Abstract
Background
This article systematically reviews the current evidence regarding inflammation in Tendinopathy with the aim to increase understanding of a potential common pathophysiology.
Methods
Following the PRISMA statements, the terms: (tendinopathy OR (tendons AND rupture)) AND (inflammation OR (inflammation AND cells) OR immune system OR inflammation mediators OR bacteria) were used. One thousand four hundred thirty-one articles were identified which was screened down to 53.
Results
39/53 studies mentioned inflammatory cells but had contradicting conclusions. Macrophages were the most common cell type and inflammatory markers were detectable in all the articles which measure them.
Conclusions
The included studies show different conclusions, but this heterogeneity is not unexpected since the clinical criteria of ‘tendinopathy’ encompass a huge clinical spectrum.
Different ‘tendinopathy’ conditions may have different pathophysiology, and even the same clinical condition may be at different disease stages during sampling, which can alter the histological and biochemical picture. Control specimen sampling was suboptimal since the healthy areas of the pathological-tendon may actually be sub-clinically diseased, as could the contralateral tendon in the same subject.
Detection of inflammatory cells is most sensitive using immunohistochemistry targeting the cluster of differentiation markers, especially when compared to the conventional haematoxylin and eosin staining methods. The identified inflammatory cell types favour a chronic inflammatory process; which suggests a persistent stimulus. This means NSAID and glucocorticoids may be useful since they suppress inflammation, but it is noted that they may hinder tendon healing and cause long term problems.
This systematic review demonstrates a diversity of data and conclusions in regard to inflammation as part of the pathogenesis of Tendinopathy, ranging from ongoing or chronic inflammation to non-inflammatory degeneration and chronic infection. Whilst various inflammatory markers are present in two thirds of the reviewed articles, the heterogenicity of data and lack of comparable studies means we cannot conclude a common pathophysiology from this systematic review.
Keywords: Tendinopathy, Tendon rupture, Inflammation, Immune system, Inflammatory mediators
Background
Tendinopathy affect millions of people in both the athletic and general population, causing great socioeconomical impacts [1]. Despite the presence of various modalities of conservative and surgical treatments, more than one third of the patients do not respond and continue to present with persistent pain and disability [2, 3]. Anti-inflammatory treatments including NSAIDs and glucocorticoids form the back-bone of conservative treatments for tendinopathy. However, there is still an ongoing debate on the presence of active inflammation in this chronic disorder. Whether inflammation play an important role in tendinopathy is currently unclear. By reviewing the presence and pattern of inflammation in tendinopathic tendons, the current management strategies can be re-assessed.
There have been an ongoing debate on whether an active inflammation is present in chronic tendinopathy. Tendon healing was proposed to be separated into 3 overlapping steps including inflammation, proliferation, and remodelling. The inflammatory phase typically lasts for no longer than weeks, and the presence of a functional, regulated inflammatory process is crucial in maintaining the integrity of tendon tissues [4, 5].
Previously, the term “tendinitis” have been commonly used to describe the clinical symptom of pain and disability of a tendon [6]. However, as argued by an editorial published in 1998, it was suggested that the usage of the term “tendinitis” should be limited to histological findings, and “tendinopathy” is a more suitable vocabulary to describe pain and deranged function of the tendon in a clinical setting [7]. It was also suggested in this editorial that using “tendinitis” to describe chronic tendon disorders is again inaccurate and misleading since tendinopathic tendons were said to present as a degenerative lesion with an absence of inflammatory cells [7, 8].
Contrarily, inflammatory cells and markers have been reported to be present or increased in tendinopathic tendons in recent studies [9]. It was suggested that defects may occur in the cellular responses regulating the inflammatory process, leading to a poor resolution of inflammation. It was further suggested that chronic inflammation may persist in the injured tendon, leading to possible further damage and ultimately the degenerative changes observed in chronic tendinopathy [10]. A recent systematic review of 5 studies have suggested the presence of inflammatory cells in painful tendinopathy [6]. However, the character of inflammation present in tendinopathic tendons is yet to be identified, and current theories of pathogenesis could not satisfactorily explain the varying presentation of inflammation observed in tendinopathic tendons. It is strongly suspected that more studies could be reviewed to facilitate the discussion of this topic.
The aim of this study is to systematically review whether tendinopathy involves an on-going inflammatory process in terms of the presence of inflammatory cells. Any reported changes in inflammatory markers will also be assessed. We would also take an ambitious step to discuss whether the current anti-inflammatory approach to conservatively manage tendinopathy is appropriate, provide a discussion on why the current management outcomes are sub-optimal, and suggest how we may be able to tackle this clinical problem alternatively. A secondary aim is to identify possible initiators of inflammation, such as trauma, mechanical stresses, inflammatory diseases or infections.
Methods
Systematic searches were carried out in November 2017 using PubMed, Scopus, Web of Science and Embase. An updated search was conducted in the 4 databases in December 2018. No limits or filters were used. No restrictions were made on language, publication date, and publication status.
The PRISMA statements [11] was used as guidelines in the performance of this systematic review. The keywords in combination with search operants were as follows: (tendinopathy OR (tendons AND rupture)) AND (inflammation OR (inflammation AND cells) OR immune system OR inflammation mediators OR bacteria).
Eligibility criteria
The inclusion criteria for studies in this systematic review consisted of the following:
Clinical studies investigating the presence of inflammation in tendinopathic tendons such as cross-sectional studies, case-control studies, prospective observational studies, randomized controlled trials. In vitro studies using tendon tissue, or cells derived from tendinopathic tendons were also included. Included studies were restricted to studies with an evidence level of 3 or better. In vitro studies using tissue, or cells that have been treated with cytokines or other agents or modified mechanically were excluded.
Studies with participants of any age presenting with tendinopathy were included. Specimens from spontaneous tendon ruptures were included considering the assumption that only tendinopathic tendons are prone to spontaneous ruptures. Diagnostic criteria of the above diseases include a clinical presentation of chronic pain or loss of function, confirmed with imaging modalities such as magnetic resonance imaging or ultrasound. Studies investigating the presence of inflammatory cells, immune cells, inflammatory markers in tendinopathic tendons, and tendon ruptures were included. Tendinopathy was defined as pain, diffuse or localized swelling, and impaired performance of the tendon. Tendon rupture was defined as a tear, visible with medical imaging, such as MRI or ultrasound, or macroscopically visible. Inflammatory, and immune cells were defined as leukocytes, neutrophils, eosinophils, basophils, mast cells, macrophages, monocytes, T-lymphocytes, B-lymphocytes, NK-cells, and dendritic cells. Inflammation markers were defined as fibroblast growth factors (FGF), platelet-derived growth factor (PDGF), transforming growth factor beta superfamily proteins (TGF-beta superfamily proteins), eicosanoids, COX-1, COX-2, and cytokines. Studies investigating the presence of possible initiators of inflammation, such as bacteria, trauma, mechanical stresses, inflammatory diseases, or other proposed factors were also included.
