Abstract
Purpose:
Ixabepilone (Ixempra®; BMS-247550) is an epothilone B analog and non-taxane microtubule-stabilizing compound with clinical activity in a range of solid tumors. This phase II study was conducted to assess the efficacy and safety of ixabepilone in patients with metastatic renal cell carcinoma (mRCC).
Experimental Design:
Patients with mRCC who had measurable disease and had not received prior cytotoxic or targeted therapy were treated with 6 mg/m2 ixabepilone intravenously daily for 5 days every 3 weeks. Levels of glu-terminated and acetylated - tubulin, markers of microtubule stabilization, were assessed by Western blot. VHL gene mutation status was determined by sequencing.
Results:
Eighty-seven patients received a total of 590 cycles with a median of 5 cycles (range 1-29). The overall response rate was 13% (RECIST). One patient had a complete response, 10 patients had partial responses, and 59 patients had stable disease. The median duration of response was 5.5 months. The median overall survival (OS) of RCC Motzer grade 0 and 1 patients with clear cell histology was 19.25 months. Treatment-related adverse events were primarily alopecia, gastrointestinal toxicity, neuropathy and fatigue. Biopsies were performed at baseline and after five doses of ixabepilone. Microtubule target engagement was achieved in 84.6 to 92.3 percent of patients evaluated. No correlation was identified between the target engagement, VHL gene mutation status and clinical response.
Conclusion:
Ixabepilone can cause tumor regression in some patients with mRCC and could be considered in combination regimens with other therapies.
Keywords: Metastatic renal cell carcinoma, ixabepilone, Ixempra®, epothilones, phase II clinical trial, VHL, Von Hippel-Lindau, RCC, mRCC, targeted therapies, sorafenib, Nexavar®, sunitinb, Sutent®, temsirolimus, Torisel®
Condensed abstract:
A phase II study in 87 patients with mRCC was conducted to assess the efficacy and safety of Ixabepilone (Ixempra®), an epothilone B analog and non-taxane microtubule-stabilizing compound. Ixabepilone caused tumor regression in some patients with mRCC and could be considered in combination regimens with other therapies.
INTRODUCTION
In 2007 in the United States, approximately 51,000 people had a diagnosis of renal cell carcinoma (RCC) and almost 13,000 died from metastatic RCC (mRCC) 1. The incidence of RCC has increased over time 2-4. Historically, no single “cytotoxic” agent or combination has consistently produced responses that justify their routine use in this group 5,6. For the past twenty years, prior to the approval of “targeted” therapies, interleukin-2 (IL-2) and interferon-alpha (IFN-α), alone or in combination have been the main treatments for mRCC. Response rates with these cytokines are low (5 to 20%) and median OS is approximately 12.0 to 17.5 months 7-12. More recently tumor responses have been reported with sorafenib (Nexavar® Bayer, Westhaven, CT), sunitinib (Sutent®, Pfizer, NY, NY), temsirolimus (Torisel®, Wyeth, Philadelphia, PA), and everolimus (Affinitor®, Novartis ) with increases in progression free survival (PFS) and or with modest improvement of overall survival 11, 13 - 15. However, because none of these therapies can be considered curative, there remains a need to develop alternative strategies.
Ixabepilone is a semisynthetic analog of the natural product, epothilone B, a member of a novel class of non-taxane microtubule stabilizing agents. It exerts anti-proliferative effects by binding tubulin and stabilizing microtubules, effecting mitotic arrest and also impairs microtubule trafficking 16-18. Epothilones are poor substrates for P-glycoprotein and exhibit activity in paclitaxel-resistant cell lines and paclitaxel-resistant tumor models 16, 19-21. A prior phase I study established ixabepilone administered at a dose of 6 mg/m2 for 5 consecutive days every 3 weeks as the recommended phase II dose (RPTD). Neutropenia was dose limiting. Peripheral neuropathy was mild, even after multiple cycles of therapy and was not dose limiting22. Because of encouraging results and tolerable toxicity profiles in these phase I studies, this phase II trial was initiated to determine the efficacy and safety of ixabepilone in patients with mRCC.
