Abstract
Aged garlic extract (AGE) contains various biologically active sulfur-containing amino acids, such as S-allylcysteine (SAC), S-1-propenylcysteine (S1PC) and S-allylmercaptocysteine (SAMC). These amino acids have been demonstrated to lower hypertension, improve atherosclerosis and enhance immunity through their anti-inflammatory and antioxidant activities. It was recently reported that the administration of AGE alleviated gingivitis in a clinical trial. In this study, to gain insight into this effect of AGE, the authors examined whether AGE and the three above-mentioned sulfur compounds influence the effects of tumor necrosis factor-α (TNF-α) in inducing intercellular adhesion molecule-1 (ICAM-1) expression and interleukin-6 (IL-6) secretion in Ca9-22 human gingival epithelial cells. It was found that S1PC reduced the level of ICAM-1 protein induced by TNF-α possibly through post-translational levels without affecting the TNF-α-induced mRNA expression. However, SAC and SAMC had no effect. It was also confirmed the inhibitory effect of an antimicrobial peptide [human-β defensin-3 (hβD3)] and found that the inhibitory effects of hbD3 and S1PC were synergistic. On the other hand, the TNF-α-induced IL-6 secretion was attenuated by SAC and SAMC in a dose-dependent manner, whereas S1PC was ineffective. In addition, SAC and SAMC, but not S1PC inhibited the phosphorylation of the transcription factor nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), which is involved in the expression of inflammatory molecules, suggesting that the anti-inflammatory effects of SAC and SAMC are mediated, at least partly, by NF-κB. On the whole, the findings of this study suggest that the three sulfur amino acids in AGE function synergistically in alleviating inflammation in human gingival epithelial cells.
Keywords: aged garlic extract, gingival epithelial cells, ICAM-1, IL-6, inflammation, sulfur-containing amino acid
Introduction
Gingivitis is a gingival inflammation triggered by periodontal pathogens present in oral biofilms (1). Unless some physical treatments, such as plaque control and scaling are administered, gingivitis becomes more severe and develops into periodontitis with more severe inflammation, leading to the destruction of connective tissues, the absorption of alveolar bones and tooth loss (1,2). It has been demonstrated that excessive inflammation in the gingival tissue, as well as an immune reaction triggered by the increased number of periodontal pathogens, such as Porphyromonas gingivalis (P.g.), plays an important role in the severity and progression of periodontal diseases (3,4). Thus, the control of inflammation may help to delay disease progression or recover severe symptoms in periodontal diseases.
Aged garlic extract (AGE) is prepared by aging garlic (Allium sativum L.) for a period of >10 months in aqueous ethanol and contains pharmacologically active sulfur-containing amino acids, such as S-allylcysteine (SAC), S-1-propenylcysteine (S1PC) and S-allylmercaptocysteine (SAMC). In human and animal studies, AGE and these sulfur compounds have been shown to exert beneficial effects, such as the improvement of atherosclerosis (5,6), decrease of high blood pressure (7,8) and the modulation of immunity (9,10). These effects are possibly ascribed, at least in part, to the anti-inflammatory (9,11) and/or antioxidant (12,13) activities of sulfur compounds in AGE.
It has been reported that several naturally occurring substances, such as curcumin and theaflavin alleviate the absorption of alveolar bones in animal periodontitis models (4,14). It has also been demonstrated that one of the sulfur-containing compounds present in garlic, diallylsulfide (DAS), reduces inflammatory reactions elicited by P.g.-derived lipopolysaccharide (LPS) in human gingival fibroblast cells (15). Furthermore, DAS and another garlic sulfur compound, alliin, have been shown to inhibit the growth of periodontal microbials (16-18). These findings suggest that sulfur compounds in garlic attenuate moderate or severe inflammation occurring in gingival tissues of gingivitis and periodontitis. Recently, a clinical study demonstrated that the intake of AGE significantly improved gingivitis (1). However, the mechanisms through which AGE attenuates gingivitis remain to be fully elucidated. In this study, the authors examined whether SAC, S1PC and SAMC in AGE exert anti-inflammatory effects against tumor necrosis factor-α (TNF-α)-induced inflammatory reactions in the human gingival epithelial cell line, Ca9-22.
Materials and methods
Aged garlic extract (AGE) and sulfur compounds
AGE was prepared as previously described (12). Briefly, AGE was manufactured according to the following steps: Originally grown raw garlic (A sativum L.) was cut into slices, immersed in aqueous ethanol, and extracted for >10 months at room temperature (12). The sulfur-containing amino acids, S1PC, SAC and SAMC, are produced during the aging process of raw garlic in 50% ethanol solution for >10 months (19). The purification and structure determination of sulfur compounds in AGE was performed by high performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS) systems as previously described (9).
Reagents
Dulbecco's modified Eagle's medium (DMEM, D6046) and WST-1 reagent (501594401) were purchased from Sigma-Aldrich. Fetal bovine serum (FBS, 10270), 0.05% trypsin/EDTA solution (25300-054), Halt protease and phosphatase inhibitor single-use cocktail (1861280) and TRIzol reagent (15596018) were from Thermo Fisher Scientific. Penicillin/streptomycin (164-25251) and recombinant human TNF-α (207-15261) were from Wako Pure Chemical Industries. Human β-defensin-3 (hβD3, 4382-s) was from Peptide Institute Inc. Anti-intercellular adhesion molecule-1 (ICAM-1) (4915S), anti-phosphorylated nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) p65 (3033) antibodies and a horseradish peroxidase (HRP)-conjugated rabbit immunoglobulin G (IgG) (7074S) were from Cell Signaling Technologies. An anti-NF-κB p65 antibody (sc-109X) was from Santa Cruz Biotechnology. RIPA lysis buffer (20-188) was purchased from Merck Millipore. An HRP-conjugated anti-β-actin antibody (PM053-7) was from MBL Life Science.
Cells and cell culture
The human gingival epithelial cells, Ca9-22 (JCRB0625, Lot. 11182016, Biomedical Innovation, Health and Nutrition Research Institute) were cultured in DMEM containing 10% fetal bovine serum, 100,000 units/ml penicillin and 100 µg/ml streptomycin in 5% CO2 at 37˚C. The Ca9-22 cells were subcultured 2 or 3 times per week. For pharmacological experiments, the cells were seeded onto a 6- or 12-well plate at the density of 1 to 2x105 cells per well and cultured for 36 to 48 h. When they reached full confluency, the cells were treated with TNF-α (100 ng/ml) in the absence or presence of AGE (0.01-1 mg/ml), S1PC, SAC and SAMC (each at 1 to 100 µM) for the 3, 6, 12 or 24 h. In the experiment with cycloheximide (CHX, Fig. 4), the cells were pre-treated with TNF-α (100 ng/ml) for 12 h, and then treated with S1PC (10 µM) in the absence or presence of CHX (10 µM) for 6 h.
Figure 4.
