Abstract
Importance of the field
Immunotherapeutic approaches to treating NSCLC via either adoptive transfer of immunity or stimulation of the endogenous immune system have shown increasing promise in recent years.
Areas covered in this review
Talactoferrin alfa is an oral immunomodulatory agent currently in late-stage clinical trials that acts through dendritic cell recruitment and activation in the gut-associated lymphoid tissue.
What the reader will gain
Talactoferrin is a recombinant human lactoferrin that is a member of the transferrin family of iron-binding glycoproteins. Lactoferrins have multiple known biological activities including cancer protection, cellular growth and differentiation and antimicrobial and anti-inflammatory properties. This review discusses the proposed mechanism of action of talactoferrin-alfa and outlines the pre-clinical, Phase I and II data in NSCLC. The ongoing Phase III trials are discussed.
Take home message
The current role of Talactoferrin alpha in the treatment of NSCLC is described and we explore potential future roles for this drug in both early stage and advanced stage disease.
Keywords: dendritic cell, immunotherapy, non small cell lung cancer, talactoferrin alpha
1. Introduction
Talactoferrin alfa (also known as recombinant human lactoferrin) is a recombinant version of the glycoprotein expressed in and purified from Aspergillus niger var. awamor (Box 1) [1]. Talactoferrin is structurally and functionally similar to native human lactoferrin purified from human milk and is known to differ only in the nature of glycosylation [2]. Lactoferrin is a member of the transferrin family of non-heme iron-binding proteins and is found in mammalian serum and exocrine secretions such as breast milk, seminal fluid, intestinal secretions, tears, sweat, saliva and nasal secretions and in secretory granules of neutrophils [3]. Lactoferrins have been demonstrated to have anti-inflammatory [4], anti-infective [5] and anti-tumor properties [6]. Lactoferrins have a wide array of immune-modulatory functions including activation of NK and lymphokine-activated killer cells [7], and enhancement of polymorphonuclear cells (PMNs) and macrophage cytotoxicity [8]. In preclinical studies lactoferrins have been shown to have anti-cancer activity in a broad range of tumor types [7].
Box 1. Drug summary.
| Drug name | Talactoferrin alfa |
| Phase | Two ongoing Phase III trials in lung cancer |
| Indication | Non-small cell lung cancer |
| Renal cell carcinoma | |
| Pharmacology description/mechanism of action | Talactoferrin is an orally active immune-modulatory protein that specifically binds receptors on cells lining the upper gastrointestinal tract, initiating an immune-stimulatory cascade in the gut associated lymphoid tissue (GALT) |
| Route of administration | Oral |
| Pivotal trials | Phase II NSCLC [27] |
| Phase II NSCLC [28] | |
| Phase II RCC [30] | |