Study selection and data collection
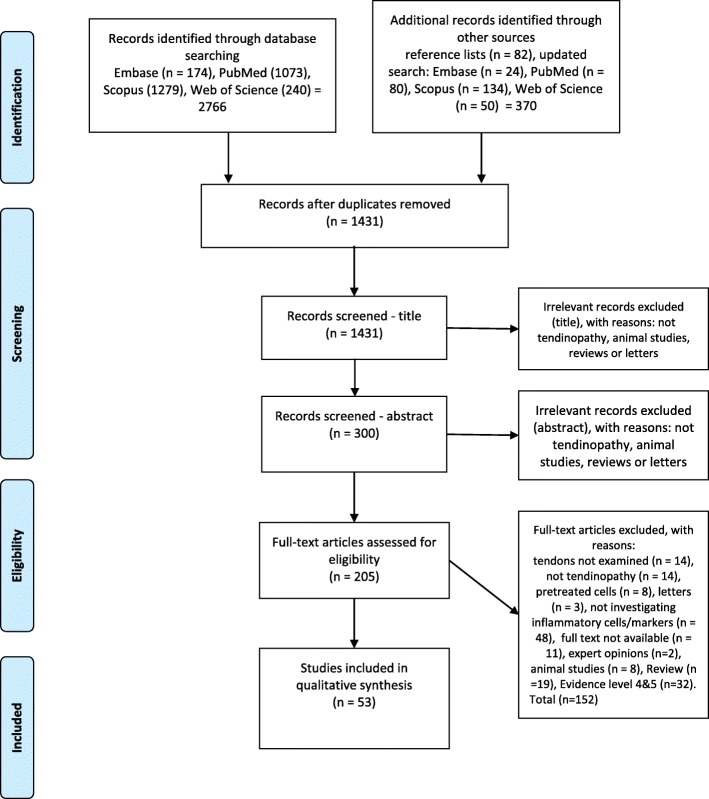
Studies from the search were merged in EndNote, and duplicates were removed. Application of exclusion, and then inclusion criteria were made by screening the titles and then the abstracts. The full texts were then obtained for the identified articles in order for data extraction. A PRISMA-flowchart of the study selection process is shown in Fig. 1. Studies were also identified by screening the reference lists.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart showing results of database search in PubMed, Scopus, Embase and Web of Science
Data collection process & data items
A data extraction table was created. The data extraction for half of the studies was performed by one review author (GJ). The data extraction for the other half of the studies was performed by the second review author (CK). Control of the data extracted were performed in the same way.
Assessment of study quality and risk of bias in individual studies
Critical Appraisal Skills Programme (CASP) [12] appraisal form was used to assess the quality of included studies. The assessment of study quality was performed in an unblinded standardized manner independently by one reviewer (GJ) and control of the assessment was then assessed by a second reviewer (CK).
Statistics
Due to the heterogeneity of the studies, e.g. study type, and outcome measures a meta-analysis could not be performed.
Results
Studies included
Using the search method mentioned above, 53 studies were included in this review. A total of 2306 tendinopathic tendon specimens were assessed. Tendon specimens were heterogenous in terms of location and presentation of tendinopathy. The details of the included studies will be included in the Table 1.
Table 1.
Data extraction table
| Study | Sample size | Presentation of tendinopathy | Additional factors associated with inflammationa | Site | QA | Method (cells) | Inflammatory cell + b | Cell types | Method (markers) | Inflammatory markers |
|---|---|---|---|---|---|---|---|---|---|---|
| Campbell 2014 [13] | 18 | Partial tear | Mean number of steroid injections 1.5 | RC | 8/11 | IHC | 100% | MP | qPCR | IL-21 receptor protein and mRNA |
| Cetti 2003 [14] | 60 | Rupture | High activity level | AT | 8/11 | IHC | 100% | NP | n/a | n/a |
| Dakin 2015 [15] | 32 | Pain or partial tear | n/a | RC | 9/11 | IHC | 100% | MP | n/a | n/a |
| Dakin 2017 [16] | 17 | Pain or rupture | n/a | AT | 7/11 | IHC | 100% | MP | qPCR, IF | IL-8 mRNA, protein |
| Hackett 2016 [17] | 39 | Calcific | n/a | RC | 9/11 | IHC | 100% | MP, TC, MC | n/a | n/a |
| Klatte-schulz 2018 [18] | 26 | Pain or ruptures | High activity level | AT | 8/11 | IHC | 88% | MP, NS | qPCR | IL-6, IL-10, IL-33. IL1B, TNFa, TGFB1, COX-2 |
| Kragsnaes 2014 [19] | 50 | Pain | 44% received steroid injection | AT | 9/11 | IHC | 96% | MP, TC, MC, NKC | n/a | n/a |
| Matthews 2006 [20] | 38 | Rupture | Mean number of steroid injections 1.8 | RC | 8/11 | IHC | 100% | MP, TC, MC | n/a | n/a |
| Millar 2010 [21] | 20 | Pain or partial tear | High activity level with mean number of 1.5 steroid injections | RC | 8/11 | IHC | 100% | MP, TC, MC | n/a | n/a |
| Millar 2012 [22] | 15 | Pain or partial tear | High activity level with mean number of 1.6 steroid injections | RC | 8/11 | IHC | 100% | MP, TC, MC | IHC, qPCR | IL-6, IL-8 protein and mRNA |
| Millar 2016 [23] | 10 | Pain or partial tear | High activity level with mean number of 1.7 steroid injections | RC | 7/11 | IHC | 100% | MP, TC, MC | qPCR | IL-17A mRNA |
| Mosca 2017 [24] | 13 | Pain | n/a | RC | 9/11 | IHC | 100% | MP | qPCR | IL-33 protein |
| Pecina 2010 [25] | 34 | Pain | n/a | PT | 8/11 | IHC | 100% | NS | n/a | n/a |
| Schubert 2005 [26] | 10 | Pain or rupture | 40% received steroid injection | AT | 6/11 | IHC | 80% | MP, TC, GC | n/a | n/a |
| Scott 2008 [27] | 22 | Pain | n/a | PT | 7/11 | IHC | 23% | MC | n/a | n/a |
| Thankam 2017 [28] | 15 | Pain or partial tear | Glenohumeral arthritis observed | LHBT | 7/11 | IHC | 27% | MP, NP | n/a | n/a |
| Åström 1995 [29] | 145 | Pain or partial tear | n/a | AT | 6/11 | H&E | 9% | NS | n/a | n/a |
| Gaida 2012 [30] | 23 | Pain | n/a | AT | 8/11 | H&E | 0% | / | ELISA | TNFa protein |
| Gumina 2006 [31] | 38 | Partial tear | n/a | RC | 10/11 | H&E | 100% | MP, LC | n/a | n/a |
| Kannus 1991 [32] | 891 | Rupture | n/a | Various | 9/11 | H&E | 0% | / | n/a | n/a |
| Khan 1996 [33] | 28 | Pain | n/a | PT | 9/11 | H&E | 0% | / | n/a | n/a |
| Lian 2007 [34] | 23 | Pain | n/a | PT | 7/11 | H&E | 0% | / | n/a | n/a |
| Ljung 1999 [35] | 6 | Pain | n/a | ECRB | 6/11 | H&E | 0% | / | n/a | n/a |
| Longo 2008 [36] | 88 | Rupture | n/a | RC | 8/11 | H&E | 0% | / | n/a | n/a |
| Longo 2009 [37] | 51 | Rupture | n/a | LHBT | 8/11 | H&E | 0% | / | n/a | n/a |
| Popp 1997 [38] | 11 | Pain | n/a | PT | 5/11 | H&E | 0% | / | n/a | n/a |
| Potter 1995 [39] | 20 | Pain | n/a | ECRB | 7/11 | H&E | 0% | / | n/a | n/a |
| Rolf 1997 [40] | 60 | Pain | 72% received NSAIDs and 27% received steroid injection | AT | 8/11 | H&E | 0% | / | n/a | n/a |
| Rolf 2017 [41] | 20 | Rupture |
Sign of infection in 40% (bacteria), 5% received steroid injection 7/20 high activity level |
AT | 9/11 | H&E | 50% | MP, TC, MC | n/a | n/a |
| Shalabi 2002 [42] | 15 | Pain | n/a | AT | 8/11. | H&E | 0% | / | n/a | n/a |
| Singaraju 2008 [43] | 6 | Pain | n/a | LHBT | 6/11 | H&E | 100% | NS | n/a | n/a |
| Tillander 2002 [44] | 23 | Pain, partial tear or rupture | n/a | RC | 6/11. | H&E | 0% | / | n/a | n/a |
| Zabrzynski 2017 [45] | 35 | Pain | n/a | LHBT | 9/11 | H&E | 9% | NS | n/a | n/a |
| Ackermann 2013 [46] | 18 | Rupture | n/a | AT | 7/11 | n/a | n/a | / | MD | IL-12, IL-17, IL-1B, IL-6, IL-8, IL-10 protein |
| Alfredson 2000 [47] | 4 | Pain | n/a | ECRB | 6/11 | n/a | n/a | / | MD | PGE2 |
| Alfredson 2003 [48] | 10 | Pain | n/a | AT | 7/11 | n/a | n/a | / | MA | IL 1–6, 10–15 mRNA |
| Alfredson 2001 [49] | 10 | Pain | n/a | PT | 7/11 | H&E | n/a | NS | MD | PGE2 |