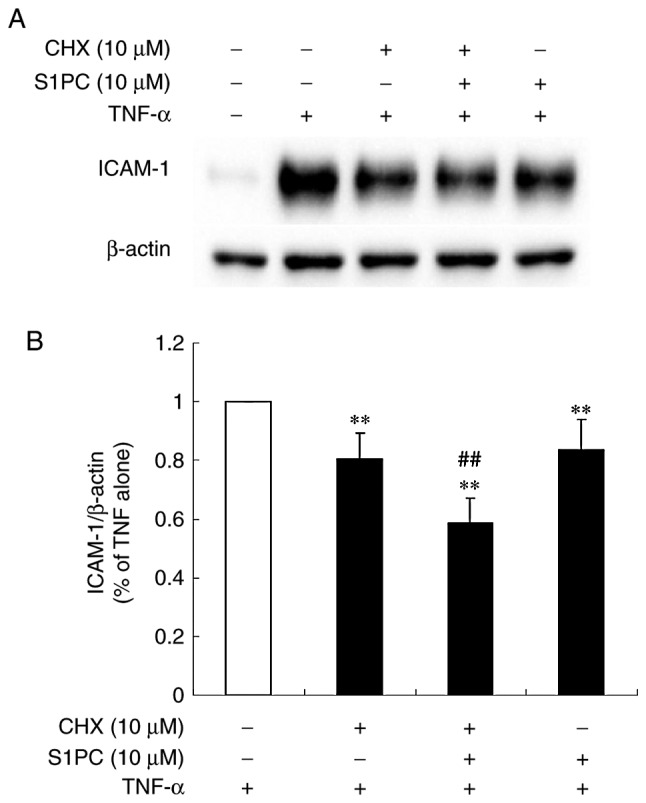
Effect of S1PC on the level of ICAM-1 protein induced by TNF-α in the presence of cycloheximide (CHX). (A) The cells were left untreated (control) or pretreated with TNF-α (100 ng/ml) for 12 h in the absence or presence of CHX (10 µM) and/or S1PC (10 µM) for 6 h and total protein was extracted. ICAM-1 proteins in the extract were detected by western blot analysis. (B) The quantitative analysis of the band intensity relative to β-actin. Each bar in the graph represents the mean ± SD (n=3). **P<0.01 in comparison to control and ##P<0.01 in comparison to TNF-α alone (Tukey's multiple comparisons test). S1PC, S-1-propenylcysteine; hβD3, human β-defensin-3; TNF-α, tumor necrosis factor-α; ICAM-1, intercellular adhesion molecule-1.
Interleukin (IL)-6 secretion
Following treatment of the cells with TNF-α (100 ng/ml) in the absence or presence of AGE (0.01-1 mg/ml), S1PC, SAC or SAMC (each at 1 to 100 µM) for 6 h, an aliquot of culture medium was collected and frozen at -80˚C until use. The IL-6 protein amount in the medium was determined with a human IL-6 Uncoated enzyme-linked immunosorbent assay (ELISA) kit (88-7066-22, Thermo Fisher Scientific).
Western blot analysis
Cells were washed with phosphate-buffered saline (PBS) 5 times and total protein was extracted with RIPA lysis buffer containing protease inhibitor cocktail. The protein amount in the extract was determined with the Pierce BCA Protein Assay kit (23225, Thermo Fisher Scientific) with bovine serum albumin (BSA, A7030, Sigma) as a standard. The protein extract (10 µg) was denatured by the addition of sample buffer [5% sodium dodecyl sulfate, 12.5% glycerol and 50 mM dithiothreitol in 100 mM Tris-hydrochloric acid (HCl) buffer, pH 6.8], and was heated at 95˚C for 5 min. The samples were separated by electrophoresis and were blotted onto a nitrocellulose membrane (1620090, Bio-Rad). The blotted membrane was blocked with 5% non-fat dry milk (999S, Cell Signaling Technology) in Tris-buffered saline (TBS)-T buffer (0.1% Tween-20, pH 7.6) for 1 h, and was subsequently incubated in the presence of the rabbit anti-ICAM-1 (4915S, Cell Signaling Technology), anti-NF-κB (sc-109X, Santa Cruz Biotechnology) or anti-phosphorylated NF-κB (3033, Cell Signaling Technology) antibody at a 1:2,000 dilution overnight at 4˚C. The membrane was washed 3 times and incubated in the presence of the HRP-conjugated anti-rabbit IgG antibody (7074S, Cell Signaling Technology) for 1 h at room temperature. On the other hand, another blotted membrane was incubated with the HRP-conjugated anti-β-actin antibody (PM053-7, MBL Life science) at a 1:2,000 dilution for 1 h at room temperature, in order to quantitatively normalize the immunoreactive ICAM-1 protein levels. After washing, immunoreactive proteins on the nitrocellulose membrane were visualized with Armasham ECL Prime peroxidase solution (RPN2232V, GE Healthcare), by using a luminoimage analyzer (ChemiDoc™ MP, Bio-Rad). The density of each immunoreactive band was analyzed with Image Lab™ software ver. 4.1 (Bio-Rad).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells with TRIzol reagent. Contaminated genomic DNA in the total RNA was removed by incubation with gDNA eraser (RR047A, Takara) for 5 min at room temperature, and total RNA was reverse transcribed into complementary DNA (cDNA) with a PrimeScript RT reagent kit with genomic DNA Eraser (RR047A, Takara). cDNA was amplified with primer pairs using a SYBR Premix Ex Taq II (RR820A, Takara). The level of gene expression relative to the internal control (β-actin) was calculated by performing a quantitative (real-time) PCR with a Real-time PCR system (PikoReal 96, Life Technologies; Thermo Fisher Scientific). The reaction was run using the following program: 30 sec at 95˚C, 40 repeats of 10 sec at 95˚C, 10 sec of 63˚C and 15 sec of 72˚C. The sequences of the specific primers were as follows: human ICAM-1 forward, 5'-TCGGCACAAAAGCACTATATG-3' and reverse, 5'-ACAGGACAAGAGGACAAGGC-3'; human IL-6 forward, 5'-TCTCCACAAGCGCCTT-3' and reverse, 5'-CTCAGGGCTGAGATGC-3'; and β-actin forward, 5'-CGCGAGAAGATGACCCAGAT-3' and reverse, 5'-GGTGAGGATCTTCATGAGGTAGTC-3'. The fold change in the relative mRNA level to β-actin was calculated based on the Cq (ΔΔCq) method (20).
Cellular IL-6 protein content
Following treatment of the cells with TNF-α (100 ng/ml) in the absence or presence of AGE (1 mg/ml) or SAC (100 µM) for 3 h, the total protein was extracted with RIPA buffer. The amount of IL-6 protein in the extract was determined by ELISA, as described above. The cellular IL-6 protein content is expressed relative to 1 mg total protein.
Statistical analysis
Data are expressed as the means ± standard deviation (SD). Statistical significance was evaluated using the Student's t-test for differences between 2 groups or one-way analysis of variance (ANOVA), followed by Tukey's post hoc test for differences between >3 groups. A P-value <0.05 was statistically significant in comparison to control or the effect of TNF-α alone.