| Phase II RCC [32] |
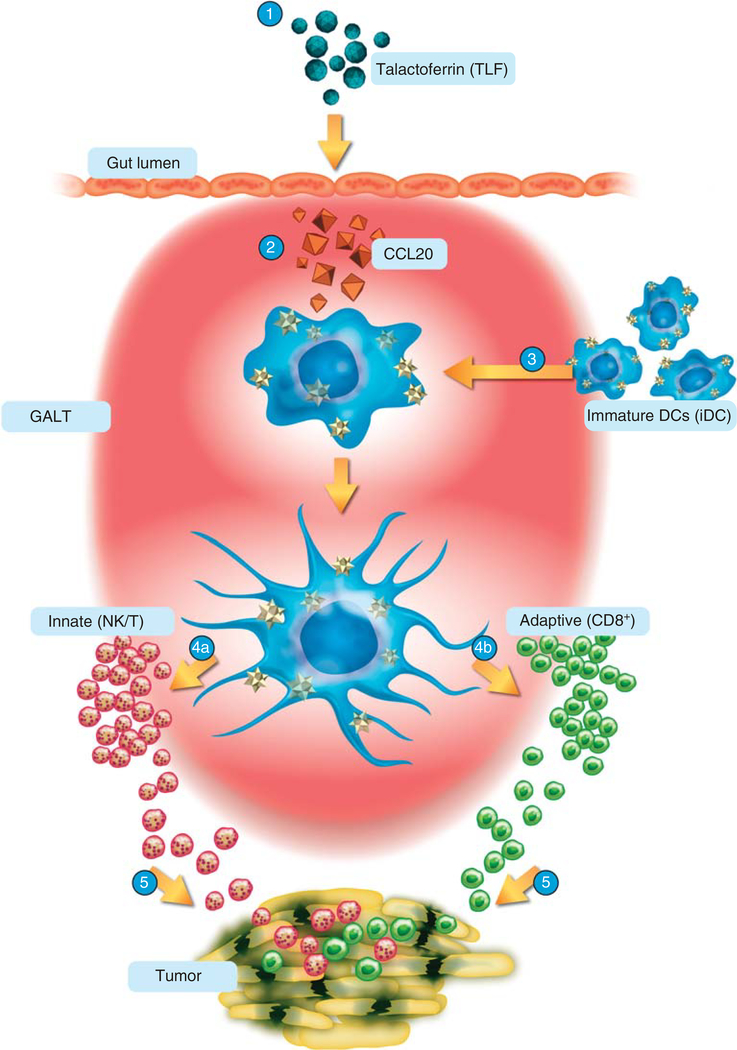
Talactoferrin is an orally active immune-modulatory protein that specifically binds receptors on cells lining the upper gastrointestinal tract, initiating an immune-stimulatory cascade in the gut associated lymphoid tissue (GALT) [9]. Talactoferrin acts as a chemokine, binding chemokine receptors and inducing the chemotaxis of immune cells including effector cells such as lymphocytes and antigen-presenting cells (APCs). Once administered, talactoferrin is transported by M-cells into Peyer’s patches in the small intestine. It is here that dendritic cells bearing tumor antigens to GALT are recruited and where their maturation occurs. Talactoferrin also induces the production of key cytokines including IL-18 and IFN-γ in the GALT [9]. These cytokines play an important role in stimulating both the innate and the adaptive immunity, including promoting the maturation and proliferation of immune cells such as anti-cancer NK cells, CD8+ lymphocytes and NK-T cells. In preclinical experiments following oral administration of talactoferrin there has been an increase in the total cellularity of small intestinal Peyer’s patches, including an increase in the numbers of NK-T cells and CD8+ T-lymphocytes. The hypothesis is that talactoferrin can result in an enhanced activation of tumor-draining lymph nodes leading to cellular infiltration of tumors and ultimately tumor-cell death (Figure 1).
Figure 1. Proposed mechanism of action of talactoferrin*.
1. TLF taken orally. 2. TLF acts on GI epithelium to release key chemokines (e.g., CCL20). 3. iDC are recruited by chemokines and undergo maturation (activation). 4a. Activated DCs initiate tumoricidal response of NK cells. 4b. Activated DCs cross present tumor antigens to CD8+ cells. 5. Immune cells seek out, infiltrate and kill tumor cells.
*The information depicted in the schematic reflects our current understanding of the anti-cancer biological activity of TLF and is subjected to change as directed by future research. The acquisition and presentation of the tumor antigens by iDC and DCs has not been fully characterized and is currently under investigation.
2. Pre-clinical data
Despite well defined known mechanisms for direct lactoferrin inhibition of tumor growth in vitro, the specific mechanisms for growth inhibition in vivo are not well understood. Preclinical studies have shown that lactoferrin induces direct cell cycle arrest in both breast and head and neck cancer cell lines [10,11]. Lactoferrins have also been shown to decrease the cellular release of the pro-inflammatory cytokines, IL-1, IL-4, IL-6 and TNF-α. These cytokines have been implicated in maintaining cellular viability and a malignant phenotype [12,13]. Lactoferrins also decrease the activity of the transcription factor, NF-κB, which is constitutively activated in a number of tumors [12].
