Abstract
Background
Diagnosing coeliac disease (CD) in patients on a gluten-free diet (GFD) is difficult. Ingesting gluten elevates circulating interleukin (IL)-2, IL-8 and IL-10 in CD patients on a GFD.
Objective
We tested whether cytokine release after gluten ingestion differentiates patients with CD from those with self-reported gluten sensitivity (SR-GS).
Methods
Australian patients with CD (n = 26) and SR-GS (n = 18) on a GFD consumed bread (estimated gluten 6 g). Serum at baseline and at 3 and 4 h was tested for IL-2, IL-8 and IL-10. Separately, Norwegian SR-GS patients (n = 49) had plasma cytokine assessment at baseline and at 2, 4 and 6 h after food bars containing gluten (5.7 g), fructan or placebo in a previous double-blind crossover study.
Results
Gluten significantly elevated serum IL-2, IL-8 and IL-10 at 3 and 4 h in patients with CD but not SR-GS. The highest median fold-change from baseline at 4 h was for IL-2 (8.06, IQR: 1.52–24.0; P < 0.0001, Wilcoxon test). The two SR-GS cohorts included only one (1.5%) confirmed IL-2 responder, and cytokine responses to fructan and placebo were no different to gluten. Overall, cytokine release after gluten was present in 22 (85%) CD participants, but 2 of the 4 non-responders remained clinically well after 1 y on an unrestricted diet. Hence, cytokine release occurred in 22 (92%) of 24 ‘verified’ CD participants.
Conclusions
Gluten challenge with high-sensitivity cytokine assessment differentiates CD from SR-GS in patients on a GFD and identifies patients likely to tolerate gluten reintroduction. Systemic cytokine release indicating early immune activation by gluten in CD individuals cannot be detected in SR-GS individuals.
Keywords: Coeliac disease, cytokines, diagnostics, gluten-free diet, interleukin-2, interleukin-8, interleukin-10, oral food challenge
Key summary
Current knowledge
Gluten challenge for weeks or months is necessary to diagnose coeliac disease in people following a gluten-free diet.
One-off gluten ingestion increases circulating cytokines, particularly interleukin (IL)-2, 8 and 10, in coeliac disease.
What is new?
Cytokine release after bolus gluten challenge differentiates coeliac disease from self-reported gluten sensitivity.
Absence of cytokine release after gluten predicted coeliac disease patients who could resume an unrestricted diet without relapse after 1 y.
Bolus gluten challenge with serum IL-2 at 3–4 h could replace extended gluten challenges for diagnosis of coeliac disease in patients already on a gluten-free diet.
Early gluten-mediated immune activation present in coeliac disease is absent in people who self-report gluten sensitivity.
Introduction
The gluten-free diet (GFD) imposes a high treatment burden and may be unnecessary for people with self-reported ‘gluten sensitivity’ (SR-GS) and for those diagnosed with coeliac disease (CD) on the basis of equivocal findings.1,2 Current diagnosis of CD based on duodenal histology and serology is only reliable when patients are consuming gluten, and for people who have adopted a GFD, a period of at least several weeks of dietary gluten reintroduction is recommended for accurate testing.3,4 As gluten challenge can induce unpleasant symptoms, often rapid in onset and severe, it is poorly tolerated and unacceptable for many patients.5,6 A simple, less intrusive test is needed for the substantial number of people on a GFD seeking a diagnosis of CD.
It is widely accepted that an acquired, adaptive immune response to gluten underlies CD, but many reports have also described separate, innate immune effects of gluten in vitro that could account for rapid onset digestive symptoms in patients with CD and SR-GS.7,8 Recently, we observed that CD patients on a GFD elevate circulating levels of interleukin(IL)-2, IL-8 and IL-10 at 2–6 h after gluten ingestion or injecting peptides that activate gluten-specific CD4+ T cells.9 We speculated that increases in blood levels of cytokines, for example IL-2, arising from activation of gluten-specific CD4+ T cells is specific for CD and absent in those without. Further, since IL-8 release has been utilised as a marker of innate immune activation by gluten in vitro,10–12 we hypothesised that IL-8 elevations may also be present in SR-GS patients on a GFD. The aim of this study was to measure early elevations of IL-2, IL-8 and IL-10 after gluten challenge to determine the clinical utility of this approach to distinguish patients on a GFD with CD from those with SR-GS.
Materials and methods
Participants
The Melbourne CD cohort consisted of patients diagnosed on the basis of small bowel villous atrophy with supportive clinical and/or laboratory findings,13 and who had human leukocyte antigen (HLA)-DQ genotypes consistent with CD (HLA-DQ2.5, DQ8 or DQ2.2).14 The SR-GS cohort consisted of subjects who self-reported a sensitivity to gluten and had CD excluded by genetics (absence of HLA-DQ2.5, DQ8 or DQ2.2) or by normal duodenal histology and/or CD-specific serology while consuming gluten. Participants were recruited via advertisements to members of Coeliac Australia and attendees at Gluten Free Expos in Melbourne, Australia. Participants were excluded if they used immunomodulatory medication or had a wheat allergy. Screening included HLA-DQA and HLA-DQB genotyping (Gen-Probe Inc., San Diego, USA; performed by Melbourne Pathology, Victoria, Australia) and assessment of serum transglutaminase 2 (TG2)-specific immunoglobulin (Ig)A and deamidated gliadin peptide (DGP)-specific IgG (QUANTA Lite R h-tTG IgA and QUANTA Lite Gliadin IgG II, Inova Diagnostics, San Diego, USA; performed by Dorevitch Pathology, Victoria, Australia).