Results
Effect of S1PC and hβD3 on the level of ICAM-1 protein induced by TNF-α
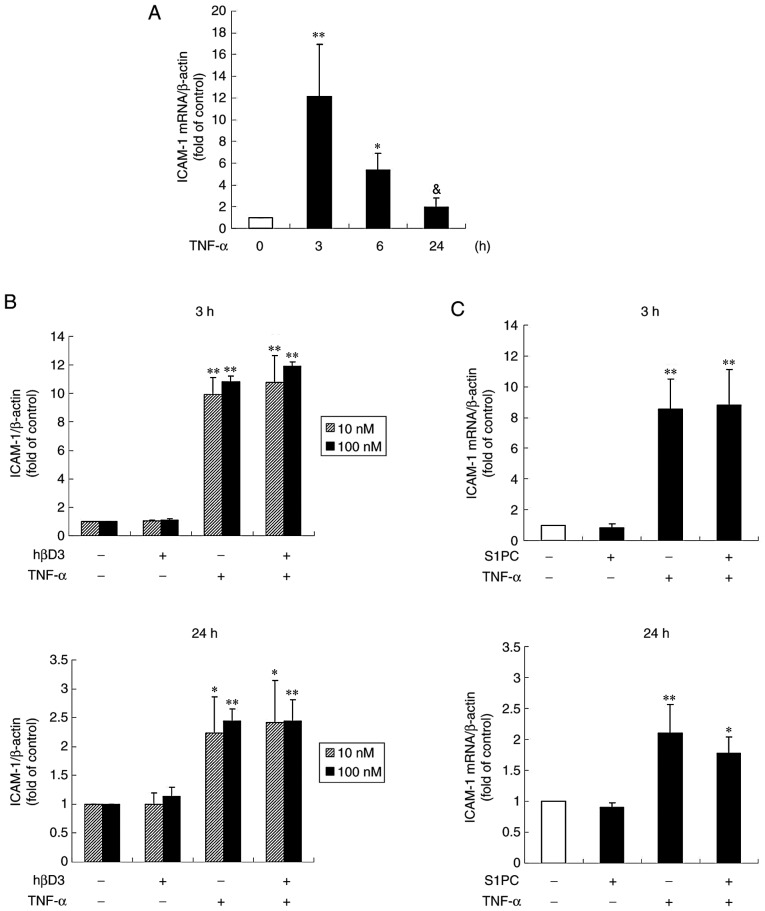
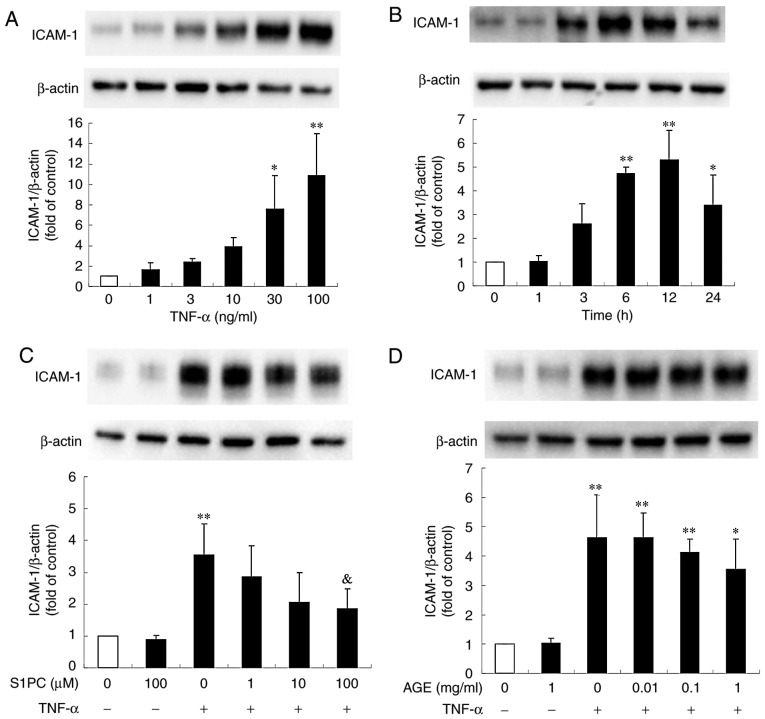
Human gingival fibroblasts and Ca9-22 cells have been shown to express ICAM-1 in response to the periodontal disease bacteria, P.g.-derived LPS or TNF-α (21-25). It was also found that ICAM-1 protein expression in the Ca9-22 cells was profoundly induced by treatment with TNF-α in a concentration-dependent manner (Fig. 1A). In addition, the ICAM-1 protein level began to increase at 3 h, reached peak levels at 12 h and remained elevated until 24 h (Fig. 1B). As shown in Fig. 1C, the presence of S1PC for 24 h inhibited the increased level of TNF-α-induced ICAM-1 protein in a concentration-dependent manner, whereas treatment with AGE exerted minimal inhibitory effects even at the highest concentration (1 mg/ml) (Fig. 1D). Both SAC and SAMC also had no significant effect (Fig. 1E and F).
Figure 1.
Effect of S1PC and hβD3 on the level of ICAM-1 protein induced by TNF-α. (A) The concentration (1 to 100 ng/ml, 12 h)- and (B) time (1 to 24 h, 100 ng/ml)-dependent induction of ICAM-1 protein stimulated by TNF-α. The cells were left untreated (control) or treated with TNF-α in the absence or presence of (C) S1PC (1 to 100 µM), (D) AGE (0.01 to 1 mg/ml), (E) SAC (10, 100 µM), (F) SAMC (10, 100 µM) or (G) hβD3 (1 to 100 nM) for 24 h and total protein was extracted. ICAM-1 proteins in the extract were detected by western blot analysis. Each bar in the graphs represents the mean ± SD of the band intensity relative to β-actin (n=3). *P<0.05, **P<0.01 in comparison to the control (Tukey's multiple comparison test). &P<0.05 in comparison to TNF-α alone (Student's t-test). S1PC, S-1-propenylcysteine; hβD3, human β-defensin-3; TNF-α, tumor necrosis factor-α; ICAM-1, intercellular adhesion molecule-1; AGE, aged garlic extract; SAC, S-allylcysteine; SAMC, S-allylmercaptocysteine.
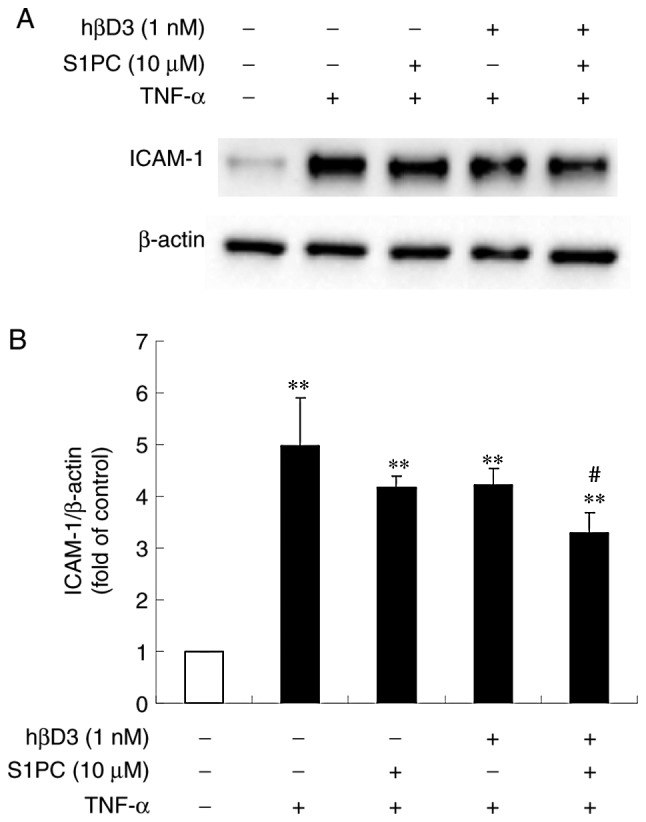
It has been previously demonstrated that the anti-microbial peptide hβD3 reduces the level of TNF-α-elicited ICAM-1 protein in human vein endothelial cells (HUVECs) (26). Thus, this study examined whether the peptide inhibits the level of ICAM-1 protein in Ca9-22 cells. As shown in Fig. 1G, the increased level of TNF-α-induced ICAM-1 protein was moderately but significantly suppressed by hβD3 at 10 nM, indicating that this peptide is also effective. Furthermore, it was found that the inhibitory effect of hβD3 was enhanced by simultaneously treating the cells in the presence of S1PC (Fig. 2), suggesting the synergistic effect of hβD3 and S1PC.