Although in vitro studies show direct growth inhibition via G1-G0 blockade, NF-κB activation, and a reduction of cellular pro-inflammatory cytokines, the mechanism of lactoferrin-induced tumor control in vivo appears to be regulated primarily by the cellular arm of the immune response [14]. Lactoferrin is known to increase TH1 response [15] and release of IL-18 from murine intestinal epithelium which in turn increases CD4+, CD8+ and NK cell activity [16,17]. Lactoferrin has also been shown to reconstitute the circulating and splenic CD4+ and CD8+ cell population in mice whom had been treated with cyclophosphamide. This increase was in functional lymphocytes as shown by demonstrating reconstitution of the type IV hypersensitivity reaction in the treated mice [18]. A number of preclinical studies have also shown that lactoferrin treatment can decrease solid tumor growth and metastases in both rats and mice [6,19,20].
The lactoferrin receptor is expressed in many tissues and specific receptors occur in different cells including the intestine, spleen, liver, heart, salivary glands, platelets, lymphyocytes and monocytes [21–23]. The deduced amino acid sequence of the lactoferrin receptor does not contain a transmembrane region. It has been suggested that the lactoferrin receptor is glycophosphatidylinositol (GPI)-anchored as an alternative to a hydrophobic transmembrane polypeptide anchor [21]. Lactoferrin receptors in the intestinal epithelium have the highest specific binding capacity when compared with lactoferrin receptors expressed at other sites [23]. The murine lactoferrin receptor has an 87% homology with the human lactoferrin receptor [24] and the presence of the lactoferrin receptor has been demonstrated in carcinoma cell lines [21].
Wolf et al. evaluated the mechanism of action of lactoferrin in cell lines of squamous cell carcinomas (SCC) of the head and neck [14]. The authors demonstrated that a dose-dependent cellular inhibition of SCC cell lines was associated with decreases in cyclin D1 and increases in P19. Lactoferrin was also found to reduce the cellular production of pro-inflammatory and pro-metastatic cytokines. In vivo studies showed that this inhibition was associated with an immunomodulatory effect characterized by a marked increase of lymphocytic infiltration into the tumors. A mouse model has shown that lactoferrin can induce growth inhibition in floor of mouth tumors (squamous cell carcinomas) on a par with the effect seen with cisplatinum. In this study oral lactoferrin was associated with increases in gut release of IL-18, NK activation and an increase in serum CD8+ cells [25].
3. Phase I data
A Phase I study enrolled 10 patients with refractory solid tumors of which 3 patients had NSCLC [9]. All patients had disease progression on conventional chemotherapy. Talactoferrin doses were increased with dosing increments ranging from 1.5 to 9 g/day administered using a 2-week-on, 2-week-off schedule. Pharmacokinetic analysis was performed to confirm talactoferrin’s lack of systemic bioavailability [9]. The results demonstrated no increase in serum talactoferrin levels following oral administration, indicating the drugs lack of systemic bioavailability. Talactoferrin does not act systemically but rather acts locally at the level of the gut whereby it can induce the production of several key cytokines, most notably IL-18 [25]. After 14 days of oral talactoferrin, pharmacodynamic assays detected a 15% increase in serum IL-18 concentration which was statistically significant (p < 0.05) in all 10 patients evaluated [9]. Circulating levels of IL-18 have also previously been demonstrated in a long-term follow up of patients with chronic hepatitis C treated with oral talactoferrin for 12 months [26]. Talactoferrin at the level of the gut induces large increases in gastrointestinal IL-18 in a dose dependent manner. Small increases in circulating IL-18 have been recorded but they are considered minimal, perhaps due to a dilution effect or increased IL-18 systemic clearance [25]. Talactoferrin has been extremely well tolerated in clinical trials to date and the drugs safety and tolerability is discussed in Section 7.
