Abstract
Context:
This study was designed to characterize the role of the dental pulp (DP) in age estimation.
Aim:
The analysis of age-related quantifiable changes in DP components such as odontoblasts, collagen fibers, and blood vessels.
Subjects and Methods:
One hundred and twenty extracted teeth from six age groups (20–30 years, 31–40 years, 41–50 years, 51–60 years, 61–70 years, and 71–80 years) were subjected to decalcification and routine histopathological processing followed by Hematoxylin and Eosin and Picrosirius Red staining. Evaluation of the number of odontoblasts, mean vessel area (MVA), mean vessel diameter (MVD), and collagen fiber thickness were done.
Statistical Analysis Used:
ANOVA test, Fisher's test, and Regression Analysis.
Results:
Reduction in the number of odontoblasts/mm of pulp-dentinal border, MVA, and MVD were seen with advancing age. Rise in collagen fiber thickness was noted with increasing age. All parameters showed strongly statistically significant differences between age groups with P < 0.001 (ANOVA test).
Conclusions:
Regression formulae derived for age estimation based on data collected demonstrated linear correlation with age. Collagen fiber thickness had the highest accuracy followed by odontoblast numbers and MVA. MVD was the least accurate among the factors considered. However, the highest accuracy of 90.9% was seen when all parameters were incorporated together in a single equation.
Keywords: Age determination by teeth, dental pulps, forensic dentistry, odontoblasts
Introduction
Aging is an inevitable process affecting all organisms characterized by homeostasis imbalance, increased susceptibility, and decreased capability for adaptation in response to all stimuli.[1]
Age-related changes in teeth begin in utero and continues throughout the life.[2] The assessment of age-related changes in teeth are based on variations in morphology and molecular makeup.[3] The aging phenomenon is influenced by normal physiologic processes and pathologic changes in response to functional and environmental factors.
The dental pulp (DP) is a soft-tissue mesenchyme present within the pulp chamber and root canals of all teeth.[4] The mature DP is rimmed by a layer of odontoblasts, at the periphery.[5] The pulp chamber is also occupied by smaller blood and lymphatic vessels, nerves, supporting fibers, ground substance, interstitial fluid, odontoblasts, fibroblasts, undifferentiated mesenchymal cells, stem cells, and defense cells such as histiocytes, mast cells, and plasma cells.[4,6]
DP undergoes regressive and reactive changes as individual ages.[7] In general, there is decreased blood flow, decreased reparative capacity, and increased calcification within the pulp.[3] Microscopically evident alterations are increased fibrosis, decreased vascularity and cellularity of pulp[5,7]
The odontoblasts are seen at the dentinal border of pulp, with processes extending into the dentinal tubules. The odontoblasts are generally believed to live as long as the tooth is viable, but inactivity and aging of the odontoblasts result in the loss of organelles and a reduction of cell size.[4,8] The young odontoblast is an elongated cell with a basally placed nucleus and a slightly basophilic cytoplasm, whereas the older cells appear stubby with little cytoplasm and a centrally placed prominent nucleus.[4] Although quantitative studies in humans have reported a reduction in total pulp cell numbers by 50% between the ages of 20 and 70 years, there is insufficient information regarding changes in the odontoblastic population with age.[2]
DP matrix is predominantly composed of Type I collagen, along with Type III fibers in moderate numbers.[9] With advancing age, there is a decrease in cellularity of pulp and the surviving fibroblasts respond by producing more fibrous matrix. DP shows both diffuse fibrillary components as well as thickened collagen fiber bundles arrayed longitudinally in the radicular pulp and diffusely in the coronal pulp. Thus, overall the connective tissue matrix in the aging pulp appears to be more fibrosed with thick bundles of collagen fibers.[4,6,9]
Vascular changes also occur in the aging DP as they do elsewhere in the body. The outer diameter of vessel walls increase as collagen fibers are deposited in the medial and adventitial layers.[5] Atherosclerotic plaques in blood vessels and perivascular calcifications may be seen. The rate of blood flow is also considerably decreased with age.[4,9]
Factors related to dental aging such as enamel attrition, secondary dentin deposition, dentinal sclerosis, and cemental apposition have been investigated and standardized for age estimation techniques in forensic odontology. However, very little is known at present regarding the role of DP in age estimation techniques although it is considered to be the best source of DNA for personal identification in forensic investigations.[10]
The aim of this study was to evaluate the role of DP in age estimation by measuring the thickness of collagen fibers, mean vessel diameter (MVD) and the area along with odontoblast density in the DP of individuals from six different age groups.