The Oslo participants consisted of those from the study reported in detail by Skodje et al. that had stored plasma from the first day of week-long food challenges with food bars containing gluten or fructan and with a matched placebo.15 Participants were aged 18–80 y and had self-instituted and strictly adhered to a GFD for at least 6 mo for SR-GS. CD was excluded in participants by genetic testing or normal duodenal histology while a gluten-containing diet was consumed. Wheat allergy was excluded by negative wheat-specific IgE.
Food challenges
In Melbourne, the unmasked challenge consisted of two and a third slices (89 g) of bread (white block loaf, Baker's Delight, Australia) over 10 min, estimated to contain 6 g gluten according to the Osborne calculation (wheat flour protein content multiplied by 0.8).16 In Oslo, the challenge vehicle was a 50 g muesli bar that was gluten-free and low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) (placebo bar). The fructan muesli bar included 2.1 g short-chain fructans/fructo-oligosaccharides (Orafti; Oligofructose, Beneo, Belgium) and the gluten muesli bar contained 7.6 g vital wheat gluten (Manildra, Gladesville, Australia), providing 5.7 g gluten protein. The fructan and gluten bars could not be distinguished from the placebo bar.
Symptom assessments
In Melbourne, participants recorded symptoms according to each of 11 items for the Celiac Disease Patient-Reported Outcome (CeD PRO) measure.17 Symptoms have previously been reported in the Oslo study.15
Cytokine assessment
IL-2, IL-8 and IL-10 were assessed in sera from 30 min before and at 3 and 4 h after gluten challenge in Melbourne, and in plasma from before and at 2, 4 and 6 h after ingesting the test agent on the first day of each of the three food challenges in Oslo. Electrochemiluminescence assays were performed at ImmusanT, Inc., according to the manufacturer's instructions (V-PLEX Proinflammatory Panel 1, Meso Scale Discovery, Rockville, USA) using a MESO Sector S600 instrument plate reader (Meso Scale Discovery). Data were analysed on Discovery Workbench 4.0 (Meso Scale Discovery) and presented as the average of triplicates. Calculated values below the lower level of quantitation (LLOQ) were reported as equal to the LLOQ. As a confirmatory test, all samples from the Oslo study from baseline and 4 h after gluten challenge were reassessed by ultrasensitive Simoa Human IL-2 immunoassay (supplied and performed by Quanterix Corporation, Lexington, USA). Sera from baseline and 3 h after gluten challenge from selected participants in the Melbourne study were also reassessed by ultrasensitive IL-2 assays: Simoa immunoassay, and S-PLEX electrochemiluminescence assay (supplied and performed by Meso Scale Discovery). Sera reassessed in the Melbourne study included those from CD non-responders for V-PLEX IL-2, IL-8 and IL-10 assays, and the one participant in the SR-GS cohort who was a responder in the V-PLEX IL-2 assay. Laboratory staff were unaware of the participants' diagnosis and food challenge status.
Statistical analysis
GraphPad Prism version 7.0d and Mathworks MATLAB version 9.4.0 were used to analyse data. Two-tailed, non-parametric tests were used (Wilcoxon test for paired, Mann-Whitney test for unpaired quantitative data). False discovery rate–adjusted P-values were estimated using the Benjamini–Hochberg method to correct for multiple comparisons. Cytokine assessments used for responder analysis were baseline, 3 and 4 h (Melbourne study), and 2, 4 and 6 h (Oslo). A participant in the Melbourne study was considered a cytokine responder if their level at 3 or 4 h after gluten challenge was >3 SD above the mean fold-change from baseline in the SR-GS cohort. A participant in the Oslo study was considered a cytokine responder if their level at 2, 4 or 6 h after food challenge was >3 SD above the mean fold-change from baseline for placebo challenge. Responder rates were compared by Fisher's exact test. No formal power calculations were undertaken.
Results
Clinical and cytokine response to gluten bread challenge in CD and SR-GS
The Melbourne study enrolled 26 CD and 18 SR-GS participants who were similar in age and duration on a GFD (Table 1). Compared to the CD cohort, the SR-GS cohort had a significantly higher baseline average overall symptom score (Figure 1). For the day of gluten challenge compared to the previous day, the CD cohort showed significant worsening in average overall symptom score (Figure 1). Two CD patients experienced vomiting. For the SR-GS cohort, one participant experienced vomiting, but CeD PRO symptom scores were not significantly different on the day of gluten challenge compared to the previous day (Figure 1).
Table 1.
Participant characteristics.
| Melbourne study |
Oslo study |
||
|---|---|---|---|
| Cohort | CD | SR-GS | SR-GS |
| Number | 26 | 18 | 49 |
| Females, n (%) | 17 (65%) | 14 (78%) | 43 (88%) |
| Median age in y (range) | 51 (20–66) | 53 (27–70) | 46 (21–72) |
| Medically diagnosed CD, n (%) | 26 (100%) | 0 | 0 |
| GFD duration in mo, median (range) | 66 (1–360) | 78 (0.5–180) | 35 (5–180) |
| GFD > 1 y, n (%) | 20 (77%) | 15 (83%) | 34 (69%) |
| Elevated baseline TG2-IgA, n (%) | 4 (15%) | 0 | 0 |
| Elevated baseline DGP-IgG, n (%) | 6 (23%) | 0 | 7 (14%) |
| HLA-DQ2.5 positive, n (%) | 25 (96%) | 6 (33%) | 18 (37%) |
| HLA-DQ2.5, 2.2, 8 & 7 negative, n (%) | 0 | 2 (11%) | 22 (45%) |
CD: coeliac disease; GFD: gluten-free diet; SR-GS: self-reported gluten sensitivity.