PATIENTS AND METHODS
Eligibility
Eligible patients had to be > 18 years, with histologically or cytologically proven RCC (clear cell, papillary, chromophobe, collecting duct and medullary), and disease that could be evaluated by RECIST (response evaluation criteria in solid tumor) 23. Additional criteria for entry included an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 – 2; a life expectancy of at least 3 months; adequate bone marrow (ANC > 1.5 × 109/L and platelet count > 100 × 109/L), hepatic and renal function. Exclusion criteria included prior chemotherapy. Of note, enrollment began in 2002, several years before either sorafenib (Nexavar® Bayer, Westhaven, CT), sunitinib (Sutent®, Pfizer, NY, NY) or temsirolimus (Torisel®, Wyeth, Philadelphia, PA) had been approved by the FDA for mRCC. Patients with brain metastasis were excluded unless they had been appropriately treated and stable for at least 6 months
Study Design and Treatment Modifications
The study was approved by the Institutional Review Board (IRB) of the Center for Cancer Research (intramural program) of the National Cancer Institute (NCI; Bethesda; Maryland). All patients signed IRB-approved written informed consent. Ixabepilone was provided by the Cancer Therapy Evaluation Program, NCI, through a cooperative agreement with Bristol-Myers Squibb (Wallingford, CT). Ixabepilone was administered at a dose of 6 mg/m2intravenously during a one-hour infusion daily for 5 consecutive days every 3 weeks. Diphenhydramine 50 mg and ranitidine 50 mg were administered intravenously 30 to 60 minutes before the administration of ixabepilone as prophylaxis against reactions to cremophor XL. Prophylactic antiemetics were not administered routinely. Adverse events were coded according the NCI Common Toxicity Criteria version 2.0 (CTC). Cycles were extended to 28 and 35 days on occasion to accommodate grade 2 neuropathy.
Response Assessment
Measurable disease was assessed by CT using RECIST guidelines 23, with baseline imaging within 4 weeks of enrollment and restaging after every two cycles.
Statistical Design and Methodology
This protocol was designed to evaluate the efficacy of ixabepilone and was conducted using a two-stage optimal design to rule out a low 5% response rate in favor of a 20% response rate 24. Patients were grouped according to histology (clear cell, papillary or other). If one or more patients had a response, accrual continued until a total of 37 evaluable patients had been enrolled (clear cell group was the only cohort expanded to 37 patients).
An amendment in 11/03 halted accrual while it sought to increase the clear cell cohort to 74 patients to examine the activity of ixabepilone in patients with wt VHL, based on an assumption (not realized) of 50% wt and 50% mutant VHL.
Correlative Studies
Patients with tumors that could safely have a biopsy performed under local anesthesia had core biopsies obtained at baseline and approximately 2-6 hours after the cycle 1 day 5 dose of ixabepilone treatment. Assessment of target engagement and sequencing of VHL were performed (See Supplemental Information).
RESULTS
Patient Characteristics
Between February 2002 and April 2007, 87 patients with metastatic renal cell carcinoma (mRCC) were enrolled onto this study. The baseline characteristics and patient’s prior therapy are summarized in Table 1. The median age was 57.1 years; the majority were male and Caucasian with ECOG PS of 0 - 1. Ninety percent had undergone a cyto-reductive nephrectomy. One half of the patients had received systemic treatment, including IL-2, IFN, thalidomide, a vaccine and other therapies while the other half had not received prior systemic treatment. Eighty-six percent of patients’ tumors were of clear cell histology. Patients had extensive tumor burden at baseline with a median of 21.7 cm of tumor (sum of longest diameters of all tumors), of which 10.5 cm was evaluated by RECIST. The mean number of metastatic sites was 3 with most patients presenting with lung involvement, as well as lymphadenopathy in the chest, abdomen or both. Other common sites of disease included the liver, bones and soft tissue. (Note: a site of metastatic disease, such as ‘lung parenchyma’, counted as one site, whether there was one nodule or 25).
Table 1:
Baseline Characteristics and Prior Therapy
| Characteristics | Number of patients | Percent |
|---|---|---|
| Gender | ||
| Male | 65 | 75 |
| Female | 22 | 25 |
| Age in years | ||
| Median (Range) | 57.1 (26 - 77) | |
| Race/ethnicity | ||
| Caucasian | 72 | 83 |
| African American | 9 | 10 |
| Asian | 5 | 6 |
| American Indian | 1 | 1 |
| ECOG performance status | ||
| 0 | 23 | 26 |
| 1 | 58 | 67 |
| 2 | 6 | 7 |
| Prior Nephrectomy | ||
| Yes | 79 | 91 |
| No | 8 | 9 |
| Prior Systemic Treatment | ||
| None | 45 | 52 |
| IL-2 | 36 | 42 |
| Interferon-alpha | 14 | 16 |
| Thalidomide | 6 | 7 |
| IL-12 | 5 | 6 |
| Bevacizumab | 3 | 3 |
| Other | 7 | 7 |
| Histology | ||
| Clear-cell | 74 | 85 |
| Papillary | 5 | 6 |
| Collecting Duct | 2 | 2 |
| Sarcomatoid + Clear | 2 | 2 |
| Papillary + Clear | 1 | 1 |
| Other | 3 | 3 |
| Site of (metastatic) disease | ||
| Lung | 71 | 82 |
| Lymph Nodes | 68 | 78 |
| Bones | 24 | 28 |
| Liver | 21 | 24 |
| Soft tissue/other | 26 | 30 |
| Primary | 8 | 9 |
| Motzer CriteriaA | ||
| 0 Risk Factors | 37 | 43 |
| 1 Risk Factor | 38 | 44 |
| 2 or 3 Risk Factors | 11 | 13 |
Karnofsky PS < 80%; Calcium > 10 mg/dL; Hgb < 13 g/dL males, 11.5 g/dL females (Motzer, Bacik et al. 2004)
Efficacy
Eighty-four of 87 patients completed two cycles. One patient with new onset hemoptysis during cycle 2/day 2 decided against further ixabepilone therapy. Another patient with significant co-morbidities including COPD and urethral obstruction had a stent placed to relieve external obstruction of the left mainstem bronchus after which his PS declined markedly without further treatment. A third patient with sepsis on cycle one/day 8 refused aggressive care, fully aware of the likely outcome, and died quickly. The total number of cycles administered was 590 with a median of five cycles per patient. All patients received a dose of 6 mg/m2 as the starting dose. Early in the conduct of the study 19 patients (43 cycles) who tolerated the starting dose of 6 mg/m2 well were advanced to a daily dose of 8 mg/m2. When it became apparent this dose often caused unacceptable fatigue, further escalations were not attempted, especially since all responses had been observed at the dose of 6 mg/m2.
