Abstract
Background:
The frequency and severity of invasive fungal infections in immunocompromised patients has increased steadily over the last 2 decades. In response to the increased incidence and high mortality rates, novel antifungal agents have been developed to expand the breadth and effectiveness of treatment options available to clinicians. Despite these therapeutic advances, the impact of the availability of new antifungal agents on pediatric practice is unknown.
Methods:
A retrospective cohort study was conducted using the Pediatric Health Information System database to describe the changes in pediatric antifungal therapy at 25 freestanding United States children’s hospitals from 2000 to 2006. All pediatric inpatients who received a charge for one or more of the following agents were included in the analysis: conventional amphotericin B (AMB), lipid amphotericin B, fluconazole, itraconazole, voriconazole, flucytosine, caspofungin, and micafungin. Underlying conditions and fungal infection status were ascertained.
Results:
A total of 62,842 patients received antifungal therapy, with prescriptions significantly increasing during the 7-year study period (P = 0.03). The most commonly prescribed antifungal agent was fluconazole (76%), followed by amphotericin preparations (26%). Prescription of AMB steadily decreased from 2000 to 2006 (P = 0.02). Prescription of voriconazole steadily increased during the study period and replaced AMB for the treatment of aspergillosis. The echinocandins steadily increased in prescription for treatment of fungal infections, particularly in disseminated/systemic candidiasis.
Conclusions:
We found that the number of pediatric inpatients requiring antifungal therapy has increased significantly and the choice of treatment has changed dramatically with the introduction of newer antifungal agents.
Keywords: antifungal therapy, pediatric, drug utilization, fungal infections
Invasive fungal infections are important causes of morbidity and mortality in immunocompromised children. As a result of advances in supportive medical care, children with life-threatening illnesses have experienced overall reductions in morbidity and mortality associated with cancer therapy and stem cell or solid organ transplantation. These same children, however, are now at increased risk for developing invasive fungal infections,1,2 the majority of which are caused by Candida and Aspergillus species and are associated with significant crude and attributable mortality.3–10
In response to the increased incidence and high mortality rates associated with invasive fungal infections, novel antifungal agents have been developed to expand the breadth and effectiveness of treatment options available to clinicians.3,11,12 Since its initial approval in 1958, conventional amphotericin B (AMB) deoxycholate has been considered the standard in treatment for invasive fungal infections.1,11,13,14 Because of the dose-limiting toxicity of conventional amphotericin B deoxycholate, lipid formulations of amphotericin (LFABs) and newer agents have been developed to potentially improve outcomes and mitigate the adverse effects associated with antifungal therapy.
Despite these therapeutic advances, the impact of the availability of new antifungal agents on pediatric practice is unknown. The objective of this study was to describe the changes in pediatric antifungal therapy in hospitalized children from 2000 to 2006.
METHODS
Data Source and Study Population
Data for this study were obtained from the Pediatric Health Information System (PHIS), an administrative database that contains inpatient data from 42 freestanding children’s hospitals in the United States. These hospitals are affiliated with the Child Health Corporation of America (CHCA, Shawnee Mission, KS), an alliance of children’s hospitals. Data quality and reliability are assured through a joint effort between CHCA and participating hospitals. The data warehouse function for the PHIS database is managed by Thomson Healthcare (Durham, NC). For the purposes of external benchmarking, participating hospitals provide discharge data, including demographics, diagnoses, and procedures. In addition, hospitals submit resource utilization data (eg, pharmaceuticals, imaging, and laboratory) to PHIS. Data are de-identified at the time of submission and are subjected to 175 reliability and validity checks before being processed into data quality reports. Information is accepted into the database once classified errors occur less frequently than a criterion threshold. If a hospital’s quarterly data are unacceptable according to these limits, the information is rejected and is eligible for resubmission and reevaluation before inclusion in the database once errors are corrected.
Study Design
Our retrospective cohort study included pediatric inpatients (age <18 years) with discharge dates between January 1, 2000 and December 31, 2006. The primary end point for this study was the prescription of an antifungal agent during hospitalization. To determine prescription of the antifungal agents of interest, we accessed the drug utilization data contained in PHIS. Data available included generic drug name, the day of service on which the drug was prescribed, and the charge associated with the drug.
The criterion for inclusion was any child with a charge for one or more of the following systemic antifungal agents: AMB, LFAB (includes liposomal amphotericin B, amphotericin B lipid complex, and amphotericin B colloidal dispersion preparations), fluconazole, itraconazole, voriconazole, flucytosine, caspofungin, and micafungin. Hospitals were excluded from the analysis if they did not contribute pharmacy data for the entire study period to allow for accurate analysis of trends or if there were limitations in data quality.