Figure 1.
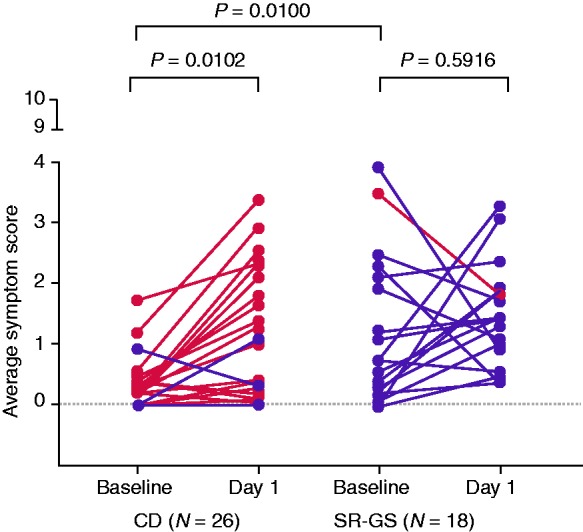
Symptomatic response to gluten challenge in coeliac disease (CD) and self-reported gluten sensitivity (SR-GS) participants. Paired changes in average self-reported symptom scores in the 11-item Celiac Disease Patient-Reported Outcome (CeD PRO) from baseline to post-challenge are shown for CD and SR-GS participants in the Melbourne study. Participants who responded with a positive cytokine release to the gluten challenge are highlighted in red. Paired changes were analysed by the Wilcoxon test. Baseline differences in symptoms between CD and SR-GS groups were analysed by the Mann–Whitney test.
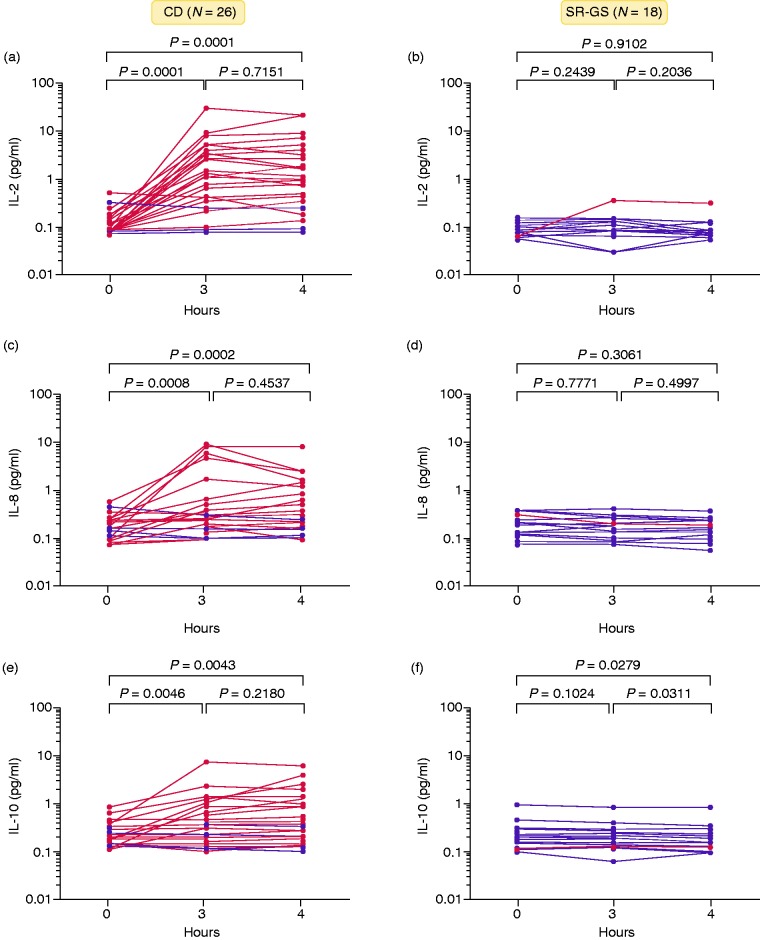
Eating gluten-containing bread significantly elevated serum concentrations of IL-2, IL-8 and IL-10 at 3 and 4 h in the CD but not in the SR-GS cohort (Figure 2, Table 2). In the CD cohort, median fold-change at 4 h relative to baseline was 8.06 for IL-2 (IQR: 1.52–24.0; P = 7.0 × 10−6, baseline versus 4 h by Wilcoxon test). Serum IL-2 at 4 h was nominally higher than at 3 h in the CD cohort but the difference did not reach statistical significance. Individual CD patient responses varied widely, so a responder analysis was performed (Table 2). Among the 26 CD participants, there were 19 (73%) IL-2 responders in the V-PLEX assay compared to only one (5%) IL-2 responder in the SR-GS cohort (P = 8.2 × 10−6, Fisher's exact test). Notably, all CD patients seropositive for TG2-IgA and/or DGP-IgG, including two participants who had commenced a GFD between 4 and 6 wk earlier, were IL-2 responders. In the SR-GS cohort, median fold-change from baseline at 3 h and 4 h for IL-2, IL-8 and IL-10 was between 0.94 and 1.0 (Table 2).