| Alfredsson 1999 [50] | 5 | Pain | n/a | AT | 6/11 | n/a | n/a | / | MD | PGE2 |
| Chaudhury 2016 [51] | 16 | Pain | n/a | RC | 8/11 | n/a | n/a | / | qPCR | IL-8 mRNA |
| Dean 2015 [52] | 9 | Pain | 100% received steroid injection | RC | 7/11 | n/a | n/a | / | qPCR | TNF-a, IL-1b mRNA |
| Fabis 2014 [53] | 9 | Rupture | n/a | RC | 7/11 | n/a | n/a | / | qPCR | TNF-a, IL-10 mRNA |
| Fu 2002 [54] | 11 | Pain | n/a | PT | 7/11 | n/a | 0% | / | IHC, WB | COX-2, TGF-b protein, PGE2 |
| Gilmer 2015 [55] | 62 | Pain or partial tear | n/a | LHBT | 9/11 | H&E | n/a | NS | n/a | n/a |
| Jelinsky 2011 [56] | 23 | Pain, tear, or rupture | 52% received steroid injection | Various | 8/11 | n/a | 0% | / | qPCR | IL13A2, FGFR1, FGFR2, IL-17D mRNA |
| Jozsa 1980 [57] | 120 | Rupture or calcific | n/a | Various | 8/11 | H&E | n/a | NS | n/a | n/a |
| Legerlotz 2012 [58] | 20 | Pain or rupture | n/a | AT PT | 7/11 | n/a | n/a | / | qPCR | COX-2, IL6, IL6R mRNA |
| Millar 2015 [59] | 17 | Pain or partial tear | n/a | RC | 8/11 | n/a | n/a | / | qPCR | IL-33 mRNA |
| Pingel 2012 [60] | 14 | Pain | 100% received steroid injection | AT | 8/11 | n/a | n/a | / | qPCR | COX-1, IL-1R, TGF-B1, bFGF mRNA |
| Pingel 2013 [61] | 27 | Pain | 100% received steroid injection | AT | 8/11 | n/a | n/a | / | qPCR | IL-1b, IL-6, IL-10, COX-2, TGF-b, TNF-a mRNA |
| Robertson 2012 [62] | 35 | Partial tear or rupture | n/a | RC | 8/11 | n/a | n/a | / | qPCR | IL-1b, IL-6, TNF-a, COX-2 mRNA |
| Shindle 2011 [63] | 24 | Partial tear or Rupture | Joint inflammation | RC | 8/11 | n/a | n/a | / | qPCR | IL-1b, IL-6, COX-2, TNF-a mRNA |
| Takeuchi 2001 [64] | 7 | Calcific | n/a | RC | 5/11 | IHC | n/a | MP | n/a | n/a |
| Waugh 2015 [65] | 10 | Pain | n/a | AT PT | 7/11 | n/a | n/a | / | MD | IL-1b, IL-2, IL-6,IL-8, IL-10 protein |
Table sorted by method used to detect the presence of inflammatory cells
Locations: AT Achilles tendon, PT patellar tendon, RC rotator cuff tendon, QT Quadriceps tendon, LHBT Long head biceps tendon, ECRB extensor carpi radialis brevis tendon, CF Common flexor tendon, CE Common extensor tendon, various (studies with specimens from more than 3 locations)
Cells: MP Macrophages, MC Mast-cells, TC T-cells, LC Lymphocytes, NP Neutrophils, GC granulocytes, NKC NK-cells, NS not specified
Detection method: H&E Hematoxylin & Eosin, qPCR quantitative polymerase chain reaction, IHC immunohistochemistry, IF immunofluorescence, MD Micro-dialysis, WB western blot, EIA enzyme immunoassay, ELISA Enzyme-Linked ImmunoSorbent Assay
a Additional factors associated with inflammation description on patient subgroup and previous treatments
bInflammatory cell + refers to the percentage of specimens showing at least 1 kind of inflammatory cells, regardless of typing
Methodological quality assessment
The results of the quality assessment are shown in the Table 1. The evidence level of included studies was assessed according to the Oxford Centre of Evidence based Medicine (OCEBM) [66]. All included studies included level 3 evidence (n = 53) regarding the presence of inflammatory cells in tendinopathic tendons. 50/53 studies scored 6 or higher out of 11 in CASP. It is notable that included studies have varying objectives, hence the results from quality assessment may not directly reflect how strictly inflammation in tendinopathic specimens were assessed.
Signs of inflammation in tendinopathic tendons
Among the 53 included studies, 39 studies showed signs of inflammation in tendinopathic tendons, including the presence of inflammatory cells or an increase in inflammatory markers. The expression of inflammation does not seem to be correlated with any obvious confounders including site, presence of rupture, chronicity, or previous treatment of corticosteroid injections.
Twenty-five studies reported the presence of inflammation in the tendon specimens, and 14 suggested the absence of inflammatory cells. By using H&E to assess for inflammatory cells, tendinopathic tendons presenting with inflammatory cells were reported to range from 0 to 100% (mean 16%) between studies. For studies adapting IHC to stain for inflammatory cells, tendinopathic tendons showing inflammatory cells were reported to be 23 to 100% between studies (mean 88%).
For the 25 studies which supported the presence of inflammatory cells, 7 of the studies did not specify which types of cells were observed. The most common cell type was macrophages, found in 16 studies. Other cells include lymphocytes in 2 studies, mast cells in 8 studies, and granulocytes in 3 studies.
Twenty-two studies performed assessment on related inflammatory markers including at least one of the markers mentioned above. All studies indicated increased levels of some inflammatory markers measured. Inflammatory markers reported include IL-1. IL-6, IL-8, IL-10, IL-17, IL-33, COX-1, COX-2, TGF-b, TNF-a, FGF and more. A full list of inflammatory mediators detected can be found in the Table 1.
Additional factors associated with inflammation
A variety of tendinopathic cases were included from the search, in terms of different locations and presentations.
It is also worth mentioning that data such as previous conservative treatments and activity levels were often non-recorded, or is inconsistent between the patients in the included studies. 19/53 studies included such additional description to the population sub-group that may influence the inflammatory status. In 12 studies, patients have undergone corticosteroid injection. In 5 studies, tendinopathic patients were reported to have experienced high activity level and overuse. In 2 studies, inflammation to the neighbouring structures such as glenohumeral arthritis or joint inflammation were identified as a source of inflammation affecting the tendon. In 1 study, bacteria was identified as a possible source of inflammation. In 1 study, there was a reported use of NSAIDs the recruited tendinopathic patients. Nevertheless, inflammation may be observed in tendinopathic tendons of any size of tear, location, or tendons previously treated with corticosteroid injection.