Determination of Underlying Condition
To identify patients with underlying conditions, we used a diagnostic classification system for pediatric complex chronic conditions (CCCs) based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. This classification system divides ICD-9-CM codes into 9 categories of conditions: neuromuscular, cardiovascular, respiratory, renal, gastrointestinal, hematologic or immunologic, metabolic, malignancy, and genetic or other congenital defects.15,16 A CCC is a medical condition expected to last at least 12 months, involve either multiple organ systems or just one that requires the utilization of pediatric specialty services, and lead to hospitalization at a tertiary care center. We determined CCC status based on the ICD-9-CM codes present on the discharge record of the hospitalization that included a prescription for antifungal therapy. In addition to CCCs, we used the All Patient Re-fined-Diagnosis Related Groups (APR-DRGs), version 20, to identify those individuals who were neonates, had received a hematopoetic stem cell transplant, or had received a solid organ transplant. The APR-DRG is a diagnostic grouping system that uses ICD-9-CM codes to categorize patients to assess and compare severity of illness and risk of mortality.17
Determination of Status of Fungal Infection
The designation of fungal infection was determined by the presence of an ICD-9-CM code in any diagnostic position for the following conditions: aspergillosis, disseminated/systemic candidiasis, mucosal/superficial candidiasis, endemic mycoses (coc-cidioidomycosis, histoplasmosis, and blastomycosis), and zygomycosis (Appendix A, http://links.lww.com/A553).
Statistical Analysis
Summary statistics were constructed using frequencies and proportions for categorical data elements and medians and interquartile ranges (IQR) for continuous variables. All analyses were conducted with SAS version 9.1 (Cary, NC) and Stata version 8.0 statistical software (College Station, TX). A P value <0.05 (2-tailed) was considered to indicate statistical significance. All statistical tests for trend were conducted in Stata 8.0 using the “nptrend” test.
Human Subjects Oversight
The conduct of this study was approved by the Committee for the Protection of Human Subjects at the Children’s Hospital of Philadelphia and CHCA.
RESULTS
Between 2000 and 2006 inclusive, data from approximately 1.8 million inpatients were reported to PHIS from 25 pediatric hospitals studied (Fig. 1, http://links.lww.com/A552) of which 62,843 (3%) were prescribed at least 1 dose of an antifungal agent.
Demographic data are displayed in Table 1. The median age of patients requiring antifungal therapy was 5.8 years (IQR: 1.2, 12.7) and the median length of stay was 11 days (IQR: 4, 31). A total of 5144 patients died during their hospitalization (8%). There were 49,411 patients (79%) who had an underlying condition as defined by CCC or APR-DRG, with 32% diagnosed with more than one condition. The most common underlying condition for the cohort was malignancy (42%), followed by hematologic or immunologic deficiency (16%), and cardiovascular condition (15%). Overall, fluconazole was the most commonly prescribed antifungal agent (76%), followed by amphotericin preparations (26%).
TABLE 1.
Demographic Characteristics of the 62,843 Inpatients Receiving Antifungal Therapy, 2000–2006
| Characteristic | Frequency Within Entire Cohort (%) |
|---|---|
| Year of discharge | |
| 2000 | 7690 (12%) |
| 2001 | 7691 (12%) |
| 2002 | 8118 (13%) |
| 2003 | 8599 (14%) |
| 2004 | 9588 (15%) |
| 2005 | 10,469 (17%) |
| 2006 | 10,688 (17%) |
| Fungal diagnosis by year of discharge | |
| 2000 | 1767 (3%) |
| 2001 | 1826 (3%) |
| 2002 | 1965 (3%) |
| 2003 | 2242 (4%) |
| 2004 | 2275 (4%) |
| 2005 | 1839 (3%) |
| 2006 | 2397 (4%) |
| Death during hospitalization | 5144 (8%) |
| At least one underlying condition* | 49,411 (79%) |
| Malignancy† | 26,548 (42%) |
| Hematologic/immunologic deficiency† | 9817 (16%) |
| Cardiovascular condition† | 9491 (15%) |
| Neonate‡ | 5839 (9%) |
| Neuromuscular condition† | 5215 (8%) |
| Respiratory condition† | 4684 (7%) |
| Other congenital condition† | 4498 (7%) |
| Bone marrow transplant‡ | 3398 (5%) |
| Metabolic condition† | 3364 (5%) |
| Gastrointestinal condition† | 2593 (4%) |
| Renal condition† | 1695 (3%) |
| Solid organ transplant‡ | 1206 (2%) |
Percentages do not add to 100 as individuals could have more than one underlying condition.