Figure 2.
Temporal cytokine response to gluten challenge in coeliac disease (CD) and self-reported gluten sensitivity (SR-GS) participants. Paired cytokine levels, pre- and post-challenge, of (a and b) IL-2, (c and d) IL-8 and (e and f) IL-10 are shown for CD and SR-GS participants in the Melbourne study. Participants who responded with a positive cytokine release to the gluten challenge are highlighted in red. Paired changes were analysed by the Wilcoxon test. Median values are listed on top of each time point for both cohorts.
Table 2.
Serum cytokine concentrations and fold-changes (median and IQR), and responder rates for cytokines after gluten challenge in the Melbourne study.
| Baseline |
3 h after gluten challenge |
4 h after gluten challenge |
|||||
|---|---|---|---|---|---|---|---|
| Conc. pg/mL | Conc. pg/mL | Fold- change | Response -rate | Conc. pg/mL | Fold-change | Response ratea | |
| IL-2 | |||||||
| CD | 0.09 (0.08–0.20) | 1.10 (0.25–3.50) | 6.7 (1.1–36.0) | 19 (73%) | 0.98 (0.25–3.40) | 8.1 (1.5–24.0) | 19 (73%) |
| SR-GS | 0.07 (0.06–0.11) | 0.08 (0.06–0.14) | 1.00 (0.9–1.3) | 1 (6%) | 0.08 (0.06–0.09) | 1.0 (1.0–1.1) | 1 (6%) |
| IL-8 | |||||||
| CD | 9.00 (6.72–11.8) | 12.6 (8.95–21.4) | 1.2 (1.1–2.4) | 14 (54%) | 14.0 (8.93–31.9) | 1.6 (1.1–2.9) | 14 (54%) |
| SR-GS | 8.13 (6.57–12.8) | 9.04 (6.37–11.7) | 1.0 (0.9–1.1) | 0 (0%) | 9.03 (6.59–11.4) | 1.0 (0.9–1.1) | 0 (0%) |
| IL-10 | |||||||
| CD | 0.25 (0.14–0.38) | 0.36 (0.22–0.91) | 1.1 (0.9–2.7) | 12 (46%) | 0.38 (0.19–1.07) | 1.2 (1.0–3.3) | 12 (46%) |
| SR-GS | 0.22 (0.15–0.31) | 0.20 (0.14–0.26) | 0.9 (0.9–1.0) | 0 (0%) | 0.19 (0.14–0.23) | 0.9 (0.8–1.0) | 0 (0%) |
V-PLEX assays.
Cutoffs for responder analyses were 2.0 for IL-2, 1.5 for IL-8, and 1.5 for IL-10 for peak fold-change at 3 h or 4 h.
CD: coeliac disease; Conc.: concentration; SR-GS: self-reported gluten sensitivity.
Bread challenge also elevated serum IL-8 (1.63 fold-change, IQR: 1.09–2.93; P = 7.0 × 10−5) and IL-10 (1.15 fold-change, IQR: 0.96–3.25; P = 3.2 × 10−3) compared to baseline in the CD but not in the SR-GS cohort (Figure 2). Responder analysis showed that the CD cohort comprised 14 (54%) responders to IL-8 and 12 (46%) responders to IL-10 compared to none in the SR-GS cohort for IL-8 (P = 0.0001) and none for IL-10 (P = 0.0005) (Table 2). Altogether, there were 6 (23%) of 26 CD participants who were non-responders for IL-2, IL-8 or IL-10 after gluten challenge (one responded to IL-8 and IL-10, but not IL-2), and 1 (6%) of 18 SR-GS participants who was a responder (IL-2 only) according to the V-PLEX assays.
Sera from blood at baseline and 3 h from the CD non-responders and SR-GS responder were re-evaluated with other CD and SR-GS samples by two separate ultrasensitive IL-2 assays and responder analyses performed (Table 3). Accordingly, two CD patients were reclassified as IL-2 responders, but four CD patients remained non-responders, and one SR-GS participant remained a ‘responder’. The four CD non-responder patients underwent clinical re-evaluation. The sole responder in the SR-GS cohort declined to be formally reassessed by extended gluten challenge. This individual was the only member of the SR-GS cohort who vomited after gluten challenge and her genotype (HLA-DQ7 and DQ9.2) has been implicated in rare cases of CD.14,18
Table 3.
Ultrasensitive assays of serum IL-2 after gluten challenge in the Melbourne study.
| Baseline pg/mL | 3-h pg/mL | 3-h fold-change | Respondersa |
||
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Number (%) | P-value | |
| CD (n = 21) | |||||
| V-PLEX | 0.1 (0.1–0.2) | 1.0 (0.2–4.1) | 8.1 (1.1–44.1) | 15 (71%) | 4.89 × 10−5 |
| S-PLEX | 0.1 (0.0–0.1) | 0.4 (0.1–1.7) | 10.1 (1.5–33.0) | 17 (81%) | 3.18 × 10−6 |
| Simoa | 0.2 (0.2–0.3) | 2.1 (0.6–11.0) | 13.0 (1.7–52.9) | 17 (81%) | 3.18 × 10−6 |
| SR-GS (n = 17) | |||||
| V-PLEX | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 1.0 (1.0–1.3) | 1 (6%) | NA |
| S-PLEX | 0.0 (0.0–0.1) | 0.0 (0.0–0.1) | 1.0 (1.0–1.1) | 1 (6%) | NA |
| Simoa | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 1.0 (1.0–1.0) | 1 (6%) | NA |
Cutoffs for responder analyses were 1.3 fold-change for V-PLEX, 1.3 for S-PLEX, and 1.4 for Simoa (mean + 3 × SD). P-value was estimated using Fisher's exact test to test the significance of number of responders in CD versus SR-GS cohorts.