Discussion
Limitations
Heterogenicity of tendinopathic cases is a major limitation of the current review. In this review we included cases with a range of presentations from chronic pain to ruptured cases. Chronicity of the tendon disorder also varied greatly, which when combined may have a great impact on the presentation of inflammation in these specimens included.
Heterogenicity of the detection methods in both the presence of inflammatory cells and inflammatory markers were also significant in this review, making included studies less comparable. However, by comparing the results from different detection methods, we were able to identify a possible explanation in the emergence of the debate on whether chronic inflammation is present in tendinopathic tendons. This will be further discussed in the following section.
Heterogeneity of control specimens is also a limitation of the consistency between studies. In some cases, specimens from macroscopically healthy areas of tendinopathic tendons have been used as controls [60]. This practice is not recommended since it is possible that the whole tendon is affected by tendinopathy [67]. In other studies, the contralateral tendon has been used as control, even though some evidence suggests that unilateral rupture is preceded by bilateral damage [14]. A seemingly healthy tendon could be subclinical and asymptomatic. Tendons from a different anatomical location have also been used as controls [67]. There is a possibility that tendons found on different anatomical localization have a divergent biomechanical construction, due to the fact that the tendons are involved in different movements. Lastly, for several studies, specimens from cadavers have been used as controls. Tendon specimens from this group most likely represents a true healthy tendon. The inconsistency in control groups may have a particularly high impact when comparing inflammatory markers presented in tendinopathic tendons.
The sampling of tendinopathic specimens presenting in different stages was also difficult. Specimens from the debridement of ruptured tendons is common, but these specimens could only represent cases of chronic tendinopathy with an acute insult of tendon rupture. Some studies identified earlier stages of tendinopathy with various methods. There were articles which sampled the macroscopically intact sub-scapularis tendon beside a ruptured supraspinatus [21, 22, 59], and there was another study which defined early pathology as an impinged tendon sampled during acromial decompression [15]. Tendon specimens obtained from the listed protocols mostly represent cases which have likely received and failed other modalities of conservative treatment. Tendinopathy that would respond to conservative treatment also comprise a large part of the total cases. However, tendon specimens were never obtained for these cases due to ethical reasons.
Another limitation is that the demographic data of tendinopathic patients were not reported consistently between studies. For example, it is suspected that the usage of NSAIDs or corticosteroid injections could have a direct effect on the presentation of local inflammation within the tendon. Activity level also have potential effects on the presentation. However, these information is often neglected.
These concerns however reflect the current standpoint in this area of research. With the lack of studies with higher evidence level, a systematic review of highest quality is currently not feasible.
Publication bias
With a previous understanding that tendinopathy is a degenerative disease with an absence of inflammation, it is possible that studies showing an inconsistent result will more likely be published. However, due to the great heterogenicity of the studies included in this review, plotting a funnel plot could not effectively identify the presence or absence of existing publication bias. Only publications in databases have been covered by our study, therefore the coverage of “grey literature” has not been evaluated [68].
Signs of inflammation were present in the majority of tendinopathic tendons
According to our search results, signs of inflammation, including either the presence of inflammatory cells or an increase in inflammatory markers, were observed in 39 out of 53 studies. One common feature of the studies which reported an absence of inflammation was that the only method used was to identify inflammatory cells with H&E staining.
Considering the comparable clinical presentation, sampling method and staining method shared across the studies, we highly suspect the diagnosing accuracy of using H&E staining in the identification of inflammatory cells. As mentioned in a previous study [13], though there was an increase in macrophages present in chronic tendinopathic tendons, tenocytes still make up the majority of cells within the tendon. As macrophages make up less than 10% of the total population, it can be difficult to identify inflammatory cells in a crowded background of tenocytes. The previous thought that inflammation is absent in chronic tendinopathy could be a result of sub-optimal detection with an inappropriate method.
Inflammatory cell types indicate chronic inflammation in tendinopathic tendons
Inflammatory cells observed in chronic tendinopathy were macrophages, lymphocytes, mast cells, and in some rare occasions, granulocytes. Except for granulocytes, the cell types indicate the state of inflammation as a chronic inflammation.
Macrophages are well known for its role in phagocytosis of infectious organisms [69]. However, the contribution of this cell type extends to many other systems including bone remodelling, erythropoiesis, brain and lung development [69]. It was also reported that macrophages play an important role in the regulation of inflammation [70]. Resident macrophages initiate the inflammatory response towards injury by recognizing damage associated molecular patterns (DAMPs) [69]. The process is followed by secretion of cytokines and eicosanoids, resulting in recruitment of inflammatory cells, with neutrophils being the first to enter the site [69]. The resolution of inflammation is also closely related to macrophage activity. The change in phenotype from M1 to a M2-like phenotype macrophage leads to a phenomenon known as the lipid mediator class switch [70].
Mast cells could have a significant role in tissue remodelling too. As reported in a previous review on mast cell physiology, it was reported that mast cell deficient mice initially had intact hair growth and bone density. However, defects were observed in case of injury, and tissue remodelling of the hair follicles and bone tissues were not comparable to that of healthy samples [71]. In another animal model on tendon injury, it was found that there were an increased expression of mast cells and myofibroblasts during the tendon healing process [72].
Lymphocytes are also known to be present in many autoimmune, inflammatory diseases such as Hashimoto thyroiditis and psoriasis [73]. It was hypothesized that the exaggerated recruitment of these cell types lead to un-controlled activation of macrophages, leading to excessive damage in cells and architecture [73]. It is possible that the same mechanism applies to tendons, where excessive lymphocyte activity could damage the extracellular matrices.
Granulocytes including neutrophils and eosinophils were reported in rare occasions. As mentioned above, neutrophils are the first cells to be recruited upon macrophage activation [69]. The presence of this cell type is indicative of an acute inflammatory state, reported only in cases of rupture.
Inflammatory markers show an inconclusive pattern with current information
Unlike the information provided by the presence of inflammatory cells, current evidence on the inflammation markers shown in tendinopathic tendons do not show a consistent picture. As summarized in a review in 1997 [74], inflammatory mediators contribute to inflammation via complex pathways, but common mediators can be categorized to be present in acute inflammation, chronic inflammation, or both. In this review, the inflammatory mediators reported were a mixture of mediators in the acute, chronic and common group. It is also notable that inflammatory markers assessed vary greatly between studies. It is therefore very difficult to deduce the character of inflammation in chronic tendinopathy with the current information on detectable inflammatory markers.
However, study of the inflammatory markers in tendinopathic patients could potentially be of high importance. As mentioned in the limitations, the sampling of tendinopathic tendons in different stages could be challenging due to the invasive nature of tendon sampling. However, with increasing evidence that tendinopathy is associated with chronic inflammation, there may be systemic changes detectable as inflammatory markers in more assessible specimens, such as blood cells. Investigation on the systemic changes in tendinopathic patients could be a rewarding field of study.