Determined by CCC.
Determined by APR-DRG v20.
Trends
During the study period, there was a significant increase in the number of inpatients who were prescribed antifungal therapy, from 32 per 1000 hospitalizations in 2000 to 38 per 1000 hospitalizations in 2006 (P = 0.03).
There was a significant decrease in the utilization of AMB, itraconazole, and flucytosine (P = 0.02, P = 0.03, and P = 0.02, respectively) (Fig. 2). The utilization of both voriconazole and the echinocandins (caspofungin and micafungin) significantly increased (P = 0.02 and P = 0.02, respectively). The increase in echinocandin utilization was almost entirely attributable to the use of caspofungin during this study period, as the first prescriptions of micafungin were reported in 2006. Prescribing of fluconazole remained relatively constant, and LFAB use also increased, although this increase was not statistically significant (P = 0.20).
FIGURE 2.
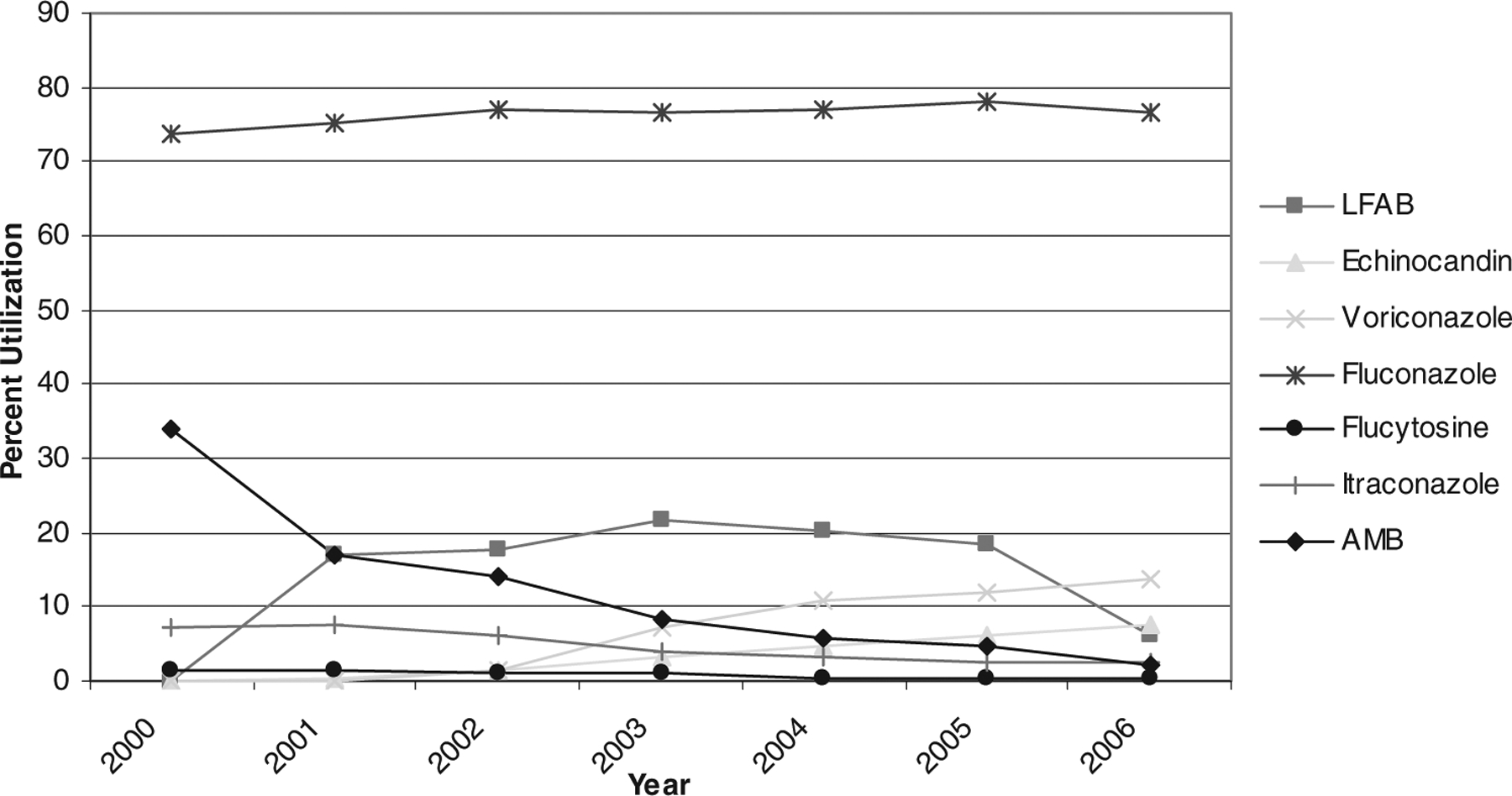
Trends in antifungal therapy among 62,843 pediatric inpatients, 2000–2006.
