Abstract
Background
Disease heterogeneity, according to the age at onset, has been reported in Crohn's disease (CD).
Objective
This study aimed to compare natural history in CD patients diagnosed ≤17 (early onset (EO)) versus ≥60 (late onset (LO)) years old.
Methods
EO CD and LO CD patients referred to two Italian inflammatory bowel disease (IBD) centres were included. Relevant data comprised sex, current smoking, disease location and behaviour, IBD family history, extra-intestinal manifestations and use of medical/surgical therapy during the follow-up period.
Results
Among 2321 CD patients, 160 met the inclusion criteria: 92 in the EO and 68 in the LO group (mean follow-up 11.7 ± 7.7 years). Family history of IBD was more frequent in EO compared to LO CD (26% vs. 4%; p < 0.0001). Ileocolonic, upper gastrointestinal and perianal involvement occurred more frequently in EO compared to LO CD (56% vs. 21%, p < 0.0001; 17% vs. 3%, p < 0.01; and 38% vs. 19%, p < 0.01, respectively). Progression to complicated disease occurred more frequently in EO CD (40% vs. 10% p < 0.005), with an increased use of corticosteroids and anti-tumour necrosis factor alpha agents within 10 years since diagnosis (81% vs. 58%, p = 0.004, and 36% vs. 16%, p = 0.01, respectively), while the cumulative probability of surgery did not differ between the two groups.
Conclusions
Patients with EO CD are more likely to develop a more aggressive disease with perianal involvement and a greater use of drug treatment compared to those with LO CD, without carrying an increased need for surgery.
Keywords: Clinical outcomes, Crohn's disease, inflammatory bowel disease
Introduction
The incidence of inflammatory bowel disease (IBD) is increasing worldwide, especially in newly industrialised countries.1,2 Although Crohn's disease (CD) generally affects the adult population, 7–20% of patients develop the disease before the age of 17 years (paediatric onset)3,4 and 10–15% after the age of 60 years (elderly onset).1 According to epidemiological studies, the incidence of paediatric onset IBD has increased, particularly for CD, over the last 10 years.5–7 On the other hand, the progressive ageing of the general population makes elderly-onset IBD an emerging and growing problem that poses particular challenges, especially due to the frequently concurrent multi-morbidity8 and a possible greater diagnostic delay.9 Disease heterogeneity according to the age at CD onset has been hypothesised. In fact, preliminary data suggest that disease phenotype in paediatric patients might be more severe compared to adult CD10,11 – possibly related to the greater genetic penetrance of younger patients – with unusual presentation and atypical endoscopic and histological findings.12 For this reason, the Montreal classification was modified and specifically adapted for the paediatric population, dividing IBD onset into five subcategories: <17 years (paediatric-onset IBD), <10 years (early onset IBD), <6 years (very early onset IBD), <2 years (infantile IBD) and within 28 days since birth (neonatal IBD).13 Similarly, clinical presentation in the elderly might be more subtle and unspecific, while the natural course of disease and impact of treatments still need to be ascertained. Until then, physicians caring for this population must face a number of age-specific problems, including misdiagnoses and the identification of the risk–benefit profile of the available medical and surgical therapeutic options.14 From both a pathogenic and a clinical point of view, early onset (EO) CD and late onset (LO) CD may represent two opposite ends of CD spectrum, in which genetic and environmental factors play different roles. Moreover, it is interesting to explore EO CD in Italy, as the only available data on an Italian population are confined to a paediatric setting without extensive follow-up,15 and there are no studies focusing on LO CD in Italy, hence the need for comparing these two peculiar populations in terms of disease natural course and medical needs.
On this basis, the primary aim of the present study was to compare EO patients (i.e. those <17 years of age) to LO patients (i.e. those >60 years of age) in a large CD Italian cohort over a 10-year follow-up period.