Subjects and Methods
The study sample consisted of physiologically sound teeth extracted from healthy patients within the age group of 20–80 years. The patients reported for extraction of normal teeth either due to periodontitis or as part of orthodontic treatment. The study was approved by an institutional review board and informed consent was obtained from all the study participants. The patients name, age, gender, and medical history were recorded before routine oral examination. One hundred and twenty samples were equally divided into six groups, based on the age of the patients. Group A: 20–30- year-old patients (n = 20), Group B: 31–40- year-old patients (n = 20), Group C: 41–50- year-old patients (n = 20), Group D: 51–60-year-old patients (n = 20), Group E: 61–70- year-old patients (n = 20), Group F: 71–80-year-old patients (n = 20) [Tables 1 and 2].
Table 1.
Age group distribution in the study sample
| Group (years) | Samples (%) |
|---|---|
| 20-30 | 20 (16.7) |
| 31-40 | 20 (16.7) |
| 41-50 | 20 (16.7) |
| 51-60 | 20 (16.7) |
| 61-70 | 20 (16.7) |
| 71-80 | 20 (16.7) |
| Total | 120 (100) |
| Mean±SD | 51.35±17.15 |
SD: Standard deviation
Table 2.
Gender distribution within age groups
| Group (years) | Gender (%) | Total cases (%) | |
|---|---|---|---|
| Female | Male | ||
| 20-30 | 10 (17.5) | 10 (15.9) | 20 (16.7) |
| 31-40 | 8 (14) | 12 (19) | 20 (16.7) |
| 41-50 | 10 (17.5) | 10 (15.9) | 20 (16.7) |
| 51-60 | 8 (14) | 12 (19) | 20 (16.7) |
| 61-70 | 10 (17.5) | 10 (15.9) | 20 (16.7) |
| 71-80 | 11 (19.3) | 9 (14.3) | 20 (16.7) |
| Total | 57 (100) | 63 (100) | 120 (100) |
Permanent, erupted, unerupted, anterior and posterior teeth were included in the study. Teeth with deep dental caries, nonvital, restored teeth, teeth with severe attrition, abrasion, erosion, dental fluorosis, severe periodontitis (associated with abscess formation), as well as teeth from participants with collagen vascular disorders, autoimmune disorders, cardiovascular disorders and uncontrolled diabetes mellitus were excluded from the study sample.[11]
Immediately after extraction, the teeth were fixed in 10% neutral buffered formalin for a maximum of 5 days. The samples were then subjected to decalcification in a solution containing 12% nitric acid with accelerators (EDTA, sodium tartrate and potassium tartrate) for up to 5 days. The decalcified teeth were processed and embedded in paraffin to obtain tissue blocks. The tissue blocks were trimmed to expose DP, and two sets of 4 μ thick sections were made. The sections were stained with hematoxylin and eosin (H and E) and picrosirius red (PSR).[12,13] PSR staining was done as follows: Paraffin sections were dewaxed and hydrated. Nuclear staining with Weigert's Hematoxylin (Nice Chemicals) for done for 8 min and subsequently washed in running tap water for 10 min. The sections were then stained in PSR solution (0.1 g of Direct Red 80, Sigma-Aldrich in 100 ml of saturated aqueous solution of picric acid) for 1 h and later washed in two changes of acidified water (5 ml of glacial acetic acid in 1 liter tap water). The sections were then dehydrated, cleared, and mounted in DPX media.[13]
H and E stained sections were analyzed under the light microscope, whereas PSR stained sections were observed under circularly polarized light.