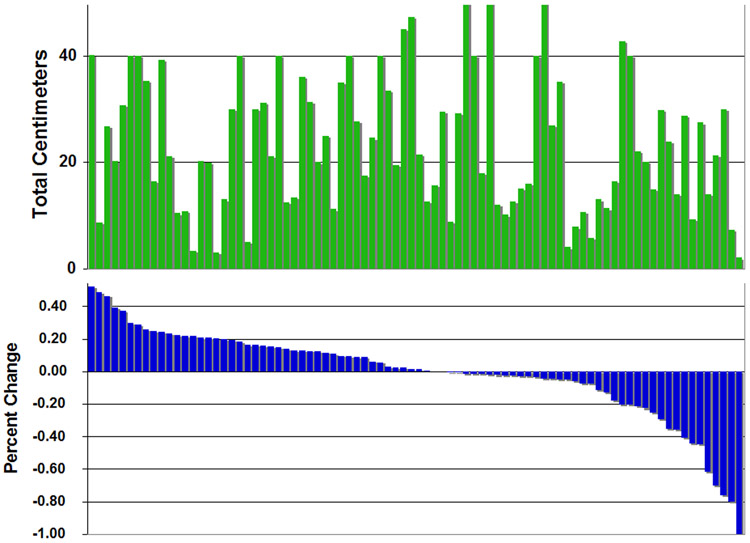
Among the 87 patients enrolled on study, the overall response rate was 12.6%. As shown in Table 2, one patient had a complete response, 10 patients had partial responses, and a best response of stable disease for at least 4 cycles per RECIST criteria was confirmed in 33 patients (37.9%). Measurable responses were observed in lung, liver, soft tissue and lymph nodes. Improvements were also seen in non-target lesions, including bones, pleura and skin. The waterfall plot of best tumor response in percentage change from baseline for the 84 patients who received at least two cycles and had a repeat imaging study patients is shown in Figure 1. The graph directly above the response graph depicts the total tumor burden in each patient. Together these graphs demonstrate that the extent of response is independent of the total tumor burden or the disease measured in the RECIST evaluation (See also Supplemental Data for correlation with RECIST measurements). Tumor shrinkage was not confined to those with small volume disease. Stable disease was not only seen in patients with a large amount of disease, where an increase of 20% might be more difficult to attain, given the large tumor burden at the start (Figures 1 and 2).
Table 2:
Best Response to Ixabepilone per RECIST (N = 87)
| Number of patients | Percentage | |
|---|---|---|
| Complete Response | 1 | 1.2 |
| Partial Response | 10 | 11.5 |
| Stable Disease ≥ 4 cycles | 33 | 37.9 |
| Progressive Disease | 40 | 46 |
| Not evaluable | 3 | 3.4 |
Figure 1:
(A) Tumor burden as represented by the sum of the size of all tumor masses larger than one centimeter. All patients shown with a tumor burden of 40 or 50 centimeters actually had a larger burden, but these consisted of variable number of small metastases that were not measured. (B) Waterfall plot of tumor response calculated using RECIST criteria. The bars depict the percentage change from baseline.