Treatment of Fungal Infections
Among 62,843 inpatients who received an antifungal agent, there were 14,262 patients (23%) diagnosed with a fungal infection: mucosal/superficial candidiasis (14%), disseminated/systemic candidiasis (5%), aspergillosis (1%), endemic mycoses (1%), zygomycosis (<1%), and other fungal infections (2%). There was no statistical difference in the number of fungal infections that occurred during the study period. AMB was more commonly given to those who had a fungal infection diagnosis (16% versus 10%, P < 0.0001). Prescriptions for LFAB increased during the study period for those with a diagnosis of fungal infection (14% in 2000 –2001 compared with 17% in 2005–2006, P = 0.0001).
There were 48,581 patients that were prescribed antifungal agents who did not receive a fungal diagnosis. Although we are unable to determine the indication for prescription, we assume the use was either preemptive or prophylactic.
Candidiasis.
A total of 11,671 pediatric patients (age <18 years) (19%) were diagnosed with a form of candidiasis during the study period.
Mucosal/Superficial Candidiasis.
There were 8715 patients (14%) diagnosed with mucosal/superficial candidal infections. The most commonly prescribed agent for these patients was fluconazole (89%), followed by LFAB (11%). During the study period, use of AMB for the treatment of mucosal/superficial candidiasis decreased from 23% in 2000–2001 to 2% in 2005–2006 (P < 0.0001).
Disseminated/Systemic Candidiasis.
There were 2956 patients (5%) diagnosed with disseminated/systemic candidiasis. Fluconazole was the most commonly prescribed antifungal agent for inpatients with disseminated/systemic candidiasis (61%), followed by LFAB (42%). During the study period, there were significant changes in prescribing