Figure 2.
Effect of S1PC on the level of ICAM-1 protein induced by TNF-α in the presence of hβD3. (A) The cells were left untreated (control) or treated with TNF-α (100 ng/ml) in the absence or presence of S1PC (10 µM) and/or hβD3 (1 nM) for 24 h and total protein was extracted. ICAM-1 proteins in the extract were detected by western blot analysis. (B) Quantitative analysis of the band intensity relative to β-actin. Each bar in the graph represents the mean ± SD (n=3). **P<0.01 in comparison to the control and #P<0.05 in comparison to TNF-α alone (Tukey's multiple comparisons test). S1PC, S-1-propenylcysteine; hβD3, human β-defensin-3; TNF-α, tumor necrosis factor-α; ICAM-1, intercellular adhesion molecule-1.
Effect of hβD3 and S1PC on ICAM-1 gene expression induced by TNF-α
It is conceivable that the inhibitory effect of S1PC on the level of ICAM-1 protein was due to the suppression of ICAM-1 gene transcriptions. Thus, this study then examined the effects of S1PC as well as those of hβD3 over a period of 3 or 24 h treatment on the TNF-α-induced ICAM-1 gene expression by RT-qPCR. As shown in Fig. 3A, TNF-α significantly enhanced the basal ICAM-1 mRNA transcription level with a peak at 3 h. Treatment with S1PC or hbD3 for either 3 or 24 h had no effect on the mRNA expression level of ICAM-1 increased by TNF-α (Fig. 3B and C). These results suggested that the actions of S1PC and hβD3 occurred at the post-transcriptional level, since these compounds did not affect ICAM-1 expression even at the concentrations at which induced a decrease in the protein level.
Figure 3.
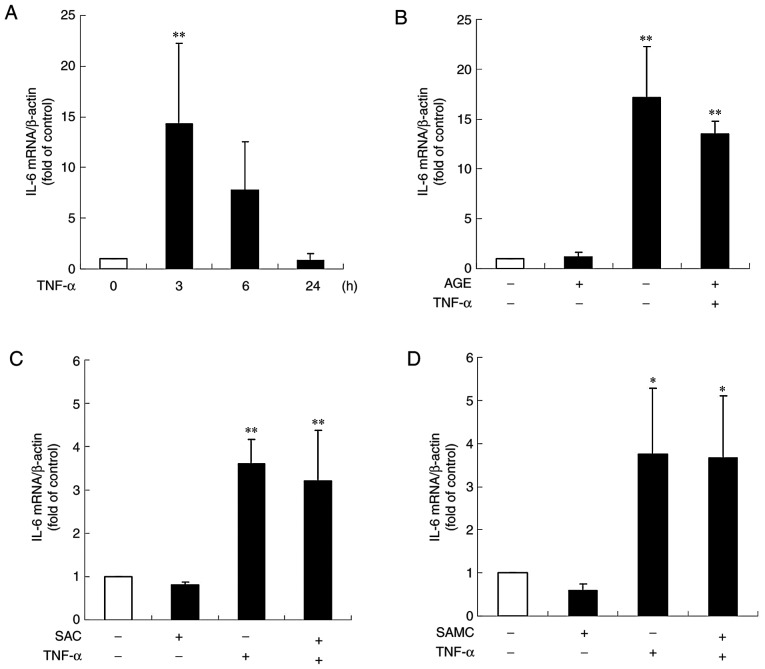
Effect of hβD3 and S1PC on the ICAM-1 gene expression induced by TNF-α. The cells were untreated (control) or treated with (A) TNF-α (100 ng/ml) alone for 3, 6 or 24 h or in the presence of (B) hβD3 (10, 100 nM) or (C) S1PC (100 µM) for 3 h or 24 h, and total RNA was extracted. The ICAM-1 mRNA expression level in total RNA was evaluated by RT-qPCR. Each bar in graphs represents the mean ± SD (n=3-5). *P<0.05, **P<0.01 (Tukey's multiple comparison test) or &P<0.05 (Student's t-test) in comparison to the control. S1PC, S-1-propenylcysteine; hβD3, human β-defensin-3; TNF-α, tumor necrosis factor-α; ICAM-1, intercellular adhesion molecule-1.
Effect of S1PC on the level of ICAM-1 protein induced by TNF-α in the presence of cycloheximide (CHX)
Since S1PC did not affect ICAM-1 gene expression, we then examined their effect at the translational level using CHX, an inhibitor of protein synthesis. As shown in Fig. 4, the inhibitory effects of CHX on the protein level of ICAM-1 were enhanced in the presence of S1PC in cells treated with TNF-α. This result suggested that the effects of S1PC occurred at the post-translational level, since this compound induced a further reduction in the ICAM-1 protein level when total protein synthesis was blocked.
Effect of AGE, SAC and SAMC on IL-6 secretion induced by TNF-α
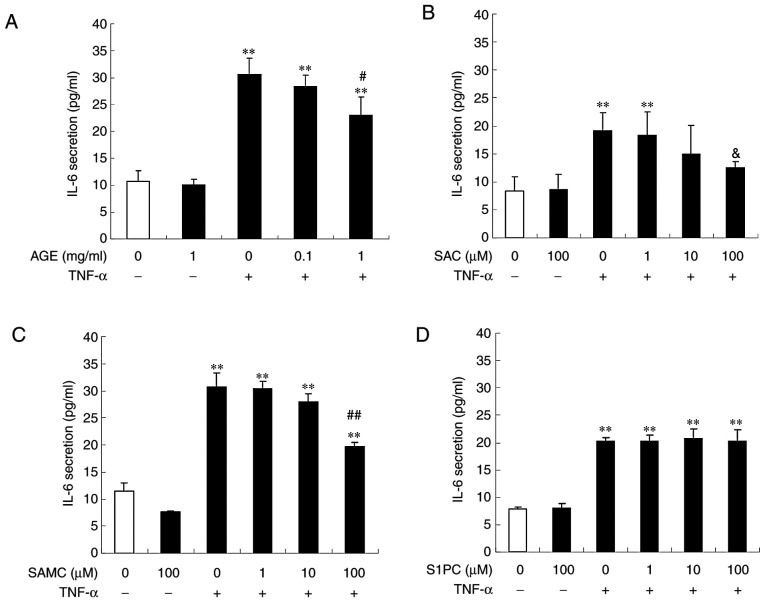
IL-6 is produced in human gingival epithelial cells in response to LPS or TNF-α, leading to the activation of osteoclasts via the upregulation of receptor activator of NF-κB ligand (RANKL) in osteoblasts (21). This study thus examined the effects of TNF-α on IL-6 secretion in Ca9-22 cells. As shown in Fig. 5A, TNF-α stimulated basal IL-6 secretion during 6 h of culture. The enhanced IL-6 secretion induced by TNF-α was reduced by simultaneous treatment with AGE, SAC or SAMC in a concentration-dependent manner (Fig. 5A-C). On the other hand, S1PC had no effect on IL-6 secretion even at the concentration of 100 µM (Fig. 5D).
