Abstract
Background:
Diagnosing invasive aspergillosis is difficult but might be improved by detection of circulating galactomannan. Although galactomannan antigenemia has been well studied in the detection of invasive aspergillosis in adult patients, little is known about the expression of circulating galactomannan in immunocompromised children with invasive aspergillosis.
Methods:
We studied the expression of galactomannan antigen by enzyme immunoassay (EIA) in 990 serum samples from 56 pediatric oncology patients (ages 3 months to 18 years) of whom 17 had proven or probable invasive aspergillosis defined by the European Organization for Research and Treatment of Cancer-Mycoses Study Group criteria. Any sample with a galactomannan EIA Galactomannan index value of ≥0.5 was considered positive.
Results:
At least 1 serum sample was positive for 11 of 17 pediatric oncology patients (65.7% sensitivity, 95% confidence interval: 38.3–85.7) with invasive aspergillosis. Galactomannan EIA was positive in 99 of 304 samples from patients with proven or probable invasive aspergillosis, and 7 of 686 (1.0%) samples from 39 control subjects resulted in a positive galactomannan EIA result. At least 1 sample tested positive in 5 of the 39 controls (12.8%, 95% confidence interval: 4.3–27.4). No significant association between accuracy and patient age was observed. Among the 7 evaluable galactomannan-positive patients with IA, the galactomannan EIA produced a positive result before clinical or radiographic evidence of infection in 6 cases, with a lead-time to diagnosis ranging from 1 day to 34 days (median: 10 days). In the remaining case, a positive galactomannan was observed on the same day as diagnosis by non-EIA methods.
Conclusions:
The presence of circulating galactomannan is predictive of invasive aspergillosis in most pediatric oncology patients. Galactomannan antigenemia may precede clinical, microbiologic, or radiographic evidence of invasive aspergillosis.
Keywords: aspergillosis, galactomannan, pediatric oncology
Invasive aspergillosis is an important cause of morbidity and mortality in immunocompromised pediatric patients, including patients with hematologic malignancies, hematopoietic stem cell transplant recipients, solid organ transplant recipients, and those with primary or acquired immunodeficiencies.1–7 Several new antifungal agents offer hope for improved outcome in treating many of these infections.8–13 Such therapies are often initiated after infection has become deeply invasive, thus reducing the chances for successful recovery from the primary infection while increasing the risk of complications and other comorbidities. Prompt therapy for invasive aspergillosis improves outcome.14 Thus, methods allowing more rapid and sensitive detection of invasive aspergillosis are needed.
Lack of rapid, early, and accurate diagnosis of invasive fungal infections, particularly invasive aspergillosis, is a major limitation in treatment of these infections.15,16 Traditional culture-based methods of detection lack sensitivity and often require invasive procedures for diagnosis of invasive aspergillosis.17 Although histopathology has a high degree of specificity, it has limited sensitivity, requires the removal of tissue (sometimes not possible in critically ill patients) and may not be helpful in the early stages of infection.18
Recent efforts have focused on the use of nucleic acid amplification techniques.19,20 The results of studies of nucleic acid amplification vary depending on platform, primer sequences, and study design. Nucleic acid amplification testing for detection of invasive aspergillosis is technically demanding and a single system has not been standardized and validated in a large number of centers.
Galactomannan is a major heteropolysaccharide of the cell wall of Aspergillus spp. During the course of infection, this carbohydrate antigen is expressed in tissue, the circulation, and the tracheobronchial tree.21–27 The most recent and widely used platform for detection of galactomannan antigen in serum is an enzyme immunoassay (EIA) that uses the EB-A2 rat monoclonal antibodies.28 Most clinical studies of the expression of serum galactomannan have been performed in adult patients.15,21,22,24,26,29–32 The patterns of expression of serum galactomannan in pediatric patients are not well understood.32–35 We therefore studied expression of serum galactomannan in pediatric oncology patients with invasive aspergillosis.
MATERIALS AND METHODS
Patients and Experimental Design.