Materials and methods
Cases included all CD patients <17 years old (EO group) and those >60 years old (LO group) at the time of diagnosis. All patient data were retrieved from the available local medical records of two IBD tertiary referral centres in Rome from 1980 to 2016. Both inpatients and outpatients were included. The study area was a region of Central Italy, and most of the patients were Caucasian and were born in that area. Patients with a follow-up period of less than two years or who accessed the hospital only once were not included in the data analysis. Retrospectively collected data included age at diagnosis, sex, family history of IBD, smoking habit (current smokers), date of CD diagnosis, presence of perianal disease and extra-intestinal manifestations (EIM), CD localisation and behaviour according to the Montreal classification (L1 ileal, L2 colonic, L3 ileocolonic and L4 upper gastrointestinal disease; B1 inflammatory, B2 stricturing and B3 penetrating disease).16 B2 and B3 were pooled together and generally defined as ‘complicated behaviour’. Perianal lesions included both abscesses and/or fistulae. EIM included joint, skin, ocular and hepatobiliary manifestations. All the following medications were recorded: corticosteroids, immunosuppressants and anti-tumour necrosis factor alpha (TNF-α) agents. The time lapse between diagnosis and the first use of any medication was calculated. Only small-bowel resection, partial or total colectomy, definitive stoma or stricturoplasty were considered as surgical interventions.
The study was performed as a clinical, retrospective audit with anonymised data, and as such it is exempt from the need for written informed consent. The study protocol was approved by the local ethics committee and conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution's human research committee.
Statistical analysis
Qualitative variables were evaluated as absolute frequencies and percentages, while quantitative variables were summarised with means and standard deviations. Global comparison according to age at diagnosis was performed using the chi-square test. Statistical significance was considered with p < 0.05. Most relevant Kaplan–Meier event-free survival curves, including corticosteroids, immunosuppressants, anti-TNF-α agents and surgery, were analysed. Univariate and multivariate analyses for possible variables associated with EO and LO CD were computed after correction by time of follow-up. Cumulative probabilities of events were evaluated within both 2 and 10 years since diagnosis. A sub-analysis of the EO and LO groups diagnosed after 1998 (year of approval of infliximab for CD treatment) was performed. All the analyses were performed with StatsDirect (StatsDirect Ltd, Cambridge, UK).
Results
Baseline characteristics of the study populations
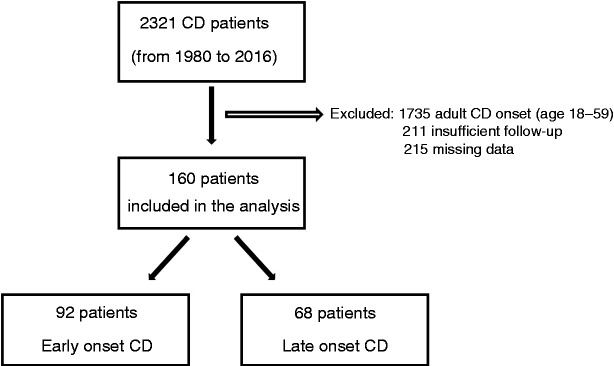
The flow chart of the study is shown in Figure 1. Of the entire cohort of 2321 CD patients, 160 met the inclusion criteria. Of these, 92 belonged to the EO cohort (median age 15 years; range 0–17 years) and 68 to the LO cohort (median age 66 years; range 60–85 years). The mean overall follow-up was 11.7 ± 7.7 years, with no difference between the two study populations (13.6 ± 8.6 for EO CD and 11.7 ± 7.8 for LO CD; p = 0.15). At maximum follow-up, none of the patients in the EO cohort died. Conversely, 12/68 (17.6%) patients in the LO cohort died of causes unrelated to CD. The baseline characteristics of the EO and LO cohorts are shown in Table 1.
Figure 1.
Flow chart of the study. As per study design, adult patients (age range 18–59 years) were excluded. CD: Crohn's disease.
Table 1.