Figure 2:
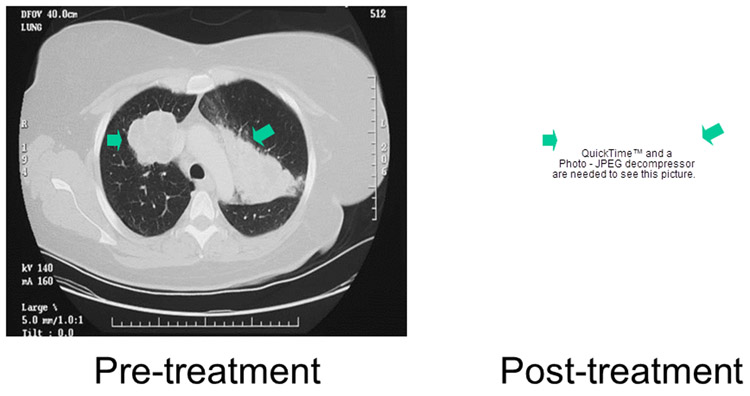
Scans demonstrating response in a patient with mRCC after treatment with ixabepilone. Prior therapies included nephrectomy, metastasectomy, radiation therapy to L1, high dose IL-2 and experimental therapy with FLT3/CD40L . The baseline scan is shown on the left with a scan obtained after 17 cycles shown on the right. According to RECIST criteria a PR was achieved after 8 cycles but the tumor continued to shrink for 21 cycles before demonstrating evidence of progression on cycle 26.
The median time to progression was 19 weeks. For the 11 patients who achieved complete response or partial response, the median duration of response was 5.5 months. The median OS of the 74 patients with RCC Motzer grade 0 and 1 patients and clear cell histology was 19.25 months. This analysis was performed to allow for a comparison to the published trials with sorafenib and sunitinib. Because 19 patients (25.6%) subsequently went on to receive sorafenib or sunitinib, any patient who received either of these agents had their survival arbitrarily censored one month after the initiation of sunitinib or sorafenib. In this way we were sure to avoid assigning any benefit on survival to ixabepilone that could have come from other therapies.
Toxicities
Toxicities are summarized in Table 3. The median and mean absolute neutrophil count (ANC) in cycle one was 2945 and 2880, respectively. The median and mean ANC in all cycles were 2879 and 2735, respectively. Only 9 out of 87 patients experienced G4 neutropenia at the dose of 6 mg/m2. Only 1 patient had G3 thromobocytopenia at that dose level. Neuropathy was observed in 59% of patients. The majorities of these events were neurosensory and grade 1 or 2, with only 4 of 87 patients developing grade 3 sensory neuropathy or motor neuropathy. Two patients developed what appeared clinically to be an autonomic neuropathy manifested by orthostatic hypotension and syncope. Both had gradual improvement in symptoms over time after ixabepilone was discontinued. The other notable adverse events attributable to ixabepilone include alopecia (70%), fatigue, nail changes, anorexia, nausea, taste disturbance and diarrhea. Only eight patients were removed from study due to toxicities. One episode of Grade 5 febrile neutropenia (discussed above) occurred during cycle one in a 55 year-old gentleman with extensive disease. A second patient died while on study, as a result of a brain-stem infarct during a vertebral body embolization procedure performed at another institution.
Table 3:
Adverse Events: All Cycles (N = 590) / All Patients (N = 87)
| CTC GradeA | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Number of Patients |
% | Number of Patients |
% | Number of Patients |
% | Number of Patients |
% | |
| Toxicity | ||||||||
| HEMATOLOGIC | ||||||||
| Neutropenia | 15 | 17 | 9 | 10 | 12 | 14 | 17 | 20 |
| Hemoglobin | 28 | 32 | 39 | 45 | 1 | 1.1 | ||
| Thrombocytopenia | 25 | 29 | 1 | 1.1 | 1 | 1.1 | ||
| Febrile neutropenia | 2 | 2.3 | 1B | 1.1 | ||||
| NON-HEMATOLOGIC | ||||||||
| Fatigue | 25 | 29 | 35 | 40 | 14 | 16 | ||
| Sensory neuropathy | 28 | 32 | 18 | 21 | 3 | 3.4 | ||