This study included 990 serum samples from 56 pediatric oncology patients (age ≤18 years) from St. Jude Children’s Research Hospital and the Pediatric Oncology Branch, National Cancer Institute. The demographic characteristics of this population are described in Table 1. We studied 686 serum samples from 39 patients without evidence of invasive aspergillosis (control patients), and 304 serum samples from 17 patients with proven or probable invasive aspergillosis. Among these 686 samples, blinded, retrospective galactomannan testing was performed on 536 frozen, excess, serum samples from 36 patients, remaining after routine diagnostic testing; the remaining 150 samples from 3 patients were tested prospectively, for patient care purposes, with results reviewed retrospectively. Samples were collected at least once weekly during the period of neutropenic or immunosuppressive risk. Review of the medical record was performed to categorize patients according to EORTC/IFICG (European Organization for Research and Treatment of Cancer/Invasive Fungal Infection Cooperative Group) and NIAID/MSG (National Institute of Allergy and Infectious Disease/Mycoses Study Group) criteria as having proven, probable or possible aspergillosis, or as having no evidence of aspergillosis.37 Chart review was conducted in a blinded manner without awareness of patients’ galactomannan antigen data. Analysis for serum galactomannan, data collection, and data analysis were performed after institutional review board approval. As the study posed no risk to patients, signed informed consent was not required.
TABLE 1.
Demographic Characteristics of Patients
| Demographic Variable | Aspergillosis (n = 17)* | Controls† (n = 39) | Total (n = 56) |
|---|---|---|---|
| Age | |||
| Mean | 10.0 | 8.0 | 8.3 |
| Range | 1.0–17.0 | 0.3–18.0 | 0.3–18.0 |
| Primary diagnosis | |||
| Leukemia/MDS‡ | 14 | 22 | 36 |
| Lymphoma | 3 | 6 | 9 |
| Solid tumor | 1 | 10 | 11 |
| Hematopoietic stem cell transplant recipients | 8 | 14 | 22 |
| Treatment with antifungal agents§ | 10 | 17 | 27 |
Proven or probable aspergillosis by EORTC/IFICG and NIAID/MSG criteria.
No clinical or laboratory evidence of aspergillosis.
Myelodysplastic syndrome.
Antifungal agents included treatment for proven or probable aspergillosis, empirical therapy, or for prophylaxis.
Antigen Determination.
All assays were conducted using the Platelia Aspergillus EIA test (Bio-Rad Laboratories, Marnes, France). Reagents and test kits were provided by Bio-Rad. Testing was performed at the 2 study locations using identical methodology, as outlined in the manufacturer’s instructions.
Galactomannan index (GMI) value was calculated for the positive and negative controls, as well as for each patient specimen (a mean GMI value was calculated for samples run in duplicate). GMI values were calculated by dividing the optical density of the sample by the mean optical density of the 2 cutoff control replicates. The positive control was expected to have a GMI value of greater than 2 for a run to be considered valid. Negative control samples were expected to have a GMI value of <0.4, and the cutoff control values were acceptable when ≥0.3 and ≤0.8. Patient samples with a calculated GMI value of ≥0.5 were considered positive for galactomannan.
Statistical Analysis.
The binomial distribution was used to compute confidence intervals for the proportion of patients having at least one positive result and for the proportion of patients with a positive result that was achieved before clinical diagnosis. Fisher exact test was used to compare sensitivity of the assay across the 2 institutions. Classic logistic regression38 was used to explore the association of age with having at least one positive sample. Logistic regression based on generalized estimating equations was used in separate univariate models to explore the association of a positive result with age or antifungal therapy. The generalized estimating equation model treated multiple samples from the same subject as exchangeable repeated measurements.
RESULTS
Galactomannan Antigen Expression in Control Patients.
Seven (1.0%) of 686 samples from 39 control subjects produced a positive galactomannan EIA result (Table 2). All serum samples tested negative in 34 of 39 control subjects, giving a subject-level specificity of 87.2% [95% confidence interval (CI), 72.5–95.7]. Based on the probability of a control subject having at least 1 positive sample, age was not associated with false positive results (P = 0.9580). Figure 1 shows the distribution of GMI values in samples from control patients. The 7 false-positive tests among the 5 patients gave a GMI value of ≤0.8, with 3 values above 0.6. Most values in the control group (98.4%) were ≤0.4 and 95.5% were ≤0.3.
TABLE 2.