Photomicrographs were captured using Olympus B × 51 Research Microscope with ProgRes CT3 camera attachment and Image Pro software, in.jpg format. Photomicrographs at × 100 were captured with a preset calibration of 1020.40 pixels = 700 μm2 and photomicrographs at × 400 were captured with a set calibration of 4080 pixels = 170 μm2.
Morphometric assessment, i.e., the calculation of the area and perimeter, was made using ImageJ Software.[14,15,16]
Assessment of number of odontoblasts were done as follows:[17,18]
In each sample, five microscopic examination fields in the mid-coronal part of the DP were assessed at × 400 for the odontoblastic layer
Photomicrographs were captured, and the total area was measured [Figure 1]
Subsequently, the number of odontoblasts were counted in each image
Finally, the average number of odontoblasts per square micrometer was calculated and conversion to number of odontoblasts per square millimeter was done.
Figure 1.
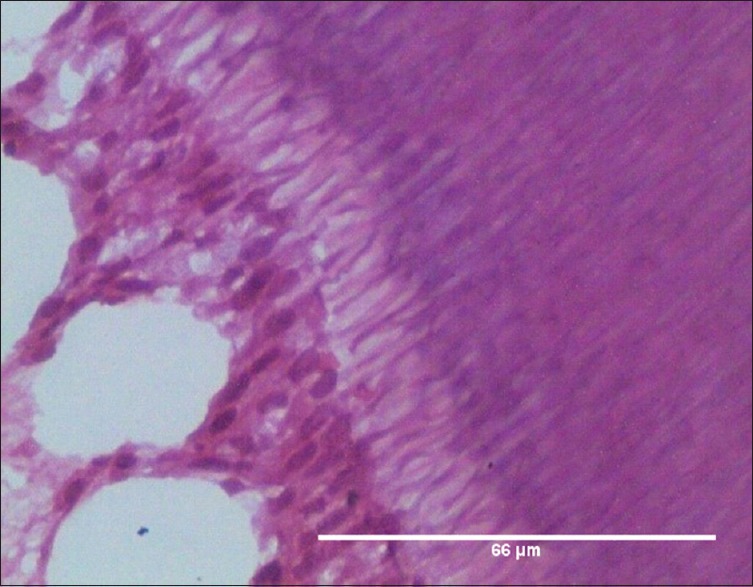
Odontoblasts in Group A (20–30 years), H and E, ×400
Assessment of mean vessel area and mean vessel diameter were done as follows:[19]
Photomicrographs of the pulp at mid-coronal level were captured at × 100 [Figure 2]
An area of 0.25 mm2 was considered, and all the blood vessels within the said area were traced
Total vessel area and total vessel diameter were calculated using the software.