4. Phase II data
Two company sponsored trials (Agennix) have been conducted in patients with NSCLC. The first trial (LF-0201) was a randomized, double-blind, placebo-controlled, Phase II study of talactoferrin monotherapy in patients with locally advanced or metastatic NSCLC (Stage IIIB/IV NSCLC) who had failed first- or second-line chemotherapy [27]. A total of 100 patients were enrolled at 10 cancer centers in India and received one vial twice a day of either talactoferrin (1.5 g) or placebo given for up to three 14-week cycles of 12 consecutive weeks on followed by two weeks off study drug. The primary endpoint was overall survival (OS). The talactoferrin and placebo arms enrolled 47 and 53 patients, respectively. All patients had previously received a first-line platinum-based regimen; 26 had also received second-line therapy. There was a 55% increase (2.1 months; p < 0.05) in median OS from 3.7 months for the placebo to 5.8 months for the talactoferrin arm. There was a trend towards an improvement in progression-free survival (PFS). Talactoferrin was very well tolerated in this study.
A Phase II (LF-0206) double blind, placebo controlled multi-centered randomized controlled study evaluated talactoferrin in combination with carboplatin and paclitaxel in patients with locally advanced and/or metastatic NSCLC [28]. A total of 110 chemo-naïve Indian patients with stage IIIB/IV NSCLC were randomized (1:1) to carboplatin (AUC5)/paclitaxel (175mg/m2) (C/P) plus talactoferrin (TLF) or placebo. Patients took TLF (1.5 g twice per day) or placebo the day after chemotherapy in cycles 1, 3 and 5. TLF was administered in 35-day cycles up to three cycles or until disease progression. The primary endpoint of this study was response rate (RR; partial response + complete response [PR + CR]) by Response Evaluation Criteria In Solid Tumors RECIST. Secondary endpoints included PFS and OS. The RR in the 110 intent to treat (ITT) patient population increased from 27% in the placebo group to 42% in the talactoferrin group (15% absolute improvement, 56% relative improvement; p = 0.08). In the 100 evaluable patients whom had at least one CT scan after starting treatment the RR increased from 29 to 47% (p = 0.05) (18% absolute and 62% relative improvement). Median PFS in the ITT group increased by 2.8 months (67%). Median OS in the ITT population increased from 8.5 months (placebo) to 10.4 months for the talactoferrin group (22% improvement).
Cytokine levels were evaluated in the peripheral blood of patients following talactoferrin in the Phase II 110 patient combination study and in two single-agent studies in various solid tumors -- a 34 patient pilot Phase II trial and in 10 patients in the Phase I portion of a Phase I/II study [29]. Serum cytokines were measured by ELISA at baseline and at subsequent timepoints. In the combination study involving C/P with or without talactoferrin, the baseline cytokine levels were similar in both groups but thereafter increased levels of cytokines involved in TH1 cellular immune response (IL-8, IL-12, IL-18 and GM-CSF) relative to the placebo group were demonstrated in those receiving talactoferrin and chemotherapy. Of these cytokines the increase in GM-CSF reached statistical significance. The single-agent talactoferrin trials were also reported to show similar increases in cytokine levels but no increase in TH2 cytokines (IL-4, IL-6 and IL-10) have been shown [29]. These trial results are consistent with results observed in preclinical experiments and help support the postulated mechanism of action, consisting of dendritic cell recruitment and activation.
A single-arm open-label pilot study evaluated talactoferrin in HLA-A2-positive stage IIIB/IV NSCLC patients. The primary objective of this trial was to evaluate the effect of talactoferrin on the immune system of patients with NSCLC as measured by quantitative and functional changes in CD4, CD8, NK, and Treg populations in PBMC and on the levels of cytokines and chemokines in serum. Ten patients were enrolled and nine received daily oral talactoferrin (1.5 g/bid) for up to 12 weeks. Patients who benefited from treatment were able to continue on a 12-weeks-on 2-weeks-off schedule until progression. Radiological evaluation was performed every 3 weeks by CT scanning and immunologic studies (including apheresis) were performed at baseline, week 6 and week 12. Preliminary results for this trial are pending.