Expression of Serum Galactomannan Antigen
| Aspergillosis* | Controls | Total | |
|---|---|---|---|
| Patients | |||
| Positive | 11 | 5 | 16 |
| Negative | 6 | 34 | 40 |
| Total | 17 | 39 | 56 |
| Samples | |||
| Positive | 99 | 7 | 106 |
| Negative | 205 | 679 | 884 |
| Total | 304 | 686 | 990 |
Patient-level sensitivity = 65%; Patient-level specificity = 87%; Specimen-level sensitivity = 33% (not all specimens are expected to be positive early in the course of infection); Specimen-level specificity = 99%; As the study was not designed to determine prevalence of invasive aspergillosis, positive and negative predictive values are not calculated.
Proven or probable invasive aspergillosis by EORTC-BAMSG criteria.37
FIGURE 1.
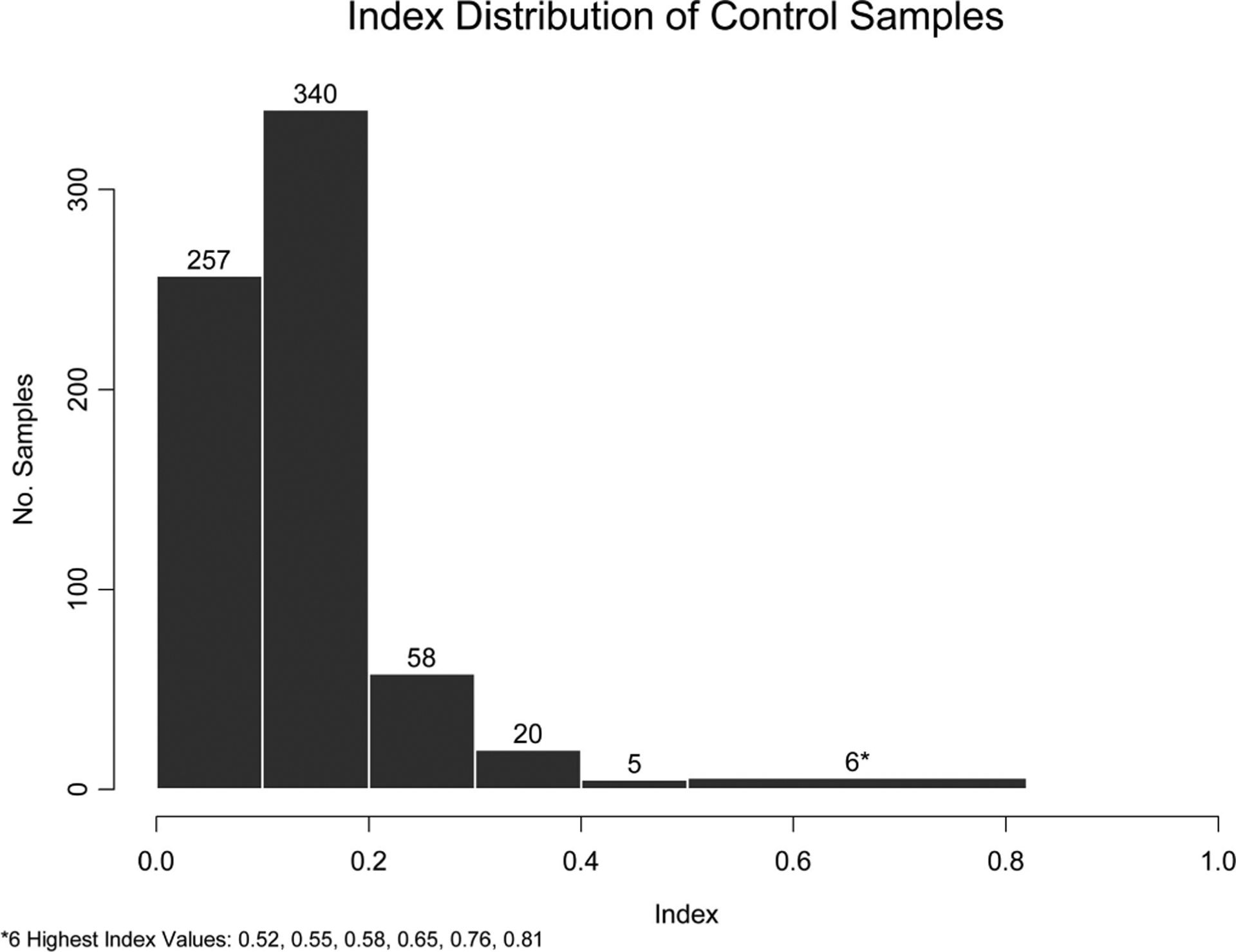
Distribution of galactomannan indices from 686 samples among 39 pediatric oncology patients without clinically overt invasive aspergillosis. Seven (1.0%) of 686 samples expressed a positive galactomannan index. Numbers located at the top of each column indicate the number of samples within each index group.