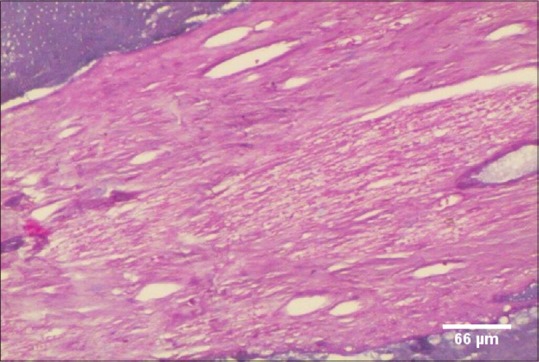
The values were expressed as percentage mean vessel area (MVA) and MVD/mm2 Assessment of Collagen Fiber Thickness:[14,20,21]
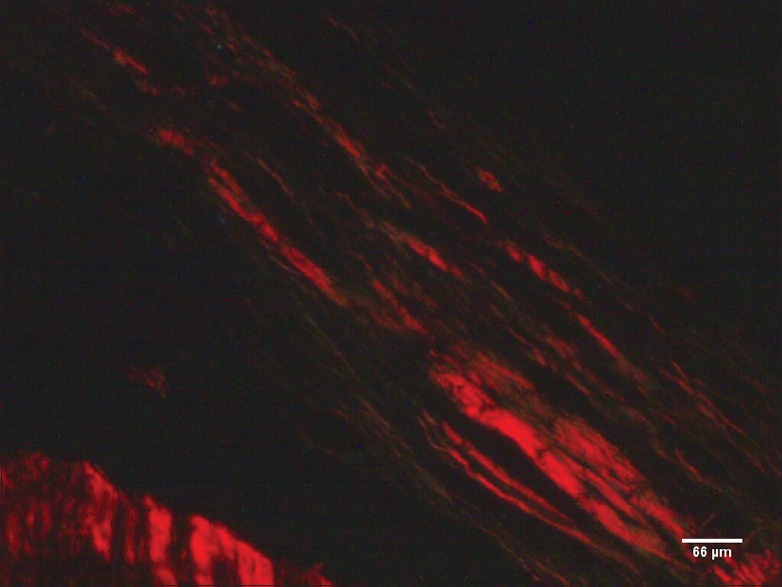
Photomicrographs of the pulpal core were captured at × 100 [Figure 3]
The observations were limited to mid coronal pulp
The cross-sectional thickness of five largest collagen fiber bundles was measured and expressed in micrometers.
Figure 2.
Collagen fiber bundles of dental pulp in Group F (71–80 years), PSR, ×100
Figure 3.
Blood vessels of dental pulp in Group F (71–80 years), H and E, ×100
Results
The patients age ranged between 20 and 80 years. Each age cohort made up 16.7% of the total sample. The mean chronological age in the present study sample was 51.35 ± 17.15 years [Table 1]. A total of 57 females and 63 males were included in the study with a percentage distribution of 47.5% and 52.5%, respectively. There was no significant difference between genders within age groups with P = 0.913, Chi-square test [Table 2].
After morphometric and quantitative analysis, we found odontoblast density to be highest in the youngest age group with 75% of cases having 301–400 cells and only 5% of the group showing odontoblast cell numbers between 201 and 300, based on measured values for number of odontoblasts/mm of pulp-dentinal border. The 31–40 years old showed odontoblast density between 201–300 and 301–400 cells with equal distribution. Sixty percent of samples in the 41–50 years age group have odontoblast cell density between 201 and 300 cells and 40% of samples demonstrate the high number of odontoblasts/mm of pulp-dentinal border, i.e., 301–400 cells. In the 51–60 years age group, 65% of samples have odontoblast density between 201 and 300 cells and 35% of samples showed 301–400 cells. The incidence of lower odontoblast density between 101 and 200 cells was first observed in the 61–70 years old involving 45% of cases, whereas 55% of cases demonstrate odontoblast cell density between 201 and 300 cells. About 70% of cases in the 71–80 years old age group show 101–200 odontoblasts/mm of pulp-dentinal border and 20% of the samples analyzed in the same age group showed extremely low odontoblast numbers between 0 and 100. Remaining 10% of cases had relatively higher odontoblast density, i.e., 201–300 cells [Figure 4]. The number of odontoblasts was highest in the age group of 20–30 years with a mean ± standard deviation (SD) of 335.40 ± 33.04. A progressive decline in numbers was seen in each decade with a mean ± SD of 301.40 ± 36.51, 286.35 ± 36.26, 288.25 ± 47.35, 204.40 ± 41.90, and 130.10 ± 61.09 in age groups of 31.40 years, 41–50 years, 51–60 years, 61–70 years, and 71–80 years, respectively [Table 3]. A significant difference was seen in the number of odontoblasts/mm of pulp-dentinal border with advancing age.
Figure 4.