5. Phase III data
Two ongoing Phase III trials are currently evaluating talactoferrin in patients with advanced NSCLC. FORTIS-M is a randomized, double-blind, placebo-controlled multicenter study. In this trial oral talactoferrin is given in addition to best supportive care to patients with stage IIIB/IV NSCLC who have failed two or more prior treatment regimens. This study opened in October 2008 and is expected to enroll 720 patients by its estimated primary completion date of June 2010. Patients are randomized at a ratio of 2:1 to the talactoferrin or placebo arm, respectively. The primary endpoint of this trial is to determine OS. The secondary endpoints are to determine the 6-month and 1-year survival rate, PFS, objective tumor response rate, disease stabilization rate, safety and tolerability and to assess quality of life. In arm one, patients receive 1.5 g of oral talactoferrin twice daily and in arm two patients receive placebo twice daily orally. Each talactoferrin or placebo cycle lasts 12 weeks (84 days) on study drug and 2 weeks (14 days) off study drug. Patients receive talactoferrin or placebo for up to a maximum of five 14-week cycles (70 weeks), until the occurrence of progressive disease, start of a next-line therapy for NSCLC, unacceptable toxicity, withdrawal of consent or withdrawal by investigator, whichever occurs first. Patients are evaluated at baseline, week 7, week 14 and then every cycle (14 weeks) with CT of chest and abdomen. The study is designed to have approximately 85% power to detect a 30% improvement in median OS in patients in the intent to treat population, from 4.6 months in the placebo arm to 6.0 months in the talactoferrin arm with a two sided p value of 0.05.
The FORTIS-C trial is another ongoing randomized, double-blind, placebo-controlled study. In this trial patient’s with locally advanced or metastatic NSCLC receive first line carboplatin and paclitaxel with or without talactoferrin or placebo. The purpose of this study is to determine whether the combination of talactoferrin, carboplatin and paclitaxel improves PFS and OS in patients with NSCLC compared with the combination of paclitaxel and carboplatin alone. Secondary endpoints include an objective response and disease stabilization rate and safety and tolerability analysis. The study commenced in February 2009 and is expected to enroll 1100 treatment-naïve patients with unresectable advanced or metastatic lung cancer prior to its completion date of March 2013.
6. Talactoferrin in other solid tumors
The only published Phase II trial involving talactoferrin is in patients with renal cell carcinoma (RCC). A total of 44 adult patients with progressive advanced or metastatic RCC who had failed prior systemic therapy received talactoferrin at a dose of 1.5 g twice daily on a 12-week-on 2-week-off schedule [30]. The trials primary endpoints were to detect a PFS rate of ≥ 40% at 14 weeks or a 12.5% response rate. The study met its pre-defined target with a 14-week PFS of 59%. The response rate was 4.5%, with 70.5% of patients demonstrating stable disease for at least 8 weeks. The median PFS was 6.4 months (one-sided 95% CI of 4.7). The median OS was 21.2 months (one-sided CI of 19.5) and the 1-year survival rate was 77% (Table 1).
Table 1.
Published trial results for talactoferrin in solid tumors.
| Tumor type | RR/OS/PFS | Phase (n) | Ref. |
|---|---|---|---|
| NSCLC | 55% increase in OS (2.1 months) | Phase II (100) | [27] |
| NSCLC | RR 42% PFS 2.8 months OS improved by 18% |
Phase II (110) | [28] |
| Renal cell | RR 4.5% PFS 6.4 months OS 21.1 months |
Phase II (44) | [30] |
| Renal cell | PFS 21 weeks OS not reported |
Phase II (44) | [32] |
n: Number of patients, OS: Overall survival, PFS: Progression-free survival, RR: Response rate.
7. Safety and tolerability
Talactoferrin is extremely well tolerated. Preclinical evaluation in both mouse and primate safety studies observed no toxicities using doses as high as 1000 mg/Kg. Based on previous animal work [25] the maximally efficacious dose for humans was estimated at between 4.5 and 9 g/day. In a Phase I clinical trial the dose escalation was stopped at 9 g/day despite the lack of a dose limiting toxicity (DLT) and no MTD being established. In this same study an additional 24 patients with heavily pretreated advanced refractory solid tumors were randomized to receive either 4.5 g/day or 9 g/day, which were the two highest dose groups. No toxicity was seen in these dose cohorts [9].