Baseline characteristics and comparison of all variables of the two study populations.
| Overall patients | EO (≤17 years) | LO (≥60 years) | ||
|---|---|---|---|---|
| N = 160 | N = 92 | N = 68 | p | |
| Sex (F/M) | 82/78 (51%/49%) | 43/49 (47%/53%) | 39/29 (57%/43%) | 0.2 |
| Family history | 27 (17%) | 24 (26%) | 3 (4%) | <0.001 |
| Current smokers | 37 (23%) | 26 (28%) | 11 (16%) | 0.1 |
| Localisation at diagnosis | ||||
| L1 | 64 (40%) | 30 (33%) | 34 (50%) | 0.0001 |
| L2 | 30 (19%) | 10 (11%) | 20 (29%) | |
| L3 | 66 (41%) | 52 (57%) | 14 (21%) | |
| L4 | 18 (11%) | 16 (17%) | 2 (3%) | <0.01 |
| Behaviour at diagnosis | ||||
| B1 | 96 (60%) | 58 (63%) | 34 (56%) | <0.05 |
| B2 | 52 (33%) | 24 (26%) | 20 (41%) | |
| B3 | 12 (7%) | 10 (11%) | 14 (3%) | |
| Progression to complicated behaviour (B2+B3) during follow-up | 27/96 (28%) | 23/58 (40%) | 4/38 (10%) | <0.005 |
| Perianal disease | 48 (30%) | 35 (38%) | 13 (19%) | <0.01 |
| Extra-intestinal manifestation | 40 (25%) | 27 (29%) | 13 (19%) | 0.2 |
EO: early onset; LO: late onset.
Comparison of EO versus LO CD
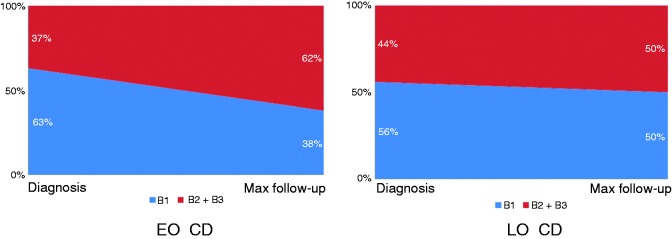
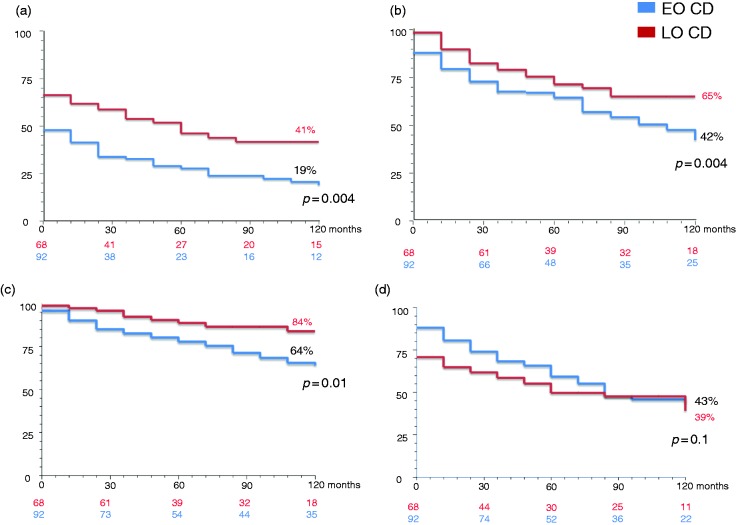
A family history of IBD occurred more frequently in the EO group compared to the LO group. Conversely, no significant difference was seen in the percentage of active smokers in the EO group compared to the LO group. A significantly higher ileocolonic localisation (L3) and upper gastrointestinal involvement (L4) occurred more frequently in the EO group compared to the LO group. Disease behaviour at diagnosis was mainly inflammatory (B1) in both groups. However, the behaviour frequency distribution was statistically different. The univariate comparison of all relevant clinical characteristics of the two study populations is summarised in Table 1. At multivariate analysis, family history of IBD, disease localisation and perianal disease maintained statistical significance (p < 0.001). In addition, progression to complicated disease during follow-up occurred more frequently in the EO group compared to the LO group (40% vs. 10%, p < 0.005; Figure 2). During the disease course, patients in the EO group were more likely to manifest with perianal lesions (fistulae or abscesses) compared to those in the LO group. The use of medical or surgical therapy within the first two years since diagnosis is reported in Table 2. In particular, the EO group had a significantly increased use of any medical therapy, including corticosteroids, immunosuppressants and anti-TNF-α agents compared to the LO group. The cumulative probabilities of receiving corticosteroids, immunosuppressants and anti-TNF-α agents within 10 years since diagnosis in the EO group and in the LO group were 81% versus 58% (p = 0.004), 58% versus 35% (p = 0.04) and 