| Neuromotor | 1 | 1.1 | 1 | 1.1 | ||||
| Syncope | 2 | 2.3 | ||||||
| Nausea | 22 | 25 | 16 | 18 | 4 | 4.6 | ||
| Vomiting | 18 | 21 | 8 | 9 | 3 | 3.4 | ||
| Diarrhea | 17 | 20 | 15 | 17 | 7 | 8 | ||
| Constipation | 7 | 8 | 13 | 15 | ||||
| Alopecia | 36 | 41 | 25 | 29 | ||||
| Dysgeusia | 29 | 33 | 11 | 13 | ||||
| Nail change | 19 | 22 | 19 | 22 | ||||
| Arthralgias | 19 | 22 | 4 | 4.6 | 1 | 1.1 | ||
| Myalgias | 8 | 9 | 2 | 2.3 | 1 | 1.1 | ||
National Cancer Institute Common Toxicity Criteria (NCI CTC)
One patient had Grade 5 febrile neutropenia during cycle 1
Correlative studies
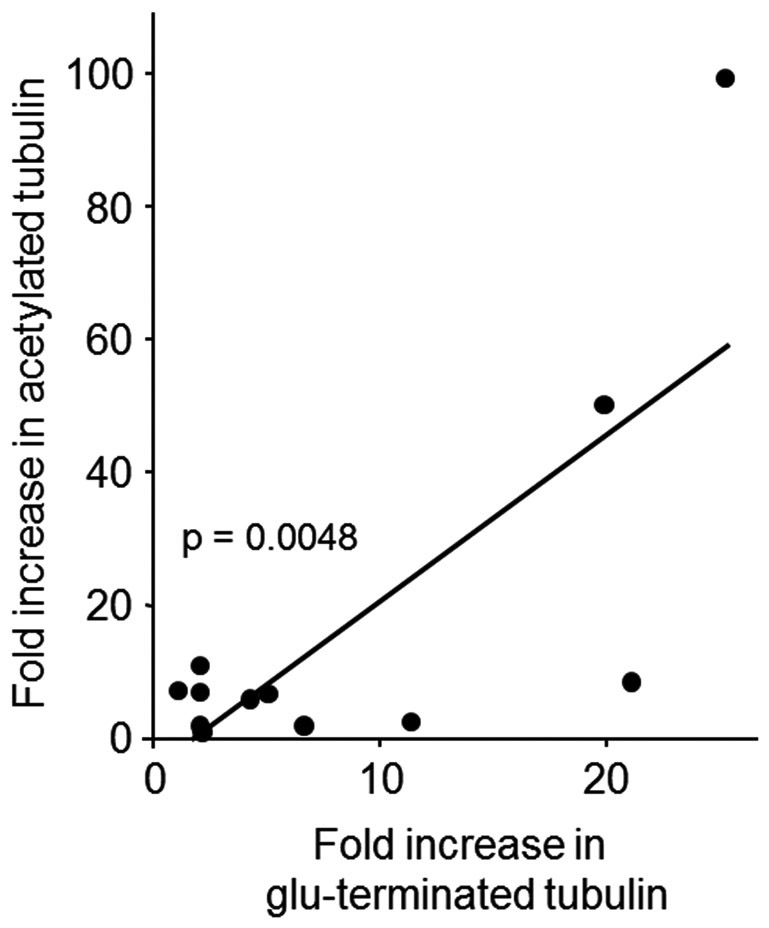
We have previously reported target engagement with two markers of stable tubulin before and after ixabepilone treatment in cycle 1 25. As shown in Figure 3 the increases in these two markers are closely correlated, confirming the target engagement by ixabepilone (See Supplemental Information).
Figure 3:
Increased levels of two markers of stable tubulin - acetylated- and glu-terminated-alpha tubulin - were observed after the administration of ixapebilone in 84 to 92% of serial biopsies. The increased levels of these two markers were closely correlated, confirming with two different antibodies the occurrence of target engagement by ixabepilone.
Finally, based largely on theoretical considerations that VHL mutation status could affect response to ixabepilone 26, a decision was made after some activity was observed early in the study to double the accrual, expecting that about one half of the patients would present with a mutation in the VHL. The coding sequence of the VHL gene in 32 patients was sequenced for mutations. The primers used in the PCR and sequencing reactions are listed in Supplemental Table 1 and shown schematically in Supplemental Figure 3. To validate our assay we isolated DNA from 8 RCC cell lines (4 with wild type VHL and 4 with mutant VHL), and from 3 RCC tumors and 3 pheochromocytoma tumors from patients with a diagnosis of VHL. Sequencing of RT PCR products from these cell lines and tumors identified all 10 mutations. By comparison we detected a VHL mutation in only 1 of 32 patient samples - a 1 bp deletion at nucleotide 317 (relative to start of translation) resulting in a premature stop codon at amino acid 158 (See Supplemental Section).
DISCUSSION
In the present study we report on the activity of the microtubule-stabilizing agent, ixabepilone, in patients with mRCC. The objective response rate as assessed by RECIST is 12.6% with a median OS of 19.25 months in the 74 patients with Motzer grade 0/1 and clear cell histology. While these results must be confirmed, they cannot be ascribed to enrollment of a select group of patients with a favorable prognosis given the large number of patients, and the advanced disease at presentation with a median tumor burden of more than 21 cm, and an average of 3 sites of metastatic disease. With the approval of three agents for mRCC in the past three years, and the knowledge that at least two, if not all three, have very similar mechanisms of action, the identification of an agent from a totally distinct class of chemotherapeutics, is in our opinion, of interest and worthy of further evaluation.