Possible explanations to varying presentation of inflammation between tendinopathic tendons
Causes of inflammation in tendinopathic tendons is currently unclear, however, 19/53 studies mentioned additional description to the recruited tendinopathic population, which may provide insights on whether the presentation of inflammation maybe limited to certain sub-groups. From our observations, inflammation is a character that often occur in chronic tendinopathy, regardless of location and chronicity. The presence of inflammation was also independent with previous treatments including steroid injection and NSAIDs. It was also directly mentioned in one included study that there were no association between steroid injections and the presentation of inflammation [20]. The previous understanding that chronic tendinopathy is an inflammation free disease may be due to low sensitivity of classical staining techniques using H&E. However, attention is drawn to two studies which reported a mere 20% rate of the presence of inflammatory cells in tendinopathic tendons despite adapting IHC for detection of related CD markers [27, 28]. The pattern is yet to be explained regarding the varying presentation of inflammation between tendinopathic tendons with similar clinical presentation.
One cause of inconsistency could be the existence of different stages to tendon injury. In some reports of acute tendon rupture, neutrophils and other granulocytes were reported present, likely acting as an acute response to trauma. However, current literature could not facilitate an educated deduction of factors associated with the presentation of inflammation. The cause, stage, and conservative managements received to treat tendinopathy could be some factors associated with the presentation of inflammation. Future studies with high quality are necessary to identify these variables.
The involvement of bacteria was also mentioned in some cases of tendinopathy. Although rarely assessed, there is a possibility that bacterial infection may play an important role in the presentation of chronic inflammation. Three studies which identified the presence of bacteria were excluded due to evidence level lower than 3. The Bacterial species identified in these studies were Mycobacterium tuberculosis [75, 76] and Borrelia [77]. One of the included studies identified staphylococcus genus as a possible initiating factor of inflammation [41]. In this study, the blood samples from the patients were negative for bacteria contrary to the presence of bacteria in the tendons. This suggests that the presence of bacteria was local to the AT. However, it is important to emphasize that the tendinopathic changes could favour the presence of bacteria and consequently the presence of bacteria in tendinopathic tissue may be secondary, as a result of the favouring environment. Further investigation into this topic may be a rewarding frontier.
The involvement of metabolic diseases associated with tendinopathy was also mentioned in several excluded case reports [78, 79]. The significance of such underlying disorders may also contribute to the varying presentation of inflammation observed. This concept is supported by previous reports that metabolic disorders including diabetes [80], obesity [81], gout [82], and hypothyroidism [83] can increase the risk of the development of tendinopathy. A systematic review in 2016 showed that various hormone receptors in tenocytes can be affected by hormonal imbalances of insulin, estrogens, thyroid hormones and growth hormone [84]. Hormone profile greatly impacts the inflammatory pathway, and one of such impacts is illustrated in a review describing inflammatory changes in diabetic and obese patients. Migration of inflammatory cells to adipose tissues lead to decreased availability and less effective healing in the tendon. Glycation of collagen and impaired cross linkage also contribute to sub-optimal healing [85]. As metabolic disorders impact on the hormonal profile differently, it is expected that inflammation following the metabolic insults may also vary.
Consistency and inconsistency with existing literature
According to an editorial in 1998 [7], the most prominent lesion in chronic tendinopathies is a degenerative process with an absence of inflammatory cells [7]. It was acknowledged that histological findings were inconsistent, and that signs of inflammation were present in some of the tendon specimens. The explanation towards this phenomenon was that inflammation could be the “primum movens” of the development of tendinopathy. It was believed that the transient inflammatory state would ultimately lead to the typical presentation of tendinosis, with an absence of inflammation [7]. In simplified terms, it was suggested in this study that active inflammation, if any, should only occur at early stages of the development of tendinopathy. Search results from this systematic review do not fully agree with the classical study. Presence of inflammatory cells were reported in chronic tendinopathy in the included studies.
It is acknowledged that the current review is not the first to discuss on the presence of inflammation in chronic tendinopathy. A recent systematic review published in 2016 [86] also reached a similar conclusion. Quoting from the review, “the absence of inflammation in tendinopathy were more based on belief rather than scientific data”. The current review agrees with the idea that inflammation may be present, but the findings suggest that its presentation may not be as straightforward. By performing a more sophisticated search with more included, relevant studies, we were able to more subjectively assess the inconsistent presentation of inflammation in chronic tendinopathy between studies. Chronic inflammation is present in the majority of chronic tendinopathy using specific staining techniques, but there are exceptions which do not show positive staining of any inflammatory cells.
Clinical significance
Tendinopathy is previously understood as a degenerative disorder with an absence of inflammation [37]. According to this review, it is likely that a chronic inflammation can be present in tendinopathic tendons. Therefore, the anti-inflammatory approach may well be supported in the conservative management of tendinopathy.
However, this does not mean that the current management strategy is flawless. Publications on the efficacy of current management strategies on tendinopathy have also shown that anti-inflammatory treatments like NSAIDs or corticosteroids only provide a short term relief of symptoms, and may have a negative impact on its structural healing [87, 88]. This result is consistent to the current finding since anti-inflammatory drugs suppress the inflammatory state to achieve pain relief. However, the effects do not last since chronic inflammation can be caused by the presence of a persistent stimulus, or a deranged cellular function to resolve inflammation.
The inhibition of the inflammatory process may also negatively impact the natural healing process of tendon healing, leading to further degenerative changes. This concept is supported by previous literature on the balance of metalloproteinases (MMPs) and its significance in maintaining healthy tendon homeostasis. MMP levels are regulated by inflammation [89], and it was mentioned that balanced MMP activity play an important role in maintaining structural integrity of tendons through constant extra-cellular matrix (ECM) remodelling [90, 91]. Inhibition to inflammation may in turns inhibit tendon homeostasis, leading to poor integrity in long term.
The cause leading to chronic inflammation could thus be multifactorial and varying among tendinopathic patients. However, rather than straightforward inhibition to the inflammatory process with NSAIDs or glucocorticoids, identification of factors leading to chronic inflammation, and corresponding targeted treatment could be the key in solving the burden caused by this common but chronic disease.
Future studies
Current literature is insufficient to deduce a common pattern in regard to the presence of inflammation in chronic tendinopathy. To improve our understanding on this issue, more high quality studies with a large sample size and comparable detection methods is required. Known confounding factors such as activity level, previous history of anti-inflammatory treatment, and the chronicity of the tendon disorder must also be consistently documented in future for valuable comparison.
Also, it is acknowledged that the sampling of tendon samples with different stages of tendinopathy and confounding factors such as metabolic disease could be challenging. A variety of potentially triggering factors of inflammation in tendinopathy have been shown in this study, such as overuse, associated inflammatory and metabolic diseases, and bacterial involvement.
Conclusion
This review suggest that inflammatory cells are observed in a proportion of tendinopathic tendons but not in all. Further controlled studies using comparable methods and sufficient sample sizes for various phases of tendon symptomatology are needed to allow any firm conclusion in regard to a potential common presentation of inflammation, and common pathway for the development of Tendinopathy.
Acknowledgements
Special appreciation to Norseen Malki for the translation of manuscripts in a foreign language.
Abbreviations
- CASP
Critical Appraisal Skill Program
- CD
Cluster of differentiation
- CEBM
Oxford Centre of Evidence based Medicine
- COX
Cyclooxygenase
- DAMP
Damage associated molecular pattern
- FGF
Fibroblast growth factor
- H&E
Hematoxylin and eosin (stain)
- IHC
Immunohistochemistry
- IL
Interleukin
- MRI
Magnetic Resonance Imaging
- NSAID
Non-steroidal anti-inflammatory drug
- PDGF
Platelet derived growth factor
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- TGFb
Transforming growth factor beta
Authors’ contributions
GJ designed the study with supervision from SCF. GJ and CKK performed data collection and analysis with supervision from SCF. CKK and GJ drafted the text with supervision from SCF. SKKL drafted the abstract. SCF, KMC, PSHY and CR read, correct and approved the various drafts up to the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors, and no material support of any kind was received.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
George Jomaa and Cheuk-Kin Kwan contributed equally to this work.