Galactomannan Antigen Expression in Patients With Proven or Probable Invasive Aspergillosis.
At least 1 serum sample was positive in 11 of 17 pediatric oncology patients (65.7% subject-level sensitivity, 95% CI: 38.3–85.8) with invasive aspergillosis. Galactomannan EIA was positive in 99 of 304 samples from these patients with proven or probable invasive aspergillosis. Piperacillin-tazobactam was not used in the patients in this study.
Fifteen (88%) of 17 patients in this study had proven aspergillosis and 2 (12%) had probable invasive aspergillosis. Ten (67%) of 15 patients with proven invasive aspergillosis tested positive for galactomannan (95% CI: 38.3–88.2). Positive results were obtained in 98 of 283 (34.6%) samples taken from these patients. One of 2 patients with probable invasive aspergillosis yielded positive galactomannan results with 1 (4.8%) of 21 of samples in these subjects testing positive.
Dates of clinical and radiographic diagnosis and sample collection were available for 7 of 11 patients testing positive for galactomannan. A positive result on or before the date of clinical diagnosis was present in all 7 (100%; 95% CI: 59.0–100) of those patients. Galactomannan was detected a median of 10 days before clinical diagnosis. Among 6 of these patients, the positive result occurred 34, 25, 12, 9, 6, and 1 days before the date of clinical diagnosis. In 1 patient, the positive result was obtained on the date of diagnosis.
Galactomannan antigen expression was assessed in relationship to patient age, history of transplantation, and the use of antifungal therapy, including prophylactic and empirical usage. There was no statistically significant association of age with the probability of detecting galactomannan in at least 1 sample for a subject with proven or probable invasive aspergillosis (P = 0.23). The probability of testing positive in at least 1 sample was not significantly associated with history of hematopoietic stem cell transplant among subjects with proven or probable aspergillosis (P = 0.24) or among control subjects (P = 0.67). A positive result was obtained in at least 1 sample for 4 of 9 (44.4%) subjects receiving antifungal therapy and in 7 of 8 (87.5%) of subjects who did not receive such therapy (P = 0.13).
DISCUSSION
This study found that serum galactomannan is frequently expressed in pediatric oncology patients with invasive aspergillosis and infrequently detected above interpretative cut-off points in control populations. The sensitivity and specificity of this assay were comparable with that seen in several adult studies and with that reported in a recent meta-analysis.21,22,24,29–32 In addition, a positive signal was obtained in most cases before clinical or radiographic evidence of infection.
Few studies have evaluated the use of galactomannan for detection of invasive aspergillosis in pediatric patients. Some studies have suggested a much lower degree of specificity of galactomannan in children.24,39–42 These earlier studies consisted of small numbers of pediatric patients, including low-birth-weight infants who are not usually considered to be patients at high risk for invasive aspergillosis. Whether these earlier studies considered the effects of antimicrobial agents in causing false-positive results is not apparent.43–46 Recent studies suggest that mannoproteins from milk or lipoglycans from some enteric bacteria, such as Bifidobacterium spp., may contribute to falsely positive serum galactomannan values.40,41 The specificity observed in this study is comparable to that of a recent report of serum galactomannan in children.48
When the specimen-based false-positive rate is closely examined, the levels of the GMI were only slightly elevated above the interpretive cutoff point of 0.5. Given the relatively large number of samples, these slightly elevated values are compatible with the analytic variability expected around this value.
The sensitivity demonstrated in the current study is in accordance with some reports from the adult literature,24,31 but it is also somewhat lower compared with that of some early reports.29,48 However, these studies detected galactomannan in serum of adult patients with advanced and fatal invasive aspergillosis. Studies in experimental invasive pulmonary aspergillosis demonstrate that expression of galactomannan in serum varies directly with the tissue burden in lungs, histologic extent of infection, and mortality.23 Laboratory animal studies and clinical data also demonstrate that concomitant use of antifungal agents reduces serum galactomannan levels.49–51
Finally, several studies in immunocompromised adult patients have demonstrated the potential value of prospective screening for expression of serum galactomannan for early diagnosis of invasive aspergillosis at times before clinical, radiographic, or microbiologic findings.30,33,36,47,49,52 The findings presented here demonstrate the utility of serum galactomannan as a biologic marker that complements existing diagnostic modalities for early detection of invasive aspergillosis in immunocompromised pediatric oncology patients.