36% versus 16% (p = 0.01), respectively. There was no significant difference between the two groups regarding the need for both first and second surgery during the first two years since diagnosis. Even cumulative probability of surgery within 10 years was not different between the two groups. At maximum follow-up, 22.8% of the EO group and 13.2% of the LO group had received more than one surgical intervention. Definitive stoma was performed in 5.4% of the EO group and in 2.9% of the LO group. Supplemental Tables S1 and S2 report the comparison of relevant clinical characteristics and the use of medical or surgical therapy in the two subgroups of patients diagnosed after 1998. Finally, Figure 3 reports Kaplan–Meier curves for both EO and LO groups regarding corticosteroid-free (Figure 3(a)), immunosuppressant-free (Figure 3(b)), anti-TNF-α agent-free (Figure 3(c)) and surgery-free (Figure 3(d)) survival. Regarding anti-TNF-α agent-free survival, no statistical difference was seen in the two subgroups of patients diagnosed after 1998 (data not shown). The mean time-free survival intervals (months) in the EO group versus the LO group were: 36.2 (95% confidence interval (CI) 26.2–46.4) versus 61.6 (95% CI 48.2–75.0) for corticosteroids; 77.7 (95% CI 67.7–87.7) versus 108.7 (95% CI 100.1–117.2) for immunosuppressants; 94.2 (95% CI 86.3–103.4) versus 116.6 (95% CI 110.1–123.1) for anti-TNF-α agents; and 97.4 (95% CI 87.7–107.0) versus 78.3 (95% CI 64.4–92.0) for surgery. The cumulative probabilities of receiving corticosteroids, immunosuppressants and anti-TNF-α agents within 10 years since diagnosis in the EO and LO groups were 81% versus 58% (p = 0.004), 58% versus 35% (p = 0.04) and 36% versus 16% (p = 0.01), respectively. The cumulative probability of surgery within 10 years did not statistically differ between the two groups. Most patients with perianal disease were treated with surgery, regardless of concurrent medical therapy and age group. In nine cases, this datum was missing.
Figure 2.
Evolution of CD behaviour from its diagnosis to the maximum follow-up period, according to the age of onset. B2 and B3 were pooled together and considered as complicated behaviour. EO: early-onset; LO: late-onset.
Table 2.
Use of medical or surgical therapy during the first two years since Crohn's disease diagnosis.
| Overall patients | EO (≤17 years) | LO (≥60 years) | ||
|---|---|---|---|---|
| N = 160 | N = 92 | N = 68 | p | |
| Corticosteroids | 89 (56%) | 61 (66%) | 28 (41%) | <0.005 |
| Immunosuppressants | 37 (23%) | 25 (27%) | 12 (18%) | 0.2 |
| Anti-TNF-α agents | 17 (11%) | 14 (15%) | 3 (4%) | 0.05 |
| First surgery | 50 (31%) | 24 (26%) | 26 (38%) | 0.1 |
| Second surgery | 5 (3%) | 3 (3%) | 2 (3%) | 0.9 |
TNF-α: tumour necrosis factor alpha.
Figure 3.