In the Phase II trial of talactoferrin in previously treated patients with metastatic RCC the safety analysis showed the drug to be extremely well tolerated [30]. At least one adverse event (AE) was reported by 42 patients (96%), and 23 (52%) reported at least one related AE. Of the related AEs, the most common were flatulence, abdominal pain/distension, diarrhea, fatigue, nausea and constipation. There were no drug-related serious AEs reported. In both Phase II lung cancer trials reported to date as abstracts, talactoferrin was very well tolerated. When talactoferrin was added to first line carboplatin/paclitaxel fewer AEs were observed in the talactoferrin arm than in the placebo arm, 346 and 432 AEs, respectively (p = 0.0023). The number of grade 3/4 AEs was also lower in the TLF arm, 60 versus 91 (p = 0.0144) [28]. The second Phase II placebo controlled single-agent trial similarly reported only mild AEs. No drug-related serious AEs were reported. The incidence of AEs and grade 3 AEs were similar in both treatment arms [27].
8. Conclusion
Talactoferrin alpha is a novel immunomodulatory molecule that has shown promising antitumor activity in NSCLC in preclinical and clinical trials. Patients with advanced heavily pretreated lung cancer represent a very difficult patient population to treat, secondary to general debilitation and chemotherapy-induced sequelae, which limits the continued use of toxic agents. Talactoferrin is well tolerated and its oral route of administration is patient friendly. Trials to date suggest that talactoferrin has some cytostatic activity and two ongoing Phase III studies will ultimately decide the efficacy and future role of this drug in NSCLC.
9. Expert opinion
Immunotherapy provides a unique approach to potentially consolidating surgery and/or standard chemotherapy in NSCLC. Stimulating the immune system by inducing a cellular immune response that harnesses CD4+ and CD8+ cytotoxic T lymphocytes (CTLs) capable of selectively destroying cancer cells by targeting tumor-associated antigens (TAAs) could result in tumor eradication. Up to recently therapeutic cancer vaccines have demonstrated immunologic activity in several clinical trials, but have not shown definitive patient benefit. Many problems exist using vaccination approaches including, finding the correct immunostimulatory protein epitopes, establishing optimal delivery platforms, and developing reliable immunological endpoints that approximate clinical benefit.
The postulated ability of talactoferrin to induce the maturation of gastrointestinal immature dendritic cells (iDCs) and dendritic cells derived from monocytes (MoDCs) represents a new and important modulatory function. Restoration of the immune system has been viewed as an important additional modality and may ultimately help responders to remain disease-free for an extended period of time. Talactoferrin is not systemically bioavailable but achieves relevant cytokine increases by acting directly at the largest immune organ in the body (GALT).
Our clinical experiences to date with talactoferrin have confirmed that it is an extremely well tolerated drug with limited to no side effects. Its lack of systemic adverse events makes it an attractive agent in patients with advanced disease or poor performance status and this has been the population that has been studied the most in our clinic. The ongoing Phase III clinical trials will ultimately define the efficacy of this drug and whether it has a role as a direct anticancer agent.
Potential future roles for talactoferrin that require investigation are that of maintenance therapy following disease response or stable disease in the metastatic setting and as adjuvant therapy post surgical resection. The ongoing FORTIS-C trial involving carboplatin and paclitaxel with or without talactoferrin or placebo has maintenance talactoferrin as an option in patients with non progressive disease. Two separate ongoing Phase III studies in NSCLC are investing the role of immunotherapy in these settings. These trials include vaccines to melanoma-associated antigen (MAGE-A3) and belagenpumatucel-L (Lucanix™) which is a mixture of four allogeneic NSCLC cell lines genetically modified to secrete an antisense oligonucleotide to TGF-β2. The MAGE-A3 as adjuvant non-small cell lung cancer immunotherapy (MAGRIT) study is currently recruiting patients with resected stage IB to IIIA MAGE-A3+ NSCLC after they have received adjuvant chemotherapy. A Phase III trial is also investigating belagenpumatucel-L (Lucanix™) as maintenance therapy in patients with unresectable stage III/IV NSCLC who have responded to or have stable disease after first-line platinum-based chemotherapy. Talactoferrin, due to its low toxicity and convenient route of administration, may be a useful drug to investigate in the maintenance setting for both early and advanced NSCLC. Talactoferrin may also potentially be used as a vaccine adjuvant when it is given in combination with other cancer vaccines to help induce a more effective and sustained immune response. Its oral administration may improve MoDC differentiation and ultimately lead to a more effective TH1 cell response. Another possibility for talactoferrin is that of a potential adjunct to anti-vascular therapies. The immunologic response of patients to cancer is decreased by high levels of circulating VEGF [31] and it has been suggested that the addition of talactoferrin to a small molecule VEGF tyrosine kinase inhibitor may enhance efficacy [30].