ACKNOWLEDGMENTS
The authors thank Diane Brand for assistance in clinical data extraction and the staff of the Clinical Microbiology Laboratory of St. Jude Children’s Research Hospital. This study was supported, in part, by the American Lebanese Syrian Associated Charities (ALSAC) and by the Intramural Research Program of the National Cancer Institute, Bethesda, MD.
REFERENCES
- 1.Benjamin DK Jr, Miller WC, Bayliff S, Martel L, Alexander KA, Martin PL. Infections diagnosed in the first year after pediatric stem cell transplantation. Pediatr Infect Dis J. 2002;21:227–234. [DOI] [PubMed] [Google Scholar]
- 2.Groll AH, Kurz M, Schneider W, et al. Five-year-survey of invasive aspergillosis in a paediatric cancer centre. Epidemiology, management and long-term survival. Mycoses. 1999;42:431–442. [DOI] [PubMed] [Google Scholar]
- 3.Steinbach WJ, Walsh TJ. Mycoses in pediatric patients. Infect Dis Clin North Am. 2006;20:663–678. [DOI] [PubMed] [Google Scholar]
- 4.Almyroudis NG, Holland SM, Segal BH. Invasive aspergillosis in primary immunodeficiencies. Med Mycol. 2005;43(Suppl 1):S247–S259. [DOI] [PubMed] [Google Scholar]
- 5.Robinson MR, Fine HF, Ross ML, et al. Sino-orbital-cerebral aspergillosis in immunocompromised pediatric patients: a report of three cases and review. Pediatr Infect Dis J. 2001;19:1197–1203. [DOI] [PubMed] [Google Scholar]
- 6.Zaoutis TE, Heydon K, Chu JH, Walsh TJ, Steinbach WJ. Epidemiology, outcomes, and costs of invasive aspergillosis in children in the United States, 2000. Pediatrics. 2006;117:711–716. [DOI] [PubMed] [Google Scholar]
- 7.Shetty D, Giri N, Gonzalez CE, Pizzo PA, Walsh TJ. Invasive aspergillosis in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1997;16:216–221. [DOI] [PubMed] [Google Scholar]
- 8.Pannaraj PS, Walsh TJ, Baker CJ. Advances in antifungal therapy. Pediatr Infect Dis J. 2005;24:921–922. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Lutsar I, Driscoll T, et al. Voriconazole in the treatment of aspergillosis, scedosporiosis, and other invasive fungal infections in children. Pediatr Infect Dis J. 2002;21:240–248. [DOI] [PubMed] [Google Scholar]
- 10.Grigull L, Kuehlke O, Beilken A, et al. Intravenous and oral sequential itraconazole antifungal prophylaxis in paediatric stem cell transplantation recipients: a pilot study for evaluation of safety and efficacy. Pediatr Transplant. 2007;11:261–266. [DOI] [PubMed] [Google Scholar]
- 11.Segal BH, Barnhart LA, Anderson VL, Walsh TJ, Malech HL, Holland SM. Posaconazole as salvage therapy in patients with chronic granulomatous disease and invasive filamentous fungal infection. Clin Infect Dis. 2005;40:1684–1688. [DOI] [PubMed] [Google Scholar]
- 12.Merlin E, Galambrun C, Ribaud P, et al. Efficacy and safety of caspo-fungin therapy in children with invasive fungal infections. Pediatr Infect Dis J. 2006;25:1186–1188. [DOI] [PubMed] [Google Scholar]
- 13.Walsh TJ, Seibel NL, Arndt C, et al. Amphotericin B lipid complex in pediatric patients with invasive fungal infections. Pediatr Infect Dis J. 1999;18:702–708. [DOI] [PubMed] [Google Scholar]
- 14.Aisner J, Wiernik PH, Schimpff SC. Treatment of invasive aspergillosis: relation of early diagnosis and treatment to response. Ann Intern Med. 1977;86:539–543. [DOI] [PubMed] [Google Scholar]
- 15.Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–622. [DOI] [PubMed] [Google Scholar]
- 16.Roilides E Early diagnosis of invasive aspergillosis in infants and children. Med Mycol. 2006;44(Suppl):199–205. [DOI] [PubMed] [Google Scholar]
- 17.Latgé J-P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stergiopoulou T, Meletiadis J, Roilides E, et al. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am J Clin Pathol. 2006;127:349–355. [DOI] [PubMed] [Google Scholar]