Figure 5.
Effect of AGE, SAC and SAMC on IL-6 secretion induced by TNF-α. The cells were left untreated (control) or treated with TNF-α (100 ng/ml) in the absence or presence of (A) AGE, (B) SAC, (C) SAMC or (D) S1PC (each at 1 to 100 µM) for 6 h and culture medium was collected. The IL-6 concentration in the medium was determined by ELISA. Each bar in graphs represents the mean ± SD (n=3-4). **P<0.01 in comparison to the control and #P<0.05, ##P<0.01 (Tukey's multiple comparison test), &P<0.05 in comparison to TNF-α alone (Student's t-test). S1PC, S-1-propenylcysteine; hβD3, human β-defensin-3; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; AGE, aged garlic extract; SAC, S-allylcysteine; SAMC, S-allylmercaptocysteine.
Effect of AGE, SAC and SAMC on IL-6 gene expression induced by TNF-α
It was possible that SAC and SAMC downregulated IL-6 gene transcription induced by TNF-α. Thus, the effectσ of these sulfur compounds on the IL-6 gene expression level were then examined by RT-qPCR. It was found that TNF-α significantly enhanced the basal IL-6 mRNA transcription level with a peak at 3 h (Fig. 6A). As shown in Fig. 6B-D, treatment with AGE, SAC or SAMC for 3 h had a minimal effect on the enhanced IL-6 mRNA expression level induced by TNF-α.
Figure 6.
Effect of AGE, SAC and SAMC on the IL-6 gene expression induced by TNF-α. The cells were untreated (control) or treated with (A) TNF-α (100 ng/ml) alone for 3, 6 or 24 h or in the presence of (B) AGE (1 mg/ml) or (B) SAC, (C) SAC, (D) SAMC (each at 100 µM) for 3 h and total RNA was extracted. The IL-6 mRNA expression level in total RNA was evaluated by RT-qPCR. Each bar in graphs represents the mean ± SD (n=3-6). *P<0.05, **P<0.01 (Tukey's multiple comparison test) in comparison to the control. S1PC, S-1-propenylcysteine; hβD3, human β-defensin-3; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; AGE, aged garlic extract; SAC, S-allylcysteine; SAMC, S-allylmercaptocysteine.
Effect of AGE, SAC and SAMC on the intracellular IL-6 protein content
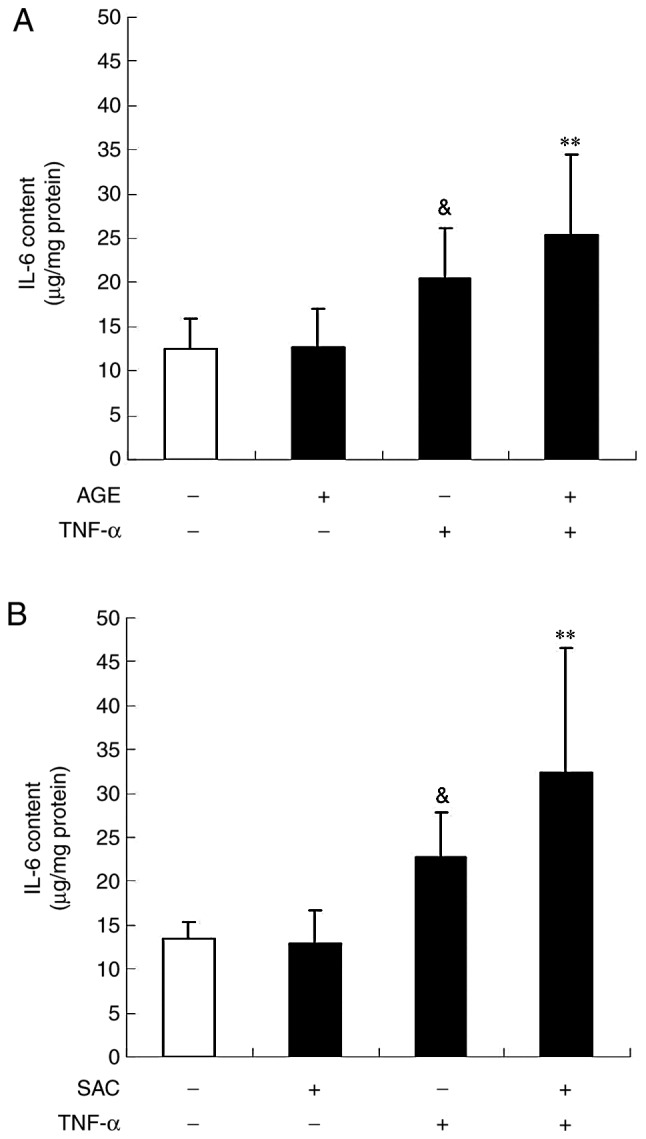
In order to examine whether AGE and sulfur compounds affect the IL-6 secretion process, the cellular IL-6 protein content was measured during TNF-α stimulation by ELISA. Treatment with AGE (1 mg/ml) or SAC (100 µM) for 3 h exhibited a tendency to enhance the effects of TNF-α on the cellular IL-6 content, although their effects were not statistically significant (Fig. 7).
Figure 7.
Effect of AGE and SAC on the intracellular IL-6 content induced by TNF-α. The cells were left untreated (control) or treated with TNF-α (100 ng/ml) in the absence or presence of (A) AGE or (B) SAC (100 µM) for 3 h and total protein was extracted. The IL-6 content in the protein extract was determined by ELISA. Each bar in the graphs represents the mean ± SD (n=6). **P<0.01 (Tukey's multiple comparison test), &P<0.05 (Student's t-test) in comparison to the control. TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; AGE, aged garlic extract; SAC, S-allylcysteine.
