associated with tuberculous meningitis: A rare complication of a common disease. Acta Neurochir (Wien) 2015; 157: 1679–1680. [DOI] [PubMed] [Google Scholar]
- 34.Dhawan SR, Chatterjee D, Radotra BD, et al. A child with tuberculous meningitis complicated by cortical venous and cerebral sino-venous thrombosis. Indian J Pediatr 2019; 86: 371–378. [DOI] [PubMed] [Google Scholar]
- 35.Wasay M, Khatri IA, Kaul S. Stroke in South Asian countries. Nat Rev Neurol 2014; 10: 135–143. [DOI] [PubMed] [Google Scholar]
- 36.Kalita J, Misra UK, Nair PP. Predictors of stroke and its significance in the outcome of tuberculous meningitis. J Stroke Cerebrovasc Dis 2009; 18: 251–258. [DOI] [PubMed] [Google Scholar]
- 37.Chan KH, Cheung RT, Lee R, et al. Cerebral infarcts complicating tuberculous meningitis. Cerebrovasc Dis 2005; 19: 391–395. [DOI] [PubMed] [Google Scholar]
- 38.Lu T, Zou Y, Jiang T, et al. Intracranial artery injury in HIV-negative tuberculous meningitis: A high-resolution vessel wall imaging study. Clin Neuroradiol. Epub ahead of print 3 May 2019. DOI: 10.1007/s00062-019-00766-4. [DOI] [PubMed] [Google Scholar]