Bibliography
- 1.Anderson BF, Baker HM, Norris GE, et al. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 Å resolution. J Mol Biol 1989;209:711–34 [DOI] [PubMed] [Google Scholar]
- 2.Sun XL, Baker HM, Shewry SC, et al. Structure of recombinant human lactoferrin expressed in Aspergillus awamori. Acta Crystallogr D Biol Crystallogr 1999;55:403–7 [DOI] [PubMed] [Google Scholar]
- 3.Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica 1995;80:252–67 [PubMed] [Google Scholar]
- 4.Bellamy W, Takase M, Wakabayashi H, et al. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J Appl Bacteriol 1992;73:472–9 [DOI] [PubMed] [Google Scholar]
- 5.Legrand D, Elass E, Carpentier M, Mazurier J. Interactions of lactoferrin with cells involved in immune function. Biochem Cell Biol 2006;84:282–90 [DOI] [PubMed] [Google Scholar]
- 6.Bezault J, Bhimani R, Wiprovnick J, Furmanski P. Human lactoferrin inhibits growth of solid tumors and development of experimental metastases in mice. Cancer Res 1994;54:2310–12 [PubMed] [Google Scholar]
- 7.Tsuda H, Sekine K, Fujita K, Ligo M. Cancer prevention by bovine lactoferrin and underlying mechanisms -- a review of experimental and clinical studies. Biochem Cell Biol 2002;80:131–6 [DOI] [PubMed] [Google Scholar]
- 8.Gahr M, Speer CP, Damerau B, Sawatzki G. Influence of lactoferrin on the function of human polymorphonuclear leukocytes and monocytes. J Leukoc Biol 1991;49:427–33 [DOI] [PubMed] [Google Scholar]
- 9.Hayes TG, Falchook GF, Varadhachary GR, et al. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Invest New Drugs 2006;24:233–40 [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y, Monitto CL, Minhas KM, Sidransky D. Lactoferrin down-regulates G1 cyclin-dependent kinases during growth arrest of head and neck cancer cells. Clin Cancer Res 2004;10:8683–6 [DOI] [PubMed] [Google Scholar]
- 11.Damiens E, El Yazidi I, Mazurier J, et al. Lactoferrin inhibits G1 cyclin-dependent kinases during growth arrest of human breast carcinoma cells. J Cell Biochem 1999;74:486–98 [PubMed] [Google Scholar]
- 12.Togawa J, Nagase H, Tanaka K, et al. Lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. Am J Physiol Gastrointest Liver Physiol 2002;283:G187–95 [DOI] [PubMed] [Google Scholar]
- 13.Zucali JR, Broxmeyer HE, Levy D, Morse C. Lactoferrin decreases monocyte-induced fibroblast production of myeloid colony-stimulating activity by suppressing monocyte release of interleukin-1. Blood 1989;74:1531–6 [PubMed] [Google Scholar]
- 14.Wolf JS, Li G, Varadhachary A, et al. Oral lactoferrin results in T cell-dependent tumor inhibition of head and neck squamous cell carcinoma in vivo. Clin Cancer Res 2007;13:1601–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillen C, McInnes IB, Vaughan DM, et al. Enhanced Th1 response to Staphylococcus aureus infection in human lactoferrin-transgenic mice. J Immunol 2002;168:3950–7 [DOI] [PubMed] [Google Scholar]
- 16.Kuhara T, Iigo M, Itoh T, et al. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutr Cancer 2000;38:192–9 [DOI] [PubMed] [Google Scholar]