Effect of hβD3, S1PC, AGE, SAC and SAMC on the level of phosphorylated NFkB p65 protein induced by TNF-α
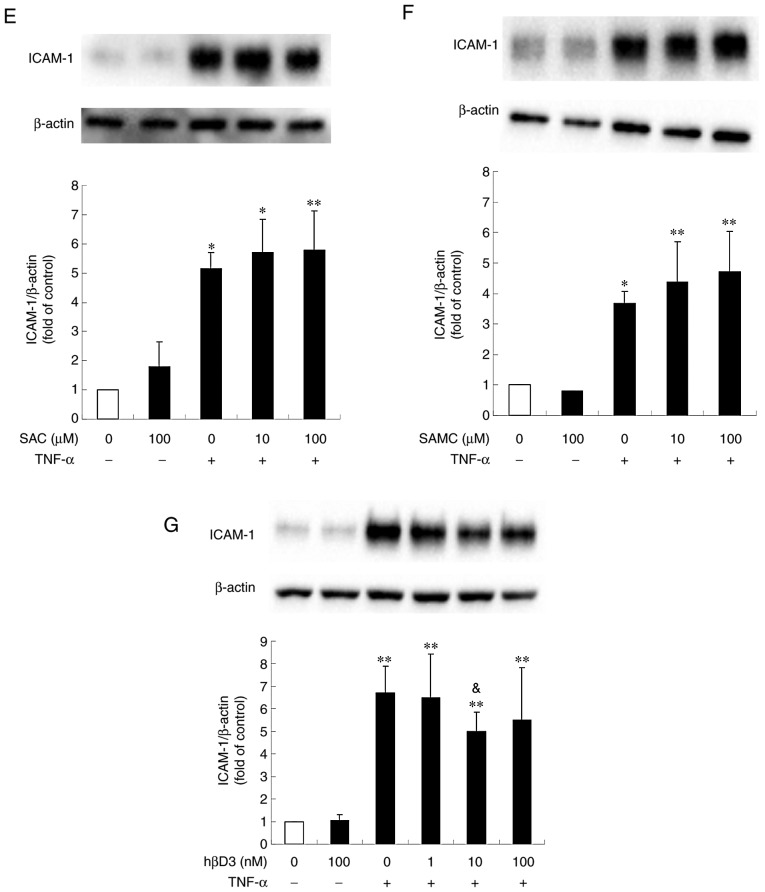
Stimulation of the cells with TNF-α has been shown to activate a variety of signaling pathways and transcription factors, such as NF-κB, which plays an essential role in the expression of inflammation-related molecules (26-28). In addition, hβD3 has been shown to alleviate periodontitis via the suppression of NF-κB (29). Thus, this study examined the effects of hβD3, S1PC, AGE, SAC and SAMC on the phosphorylation (activation) of NF-κB induced by TNF-α. As shown in Fig. 8A and C-E, hβD3 (100 nM), AGE (1 mg/ml), SAC and SAMC (each at 100 µM) inhibited NF-κB activation, whereas S1PC (100 µM) did not have any significant effect (Fig. 8B).
Figure 8.
Effect of hβD3, S1PC, AGE, SAC and SAMC on the level of phosphorylated NF-κB p65 protein induced by TNF-α. The cells were left untreated (control) or treated with TNF-α (100 ng/ml) in the absence or presence of (A) hβD3 (10, 100 nM) or (B) S1PC (100 µM) for 24 h, (C) AGE (1 mg/ml), (D) SAC or (E) SAMC (each at 100 µM) for 6 h and total protein was extracted. Total NF-κB (NF-κB) and phosphorylated NF-κB (pNF-κB) p65 proteins in the extract were detected by western blot analysis. Each bar in graphs represents the mean ± SD of the band intensity relative to β-actin (n=3-4). *P<0.05, **P<0.01 in comparison to the control and #P<0.05, ##P<0.01 in comparison to TNF-α alone (Tukey's multiple comparisons test). S1PC, S-1-propenylcysteine; hβD3, human β-defensin-3; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; AGE, aged garlic extract; SAC, S-allylcysteine; SAMC, S-allylmercaptocysteine.
Discussion
In gingivitis and periodontitis, TNF-α secreted from macrophages and neutrophils accumulates in gingival tissues during the progression of inflammation and induces cytokine production, such as IL-6 in gingival cells, leading to an increased RANKL expression in osteoblasts, the activation of osteoclasts and consequently, to the resorption of alveolar bone (30). Therefore, regulating the excess inflammation in gingiva is considered to be a key step to treat the periodontal diseases. In the present study, it was found that sulfur-containing compounds in AGE differentially inhibited the TNF-α-elicited ICAM-1 expression and IL-6 secretion, suggesting that these compounds control inflammation occurring in human gingival tissues under pathological conditions such as gingivitis.
ICAM-1 is expressed by several cell types in response to a variety of stimuli, such as LPS and pro-inflammatory cytokines, and helps to bind lymphocytes to T cells or vascular endothelial cells, leading to the activation of immune responses and the trans-endothelial migration of lymphocytes (31,32). It has been demonstrated that ICAM-1 is highly expressed in inflamed tissues, such as atherosclerotic lesions and is associated with disease progression (32). There are a number of findings indicating that ICAM-1 is highly expressed by treating HUVECs with TNF-α or LPS (33-35). Zhao et al demonstrated that a variety of intracellular signaling molecules, such as p38 MAPK acting on the MAPK pathway, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the transcription factor NF-κB, were involved in the ICAM-1 expression in HUVEC (35). In particular, NF-κB plays a crucial role in innate immune responses via enhanced transcription of inflammatory mediators, including interleukins, chemokines and cytokines (27,28). The findings of study suggest that S1PC can modulate the level of ICAM-1 protein in Ca9-22 cells independently of NF-κB. At present, the S1PC-mediated intracellular pathway remains to be elucidated.
In human gingival fibroblast cells, ICAM-1 is expressed in response to pro-inflammatory cytokines, such as TNF-α and IL-1β (21,22) or periodontal pathogens such as P. gingivalis (23) via the activation of NF-κB, p38 MAPK or nucleotide binding oligomerization domain-containing protein (NOD). On the other hand, to the best of our knowledge, there are only a few studies available to date demonstrating the TNF-α-induced expression of ICAM-1 in human gingival epithelial cells, particularly Ca9-22 cells (22,25), and the functional mechanisms have not yet been fully clarified. However, it was previously demonstrated that TNF-α also enhanced the expression or secretion of IL-6 and IL-8 proteins in another human gingival epithelial cell line (OBA9) (36) and the oral epithelial cell line, TR146(37). These findings indicate that Ca9-22 cells are also available to pharmacologically investigate the anti-inflammatory function of AGE, although Ca9-22 cells have been often used as an oral cancer model. Thus, this study first examined whether TNF-α induces the ICAM-1 expression and found that the levels of both ICAM-1 protein and mRNA were augmented by TNF-α, but only slightly by P.g.-derived LPS, indicating that Ca9-22 cells were less sensitive to LPS. It was also found that S1PC inhibited the level of TNF-α-induced ICAM-1 protein possibly through post-translational modification (Fig. 4), while AGE caused little inhibition, suggesting that AGE may contain some substances to block the S1PC action. Furthermore, it was shown that extracellular signal-regulated kinase-1/2 (ERK1/2), p38 MAPK (data not shown) and NF-κB (Fig. 8B) were not involved in the inhibition by S1PC.
The antimicrobial peptide, hβD3, is mainly produced in gingival epithelial cells and is extracellularly secreted into the oral cavity, and exhibits antimicrobial activity against Gram-positive and negative bacteria, fungi and viruses (29,38). hβD3 also plays an important role in the innate immune response via its immunomodulatory effect, suppressing the periodontal diseases progression (29,38). It has been reported that the hβD3 expression level in gingival tissues and crevicular fluid is decreased in patients with periodontitis in comparison to healthy controls (29). It has been found that hβD3 reduced the level of ICAM-1 protein and inhibits the phosphorylation of NF-κB induced by TNF-α, which is consistent with a finding obtained in HUVECs (26). Although it was not statistically significant, S1PC at 10 µM exhibited near the maximal inhibition induced by S1PC at 100 µM on the elevated ICAM-1 protein by TNF-α (Fig. 1C). Similarly, hβD3 exhibited the maximal inhibition at 10 nM (Fig. 1G). Since it seemed to be difficult to detect further inhibition when S1PC and hβD3 at the higher concentrations, such as 100 µM and 10 nM, respectively, are used, the authors used S1PC at 10 µM and hβD3 at 1 nM in the synergistic experiment. As shown in Fig. 2, it was found that the inhibitory effect of hβD3 was enhanced by simultaneous treatment with S1PC, suggesting that S1PC synergistically modulates the action of hβD3 through a signaling pathway different from that of this peptide.