for inpatients (Fig. 3A). AMB was used to treat 65% of inpatients in 2000–2001, but only 11% of cases received the drug in 2005–2006 (P < 0.0001). In 2000–2001, 24% of all amphotericin preparations prescribed for disseminated/systemic candidiasis were LFAB, whereas in 2005–2006, 77% were LFAB. The echinocandins significantly increased in prescription for the treatment of disseminated/systemic candidiasis, from 0% in 2000–2001 to 23% in 2005–2006 (P < 0.0001).
FIGURE 3.
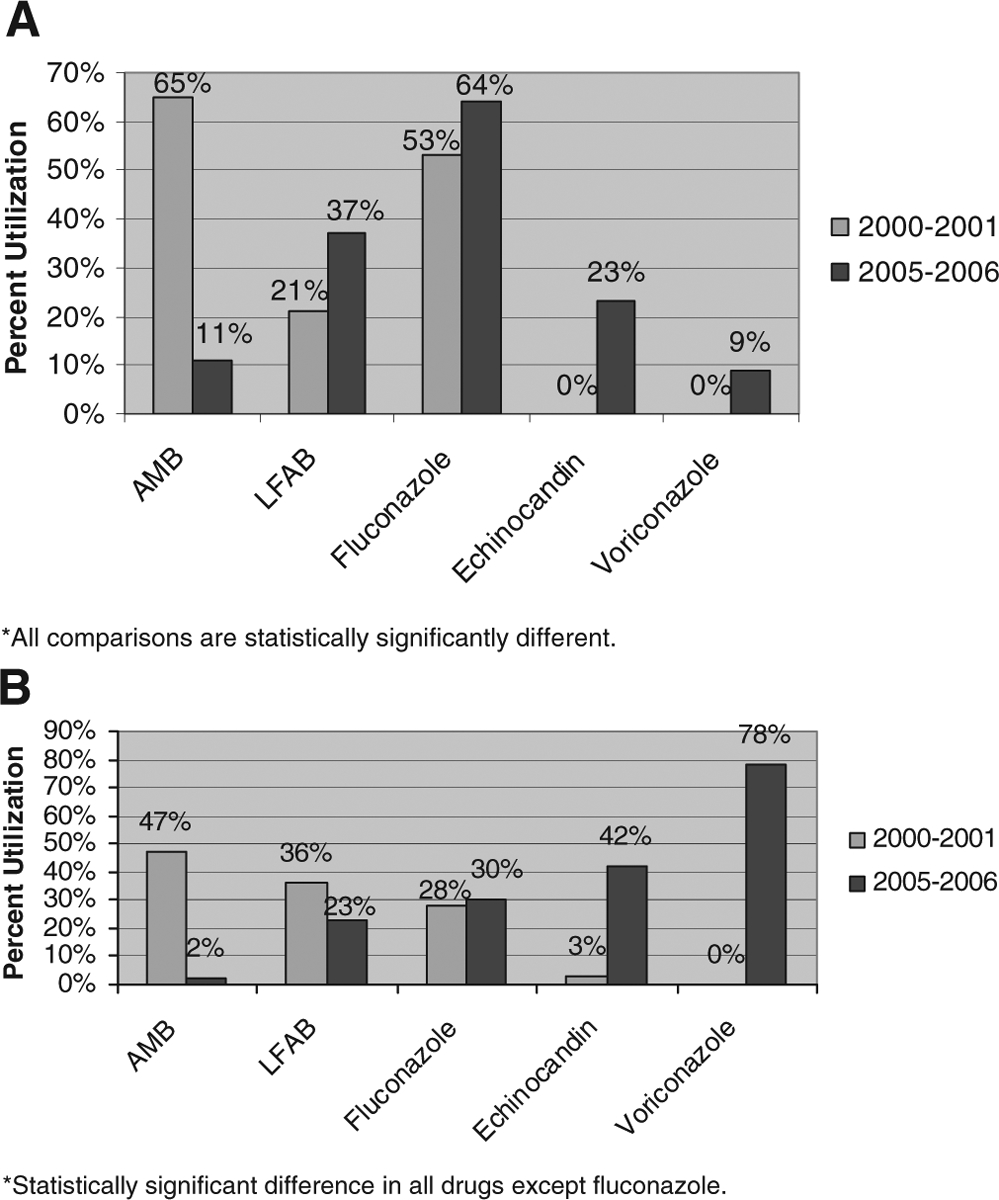
A, Trends in antifungal therapy for disseminated systemic candidiasis. B, Trends in antifungal therapy for aspergillosis.
Aspergillosis.
There were a total of 1002 patients who were diagnosed with aspergillosis (2%). The most common antifungal agents prescribed for patients with aspergillosis were voriconazole (43%) and LFAB (42%). In 2000–2001, 43% of all amphotericin preparations prescribed for aspergillosis were LFAB, while in 2005–2006, 92% were LFAB. All antifungal agents except fluconazole demonstrated significant changes in utilization over the study period (Fig. 3B).
Antifungal Utilization in Neonates
During the study period, there were 5839 neonatal hospitalizations requiring at least 1 dose of an antifungal agent. There was a significant increase in the number of neonatal patients treated with antifungal therapy during the 7-year period (P = 0.03). Fluconazole was the most commonly prescribed antifungal agent in neonates (65%), followed by AMB (30%) and LFAB (20%). The use of fluconazole and the echinocandins in neonates significantly increased during the study period (P = 0.03 and P = 0.04, respectively), whereas the use of AMB and flucytosine decreased over the same time period (P = 0.02 and P = 0.02, respectively) (Fig. 4).
FIGURE 4.