Kaplan–Meier curves for both EO (blue lines) and LO (red lines) CD groups regarding (a) corticosteroid-free, (b) immunosuppressant-free, (c) anti-tumour necrosis factor alpha agent-free and (d) surgery-free survival.
Discussion
We herein described CD natural history in a cohort of Italian patients, with the aim of comparing EO to LO CD, with an extended follow-up. Previously published studies exploring CD natural history focused on either a paediatric3,17 or an elderly population.14 Hence, it is not possible to compare the characteristics of EO versus LO CD in our cohort with other studies due to the different settings of enrolment. However, the strength of this paper lies in the direct comparison of CD at the two opposite ages of onset. Novel and interesting characteristics were found in the EO and LO groups.
Overall, EO CD was more aggressive compared to LO CD with respect to disease behaviour, complications and use of medical therapy. EO CD was characterised by the predominance of ileocolonic localisation (L3) and upper gastrointestinal involvement (L4) in a consistent rate of patients. Our findings confirm those described in other paediatric CD series, reporting a more prevalent ileocolonic localisation, with or without upper gastrointestinal involvement.3,18–20 Instead, LO CD localisation was colonic in half of patients, but the exclusively colonic localisation occurred in only 29% of cases, in contrast with the literature that described a predominantly colonic localisation of the disease in the elderly.21–25 However, this difference could be explained by the lack of ileal exploration in some of the aforementioned studies, whereas all patients included in our study underwent ileo-colonoscopy. Disease behaviour at diagnosis was inflammatory in approximately 60% of patients of both groups. Notably, progression to complicated disease (B2+B3) during follow-up occurred more frequently in the EO group than it did in the LO group. Moreover, perianal disease was mostly associated with EO CD. EO CD patients had increased use of corticosteroids and anti-TNF-α agents, especially during the first two years since diagnosis, and the cumulative probability of receiving any therapy (corticosteroids, immunosuppressants and anti-TNF-α agents) within 10 years since diagnosis was greater in EO CD compared to LO CD. Thus, the LO CD phenotype seemed to be less aggressive, with a more stable and milder course, consistent with previously reported results.14,26 From a clinical point of view, despite EO CD being the most aggressive disease, the greater extension and younger age might discourage a radical surgical approach. On the contrary, although LO CD seems to be less aggressive, the risk related to side effects of drugs, infections, tumours and drug interactions would favour elective surgery as the best strategy in the elderly subgroup. Hence, the need for surgery is not directly proportional to disease severity, and is still necessary in case of complications such as strictures or abscesses. Of note, the development of intestinal fibrosis, one of the most threatening complications of CD, is partially related to intestinal inflammation, occurs in more than one third of these patients and often requires surgery.27 Regardless of age, both medical and surgical therapies carry some risk and potential adverse effects that should be carefully evaluated and discussed with patients.
Interpreting the different use of corticosteroids, immunosuppressants, biologics and surgery has some limits that must be taken into account. In particular, even if this study reflects a tertiary referral practice, there might be a certain degree of variability among different prescribing clinicians, and marked changes in the therapeutic approach have been made in recent decades. Also, data regarding medication non-adherence, which may be responsible for a considerable rate of disease flare,28 were not available. The choice of surgery as a potential ‘gold standard’ of disease severity, as reported in previous studies, has many limitations as well, as previously mentioned. For example, the similar surgery rates between the two groups in our study may potentially reflect CD medical under-treatment, at least in a proportion of patients with concurrent multi-morbidity.
The prescription of immunomodulators, according to guidelines, has become in the more extensive last decades, especially in the paediatric age group. Since 1998, with the introduction of infliximab, the therapeutic choice for CD has changed even more. Starting from these premises, we did a sub-analysis for patients diagnosed in the biological era. We observed a significantly greater use of anti-TNF-α agents in the EO group compared to the LO group, which is in accordance with the higher efficacy of anti-TNF-α agents in the early stages of the disease.29 Conversely, the significantly lower use of corticosteroids in the older group could be explained by the higher risk of drug-related complications.