While the potential value in mRCC of ixabepilone lies in its different mechanism of action and not as an agent that competes with drugs already approved for RCC, it is useful nevertheless to compare its activity to the already approved chemotherapeutics. While the median time on therapy was 19 weeks we would note that some patients were removed from study prior to meeting the criteria for RECIST-defined progression. This occurred primarily prior to sorafenib’s approval and available information that suggested this agent was very active in mRCC. Consequently some patients were encouraged to seek out trials with sorafenib or later compassionate use sorafenib, when it appeared remaining on ixabepilone would not lead to a significant response. These patients thus came off study before meeting RECIST criteria for progression and could have remained on study for at least an additional 6 weeks until the subsequent scan. This approach was taken because time to progression was not an endpoint and was driven by what investigators saw as the patient’s best interest. Had these patients continued on study for a minimum of six additional weeks, then the median time on ixabepilone prior to progression would have risen to at least 24.9 weeks, a value that compares favorably with sorafenib data showing a median TTP of 24 weeks. More importantly, the median OS of RCC Motzer grade 0 and 1 patients with clear cell histology – a cohort comparable to patients enrolled on the sorafenib and sunitinib registration trials – was 19.25 months, comparable to the 19.3 months for sorafenib 13. As noted in the Results Section, the latter value is not affected by the few patients who received sorafenib or sunitinib since if they received either drug for more than one month their “survival” was tabulated from enrolment to one month after the start of sorafenib or sunitinib. But in fact the majority of patients either did not tolerate these drugs or had minimal benefit, and only 2 patients exceeded the median OS while receiving sorafenib or sunitinib and the adjustment in “survival” was only meaningful in these two.
Although one might argue the daily times five schedule is suboptimal in terms of convenience, we found this schedule easy to administer, well-tolerated and acceptable to the patients, many of who traveled great distances to participate in this trial. Neurotoxicity was largely grade 1 or 2 with less than 5% grade 3 toxicity consistent with other studies conducted with the same schedule 27, 28 and much less than with the 30 - 40 mg/m2every 3 weeks schedule that has reported G3 neuropathy in as many as 20% or more of patients enrolled 29, 30. The lower incidence of neurotoxicity with this regimen is reminiscent of data with the taxanes that demonstrated a much lower incidence of neurotoxicity with a 96-hour infusion of paclitaxel 31 - 35. If for ixabepilone as for the taxanes the peak concentration is important in causing peripheral neuropathy, then the lower peaks achieved with a daily for five days schedule might be important 36. The lower incidence of neurotoxicity with this schedule could be considered by physicians preparing to administer ixabepilone to patients with other cancers, especially those with neurotoxicity prior to the start of ixabepilone. Also notable was the fact that severe myelosuppression was very uncommon. The median ANCs for cycle one and for all cycles were 2879 and 2735 respectively with G3 thrombocytopenia recorded in only one patient. While we recognize the patients enrolled on this clinical trial had not received prior myelosuppresive therapy, the results are nevertheless encouraging, especially if one considers this ixabepilone schedule as a building block with other agents.
Ixabepilone has been evaluated in one other phase II trial in RCC using a different schedule 37. That study evaluated a dose of 40 mg/m2 every 21 days and reported the schedule to be inactive. However, only five of twelve patients had lear cell RCC with other histologies in the remaining seven – possibly explaining the different results. Alternately, the difference in schedule, daily for five days versus once every three weeks, could potentially affect tumor response. As has been previously demonstrated in pre-clinical studies with the taxanes, longer or more frequent schedules of administration might be more active in slower growing tumors such as RCC, especially if cytotoxicity depends on where in the cell cycle the cell is when ixabepilone is administered 38, 39.
Kidney cancer has historically been refractory to “cytotoxic” agents including the taxanes 6, 40, 41. The ineffectiveness of chemotherapy can be ascribed, in part, to intrinsic resistance to chemotherapy. Over-expression of the drug transporter P-glycoprotein (Pgp) and its encoding gene, MDR-1, has been frequently cited as a mechanism of resistance. A high level of expression of MDR-1 and in vitro sensitization to vinblastine with the antagonists verapamil and quinidine have been observed in kidney cancer 42. These findings suggest Pgp expression could explain, at least in part, the resistance of RCC. The epothilones represent the first anti-microtubule agents that are not Pgp substrates. While other putative advantages of ixabepilone are being studied, to date the only advantage that has been validated is the lack of Pgp-mediated efflux and this may in part explain ixabepilone’s efficacy in RCC. That this cannot be the only explanation, but must be part of a more diversified resistance portfolio, is evidenced by the fact that target engagement was observed in the majority of tumor samples analyzed, indicating resistance mechanisms distal to engagement of the target prevented cytotoxicity. Studies are underway to try to discern other mechanisms.