- 17.Wang WP, Iigo M, Sato J, et al. Activation of intestinal mucosal immunity in tumor-bearing mice by lactoferrin. Jpn J Cancer Res 2000;91:1022–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artym J, Zimecki M, Kruzel ML. Reconstitution of the cellular immune response by lactoferrin in cyclophosphamide-treated mice is correlated with renewal of T cell compartment. Immunobiology 2003;207:197–205 [DOI] [PubMed] [Google Scholar]
- 19.Masuda C, Wanibuchi H, Sekine K, et al. Chemopreventive effects of bovine lactoferrin on N-butyl-N-(4-hydroxybutyl) nitrosamine-induced rat bladder carcinogenesis. Jpn J Cancer Res 2000;91:582–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekine K, Watanabe E, Nakamura J, et al. Inhibition of azoxymethane-initiated colon tumor by bovine lactoferrin administration in F344 rats. Jpn J Cancer Res 1997;88:523–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki YA, Shin K, Lonnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry 2001;40:15771–9 [DOI] [PubMed] [Google Scholar]
- 22.Suzuki YA, Lonnerdal B. Characterization of mammalian receptors for lactoferrin. Biochem Cell Biol 2002;80:75–80 [DOI] [PubMed] [Google Scholar]
- 23.Talukder MJ, Takeuchi T, Harada E. Characteristics of lactoferrin receptor in bovine intestine: higher binding activity to the epithelium overlying Peyer’s patches. J Vet Med A Physiol Pathol Clin Med 2003;50:123–31 [DOI] [PubMed] [Google Scholar]
- 24.Hu WL, Mazurier J, Montreuil J, Spik G. Isolation and partial characterization of a lactotransferrin receptor from mouse intestinal brush border. Biochemistry 1990;29:535–41 [DOI] [PubMed] [Google Scholar]
- 25.Varadhachary A, Wolf JS, Petrak K, et al. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int J Cancer 2004;111:398–403 [DOI] [PubMed] [Google Scholar]
- 26.Ishii K, Takamura N, Shinohara M, et al. Long-term follow-up of chronic hepatitis C patients treated with oral lactoferrin for 12 months. Hepatol Res 2003;25:226–233 [DOI] [PubMed] [Google Scholar]
- 27.Parikh PK, Wang Y, Ranade AA, et al. Oral talactoferrin extends survival in patients with refractory NSCLC in a randomized, placebo-controlled, phase 2 trial (Meeting Abstracts). J Clin Oncol 2007;25(18 Suppl):7540 [Google Scholar]
- 28.Wang Y, Raghunadharao D, Raman G, et al. Adding oral talactoferrin to first-line NSCLC chemotherapy safely enhanced efficacy in a randomized trial (Meeting Abstracts). J Clin Oncol 2006;24(18 Suppl):7095 [Google Scholar]
- 29.Hayes TG, Digumarti R, Engelmeyer J, et al. Effect of oral talactoferrin (TLF) on levels of cytokines involved in the Th1-mediated immune response in clinical studies (Meeting Abstracts). J Clin Oncol 2008;26(15 Suppl):3080 [Google Scholar]
- 30.Jonasch E, Stadler WM, Bukowski RM, et al. Phase 2 trial of talactoferrin in previously treated patients with metastatic renal cell carcinoma. Cancer 2008;113:72–7 [DOI] [PubMed] [Google Scholar]
- 31.Osada T, Chong G, Tansik R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother 2008;57:1115–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivas S, Stadler WM, Bukowski R, et al. Talactoferrin alfa may prolong progression-free survival in advanced renal carcinoma patients (Meeting Abstracts). J Clin Oncol 2006;24(18 Suppl):4600 [Google Scholar]