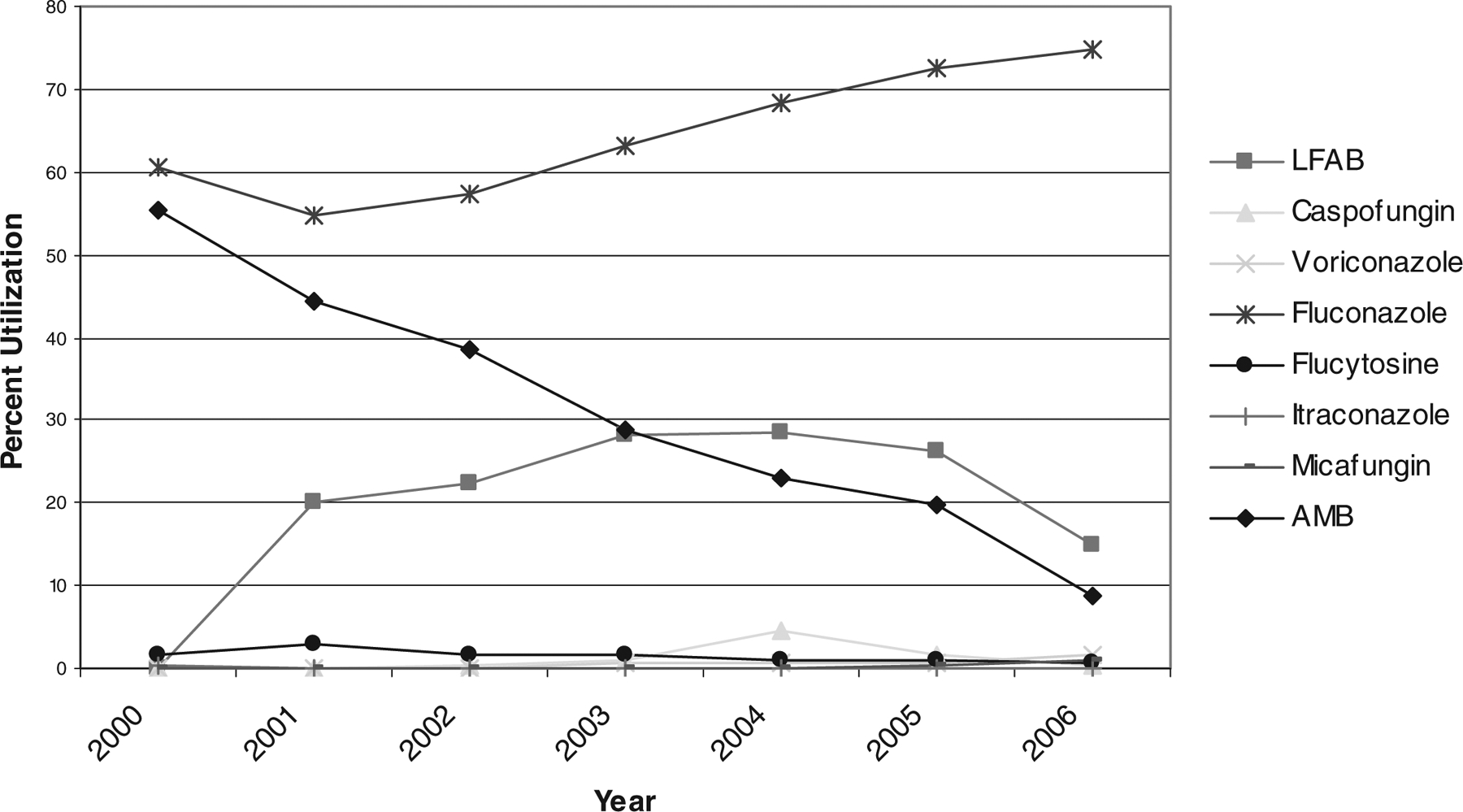
Trends in antifungal therapy for neonatal hospitalizations, 2000–2006.
A total of 1247 (21%) neonates were diagnosed with a fungal infection, most commonly a form of candidiasis (86%). Mucosal/superficial candidal infections accounted for 442 (35%) neonatal fungal infections, which were most commonly treated with fluconazole (71%), AMB (36%), and LFAB (15%). Disseminated/systemic candidiasis accounted for 631 (51%) neonatal fungal infections. AMB was used most commonly to treat these neonates (54%), followed by fluconazole (50%). Prescribing of AMB, LFAB, and the echinocandins for the treatment of neonatal disseminated/systemic candidiasis changed significantly during the study period. In 2000–2001, 14% of all amphotericin preparations prescribed were LFAB, while in 2005–2006, 63% were LFAB.