References
- 1.Hopkins C, Fu S-C, Chua E, Hu X, Rolf C, Mattila VM, et al. Critical review on the socio-economic impact of tendinopathy. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2016;4:9–20. doi: 10.1016/j.asmart.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brox JI, Gjengedal E, Uppheim G, Bøhmer AS, Brevik JI, Ljunggren AE, et al. Arthroscopic surgery versus supervised exercises in patients with rotator cuff disease (stage II impingement syndrome): a prospective, randomized, controlled study in 125 patients with a 2 1/2-year follow-up. J Shoulder Elb Surg. 1999;8(2):102–111. doi: 10.1016/S1058-2746(99)90001-0. [DOI] [PubMed] [Google Scholar]
- 3.Seitz AL, McClure PW, Finucane S, Boardman ND, Michener LA. Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech. 2011;26(1):1–12. doi: 10.1016/j.clinbiomech.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Fu S-C, Rolf C, Cheuk Y-C, Lui PP, Chan K-M. Deciphering the pathogenesis of tendinopathy: a three-stages process. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:30. doi: 10.1186/1758-2555-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andarawis-Puri N, Flatow EL, Soslowsky LJ. Tendon basic science: development, repair, regeneration, and healing. J Orthop Res. 2015;33(6):780–784. doi: 10.1002/jor.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torstensen ET, Bray RC, Wiley JP. Patellar tendinitis: a review of current concepts and treatment. Clin J Sport Med. 1994;4(2):77. doi: 10.1097/00042752-199404000-00002. [DOI] [Google Scholar]
- 7.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14(8):840–843. doi: 10.1016/S0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 8.Li HY, Hua YH. Achilles Tendinopathy: current concepts about the basic science and clinical treatments. Biomed Res Int. 2016;2016:6492597. doi: 10.1155/2016/6492597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees JD, Stride M, Scott A. Tendons – time to revisit inflammation. Br J Sports Med. 2014;48(21):1553–1557. doi: 10.1136/bjsports-2012-091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battery L, Maffulli N. Inflammation in overuse tendon injuries. Sports Med Arthrosc Rev. 2011;19(3):213–217. doi: 10.1097/JSA.0b013e31820e6a92. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadelson S, Nadelson L. Evidence-based practice article reviews using CASP tools: a method for teaching EBP. Worldviews Evid Based Nurs. 2014;1:11. doi: 10.1111/wvn.12059. [DOI] [PubMed] [Google Scholar]
- 13.Campbell AL, Smith NC, Reilly JH, Kerr SC, Leach WJ, Fazzi UG, et al. IL-21 receptor expression in human tendinopathy. Mediat Inflamm. 2014;2014:481206. doi: 10.1155/2014/481206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cetti R, Junge J, Vyberg M. Spontaneous rupture of the Achilles tendon is preceded by widespread and bilateral tendon damage and ipsilateral inflammation - a clinical and histopathologic study of 60 patients. Acta Orthop Scand. 2003;74(1):78–84. doi: 10.1080/00016470310013707. [DOI] [PubMed] [Google Scholar]
- 15.Dakin SG, Martinez FO, Yapp C, Wells G, Oppermann U, Dean BJF, et al. Inflammation activation and resolution in human tendon disease. Sci Transl Med. 2015;7(311):311ra173. doi: 10.1126/scitranslmed.aac4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dakin SG, Newton J, Martinez FO, Hedley R, Gwilym S, Jones N, et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med. 2017. 10.1136/bjsports-2017-098161. [DOI] [PMC free article] [PubMed]
- 17.Hackett L, Millar NL, Lam P, Murrell GA. Are the symptoms of calcific tendinitis due to Neoinnervation and/or neovascularization? J Bone Joint Surg Am. 2016;98(3):186–192. doi: 10.2106/JBJS.O.00417. [DOI] [PubMed] [Google Scholar]
- 18.Klatte-Schulz Franka, Minkwitz Susann, Schmock Aysha, Bormann Nicole, Kurtoglu Alper, Tsitsilonis Serafeim, Manegold Sebastian, Wildemann Britt. Different Achilles Tendon Pathologies Show Distinct Histological and Molecular Characteristics. International Journal of Molecular Sciences. 2018;19(2):404. doi: 10.3390/ijms19020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kragsnaes MS, Fredberg U, Stribolt K, Kjaer SG, Bendix K, Ellingsen T. Stereological quantification of immune-competent cells in baseline biopsy specimens from achilles tendons: results from patients with chronic tendinopathy followed for more than 4 years. Am J Sports Med. 2014;42(10):2435–2445. doi: 10.1177/0363546514542329. [DOI] [PubMed] [Google Scholar]
- 20.Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88(4):489–495. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 21.Millar NL, Hueber AJ, Reilly JH, Xu Y, Fazzi UG, Murrell GAC, et al. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010;38(10):2085–2091. doi: 10.1177/0363546510372613. [DOI] [PubMed] [Google Scholar]
- 22.Millar NL, Reilly JH, Kerr SC, Campbell AL, Little KJ, Leach WJ, et al. Hypoxia: a critical regulator of early human tendinopathy. Ann Rheum Dis. 2012;71(2):302–310. doi: 10.1136/ard.2011.154229. [DOI] [PubMed] [Google Scholar]
- 23.Millar NL, Akbar M, Campbell AL, Reilly JH, Kerr SC, McLean M, et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci Rep. 2016;6:27149. doi: 10.1038/srep27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosca MJ, Carr AJ, Snelling SJB, Wheway K, Watkins B, Dakin SG. Differential expression of alarmins-S100A9, IL-33, HMGB1 and HIF-1α in supraspinatus tendinopathy before and after treatment. BMJ Open Sport Exerc Med. 2017;3(1):e000225. doi: 10.1136/bmjsem-2017-000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pecina M, Bojanic I, Ivkovic A, Brcic L, Smoljanovic T, Seiwerth S. Patellar tendinopathy: Histopathological examination and follow-up of surgical treatment. Acta Chir Orthop Traumatol Cechoslov. 2010;77(4):277–283. [PubMed] [Google Scholar]
- 26.Schubert TEO, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64(7):1083–1086. doi: 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott A, Lian O, Roberts CR, Cook JL, Handley CJ, Bahr R, et al. Increased versican content is associated with tendinosis pathology in the patellar tendon of athletes with jumper’s knee. Scand J Med Sci Sports. 2008;18(4):427–435. doi: 10.1111/j.1600-0838.2007.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thankam Finosh G., Boosani Chandra S., Dilisio Matthew F., Agrawal Devendra K. MicroRNAs associated with inflammation in shoulder tendinopathy and glenohumeral arthritis. Molecular and Cellular Biochemistry. 2017;437(1-2):81–97. doi: 10.1007/s11010-017-3097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Astrom M, Rausing A. Chronic Achilles tendinopathy - a survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995;316:151–164. doi: 10.1097/00003086-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Gaida JE, Bagge J, Purdam C, Cook J, Alfredson H, Forsgren S. Evidence of the TNF-alpha system in the human Achilles tendon: expression of TNF-alpha and TNF receptor at both protein and mRNA levels in the Tenocytes. Cells Tissues Organs. 2012;196(4):339–352. doi: 10.1159/000335475. [DOI] [PubMed] [Google Scholar]