Finally, using a methodology that was validated with 10 samples whose mutations were unknown to the individual performing the analysis, we sought to determine the VHL mutation status of tumors. To our surprise, the majority of tumors from our patients with sporadic RCC did not harbor VHL mutations. Using primers that generated small fragments we were able to successfully clone all samples, even those for which we had only DNA isolated from micro-dissected formalin-embedded tissues. While the significance remains to be determined, we must conclude the incidence of VHL mutations in patients with aggressive, sporadic RCC is lower than previously thought.
In summary, ixabepilone is a potent microtubule-stabilizing agent that demonstrates some activity in mRCC. This cytotoxic agent could be considered in combination or sequentially with other therapies – e.g. bevacizumab, sorafenib, sunitinib – in future studies to attempt to improve the therapy of mRCC.
Supplementary Material
Statement of Translational Relevance:
This study reports the results of a clinical trial with ixabepilone (Ixempra®) in patients with advanced metastatic renal cell carcinoma (RCC). Ixabepilone is a novel microtubule-targeting agent recently approved by the FDA for the therapy of metastatic breast cancer. The current study represents a large, single institution trial in patients with RCC with mature data including overall survival. It also reports interesting translational analyses that demonstrate this agent consistently reaches its target in the cell and effects microtubule stabilization. Given the recent interest in RCC and renewed hope that better therapies may be forthcoming, this report is interesting in that it describes activity of an agent unlike any approved to date for this still very refractory disease. Its tolerability and its low toxicity make it an attractive agent to use ion combination therapies.
Abbreviations:
- RCC
renal cell carcinoma
- OS
overall survival
- VHL
Von Hippel-Lindau
- IFN-α
interferon-alpha
- Il-2
interleukin-2
- PFS
progression free survival
- RECIST
Response evaluation criteria in solid tumor
- ECOG
Eastern Cooperative Oncology Group
- PET
positron emission tomography
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA 1999;281:1628–31. [DOI] [PubMed] [Google Scholar]
- 3.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol 2001;166:1611–23. [PubMed] [Google Scholar]
- 4.Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol 2006;176(6 Pt 1):2397–400;discussion 2400. [DOI] [PubMed] [Google Scholar]
- 5.Yagoda A, Petrylak D, Thompson S. Cytotoxic chemotherapy for advanced renal cell carcinoma. Urol Clin North Am 1993;20:303–21. [PubMed] [Google Scholar]
- 6.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol 2000;163:408–17. [PubMed] [Google Scholar]
- 7.Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med 1987;316:889–97. [DOI] [PubMed] [Google Scholar]
- 8.Yang JC, Topalian SL, Parkinson D, Schwartzentruber DJ, Weber JS, Ettinghausen SE, et al. Randomized comparison of high-dose and low-dose intravenous interleukin-2 for the therapy of metastatic renal cell carcinoma: an interim report. J Clin Oncol 1994;12:1572–6. [DOI] [PubMed] [Google Scholar]
- 9.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 1995;13:688–96. [DOI] [PubMed] [Google Scholar]
- 10.Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d'Immunotherapie. N Engl J Med 1998;338:1272–8. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Murphy BA, Bacik J, et al. Phase III trial of interferon alfa-2a with or without 13-cis-retinoic acid for patients with advanced renal cell carcinoma. J Clin Oncol 2000;18:2972–80. [DOI] [PubMed] [Google Scholar]
- 12.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 2005;23:133–41. [DOI] [PubMed] [Google Scholar]
- 13.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. [DOI] [PubMed] [Google Scholar]
- 14.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–81. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449–56. [DOI] [PubMed] [Google Scholar]
- 16.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol(R)). J Biol Chem 1997;272:2534–41. [DOI] [PubMed] [Google Scholar]
- 17.Lee FY, Borzilleri R, Fairchild CR, et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res 2001;7:1429–37. [PubMed] [Google Scholar]
- 18.Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol 2004;22:2015–25. [DOI] [PubMed] [Google Scholar]
- 19.Bollag DM, McQueney PA, Zhu J, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res 1995;55:2325–33. [PubMed] [Google Scholar]