DISCUSSION
Since 2000, there have been significant changes in the prescribing of antifungal agents in hospitalized children. Although AMB has been considered the standard in antifungal therapy during the past 4 decades,1,11,13,14 pediatricians have markedly decreased their utilization of this agent. The data in this study indicate that LFAB and the newer agents, echinocandins and voriconazole, replaced AMB after their introduction. Although AMB prescriptions also decreased significantly in neonates, it is still commonly used for the treatment of candidiasis. This observation is consistent with data demonstrating that infants and young children infrequently experience adverse events associated with AMB and LFAB14; in addition, there is a lack of dosing and safety data for the prescription of newer agents in this population. The increase in the number of neonates requiring antifungal therapy over the study period likely reflects the increased use of prophylactic fluconazole to prevent invasive fungal infections in premature neonates.18,19
As the antifungal treatment options available to clinicians have increased, prescribing and treatment strategies have also significantly changed. For example, studies have shown that the echinocandins are at least as effective as AMB for the treatment of candidiasis and their use is associated with fewer side-effects.20 Although no direct comparisons have been made between AMB and the echinocandins within a large pediatric cohort, data from a prospective multicenter study show that the efficacy of the echinocandins in pediatric patients with invasive aspergillosis or invasive candidiasis is consistent with results from previous adult studies.21 Our data show that later in the study period, clinicians were more commonly prescribing the echinocandins for disseminated/systemic candidiasis and other fungal infections than AMB and LFAB. Both caspofungin and micafungin have been studied in children; caspofungin administered at 50 mg/m2/d in children provides exposure that is comparable to that after 50 mg/d doses in adults.22 Micafungin given in a dose of 4–5 mg/kg has been shown to be as effective as LFAB at treating invasive candidiasis in children.23
The most striking change in antifungal prescribing was seen in children with aspergillosis, as AMB was almost completely replaced by voriconazole. We assume that this change in practice was influenced by the large randomized clinical trial demonstrating that voriconazole is statistically superior to AMB for treatment of invasive aspergillosis. Notably, however, few children were included in this study.24 Because of the more accelerated metabolic clearance in pediatric patients, the dosages of voriconazole may need to be higher.25 A maintenance dose of 7 mg/kg twice daily in children is recommended by the European Medicines Agency for the attainment of plasma values that are comparable to those in adults. Loading regimens in pediatric populations have not been adequately studied.
The majority of patients prescribed an antifungal agent did not have a documented fungal infection by ICD-9-CM code. This finding may be due in part to the low sensitivity of ICD-9-CM codes for identifying fungal infections. However, we determined that 8% of these patients were diagnosed with “septicemia” and 5% were diagnosed with “bacteremia,” which could account for some of the antifungal treatment. In addition, patients who did not have a documented fungal infection were most commonly prescribed fluconazole (77%) and were more likely to have an underlying condition (82%) when compared with those patients who were diagnosed with a fungal infection (72%). Because of their underlying condition, these patients without documented fungal infection could have been receiving antifungal therapy as prophylaxis or empiric therapy.
Because of the comprehensive data on more than 6 million patients, the PHIS database can be a powerful tool for clinical researchers. This descriptive study included a large number of geographically diverse and representative freestanding children’s hospitals, suggesting that the results could be generalizable to other regions of the country. Our study has several limitations to be considered. First, the determination of fungal infection status and/or underlying comorbid conditions may be vulnerable to miscoding as these variables were determined by ICD-9-CM codes; however, fungal codes tend to have high specificity. Therefore the results of our study would more likely represent an underestimate of the burden of disease in the population. Additionally, the CCCs used to designate underlying condition in this analysis were previously validated by Feudtner et al.16 Although there are inherent limitations associated with the use of administrative data, we believe our results accurately describe national pediatric antifungal prescribing patterns.
In summary, we found that the number of hospitalized children prescribed antifungal therapy has increased and the choice of antifungal therapy has changed dramatically with the introduction of newer antifungal agents. This rapid shift to the use of newer agents has occurred despite the lack of adequate pharmacokinetic/pharmacodynamic, safety, or efficacy data in children. The shift likely reflects clinicians’ desire to use agents with better toxicity profiles. Further studies must be conducted to determine the optimal dosing, efficacy, and safety of these newer agents in the pediatric population.
Supplementary Material
Acknowledgments
Supported from the National Institutes of Health (1K23 AI0629753-01) and the Centers for Education and Research on Therapeutics (U18-HS103999 from the Agency for Healthcare Research and Quality) (to T.E.Z.).
Footnotes
This work was presented in part at the Interscience Conference on Antimicrobial Agents and Chemotherapy, September 2006, San Francisco, CA and the Infectious Diseases Society of America Annual Meeting, October 2006, Toronto, Ontario, Canada.
Dr. Zaoutis has received research funding from Merck. All other authors report no conflicts of interest relevant to this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text; simply type the URL address into any Web browser to access this content. Clickable links to the material are provided in the HTML text and PDF of this article on the Journal’s Web site (www.pidj.com).