- 31.Gumina S, Di Giorgio G, Bertino A, Della Rocca C, Sardella B, Postacchini F. Inflammatory infiltrate of the edges of a torn rotator cuff. Int Orthop. 2006;30(5):371–374. doi: 10.1007/s00264-006-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507–1525. doi: 10.2106/00004623-199173100-00009. [DOI] [PubMed] [Google Scholar]
- 33.Khan KM, Bonar F, Desmond PM, Cook JL, Young DA, Visentini PJ, et al. Patellar tendinosis (jumper’s knee): findings at histopathologic examination, US, and MR imaging. Victorian Institute of Sport Tendon Study Group. Radiology. 1996;200(3):821–827. doi: 10.1148/radiology.200.3.8756939. [DOI] [PubMed] [Google Scholar]
- 34.Lian O, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35(4):605–611. doi: 10.1177/0363546506295702. [DOI] [PubMed] [Google Scholar]
- 35.Ljung BO, Forsgren S, Friden J. Substance P and calcitonin gene-related peptide expression at the extensor carpi radialis brevis muscle origin: implications for the etiology of tennis elbow. J Orthop Res. 1999;17(4):554–559. doi: 10.1002/jor.1100170414. [DOI] [PubMed] [Google Scholar]
- 36.Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, et al. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med. 2008;36(3):533–538. doi: 10.1177/0363546507308549. [DOI] [PubMed] [Google Scholar]
- 37.Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, et al. Characteristics at haematoxylin and eosin staining of ruptures of the long head of the biceps tendon. Br J Sports Med. 2009;43(8):603–607. doi: 10.1136/bjsm.2007.039016. [DOI] [PubMed] [Google Scholar]
- 38.Popp JE, Yu JS, Kaeding CC. Recalcitrant patellar tendinitis. Magnetic resonance imaging, histologic evaluation, and surgical treatment. Am J Sports Med. 1997;25(2):218–222. doi: 10.1177/036354659702500214. [DOI] [PubMed] [Google Scholar]
- 39.Potter HG, Hannafin JA, Morwessel RM, DiCarlo EF, O’Brien SJ, Altchek DW. Lateral epicondylitis: correlation of MR imaging, surgical, and histopathologic findings. Radiology. 1995;196(1):43–46. doi: 10.1148/radiology.196.1.7784585. [DOI] [PubMed] [Google Scholar]
- 40.Rolf C, Movin T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot & Ankle International. 1997;18(9):565–569. doi: 10.1177/107110079701800906. [DOI] [PubMed] [Google Scholar]
- 41.Rolf CG, Fu S-C, Hopkins C, Luan J, Ip M, Yung S-H, et al. Presence of Bacteria in spontaneous Achilles tendon ruptures. Am J Sports Med. 2017;45(9):2061–2067. doi: 10.1177/0363546517696315. [DOI] [PubMed] [Google Scholar]
- 42.Shalabi A, Kristoffersen-Wiberg M, Papadogiannakis N, Aspelin P, Movin T. Dynamic contrast-enhanced mr imaging and histopathology in chronic achilles tendinosis. A longitudinal MR study of 15 patients. Acta Radiol. 2002;43(2):198–206. doi: 10.1080/028418502127347781. [DOI] [PubMed] [Google Scholar]
- 43.Singaraju VM, Kang RW, Yanke AB, McNickle AG, Lewis PB, Wang VM, et al. Biceps tendinitis in chronic rotator cuff tears: a histologic perspective. J Shoulder Elb Surg. 2008;17(6):898–904. doi: 10.1016/j.jse.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 44.Tillander B, Franzen L, Norlin R. Fibronectin, MMP-1 and histologic changes in rotator cuff disease. J Orthop Res. 2002;20(6):1358–1364. doi: 10.1016/S0736-0266(02)00057-8. [DOI] [PubMed] [Google Scholar]
- 45.Zabrzyński J, Paczesny Ł, Łapaj Ł, Grzanka D, Szukalski J. Is the inflammation process absolutely absent in tendinopathy of the long head of the biceps tendon? Histopathologic study of the long head of the biceps tendon after arthroscopic treatment. Pol J Pathol. 2017;68(4):318–325. doi: 10.5114/pjp.2017.73928. [DOI] [PubMed] [Google Scholar]
- 46.Ackermann PW, Domeij-Arverud E, Leclerc P, Amoudrouz P, Nader GA. Anti-inflammatory cytokine profile in early human tendon repair. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1801–1806. doi: 10.1007/s00167-012-2197-x. [DOI] [PubMed] [Google Scholar]
- 47.Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique--no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand. 2000;71(5):475–479. doi: 10.1080/000164700317381162. [DOI] [PubMed] [Google Scholar]
- 48.Alfredson H, Lorentzon M, Backman S, Backman A, Lerner UH. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. J Orthop Res. 2003;21(6):970–975. doi: 10.1016/S0736-0266(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 49.Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. J Orthop Res. 2001;19(5):881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- 50.Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):378–381. doi: 10.1007/s001670050184. [DOI] [PubMed] [Google Scholar]
- 51.Chaudhury S, Xia Z, Thakkar D, Hakimi O, Carr AJ. Gene expression profiles of changes underlying different-sized human rotator cuff tendon tears. J Shoulder Elb Surg. 2016;25(10):1561–1570. doi: 10.1016/j.jse.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 52.Dean BJ, Snelling SJ, Dakin SG, Murphy RJ, Javaid MK, Carr AJ. Differences in glutamate receptors and inflammatory cell numbers are associated with the resolution of pain in human rotator cuff tendinopathy. Arthritis Res Ther. 2015;17:176. doi: 10.1186/s13075-015-0691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fabis J, Szemraj J, Strek M, Fabis A, Dutkiewicz Z, Zwierzchowski TJ. Is resection of the tendon edge necessary to enhance the healing process? An evaluation of the homeostasis of apoptotic and inflammatory processes in the distal 1 cm of a torn supraspinatus tendon: part I. J Shoulder Elb Surg. 2014;23(12):1772–1778. doi: 10.1016/j.jse.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Fu SC, Wang W, Pau HM, Wong YP, Chan KM, Rolf CG. Increased expression of transforming growth factor-??1 in patellar Tendinosis. Clin Orthop Relat Res. 2002;400:174–183. doi: 10.1097/00003086-200207000-00022. [DOI] [PubMed] [Google Scholar]
- 55.Gilmer BB, DeMers AM, Guerrero D, Reid JB, Lubowitz JH, Guttmann D. Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy. 2015;31(1):29–34. doi: 10.1016/j.arthro.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 56.Jelinsky SA, Rodeo SA, Li J, Gulotta LV, Archambault JM, Seeherman HJ. Regulation of gene expression in human tendinopathy. BMC Musculoskelet Disord. 2011;12:86. doi: 10.1186/1471-2474-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jozsa L, Balint BJ, Reffy A. Calcifying tendinopathy. Arch Orthop Trauma Surg. 1980;97(4):305–307. doi: 10.1007/BF00380713. [DOI] [PubMed] [Google Scholar]
- 58.Legerlotz K, Jones ER, Screen HR, Riley GP. Increased expression of IL-6 family members in tendon pathology. Rheumatology (Oxford) 2012;51(7):1161–1165. doi: 10.1093/rheumatology/kes002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Millar NL, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, et al. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat Commun. 2015;6:6774. doi: 10.1038/ncomms7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pingel J, Fredberg U, Qvortrup K, Larsen JO, Schjerling P, Heinemeier K, et al. Local biochemical and morphological differences in human Achilles tendinopathy: a case control study. BMC Musculoskelet Disord. 2012;13:53. doi: 10.1186/1471-2474-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pingel J, Fredberg U, Mikkelsen LR, Schjerling P, Heinemeier KM, Kjaer M, et al. No inflammatory gene-expression response to acute exercise in human Achilles tendinopathy. Eur J Appl Physiol. 2013;113(8):2101–2109. doi: 10.1007/s00421-013-2638-3. [DOI] [PubMed] [Google Scholar]