We are aware of the limitations of this study. In particular, there are some selection biases, first because this is a retrospective study of patients who accessed tertiary referral centres for IBD. Hence, these data could not be transferred to other settings. Although patients diagnosed and followed in a tertiary referral centre may have a lower risk of misdiagnosis, especially in the elderly, they do not reflect the real course of CD because they generally are more complex and difficult cases to manage. Moreover, the period covered is very long, with drastic changes in medical therapy, and the sub-analysis of patients diagnosed after 1998 can only partially overcome this bias. Furthermore, data regarding multi-morbidity were not available, even if in LO CD it may influence disease course more than ageing itself, as was recently shown.30 Nonetheless, we found some distinctive aspects of EO CD in comparison to LO CD to the point that EO CD and LO CD could be even ascribed as two different diseases with different challenges.
To conclude, we have reported the largest Italian study regarding CD natural history, embracing the pre- and post-biological eras. Patients with EO CD compared to those with LO CD are more likely to have a family history of CD and could probably develop a more aggressive disease with perianal involvement and greater use of medical therapy, without carrying an increased need for surgery. The identification of early markers of disease with a prognostic value is still awaited, along with a patient-tailored approach to this condition.
Supplemental Material
Supplemental Material for Early-onset versus late-onset Crohn's disease: An Italian cohort study by Laura Cantoro, Marco Vincenzo Lenti, Rita Monterubbianesi, Michele Cicala, Diana Giannarelli, Claudio Papi# Anna Kohn#Antonio Di Sabatino in United European Gastroenterology Journal
Declaration of conflicting interests
The authors declare that there is no conflict of interests regarding the publication of this article.
Ethics approval
The study protocol was approved by the local ethics committee (San Camillo-Forlanini Hospital, 2014) and conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution’s human research committee.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Informed consent
The study was performed as a clinical, retrospective audit with anonymised data, and as such it is exempt from the need for written informed consent.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011; 140: 1785–1794. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015; 12: 720–727. [DOI] [PubMed] [Google Scholar]
- 3.Auvin S, Molinie F, Gower-Rousseau C, et al. Incidence, clinical presentation and location at diagnosis of pediatric inflammatory bowel disease: a prospective population-based study in northern France (1988–1999). J Pediatr Gastroenterol Nutr 2005; 41: 49–55. [DOI] [PubMed] [Google Scholar]
- 4.IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: recommendations for diagnosis – the Porto criteria. J Pediatr Gastroenterol Nutr 2005; 41: 1–7. [DOI] [PubMed] [Google Scholar]
- 5.Ehlin AG, Montgomery SM, Ekbom A, et al. Prevalence of gastrointestinal diseases in two British national birth cohorts. Gut 2003; 52: 1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tourtelier Y, Dabadie A, Tron I, et al. Incidence of inflammatory bowel disease in children in Brittany (1994–1997). Breton association of study and research on digestive system diseases (Abermad). Arch Pediatr 2000; 7: 377–384. [DOI] [PubMed] [Google Scholar]
- 7.Hildebrand H, Karlberg J, Kristiansson B. Longitudinal growth in children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 1994; 18: 165–173. [DOI] [PubMed] [Google Scholar]
- 8.Corazza GR, Formagnana P, Lenti MV. Bringing complexity into clinical practice: an internistic approach. Eur J Intern Med 2019; 61: 9–14. [DOI] [PubMed] [Google Scholar]
- 9.Cantoro L, Di Sabatino A, Papi C, et al. The time course of diagnostic delay in inflammatory bowel disease over the last sixty years: an Italian multicentre study. J Crohns Colitis 2017; 11: 975–980. [DOI] [PubMed] [Google Scholar]