CD: coeliac disease; SR-GS: self-reported gluten sensitivity.
Clinical follow-up in CD non-responders after gluten challenge
The four CD patients who remained cytokine non-responders after ultrasensitive IL-2 assessments resumed a diet containing at least 6 g gluten daily. After 6–9 wk, TG2-IgA and DGP-IgG serology were assessed and a gastroscopy with duodenal biopsy collection performed. In two CD patients, there was unequivocal serological and histological evidence of relapse after 6 wk in one, and after 9 wk in the other (Table 4). The remaining two patients, who had previously maintained a GFD for 4.5 or 30 y, have continued unrestricted diets for 12 mo and remain in serological, histological and clinical remission, suggesting they do not have CD. Notably, these two patients had been diagnosed on the basis of duodenal histology without supportive serology. If these two who were assessed not to have CD are excluded from the total CD cohort, cytokine release occurred in 22 (92%) of 24 ‘verified’ CD participants.
Table 4.
Clinical follow-up of coeliac disease non-responders in the Melbourne study.
| CD presentation and status at study entry | Outcome of gluten reintroduction |
|---|---|
| Male aged 40 y. GFD for 1.5 y. Diagnosis prompted by strong family history of CD; low bone mineral density. At diagnosis, elevated TG2-IgA, normal DGP-IgG; mild duodenal villous atrophy & crypt hyperplasia & intra-epithelial lymphocytosis. HLA-DQ2.5+ | Relapse at 6 wk; elevated TG2-IgA, normal DGP-IgG; partial duodenal villous atrophy, mild crypt hyperplasia & intra-epithelial lymphocytosis |
| Female aged 27 y. GFD for 3 y. Diagnosis prompted by diarrhoea. At diagnosis, elevated TG2-IgA & DGP-IgG; mild variable duodenal villous atrophy & probably crypt hyperplasia & patchy intra-epithelial lymphocytosis. HLA-DQ2.5+ | Relapse at 9 wk; elevated TG2-IgA & DGP-IgG; partial duodenal villous atrophy & intra- epithelial lymphocytosis |
| Female aged 66 y. GFD for 4.5 y. Diagnosis prompted by IBS-like symptoms. At diagnosis, normal TG2-IgA; irregular, moderate duodenal villous atrophy (crypts not reported), moderate diffuse increase in intra-epithelial lymphocytes. HLA-DQ2.5+ | No relapse at 1 y; normal TG2-IgA & DGP-IgG at 7 & 52 wk; normal duodenal histology at 7 wk |
| Female aged 53 y. GFD for 30 y. Diagnosis prompted by anaemia, lethargy, sister with CD. At diagnosis, serology not done; marked duodenal villous flattening, crypt hyperplasia & plentiful intra-epithelial lymphocytes. HLA-DQ2.5+ | No relapse at 1 y; normal TG2-IgA & DGP-IgG at 7, 20 & 52 wk; normal duodenal histology at 7 & 20 wk |
CD: coeliac disease; GFD: gluten-free diet; IBS: irritable bowel syndrome.
Plasma cytokines after gluten, fructan and placebo food challenges in SR-GS patients
Characteristics of the SR-GS cohort from Oslo are shown in Table 1. After eating gluten, fructan or placebo muesli bars, the SR-GS cohort showed no significant elevations from baseline in plasma concentrations of IL-2, IL-8 or IL-10 at 2, 4 or 6 h (Table 5). There were two (4%) responders for IL-8 and one (2%) responder for IL-10 after gluten, which was similar to the response rates after fructan challenge (1 for IL-8) and after placebo challenge (2 for IL-2 and 1 for IL-10) (Table 5).
Table 5.
Plasma cytokine concentrations and fold-changes (median and IQR), and responder rates for cytokines after gluten challenge in the Oslo study with SR-GS participants.
| Baseline |
Peak after food challengea |
||||
|---|---|---|---|---|---|
| Cytokine assay | Challenge | pg/mL | pg/mL | Fold-change | Respondersb |
| IL-2 V-PLEX | Gluten | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) | 1.0 (1.0–1.6) | 0 (0%) |
| Fructan | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) | 1.0 (1.0–1.3) | 0 (0%) | |
| Placebo | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) | 1.0 (1.0–1.5) | 2 (4%) | |
| IL-8 V-PLEX | Gluten | 4.7 (3.7–6.1) | 5.2 (4.4–6.6) | 1.0 (1.0–1.2) | 2 (4%) |
| Fructan | 5.0 (4.2–5.9) | 5.3 (4.6–6.5) | 1.0 (1.0–1.1) | 1 (2%) | |
| Placebo | 5.0 (4.0–6.5) | 5.5 (4.5–7.2) | 1.0 (1.0–1.2) | 0 | |
| IL-10 V-PLEX | Gluten | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 1.0 (1.0–1.1) | 1 (2%) |
| Fructan | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 1.0 (1.0–1.1) | 0 (0%) | |
| Placebo | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) | 1.0 (1.0–1.2) | 1 (2%) | |
| IL-2 Simoa | Gluten | 0.1 (0.1–0.2) | 0.1 (0.1–0.2) | 1.0 (1.0–1.1) | 0 (0%) |
Assessments at 2, 4 and 6 h for V-PLEX assays and at 4 h for Simoa assay.