IL-6 is transiently produced and secreted by several cell types in response to infections and tissue injuries under acute inflammation (39). It was well known that IL-6 exerts beneficial pleiotropic effects, i.e., the maturation of B cells into antibody-producing cells and development of effector T-cells (39). On the other hand, the continuous production of IL-6 in inflamed tissues results in the progression of chronic inflammation and autoimmunity (39). Thus, the precise control of IL-6 secretion is crucial to prevent severe inflammation, which is often observed under pathophysiological conditions, including rheumatoid arthritis (39).
In human gingival fibroblasts, the pro-inflammatory cytokine, IL-1β, secreted from macrophages promotes the production and secretion of IL-6(40). IL-6 facilitates inflammation at higher concentrations via the activation of matrix metalloproteases (MMPs) and osteoclasts, resulting in the gingival tissue destruction and alveolar bone resorption (40). In human gingival epithelial OBA9 cells, the IL-6 mRNA level has been shown to be augmented by stimulation with TNF-α through the phosphorylation of ERK1/2 and p38 MAPK (36). This study found that the enhanced IL-6 secretion by Ca9-22 cells stimulated with TNF-α was significantly inhibited by simultaneously treating the cells with AGE, SAC or SAMC, suggesting their involvement in the regulation of IL-6 secretion. However, these substances did not exhibit any inhibitory effect on the IL-6 mRNA expression levels (Fig. 6), suggesting the post-transcriptional mode of control. In addition, it was found that AGE and SAC tended to augment the content of IL-6 protein in cells induced by TNF-α, supporting their post-translational modifications. Based on these findings, it is possible that the reduced IL-6 release by AGE or SAC may result from the partial blockade of extracellular secretion, but not from the decreased production of the cytokine.
In conclusion, this study found that AGE and its sulfur compounds, S1PC, SAC and SAMC, inhibited inflammatory reactions induced by TNF-α in human gingival epithelial cells. Although their mechanisms of action have not been fully clarified, these data suggest that these sulfur compounds in AGE may directly reduce inflammation occurring in human gingival epithelial cells by suppressing the ICAM-1 expression and IL-6 secretion. Moreover, it is possible that the sulfur compounds in AGE ingested through systemic circulation reduced gingival inflammation to improve gingivitis.
Acknowledgements
The authors would like to thank Dr Takami Oka of Wakunaga Pharmaceutical Co., Ltd. for his helpful advice, encouragement and critical reading of the manuscript.
Funding
This study was funded by Wakunaga Pharmaceutical Co., Ltd.
Availability data and materials
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.
Authors' contributions
MO drafted the manuscript and performed the experiments. TN proposed a part of the experimental ideas and concepts, helped interpret the data and revised the manuscript. Both authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zini A, Mann J, Mazor S, Vered Y. The efficacy of aged garlic extract on gingivitis-A randomized clinical trial. J Clin Dentist. 2018;29:52–56. [PubMed] [Google Scholar]
- 2.Sojod B, Chateau D, Mueller CG, Babajko S, Berdal A, Lézot F, Castaneda B. RANK/RANKL/OPG signalization implication in periodontitis: New evidence from a RANK transgenic mouse model. Front Physiol. 2017;8(338) doi: 10.3389/fphys.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzach-Nahman R, Nashef R, Fleisigg O, Palmon A, Shapira L, Wilensky A, Nussbaum G. Oral fibroblasts modulate the macrophage response to bacterial challenge. Sci Rep. 2017;7(11516) doi: 10.1038/s41598-017-11771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu YH, Kuraji R, Taya Y, Ito H, Numabe Y. Effects of theaflavins on tissue inflammation and bone resorption on experimental periodontitis in rats. J Periodontal Res. 2018;53:1009–1019. doi: 10.1111/jre.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morihara N, Hino A, Yamaguchi T, Suzuki JI. Aged garlic extract suppresses the development of atherosclerosis in apolipoprotein E-knockout mice. J Nutr. 2016;146:460S–463S. doi: 10.3945/jn.114.206953. [DOI] [PubMed] [Google Scholar]
- 6.Zeb I, Ahmadi N, Nasir K, Kadakia J, Larijani VN, Flores F, Li D, Budoff MJ. Aged garlic extract and coenzyme Q10 have favorable effect on inflammatory markers and coronary atherosclerosis progression: A randomized clinical trial. J Cardiovasc Dis Res. 2012;3:185–190. doi: 10.3945/jn.114.206953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsutomo T, Ushijima M, Kodera Y, Nakamoto M, Takashima M, Morihara N, Tamura K. Metabolomic study on the antihypertensive effect of S-1-propenylcysteine in spontaneously hypertensive rats using liquid chromatography coupled with quadrupole-Orbitrap mass spectrometry. J Chromatogr B. 2017;1046:147–155. doi: 10.1016/j.jchromb.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Ried K, Travica N, Sali A. The effect of aged garlic extract on blood pressure and other cardiovascular risk factors in uncontrolled hypertensives: The AGE at heart trial. Integr Blood Press Control. 2016;9:9–21. doi: 10.2147/IBPC.S93335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki JI, Kodera Y, Miki S, Ushijima M, Takashima M, Matsutomo T, Morihara N. Anti-inflammatory action of cysteine derivative S-1-propenylcysteine by inducing MyD88 degradation. Sci Rep. 2018;8(14148) doi: 10.1038/s41598-018-32431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki J, Yamaguchi T, Matsutomo T, Amano H, Morihara N, Kodera Y. S-1-Propenylcysteine promotes the differentiation of B cells into IgA-producing cells by the induction of Erk1/2-dependent Xbp1 expression in Peyer's patches. Nutrition. 2016;32:884–889. doi: 10.1016/j.nut.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Anandasadagopan SK, Sundaramoorthy C, Pandurangan AK, Nagarajan V, Srinivasan K, Ganapasam S. S-Allyl cysteine alleviates inflammation by modulating the expression of NF-κB during chromium (VI)-induced hepatotoxicity in rats. Hum Exp Toxicol. 2017;36:1186–1220. doi: 10.1177/0960327116680275. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu K, Tsuneyoshi T, Ogawa T, Morihara N. Aged garlic extract enhances heme oxygenase-1 and glutamate-cysteine ligase modifier subunit expression via the nuclear factor erythroid 2-related factor 2-antioxidant response element signaling pathway in human endothelial cells. Nutr Res. 2016;36:143–149. doi: 10.1016/j.nutres.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Tsuneyoshi T, Kunimura K, Morihara N. S-1-Propenylcysteine augments BACH1 degradation and heme oxygenase 1 expression in a nitric oxide-dependent manner in endothelial cells. Nitric Oxide. 2019;84:22–29. doi: 10.1016/j.niox.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang HH, Lee HM, Raja V, Hou W, Lacono VJ, Sacduto J, Johnson F, Golub MN, Gu Y. Enhanced efficacy of chemically modified curcumin in experimental periodontitis: Systemic implications. J Exp Pharmacol. 2019;11:1–14. doi: 10.2147/JEP.S171119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu E, Tsai MC, Chin YT, Tu HP, Fu MM, Chian CY, Chiu HC. The effects of diallyl sulfide upon Porphyromonas gingivalis lipopolysaccharide stimulated proinflammatory cytokine expressions and nuclear factor-kappa B activation in human gingival fibroblasts. J Periodontal Res. 2015;50:380–388. doi: 10.1111/jre.12217. [DOI] [PubMed] [Google Scholar]