- 19.Buchheidt D, Baust C, Skladny H, Baldus M, Brauninger S, Hehlmann R. Clinical evaluation of a polymerase chain reaction assay to detect Aspergillus species in bronchoalveolar lavage samples of neutropenic patients. Br J Haematol. 2002;116:803–811. [DOI] [PubMed] [Google Scholar]
- 20.O’Sullivan CE, Kasai M, Francesconi A, et al. Development and validation of a quantitative real-time PCR assay using fluorescence resonance energy transfer (FRET) technology for detection of Aspergillus fumigatus in experimental invasive pulmonary aspergillosis. J Clin Microbiol. 2003;41:5676–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verdaguer V, Walsh TJ, Hope W, Cortez KJ. Galactomannan antigen detection in the diagnosis of invasive aspergillosis. Expert Rev Mol Diagn. 2007;7:21–32. [DOI] [PubMed] [Google Scholar]
- 22.Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J Infect Dis. 2004;190:641–649. [DOI] [PubMed] [Google Scholar]
- 23.Hope WW, Kruhlak MJ, Lyman CA, et al. Mathematical modeling of galactomannan and amphotericin B in a model of early invasive pulmonary aspergillosis. J Infect Dis. 2007;195:455–466. [DOI] [PubMed] [Google Scholar]
- 24.Herbrecht R, Letscher-Bru V, Oprea C, et al. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J Clin Oncol. 2002;20:1898–1906. [DOI] [PubMed] [Google Scholar]
- 25.Woods G, Miceli MH, Grazziutti ML, Zhao W, Barlogie B, Anaissie E. Serum Aspergillus galactomannan antigen values strongly correlate with outcome of invasive aspergillosis: a study of 56 patients with hematologic cancer. Cancer. 2007;110:830–834. [DOI] [PubMed] [Google Scholar]
- 26.Maertens J, Theunissen K, Lodewyck T, Lagrou K, Eldere JV. Advances in the serological diagnosis of invasive Aspergillus infections in patients with haematological disorders. Mycoses. 2007;50(Suppl 1):2–17. [DOI] [PubMed] [Google Scholar]
- 27.Francesconi A, Kasai M, Petraitiene R, et al. Characterization of galactomannan enzyme immunoassay and quantitative real-time PCR for the detection of Aspergillus fumigatus from bronchoalveolar lavage fluid in experimental invasive pulmonary aspergillosis. J Clin Microbiol. 2006; 44:2475–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stynen D, Sarfati J, Goris A, et al. Rat monoclonal antibodies against Aspergillus galactomannan. Infect Immun. 1992;60:2237–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maertens J, Verhaegen J, Demuynck H, et al. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematologic patients at risk for invasive aspergillosis. J Clin Microbiol. 1999;37:3223–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maertens J, Van Eldere J, Verhaegen J, Verbeken E, Verschakelen J, Boogaerts M. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. J Infect Dis. 2002;186:1297–1306. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42:1417–1427. [DOI] [PubMed] [Google Scholar]
- 32.Sulahian A, Boutboul F, Ribaud P, Leblanc T, Lacroix C, Derouin F. Value of antigen detection using an enzyme immunoassay in the diagnosis and prediction of invasive aspergillosis in two adult and pediatric hematology units during a 4-year prospective study. Cancer. 2001;91: 311–318. [DOI] [PubMed] [Google Scholar]
- 33.Hovi L, Saxen H, Saarinen-Pihkala UM, Vettenranta K, Meri T, Richardson M. Prevention and monitoring of invasive fungal infections in pediatric patients with cancer and hematologic disorders. Pediatr Blood Cancer. 2007;48:28–34. [DOI] [PubMed] [Google Scholar]
- 34.Verweij PE, Weemaes CM, Curfs JH, Bretagne S, Meis JF. Failure to detect circulating Aspergillus markers in a patient with chronic granulomatous disease and invasive aspergillosis. J Clin Microbiol. 2000;38: 3900–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Mahallawy HA, Shaker HH, Ali Helmy H, Mostafa T, Razak Abo-Sedah A. Evaluation of pan-fungal PCR assay and Aspergillus antigen detection in the diagnosis of invasive fungal infections in high risk paediatric cancer patients. Med Mycol. 2006;44:733–739. [DOI] [PubMed] [Google Scholar]