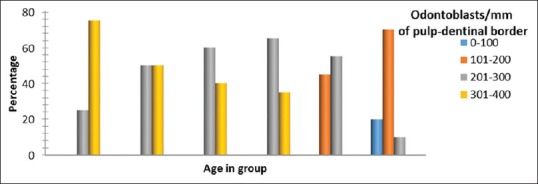
Odontoblast density across different age groups. Significant difference in odontoblast population between age groups with P < 0.001, Fisher's exact test
Table 3.
Median and range of measured parameters in different age groups
| Age (years) | Odontoblasts/mm of pulp-dentinal border | Collagen fibre thickness (in microns) | Mean vessel area (in percentage) | Mean vessel diameter/sq.mm |
|---|---|---|---|---|
| 20-30 | 350 (277-384) | 4.65 (3.45-5.54) | 9.52 (6.36-22.26) | 0.52 (0.12-7.20) |
| 31-40 | 301 (232-354) | 5.55 (4.90-5.91) | 6.68 (4.96-9.43) | 0.57 (0.32-0.91) |
| 41-50 | 282 (223 -345) | 6.58 (5.38-8.09) | 5.72 (2.52-8.12) | 0.58 (0.30-1.02) |
| 51-60 | 270.0 (225-391) | 7.98 (6.90-9.91) | 3.08 (2.60-4.89) | 0.48 (0.17-0.99) |
| 61-70 | 205 (104-275) | 11.10 (9.21-13.77) | 3.08 (2.01-4.80) | 0.41 (0.29-0.59) |
| 71-80 | 137.0 (0.0-232) | 13.57 (10.15-18.02) | 0.79 (0.38-2.0) | 0.12 (0.04-0.25) |
Thin collagen fibers, <5 μm thickness are seen in 65% of 20–30 years age group and 10% of 31–40 years old. About 35% of 20–30 years old showed moderate collagen fiber thickness between 5 μm and 15 μm. Furthermore, 90% of 31–40 years old and all 41–70 years olds along with 60% of 71–80 years old exhibited collagen fiber thickness between 5 μm and 15 μm. Thicker collagen fiber bundles, more than 15 μm in breadth are noted in 40% of cases within the 71–80 years age group [Figure 5]. A progressive increase in collagen fiber thickness was noted across age groups with a mean thickness of 4.61 ± 0.61 μm at 20–30 years. Calculated mean values of collagen fiber thickness in micrometers were 5.49 ± 0.35, 6.55 ± 0.61, 8.05 ± 0.79, and 11.11 ± 1.41 (mean ± SD) across age groups 31–40 years, 41–50 years, 51–60 years and 61–70 years, respectively [Table 3]. Maximum collagen fiber thickness was noted in 71–80 years age group with a mean ± SD of 14.06 ± 2.19.
Figure 5.
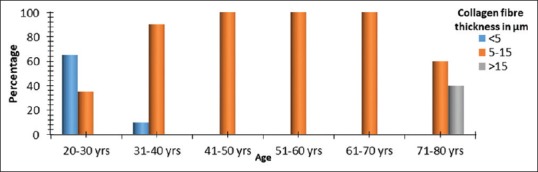
Collagen fiber thickness (in microns) across age groups. Significant difference between collagen fiber thickness across age groups with P < 0.001, Fisher's exact test
The calculated percentage MVA of the DP, in 35% of 20–30 years was more than 10%. Sixty-five percent of samples in the same age group showed pulpal MVA of 5%–10%. Almost 90% of 31–40 years old patients have 5%–10% pulpal MVA, the remaining 10% of patients have lower MVA <5%. The similar distribution pattern was seen in 41–50 years old with 5%–10% MVA seen in 60% patients and < 5% MVA in 40% patients. Lower MVA, <5% was seen in 95% of 51–60 years old patients, and all patients between 61 and 80 years of age. Only 5% of patients within the 51–60 years old sample showed high MVA of more than 10% [Figure 6].