- 16.Bachrach G, Jamil A, Noar R, Tal G, Ludmer Z, Steinberg D. Garlic allicin as a potential agent for controlling oral pathogens. J Med Food. 2011;14:1338–1343. doi: 10.1089/jmf.2010.0165. [DOI] [PubMed] [Google Scholar]
- 17.Bakri IM, Douglas CW. Inhibitory effect of garlic extract on oral bacteria. Arch Oral Biol. 2005;50:645–651. doi: 10.1016/j.archoralbio.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Velliyagounder K, Ganeshnarayan K, Velusamy SK, Fine DH. In vitro efficacy of diallyl sulfides against the periodontopathogen Aggregatibacter actinomycetemcomitans. Antimicrob Agents Chemother. 2012;56:2397–2407. doi: 10.1128/AAC.00020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodera Y, Ushijima M, Amano H, Suzuki JI, Matsutomo T. Chemical and biological properties of S-1-propenyl-L-cysteine in aged garlic extract. Molecules. 2017;22(570) doi: 10.3390/molecules22040570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2002;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. Cytokines differentially regulate ICAM-1 and VCAM-1 expression on human gingival fibroblasts. Clin Exp immunol. 2006;144:494–502. doi: 10.1111/j.1365-2249.2006.03064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato Y, Hagiwara M, Ishihara Y, Isoda R, Sugiura S, Komatsu T, Ishida N, Noguchi T, Matsushita K. TNF-α augmented Porphyromonas gingivalis invasion in human gingival epithelial cells through Rab5 and ICAM-1. BMC Microbiol. 2014;14(229) doi: 10.1186/s12866-014-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Duan J, Wang Y, Ouyang X. Intracellular adhesion molecule-1 is regulated by Porphyromonas gingivalis through nucleotide binding oligomerization domain-containing proteins 1 and 2 molecules in periodontal fibroblasts. J Periodontol. 2014;85:358–368. doi: 10.1902/jop.2013.130152. [DOI] [PubMed] [Google Scholar]
- 24.Song H, Zhao H, Qu Y, Sun Q, Zhang F, Du Z, Liang W, Qi Y, Yang P. Carbon monoxide releasing molecule-3 inhibits concurrent tumor necrosis factor-α- and interleukin-1β-induced expression of adhesion molecules on human gingival fibroblasts. J Periodontal Res. 2011;46:48–57. doi: 10.1111/j.1600-0765.2010.01307.x. [DOI] [PubMed] [Google Scholar]
- 25.Tancharoen S, Matsuyama T, Abeyama K, Matsushita K, Kawahara K, Sanqalungkam V, Tokuda M, Hashiguchi T, Maruyama I, Izumi Y. The role of water channel aquaporin 3 in the mechanism of TNF-alpha-mediated proinflammatory events: Implication in periodontal inflammation. J Cell Physiol. 2008;217:338–349. doi: 10.1002/jcp.21506. [DOI] [PubMed] [Google Scholar]
- 26.Bian T, Li H, Zhou Q, Ni C, Zhang Y, Yan F. Human β-defensin 3 reduces TNF-α-induced inflammation and monocyte adhesion in human umbilical vein endothelial cells. Mediators Inflamm. 2017;2017(8529542) doi: 10.1155/2017/8529542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nennig SE, Schank JR. The role of NFkB in drug addiction: Beyond inflammation. Alcohol Alcohol. 2017;52:172–179. doi: 10.1093/alcalc/agw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi H, Ishida Y, Hosomichi J, Suzuki JI, Usumi-Fujita R, Shimizu Y, Kaneko S, Ono T. A new approach to transfect NF-κB decoy oligodeoxynucleotides into the periodontal tissue using the ultrasound-microbubble method. Int J Oral Sci. 2017;9:80–86. doi: 10.1038/ijos.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui D, Lyu J, Li H, Lei L, Bian T, Li L, Yan F. Human β-defensin 3 inhibits periodontitis development by suppressing inflammatory responses in macrophages. Mol Immunol. 2017;91:65–74. doi: 10.1016/j.molimm.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Song Z, Dong J, Shu R. MicroRNA-142 is upregulated by tumor necrosis factor-alpha and triggers apoptosis in human gingival epithelial cells by repressing BACH2 expression. Am J Transl Res. 2017;9:175–183. [PMC free article] [PubMed] [Google Scholar]
- 31.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/S1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 32.Lyck R, Enzmann G. The physiological roles of ICAM-1 and ICAM-2 in neutrophil migration into tissues. Curr Opin Hepatol. 2015;22:53–59. doi: 10.1097/MOH.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 33.Liu CW, Sung HC, Lin SR, Wu CW, Lee CW, Lee IT, Yang YF, Yu IS, Lin SW, Chiang MH, et al. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-α-treated endothelial cells: Evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-κB pathway. Sci Rep. 2017;7(44689) doi: 10.1038/srep44689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan W, Zhao K, Jiang Y, Huang Q, Wang J, Kan W, Wang S. Role of p38 MAPK in ICAM-1 expression of vascular endothelial cells induced by lipopolysaccharide. Shock. 2002;17:433–438. doi: 10.1097/00024382-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Zhao W, Feng H, Guo S, Han Y, Chen X. Danshenol A inhibits TNF-α-induced expression of intercellular adhesion molecule-1 (ICAM-1) mediated by NOX4 in endothelial cells. Sci Rep. 2017;7(12953) doi: 10.1038/s41598-017-13072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai H, Fujita T, Kajiya M, Ouhara K, Yoshimoto T, Matsuda S, Takeda K, Kurihara H. Mobilization of TLR4 into lipid rafts by Aggregatibacter actinomycetemcomitans in gingival epithelial cells. Cell Physiol Biochem. 2016;39:1777–1786. doi: 10.1159/000447877. [DOI] [PubMed] [Google Scholar]
- 37.Hosokawa Y, Hosokawa I, Ozaki K, Matsuo T. IL-27 modulates chemokine production in TNF-α-stimulated human oral epithelial cells. Cell Physiol Biochem. 2017;43:1198–1206. doi: 10.1159/000481760. [DOI] [PubMed] [Google Scholar]
- 38.Güncü GN, Yilmaz D, Könönen E, Gürsoy U. Salivary antimicrobial peptides in early detection of periodontitis. Front Cell Infect Microbiol. 2015;5(99) doi: 10.3389/fcimb.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(a016295) doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn SH, Lee JK, Kim ND, Kim SH, Lee S, Jung S, Chay KO, Lee TH. [2-(1,2-diphenyl-1H-indol-3-yl)ethanamine] augments pro-inflammatory cytokine production in IL-1b-stimulated primary human oral cells. Int J Mol Sci. 2018;19(1835) doi: 10.3390/ijms19071835. [DOI] [PMC free article] [PubMed] [Google Scholar]