Cutoffs for responder analyses were 3.6 fold-change for IL-2, 1.7 for IL-8 and 1.6 for IL-10 in V-PLEX assays, and 3.6 for IL-2 Simoa assay (mean + 3 × SD).
Discussion
Contemporary definitions of CD stress the chronic effects of gluten on the small intestine, and that diagnosis can only be confirmed with biopsies of the duodenum.3,4 Once diagnosed, CD is considered a condition that requires a lifelong GFD. Irrespective of gluten intake, or the status of duodenal histology or TG2-IgA serology, the immunological ‘recall’ response to gluten that defines CD persists. Relapse of CD in patients on a GFD appears to be dependent on reactivation of long-lived memory CD4+ T cells specific for gluten present in the small intestinal mucosa and circulating in blood.19
This study indicates that elevations of IL-2, IL-8 and IL-10 after a one-off gluten challenge distinguish between individuals on a GFD with CD and SR-GS. These findings suggest this approach could have clinical utility in identifying patients on a GFD with CD who have not previously been medically evaluated or in reassessing CD patients on a GFD who may have been misdiagnosed. This application was highlighted by finding two patients who had been diagnosed with CD and had maintained a GFD for many years who did not elevate serum IL-2 after gluten challenge and subsequently tolerated an unrestricted diet without relapse.
SR-GS patients had adopted a GFD because they attributed symptoms to eating gluten-containing food. In the Melbourne study, SR-GS patients had elevated digestive symptoms at baseline and after bread challenge. For the SR-GS patients enrolled in the Oslo study, worsening of digestive symptoms was clearly demonstrated during a 3-d wheat bread challenge done as clinical workup of these patients.20 Prior to this open challenge, symptom load was low. Thus, they were diagnosed with ‘non-coeliac wheat sensitivity’. The current study demonstrates that although IL-8 has been utilised as an in vitro marker of innate immune activation by gluten,10–12 no alterations in plasma IL-8 after gluten challenge were evident in individuals with SR-GS. These observations provide direct evidence that rapid immune activation after gluten exposure occurs in CD patients; while postulated to also occur in SR-GS, no gluten-induced responses are detectable in the blood of SR-GS patients on a GFD.7 An alternative explanation for symptoms in SR-GS patients is likely, such as FODMAP-associated irritable bowel syndrome.21 Pest resistance proteins termed α-amylase/trypsin inhibitors (ATIs) in wheat are reported to trigger innate immune activation in vitro;11 however, controlled in vivo data is needed to determine their significance in human health. As ATIs co-purify with gluten, clear separation of gluten and ATI content is not readily achieved. Thus, a vehicle for gluten food challenge studies that can deliver gluten devoid of potential FODMAP and ATI effects is desirable22 but not yet available to directly define the clinical role of ATIs. In contrast, systemic administration of synthetic gluten peptides that activate CD4+ T cells has clearly defined their immunological effects and potential to cause digestive symptoms similar to those following gluten ingestion in CD patients.9,23
After patients eliminate dietary gluten the evaluation of CD becomes a diagnostic challenge as CD-specific serologic and histologic abnormalities resolve. Genetic testing showing HLA-DQ2.5, DQ8 and DQ2.2 are absent is the only test that can immediately rule out CD;24 but for most patients requiring diagnostic workup the only option is to reintroduce gluten for at least several weeks with the aim of reactivating gluten-specific immunity and inducing diagnostic changes in serology and histology.3,4 However, as the symptomatic, serologic and histologic response to gluten is highly heterogeneous the optimal duration and dose of gluten required to consistently induce diagnostic changes remains unresolved.6,25–27 The gluten challenge is often poorly tolerated because of intolerable symptoms occurring within hours or days of gluten reintroduction.5,6 In contrast, one-off gluten exposure is straightforward, and serum cytokine measurement provides an objective assessment that could be provided by most clinical pathology services. Among the three cytokines assessed, IL-2 was the most sensitive marker for CD. Assay optimisation and a more potent gluten challenge may further increase diagnostic sensitivity utilising IL-2 alone.
In future studies, eligibility criteria for the ‘true-positive’ CD and ‘true-negative’ cohorts should be carefully considered to avoid underestimating diagnostic performance by including patients who may be misdiagnosed with CD or in whom CD is excluded based on genetic testing. In the present study, requiring a diagnosis of CD supported by typical histology as well as explicitly requiring elevated TG2 serology would have excluded both cytokine ‘non-responders’ in the CD cohort. Similarly, including HLA-DQ7 or DQ9.2, which have occasionally been associated with CD,18,28 among the genotypes conferring susceptibility to CD would have excluded the one SR-GS responder.