Figure 6.
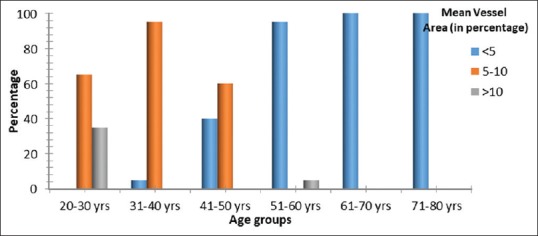
Percentage mean vessel area of dental pulp across age groups. Significant difference in percentage MVA across age groups with P < 0.001 Fisher's exact test
The analysis of percentage MVA revealed a gradual decline with increasing age. The maximum value of 10.85% ±4.32% (mean ± SD) was seen in the age group of 20–30 years. Age groups of 31–40 years, 41–50 years, 51–60 years, 61–70 years, and 71–80 years presented percentage MVA (mean ± SD) of 6.91 ± 1.29, 5.43 ± 1.49, 3.47 ± 0.81, 3.27 ± 0.86 and 0.90 ± 0.46, respectively. Significant difference in measured MVA was seen across the age groups considered.
Fifty-five percent of patients between 20 and 30 years of age have MVD between 0.4 mm and 0.8 mm, while 25% had MVD >0.8 mm, only 20% of the samples had MVD < 0.4 mm. In the 31–40 years age group, 75% of patients presented a MVD of 0.4–0.8 mm, whereas 15% and 10% of samples had MVDs of > 0.8 mm and < 0.4 mm, respectively. A similar trend was seen in the 41–50 years age group, with 60% of patients exhibiting MVD between 0.4 mm and 0.8 mm and 25% and 15% patients having MVDs of > 0.8 mm and <0.4 mm, respectively. MVD between 0.4 mm and 0.8 mm was seen in 55% of 51–60 years olds, and 60% of 61–70 years old. Thirty-five percent of the former and 40% of the latter group had low MVD, <0.4 mm while only 10% of 51–60 years old patients showed high MVD >0.8 mm. 61 –70-year-old patients had 60% samples with a mean diameter between 0.4 mm and 0.8 mm and 40% <0.4 mm. The oldest age group presented exclusively with low pulpal MVD/mm2, i.e., <0.4 mm [Figure 7].
Figure 7.
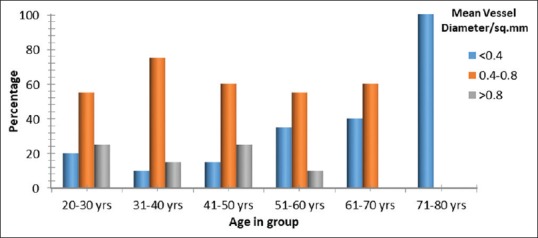
Mean vessel diameter/mm2 of dental pulp across different age groups. Significant difference in mean vessel diameter/mm2 across age groups with P < 0.001, Fisher's exact test
Scrutiny of MVD/mm2 of DP across age groups indicated decline in values with age. The highest value of 1.12 ± 1.65 was seen in the 20–30 years age group, followed by decreasing values of 0.59 ± 0.17, 0.61 ± 0.21, 0.44 ± 0.16, 0.42 ± 0.08, and 0.13 ± 0.06 from 4th to 8th decades of life.
Regression formulae for age estimation were derived from the data collected. It was found that when individual parameters were assessed, MVD was least accurate, with an R2 of 10.9% and standard error of ± 16.239 years. Measurement of odontoblasts/mm of pulp dentinal border and MVA were comparatively more precise with an accuracy of 58.7% and 66.1% and standard error of ± 11.022 and ± 10.018 years, respectively. Collagen fiber thickness appeared to be the most accurate individual parameter for age estimation with an R2 of 85.7% and a standard error of ± 6.483 years. However, when all parameters were considered together, a higher accuracy of 90.9% with a standard error of ± 5.293 years was achieved [Table 4].