- 10.Puntis J, McNeish AS, Allan RN. Long term prognosis of Crohn's disease with onset in childhood and adolescence. Gut 1984; 25: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman HJ. Long-term prognosis of early-onset Crohn's disease diagnosed in childhood or adolescence. Can J Gastroenterol 2004; 18: 661–665. [DOI] [PubMed] [Google Scholar]
- 12.Uhlig HH, Schwerd T, Koletzko S, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014; 147: 990–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011; 17: 1314–1321. [DOI] [PubMed] [Google Scholar]
- 14.Charpentier, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut 2014; 63: 423–432. [DOI] [PubMed] [Google Scholar]
- 15.Aloi M, Lionetti P, Barabino A, et al. Phenotype and disease course of early-onset pediatric inflammatory bowel disease. Inflamm Bowel Dis 2014; 20: 597–605. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Suppl A): 5A–36A. [DOI] [PubMed] [Google Scholar]
- 17.Langholz E, Munkholm P, Krasilnikoff PA, et al. Inflammatory bowel diseases with onset in childhood. Clinical features, morbidity and mortality in a regional cohort. Scand J Gastroenterol 1997; 32: 139–147. [DOI] [PubMed] [Google Scholar]
- 18.Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn's disease: a population-based cohort study. Gastroenterology 2008; 135: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 19.Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008; 135: 1114–1122. [DOI] [PubMed] [Google Scholar]
- 20.Crocco S, Martelossi S, Giurici N, et al. Upper gastrointestinal involvement in paediatric onset Crohn's disease: prevalence and clinical implications. J Crohns Colitis 2012; 6: 51–55. [DOI] [PubMed] [Google Scholar]
- 21.Heresbach D, Alexandre JL, Bretagne JF, et al. Crohn's disease in the over-60 age group: a population based study. Eur J Gastroenterol Hepatol 2004; 16: 657–664. [DOI] [PubMed] [Google Scholar]
- 22.Freeman HJ. Crohn's disease initially diagnosed after age 60 years. Age Ageing 2007; 36: 587–589. [DOI] [PubMed] [Google Scholar]
- 23.Lovasz BD, Lakatos L, Horvath A, et al. Evolution of disease phenotype in adult and pediatric onset Crohn's disease in a population-based cohort. World J Gastroenterol 2013; 19: 2217–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picco MF, Cangemi JR. Inflammatory bowel disease in the elderly. Gastroenterol Clin North Am 2009; 38: 447–462. [DOI] [PubMed] [Google Scholar]
- 25.Quezada SM, Steinberger EK, Cross RK. Association of age at diagnosis and Crohn's disease phenotype. Age Ageing 2013; 42: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakatos PL, Golovics PA, David G, et al. Has there been a change in the natural history of Crohn's disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977–2009. Am J Gastroenterol 2012; 107: 579–588. [DOI] [PubMed] [Google Scholar]
- 27.Lenti MV, Di Sabatino A. Intestinal fibrosis. Mol Aspects Med 2019; 65: 100–109. [DOI] [PubMed] [Google Scholar]
- 28.Lenti MV, Selinger CP. Medication non-adherence in adult patients affected by inflammatory bowel disease: a critical review and update of the determining factors, consequences and possible interventions. Expert Rev Gastroenterol Hepatol 2017; 11: 215–226. [DOI] [PubMed] [Google Scholar]
- 29.Naviglio S, Giuffrida P, Stocco G, et al. How to predict response to anti-tumour necrosis factor agents in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2018; 12: 797–810. [DOI] [PubMed] [Google Scholar]
- 30.Kariyawasam VC, Kim S, Mourad FH, et al. Comorbidities rather than age are associated with the use of immunomodulators in elderly-onset inflammatory bowel disease. Inflamm Bowel Dis. Epub ahead of print 29 December 2018. DOI: 10.1093/ibd/izy389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Early-onset versus late-onset Crohn's disease: An Italian cohort study by Laura Cantoro, Marco Vincenzo Lenti, Rita Monterubbianesi, Michele Cicala, Diana Giannarelli, Claudio Papi# Anna Kohn#Antonio Di Sabatino in United European Gastroenterology Journal