Larger studies with a potent, standardised gluten challenge format in patients who are ‘true-positive’ with an unequivocal diagnosis of CD and others who are ‘true-negative’ are required to establish diagnostic performance characteristics of this approach. However, based on IL-2 release in previous studies with systemic administration of gluten peptides that activate gluten-specific CD4+ T cells and also placebo-controlled gluten feeding studies, the sensitivity of IL-2 release after gluten challenge is likely to be over 90% in ‘true-positive’ CD patients on a GFD.9
Whether or how long a patient is required to be adherent with a GFD before gluten causes detectable IL-2 elevations in blood needs to be clarified. In the present study CD patients on a GFD for as little as 5 wk were IL-2 responders after challenge. Studies should also address if IL-2 assessment with gluten challenge might be a definitive test for CD when compared to histology. Duodenal histology assessment has been found to yield false positives in over 10% of community-diagnosed CD cases.29 Collectively, our findings raise the possibility that gluten challenge with IL-2 assessment could be used to address misdiagnosed CD, which may be particularly common among ‘biopsy-confirmed’ cases lacking supportive serology or in cases diagnosed with serology alone.24,30
Identifying people on a GFD without CD who do not need lifetime gluten exclusion has important clinical significance. A strict GFD is costly, socially restrictive, often higher in refined starch and fat and lower in whole grain content,31 and associated with increased coronary heart disease and metabolic syndrome.32–34 Our finding that gluten challenge does not elicit IL-8 release in patients with SR-GS adds further weight to the evidence that immune activation by gluten or related cereal proteins is not the explanation for early symptoms, and that gluten avoidance cannot be justified on these grounds. Indeed, the SR-GS patients we studied on a GFD described troubling digestive symptoms before gluten challenge, and neither the Australian nor Norwegian SR-GS cohorts showed symptomatic deterioration after gluten.15 This would be consistent with data showing that symptomatic deterioration in SR-GS can be attributed to dietary FODMAPs often present in gluten-containing cereals as opposed to gluten itself.15,35
A simple approach to identify the people following a GFD with CD who will medically benefit from a long-term GFD is an unmet need. Our findings suggest that measuring serum IL-2 before and after gluten challenge could address this requirement. Cytokine assessment shortly after acute gluten food challenge reveals that rapid immune activation is specific for patients on a GFD who have CD, and that comparable effects of gluten ingestion cannot be detected in patients with SR-GS.
Acknowledgements
The authors thank the Nutrition Outpatient Clinic at Oslo University Hospital for their contribution, and staff engineer Carina Hinrichs for invaluable assistance. We also thank Coeliac Australia for their support in volunteer recruitment and Cathy Pizzey and Brooke Flanders in collecting samples. We are indebted to all the study participants for undertaking gluten challenge and giving blood samples.
Author contributions
RPA, KEAL, LMS, LJW and JAT-D designed the studies. JAT-D, GIS and VKS conducted clinical studies and collected clinical data. AKR, SW, KEG and JLD executed immune assays. Data integration and analysis was performed by GG and RPA. Tables and figures were prepared by JAT-D, GG and RPA. LMS assisted in interpretation of results. JAT-D, KEAL and RPA wrote the manuscript. All authors reviewed and approved the manuscript, tables and figures. The authors made the decision to submit the manuscript for publication and vouch for the accuracy of the data and analyses and for the fidelity of this report to the trial protocol. RPA had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of conflicting interests
GIS, VKS and AKR have no conflicting interests. GG, LJW, RPA, KEG, SW and JLD are employees of ImmusanT, Inc. JAT-D, KEAL and LMS serve as advisors to ImmusanT, Inc. RPA and JAT-D are inventors of patents, owned or licensed by ImmusanT, Inc., relating to the diagnostic application of gluten challenge, and utilisation of gluten-derived T cell epitopes for use in therapeutics. LMS and KEAL hold a patent on methods for detection of gluten-specific T cells. LMS, privately or via his employer, serves as a scientific advisor to Bioniz Therapeutics, ActoBio Therapeutics, UCB Biopharma, Merck and Chugai Pharmaceutical. KEAL serves as a scientific advisor to Amyra AG, Bioniz Therapeutics and ActoBio Therapeutics, receives speaker fees from Takeda and from Tillots, and has a clinical trial honorarium from Dr Falk Pharma.
Ethics approval
The study in Melbourne was approved by the Human Research Ethics Committee (HREC) at the Walter and Eliza Hall Institute (HREC identifier 03/4) on 21 May 2003 and at Melbourne Health (HREC identifier 2003.009) on 19 April 2003, and the Oslo study was approved by the Regional Ethical Committee of South-East Norway (reference 2013/1237) on 17 Dec 2014. Each study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Funding
This work was funded by ImmusanT, Inc., Cambridge, MA, USA and by the following grants: Norwegian Extra Foundation for Health and Rehabilitation (project 2013-2-124) to KEAL; South-Eastern Norway Health Authority (project 2013046) to KEAL; Centre of Excellence funding scheme of the Research Council of Norway (project 179573/V40) to LMS; Stiftelsen KG Jebsen (project SKGJ-MED-017) to KEAL and LMS; and University of Oslo world-leading research program on human immunology (WL-IMMUNOLOGY) to LMS. JAT-D was supported by the Mathison Centenary Fellowship, University of Melbourne.
Informed consent
Written informed consent was obtained from each participant included in the study.