- 62.Robertson CM, Chen CT, Shindle MK, Cordasco FA, Rodeo SA, Warren RF. Failed healing of rotator cuff repair correlates with altered collagenase and gelatinase in supraspinatus and subscapularis tendons. Am J Sports Med. 2012;40(9):1993–2001. doi: 10.1177/0363546512456519. [DOI] [PubMed] [Google Scholar]
- 63.Shindle MK, Chen CCT, Robertson C, DiTullio AE, Paulus MC, Clinton CM, et al. Full-thickness supraspinatus tears are associated with more synovial inflammation and tissue degeneration than partial-thickness tears. J Shoulder Elb Surg. 2011;20(6):917–927. doi: 10.1016/j.jse.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeuchi E, Sugamoto K, Nakase T, Miyamoto T, Kaneko M, Tomita T, et al. Localization and expression of osteopontin in the rotator cuff tendons in patients with calcifying tendinitis. Virchows Arch. 2001;438(6):612–617. doi: 10.1007/s004280000367. [DOI] [PubMed] [Google Scholar]
- 65.Waugh CM, Morrissey D, Jones E, Riley GP, Langberg H, Screen HR. In vivo biological response to extracorporeal shockwave therapy in human tendinopathy. Eur Cell Mater. 2015;29:268–280. doi: 10.22203/eCM.v029a20. [DOI] [PubMed] [Google Scholar]
- 66.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–310. doi: 10.1097/PRS.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chillemi C, Petrozza V, Franceschini V, Garro L, Pacchiarotti A, Porta N, et al. The role of tendon and subacromial bursa in rotator cuff tear pain: a clinical and histopathological study. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3779–3786. doi: 10.1007/s00167-015-3650-4. [DOI] [PubMed] [Google Scholar]
- 68.Mahood Q, Van Eerd D, Irvin E. Searching for grey literature for systematic reviews: challenges and benefits. Res Synth Methods. 2014;5(3):221–234. doi: 10.1002/jrsm.1106. [DOI] [PubMed] [Google Scholar]
- 69.Gordon S, Martinez-Pomares L. Physiological roles of macrophages. Pflugers Arch. 2017;469(3):365–374. doi: 10.1007/s00424-017-1945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dakin Stephanie Georgina, Werling Dirk, Hibbert Andrew, Abayasekara Dilkush Robert Ephrem, Young Natalie Jayne, Smith Roger Kenneth Whealands, Dudhia Jayesh. Macrophage Sub-Populations and the Lipoxin A4 Receptor Implicate Active Inflammation during Equine Tendon Repair. PLoS ONE. 2012;7(2):e32333. doi: 10.1371/journal.pone.0032333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Behzad H, Sharma A, Mousavizadeh R, Lu A, Scott A. Mast cells exert pro-inflammatory effects of relevance to the pathophyisology of tendinopathy. Arthritis Res Ther. 2013;15(6):R184. doi: 10.1186/ar4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang C, Chen Y, Huang J, Zhao K, Chen X, Yin Z, et al. The roles of inflammatory mediators and immunocytes in tendinopathy. J Orthop Translat. 2018;14:23–33. doi: 10.1016/j.jot.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–d26. doi: 10.2741/A171. [DOI] [PubMed] [Google Scholar]
- 75.Franceschi F, Longo UG, Ruzzini L, Denaro V. Isolated tuberculosis of the patellar tendon. J Bone Joint Surg Br. 2007;89(11):1525–1526. doi: 10.1302/0301-620X.89B11.19624. [DOI] [PubMed] [Google Scholar]
- 76.Varshney MK, Trikha V, Gupta V. Isolated tuberculosis of Achilles tendon. Joint Bone Spine. 2007;74(1):103–106. doi: 10.1016/j.jbspin.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 77.Pandya NK, Zgonis M, Ahn J, Israelite C. Patellar tendon rupture as a manifestation of Lyme disease. Am J Orthop (Belle Mead NJ) 2008;37(9):E167–E170. [PubMed] [Google Scholar]
- 78.Alves EM, MacIeira JC, Borba E, Chiuchetta FA, Santiago MB. Spontaneous tendon rupture in systemic lupus erythematosus: association with Jaccouds arthropathy. Lupus. 2010;19(3):247–254. doi: 10.1177/0961203309351729. [DOI] [PubMed] [Google Scholar]
- 79.Benjilali L, Benhima H, Zahlane M, Essaadouni L. Spontaneous Achille’s tendon rupture as early manifestation of systemic lupus erythematosus. Rev Med Interne. 2012;33(8):e47–e48. doi: 10.1016/j.revmed.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Ranger TA, Wong AMY, Cook JL, Gaida JE. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br J Sports Med. 2016;50(16):982–989. doi: 10.1136/bjsports-2015-094735. [DOI] [PubMed] [Google Scholar]
- 81.Franceschi F, Papalia R, Paciotti M, Franceschetti E, Di Martino A, Maffulli N, et al. Obesity as a risk factor for tendinopathy: a systematic review. Int J Endocrinol. 2014;2014:670262. doi: 10.1155/2014/670262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abate M, Schiavone C, Salini V, Andia I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology (Oxford) 2013;52(4):599–608. doi: 10.1093/rheumatology/kes395. [DOI] [PubMed] [Google Scholar]
- 83.Oliva F, Berardi AC, Misiti S, Maffulli N. Thyroid hormones and tendon: current views and future perspectives. Concise review Muscles Ligaments Tendons J. 2013;3(3):201–203. [PMC free article] [PubMed] [Google Scholar]
- 84.Oliva F, Piccirilli E, Berardi AC, Frizziero A, Tarantino U, Maffulli N. Hormones and tendinopathies: the current evidence. Br Med Bull. 2016;117(1):39–58. doi: 10.1093/bmb/ldv054. [DOI] [PubMed] [Google Scholar]
- 85.Del Buono A, Battery L, Denaro V, Maccauro G, Maffulli N. Tendinopathy and inflammation: some truths. Int J Immunopathol Pharmacol. 2011;24(1 Suppl 2):45–50. doi: 10.1177/03946320110241S209. [DOI] [PubMed] [Google Scholar]
- 86.Dean BJF, Gettings P, Dakin SG, Carr AJ. Are inflammatory cells increased in painful human tendinopathy? A systematic review. Br J Sports Med. 2016;50(4):216–220. doi: 10.1136/bjsports-2015-094754. [DOI] [PubMed] [Google Scholar]
- 87.Chan K-M, Fu S-C. Anti-inflammatory management for tendon injuries - friends or foes? Sports Med Arthrosc Rehabil Ther Technol. 2009;1(1):23. doi: 10.1186/1758-2555-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andres BM, Murrell GAC. Treatment of Tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539–1554. doi: 10.1007/s11999-008-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Del Buono A, Oliva F, Osti L, Maffulli N. Metalloproteases and tendinopathy. Muscles Ligaments Tendons J. 2013;3(1):51–57. doi: 10.32098/mltj.01.2013.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Magra M, Maffulli N. Matrix metalloproteases: a role in overuse tendinopathies. Br J Sports Med. 2005;39(11):789–791. doi: 10.1136/bjsm.2005.017855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fu SC, Chan BP, Wang W, Pau HM, Chan KM, Rolf CG. Increased expression of matrix metalloproteinase 1 (MMP1) in 11 patients with patellar tendinosis. Acta Orthop Scand. 2002;73(6):658–662. doi: 10.3109/17453670209178031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.