- 20.Chou TC, O'Connor OA, Tong WP, et al. Desoxyepothilone B is curative against human tumor xenografts that are refractory to paclitaxel. Proc Natl Acad Sci U S A 1998;95:15798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou TC, O'Connor OA, Tong WP, et al. The synthesis, discovery, and development of a highly promising class of microtubule stabilization agents: curative effects of desoxyepothilones B and F against human tumor xenografts in nude mice. Proc Natl Acad Sci U S A 2001;98:8113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham J, Agrawal M, Bakke S, et al. Phase I trial and pharmacokinetic study of BMS-247550, an epothilone B analog, administered intravenously on a daily schedule for five days. J Clin Oncol 2003;21:1866–73. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 24.Simon R Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang SH, Hung YE, Hung L, et al. Evidence for microtubule target engagement in tumors of patients receiving ixabepilone. Clin Cancer Res 2007;13:7480–6. [DOI] [PubMed] [Google Scholar]
- 26.Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel-Lindau tumour suppressor protein pVHL. Nat Cell Biol 2003;5:64–70. [DOI] [PubMed] [Google Scholar]
- 27.Low JA, Wedam SB, Lee JJ, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol 2005;23:2726–34. [DOI] [PubMed] [Google Scholar]
- 28.Denduluri N, Low JA, Lee JJ, et al. Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes. J Clin Oncol 2007;25:3421–7. [DOI] [PubMed] [Google Scholar]
- 29.Roché H, Yelle L, Cognetti F, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol 2007;25:3415–20. [DOI] [PubMed] [Google Scholar]
- 30.Thomas ES, Gomez HL, Li RK, Chung HC, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 2007;25:5210–7. [DOI] [PubMed] [Google Scholar]
- 31.Wilson WH, Berg SL, Bryant G, et al. Paclitaxel in doxorubicin-refractory or mitoxantrone-refractory breast cancer: a phase I/II trial of 96-hour infusion. J Clin Oncol 1994;12:1621–9. [DOI] [PubMed] [Google Scholar]
- 32.Breathnach OS, Georgiadis MS, Schuler BS, Pizzella P, Llorens V, Kasturi V, et al. Phase II trial of paclitaxel by 96-hour continuous infusion in combination with cisplatin for patients with advanced non-small cell lung cancer. Clin Cancer Res 2000;6(7):2670–6. [PubMed] [Google Scholar]
- 33.Cocconi G, Mambrini A, Quarta M, et al. Vinorelbine combined with paclitaxel infused over 96 hours (VI-TA-96) for patients with metastatic breast carcinoma. Cancer 2000;88:2731–8. [DOI] [PubMed] [Google Scholar]
- 34.Anderson SE, O'Reilly EM, Kelsen DP, Ilson DH. Phase II trial of 96-hour paclitaxel in previously treated patients with advanced esophageal cancer. Cancer Invest 2003;21:512–6. [DOI] [PubMed] [Google Scholar]
- 35.Langer CJ, Li Y, Jennings T, et al. Phase II evaluation of 96-hour paclitaxel infusion in advanced (recurrent or metastatic) squamous cell carcinoma of the head and neck (E3395): a trial of the Eastern Cooperative Oncology Group. Cancer Invest 2004;22:823–31. [DOI] [PubMed] [Google Scholar]
- 36.Maier-Lenz H, Hauns B, Haering B, et al. Phase I study of paclitaxel administered as a 1-hour infusion: toxicity and pharmacokinetics. Semin Oncol 1997;24(6 Suppl 19):S19–16-S19–19. [PubMed] [Google Scholar]
- 37.Posadas EM, Undevia S, Manchen E, Wade JL, Colevas AD, Karrison T. A phase II study of ixabepilone (BMS-247550) in metastatic renal-cell carcinoma. Cancer Biol Ther 2007;6:490–3. [DOI] [PubMed] [Google Scholar]
- 38.Zhan Z, Scala S, Monks A, Hose C, Bates S, Fojo T. Resistance to paclitaxel mediated by P-glycoprotein can be modulated by changes in the schedule of administration. Cancer Chemother Pharmacol 1997;40:245–50. [DOI] [PubMed] [Google Scholar]
- 39.Vassileva V, Allen CJ, Piquette-Miller M. Effects of sustained and intermittent paclitaxel therapy on tumor repopulation in ovarian cancer. Mol Cancer Ther 2008;7:630–7. [DOI] [PubMed] [Google Scholar]
- 40.Einzig AI, Gorowski E, Sasloff J, Wiernik PH. Phase II trial of taxol in patients with metastatic renal cell carcinoma. Cancer Invest 1991;9:133–6. [DOI] [PubMed] [Google Scholar]
- 41.Bruntsch U, Heinrich B, Kaye SB, de Mulder PH, van Oosterom A, Paridaens R, et al. Docetaxel (Taxotere) in advanced renal cell cancer. A phase II trial of the EORTC Early Clinical Trials Group. Eur J Cancer 1994;30A:1064–7. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez M, Paull K, Monks A, et al. Generation of a drug resistance profile by quantitation of mdr-1/P-glycoprotein in the cell lines of the National Cancer Institute Anticancer Drug Screen. J Clin Invest 1995;95:2205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.