References
- 1.Lerner BA, Green PHR, Lebwohl B. Going against the grains: gluten-free diets in patients without celiac disease – worthwhile or not?. Dig Dis Sci 2019; 64: 1740–1747. [DOI] [PubMed] [Google Scholar]
- 2.Shah S, Akbari M, Vanga R, et al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am J Gastroenterol 2014; 109: 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J 2019; 7: 583–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut 2014; 63: 1210–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahdeaho ML, Maki M, Laurila K, et al. Small- bowel mucosal changes and antibody responses after low- and moderate-dose gluten challenge in celiac disease. BMC Gastroenterol 2011; 11: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruins MJ. The clinical response to gluten challenge: a review of the literature. Nutrients 2013; 5: 4614–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonard MM, Sapone A, Catassi C, et al. Celiac disease and nonceliac gluten sensitivity: a review. JAMA 2017; 318: 647–656. [DOI] [PubMed] [Google Scholar]
- 8.Jabri B, Sollid LM. T cells in celiac disease. J Immunol 2017; 198: 3005–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel G, Tye-Din JA, Qiao S-W, et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci Adv 2019, pp. 5 eaaw7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelinkova L, Tuckova L, Cinova J, et al. Gliadin stimulates human monocytes to production of IL-8 and TNF-alpha through a mechanism involving NF-kappaB. FEBS Lett 2004; 571: 81–85. [DOI] [PubMed] [Google Scholar]
- 11.Junker Y, Zeissig S, Kim SJ, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med 2012; 209: 2395–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammers KM, Khandelwal S, Chaudhry F, et al. Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR3-dependent manner only in patients with coeliac disease. Immunology 2011; 132: 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker-Smith J, Guandalini S, Schmitz J, et al. Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child 1990; 65: 909–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karell K, Louka AS, Moodie SJ, et al. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol 2003; 64: 469–477. [DOI] [PubMed] [Google Scholar]
- 15.Skodje GI, Sarna VK, Minelle IH, et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology 2018; 154: 529–539, e2. [DOI] [PubMed] [Google Scholar]
- 16.van Overbeek FM, Uil-Dieterman IG, Mol IW, et al. The daily gluten intake in relatives of patients with coeliac disease compared with that of the general Dutch population. Eur J Gastroenterol Hepatol 1997; 9: 1097–1099. [DOI] [PubMed] [Google Scholar]
- 17.Leffler DA, Kelly CP, Green PH, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology 2015; 148: 1311–1319, e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodd M, Tollefsen S, Bergseng E, et al. Evidence that HLA-DQ9 confers risk to celiac disease by presence of DQ9-restricted gluten-specific T cells. Hum Immunol 2012; 73: 376–381. [DOI] [PubMed] [Google Scholar]
- 19.Risnes LF, Christophersen A, Dahal-Koirala S, et al. Disease-driving CD4 + T cell clonotypes persist for decades in celiac disease. J Clin Invest 2018; 128: 2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skodje GI, Minelle IH, Rolfsen KL, et al. Dietary and symptom assessment in adults with self-reported non-coeliac gluten sensitivity. Clin Nutr ESPEN 2019; 31: 88–94. [DOI] [PubMed] [Google Scholar]
- 21.Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014; 146: 67–75 e5. [DOI] [PubMed] [Google Scholar]
- 22.Catassi C, Elli L, Bonaz B, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno experts' criteria. Nutrients 2015; 7: 4966–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel G, King T, Daveson AJ, et al. Epitope-specific immunotherapy targeting CD4-positive T cells in coeliac disease: two randomised, double-blind, placebo-controlled phase 1 studies. Lancet Gastroenterol Hepatol 2017; 2: 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson RP, Henry MJ, Taylor R, et al. A novel serogenetic approach determines the community prevalence of celiac disease and informs improved diagnostic pathways. BMC Med 2013; 11: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leffler D, Schuppan D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut 2013; 62: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarna VK, Skodje GI, Reims HM, et al. HLA-DQ:gluten tetramer test in blood gives better detection of coeliac patients than biopsy after 14-day gluten challenge. Gut 2018; 67: 1606–1613. [DOI] [PubMed] [Google Scholar]
- 27.Kurppa K, Koskinen O, Collin P, et al. Changing phenotype of celiac disease after long-term gluten exposure. J Pediatr Gastroenterol Nutr 2008; 47: 500–503. [DOI] [PubMed] [Google Scholar]
- 28.Karell K, Louka AS, Moodie SJ, et al. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European genetics cluster on celiac disease. Hum Immunol 2003; 64: 469–477. [DOI] [PubMed] [Google Scholar]
- 29.Arguelles-Grande C, Tennyson CA, Lewis SK, et al. Variability in small bowel histopathology reporting between different pathology practice settings: impact on the diagnosis of coeliac disease. J Clin Pathol 2012; 65: 242–247. [DOI] [PubMed] [Google Scholar]
- 30.DeGaetani M, Tennyson CA, Lebwohl B, et al. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol 2013; 108: 647–653. [DOI] [PubMed] [Google Scholar]
- 31.See JA, Kaukinen K, Makharia GK, et al. Practical insights into gluten-free diets. Nat Rev Gastroenterol Hepatol 2015; 12: 580–591. [DOI] [PubMed] [Google Scholar]
- 32.Lebwohl B, Cao Y, Zong G, et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: prospective cohort study. BMJ 2017; 357: j1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tovoli F, Negrini G, Fari R, et al. Increased risk of nonalcoholic fatty liver disease in patients with coeliac disease on a gluten-free diet: beyond traditional metabolic factors. Aliment Pharmacol Ther 2018; 48: 538–546. [DOI] [PubMed] [Google Scholar]
- 34.Tortora R, Capone P, De Stefano G, et al. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther 2015; 41: 352–359. [DOI] [PubMed] [Google Scholar]
- 35.Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013; 145: 320-328 e1–3. [DOI] [PubMed] [Google Scholar]