- 36.Baker C Serial Aspergillus antigen monitoring in pediatric bone marrow transplant patients. J Pediatr Oncol Nurs. 2006;23:300–304. [DOI] [PubMed] [Google Scholar]
- 37.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. [DOI] [PubMed] [Google Scholar]
- 38.Agretsi A Categorical Data Analysis. 2nd ed Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- 39.Siemann M, Koch-Dorfler M, Gaude M. False-positive results in pre-mature infants with the Platelia Aspergillus sandwich enzyme-linked immunosorbent assay. Mycoses. 1998;41:373–377. [DOI] [PubMed] [Google Scholar]
- 40.Mennink-Kersten MA, Klont RR, Warris A, Op den Camp HJ, Verweij PE. Bifidobacterium lipoteichoic acid and false ELISA reactivity in Aspergillus antigen detection. Lancet. 2004;363:325–327. [DOI] [PubMed] [Google Scholar]
- 41.Mennink-Kersten MA, Ruegebrink D, Klont RR, et al. Bifidobacterial lipoglycan as a new cause for false-positive platelia Aspergillus enzyme-linked immunosorbent assay reactivity. J Clin Microbiol. 2005;43:3925–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gangneux JP, Lavarde D, Bretagne S, Guiguen C, Gandemer V. Transient Aspergillus antigenaemia: think of milk. Lancet. 2002;359: 1251. [DOI] [PubMed] [Google Scholar]
- 43.Sulahian A, Touratier S, Ribaud P. False positive test for Aspergillus antigenemia related to concomitant administration of piperacillin and tazobactam. N Engl J Med. 2003;349:2366–2367. [DOI] [PubMed] [Google Scholar]
- 44.Singh N, Obman A, Husain S, Aspinall S, Mietzner S, Stout JE. Reactivity of platelia Aspergillus galactomannan antigen with piperacillin-tazobactam: clinical implications based on achievable concentrations in serum. Antimicrob Agents Chemother. 2004;48:1989–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh TJ, Shoham S, Petraitiene R, et al. Detection of galactomannan antigenemia in patients receiving piperacillin-tazobactam and correlations between in vitro, in vivo, and clinical properties of the drug-antigen interaction. J Clin Microbiol. 2004;42:4744–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mattei D, Rapezzi D, Mordini N, et al. False-positive Aspergillus galactomannan enzyme-linked immunosorbent assay results in vivo during amoxicillin-clavulanic acid treatment. J Clin Microbiol. 2004;42: 5362–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bretagne S, Marmorat-Khuong A, Kuentz M, Latge JP, Bart-Delabesse E, Cordonnier C. Serum Aspergillus galactomannan antigen testing by sandwich ELISA: practical use in neutropenic patients. J Infect. 1997;35:7–15. [DOI] [PubMed] [Google Scholar]
- 48.Steinbach WJ, Addison RM, McLaughlin L, et al. Prospective Aspergillus galactomannan antigen testing in pediatric hematopoietic stem cell transplant recipients. Pediatr Infect Dis J. 2007;26:558–564. [DOI] [PubMed] [Google Scholar]
- 49.Francis P, Lee JW, Hoffman A, et al. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar lavage D-mannitol and galactomannan as markers of infection. J Infect Dis. 1994;169:356–368. [DOI] [PubMed] [Google Scholar]
- 50.Petraitiene R, Petraitis V, Groll AH, et al. Antifungal activity and plasma pharmacokinetics of posaconazole (SCH 56592) in treatment and prevention of experimental invasive pulmonary aspergillosis: correlation with galactomannan antigenemia. Antimicrob Agents Chemother. 2001; 45:857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005;40:1762–1769. [DOI] [PubMed] [Google Scholar]
- 52.Maertens J, Theunissen K, Verhoef G, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005;41:1242–1250. [DOI] [PubMed] [Google Scholar]