REFERENCES
- 1.Steinbach WJ, Walsh TJ. Mycoses in pediatric patients. Infect Dis Clin North Am. 2006;20:663–678. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak CC, Steinbach WJ, Brown JM, Agarwal R. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2005;36: 621–629. [DOI] [PubMed] [Google Scholar]
- 3.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41:1232–1239. [DOI] [PubMed] [Google Scholar]
- 4.Zaoutis TE, Coffin SE, Chu JH, et al. Risk factors for mortality in children with candidemia. Pediatr Infect Dis J. 2005;24:736–739. [DOI] [PubMed] [Google Scholar]
- 5.Zaoutis TE, Heydon K, Localio R, Walsh TJ, Feudtner C. Outcomes attributable to neonatal candidiasis. Clin Infect Dis. 2007;44:1187–1193. [DOI] [PubMed] [Google Scholar]
- 6.Zaoutis TE, Heydon K, Chu JH, Walsh TJ, Steinbach WJ. Epidemiology, outcomes, and costs of invasive aspergillosis in immunocompromised children in the United States, 2000. Pediatrics. 2006;117:e711–e716. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin DK Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. [DOI] [PubMed] [Google Scholar]
- 8.Saiman L, Ludington E, Pfaller M, et al. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19:319–324. [DOI] [PubMed] [Google Scholar]
- 9.Smith PB, Morgan J, Benjamin JD, et al. Excess costs of hospital care associated with neonatal candidemia. Pediatr Infect Dis J. 2007;26:197–200. [DOI] [PubMed] [Google Scholar]
- 10.Steinbach WJ. Pediatric aspergillosis: disease and treatment differences in children. Pediatr Infect Dis J. 2005;24:358–364. [DOI] [PubMed] [Google Scholar]
- 11.Antachopoulos C, Walsh TJ. New agents for invasive mycoses in children. Curr Opin Pediatr. 2005;17:78–87. [DOI] [PubMed] [Google Scholar]
- 12.Blyth CC, Palasanthiran P, O’Brien TA. Antifungal therapy in children with invasive fungal infections: a systematic review. Pediatrics. 2007; 119:772–784. [DOI] [PubMed] [Google Scholar]
- 13.Steinbach WJ, Benjamin DK. New antifungal agents under development in children and neonates. Curr Opin Infect Dis. 2005;18:484–489. [DOI] [PubMed] [Google Scholar]
- 14.Zaoutis TE, Benjamin DK, Steinbach WJ. Antifungal treatment in pediatric patients. Drug Resist Updat. 2005;8:235–245. [DOI] [PubMed] [Google Scholar]
- 15.Feudtner C, Feinstein JA, Satchell M, Zhao H, Kang TI. Shifting place of death among children with complex chronic conditions in the United States, 1989–2003. JAMA. 2007;297:2725–2732. [DOI] [PubMed] [Google Scholar]
- 16.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001; 107:E99. [DOI] [PubMed] [Google Scholar]
- 17.Averill R, Goldfield N, Hughes J, et al. What are APR-DRGs? An introduction to the severity of illness and risk of mortality adjustment methodology. 2003. [Google Scholar]
- 18.Manzoni P, Stolfi I, Pugni L, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. 2007;356: 2483–2495. [DOI] [PubMed] [Google Scholar]
- 19.Parikh TB, Nanavati RN, Patankar CV, et al. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660–1666. [DOI] [PubMed] [Google Scholar]
- 20.Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347: 2020–2029. [DOI] [PubMed] [Google Scholar]
- 21.Zaoutis TE, Jafri H, Huang LM, et al. Prospective, multicenter study of caspofungin (CAS) for the treatment of documented fungal infections in pediatric patients In: Annual Meeting of the Infectious Disease Society of America; 2007; San Diego, CA. [Google Scholar]
- 22.Walsh TJ, Adamson PC, Seibel NL, et al. Pharmacokinetics, safety, and tolerability of caspofungin in children and adolescents. Antimicrob Agents Chemother. 2005;49:4536–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arrieta A, Queiroz-Tellas F, Freire A, et al. Micafungin versus liposomal amphotericin B (AmBisome®) in paediatric patients with invasive candidiasis or candidaemia. In: 17th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) & 25th International Congress of Chemotherapy (ICC); 2007; Munich, Germany. [Google Scholar]
- 24.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. [DOI] [PubMed] [Google Scholar]
- 25.Walsh TJ, Karlsson MO, Driscoll T, et al. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob Agents Chemother. 2004;48:2166–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