Table 4.
Regression analysis of the measured parameters
| Regression equation | R2 (%) | Standard error of estimate |
|---|---|---|
| Age=92.91-0.161 × Odontoblasts | 58.7 | 11.022 |
| Age=13.79+4.52 × Collagen fiber thickness | 85.7 | 6.483 |
| Age=70.55-3.74 × MVA (in percentage) | 66.1 | 10.018 |
| Age=55.665-7.95 × MVD/sq.mm | 10.9 | 16.239 |
| Age=36.27-0.014 × odontoblasts+3.155 × Collagen fiber −1.338 × MVA −1.179 × MVD | 90.9 | 5.293 |
MVD: Mean vessel diameter, MVA: Mean vessel area
Discussion
Aging is an ongoing process in all organisms with a mitochondrial genome. In somatic tissues, it is characterized by the gradual accumulation of cellular damage and compromised function. The rates at which these changes occur are affected by many factors (intrinsic and extrinsic) and vary among individuals. This gives rise to inconsistencies in biological age among entities of the same chronological age thus, rendering conventional age estimation techniques difficult.[22]
The DP is enclosed in a rigid chamber by hard tissues which provide it with support and protection.[5] Thus, the DP is fortified from extrinsic factors which influence biological aging, to some extent. Therefore, in the absence of debilitating conditions such as infections or systemic diseases, the DP can be considered a tissue, in which biological age approximates chronological age. Furthermore, Gawande et al.[23] have elucidated the postmortem DP shows well-preserved histological appearance for up to 72 h in contrast with destruction and degeneration seen in other body tissues as early as 24 h, making it a viable candidate for soft-tissue age estimation.
Vavpotic et al.[18] have demonstrated that odontoblast numbers can be used to estimate time of death for about 5 days postmortem, further perpetuating the role of DP in forensic odontology. Another application of DP in forensics has been described by Sandoval et al.[24] who validated that the Barr body test can be applied to DP fibroblasts of human remains exposed to extreme conditions of pH, humidity and salinity for gender identification with 98.9% accuracy.
The present study was conducted on teeth extracted from 120 patients, with age ranging from 20 to 80 years. Observation and quantification of morphological changes in DP in relation to age were carried out for the estimation of biological age and correlation with chronological age. The parameters considered were number of odontoblasts/mm of pulp-dentinal border, collagen fiber thickness, percentage MVA, and mean vessel density per square mm of pulpal area.
Teeth where pulpal integrity was compromised, especially teeth with deep dental caries, severely attrited abraded and eroded teeth as well as nonvital teeth were excluded from this study.
Teeth with restorations were avoided, as it would not be amenable to histopathological processing and also because literature shows conflicting reports regarding effect of dental restorations and restorative procedures on pulp. Murray et al.[17] claimed that cavity preparation and restoration variables have no significant effect on odontoblast numbers. However, Vitalariu et al.[25] postulated that clinical operative procedures such as depth of cavity preparation and use of water-cooling affect odontoblast numbers. Similarly, teeth with fluorosis were not included, as Sentut et al.[26] have demonstrated significant difference in odontoblast numbers between fluorotic and nonfluorotic teeth.
Studies by Zuza et al.[27] have demonstrated pulpal fibrosis and dystrophic calcifications in teeth affected by severe periodontitis with >5 mm attachment loss. Similarly, Caraivan et al.[28] established fibrosis, congestive changes in blood vessels and odontoblast destruction in DP of teeth with severe periodontitis. Wan et al.[29] also validated correlation between histological changes in pulp and attachment loss. Considering these findings, patients with teeth affected by severe periodontitis were exempted from the study. Periodontitis, however, mild may be a confounding factor in correlation of biological age and chronological age as evidenced by Caraivan et al.[28] Nevertheless, mild and moderate periodontitis samples with <5 mm attachment loss has been included in the study based on the supposition that, in a practical situation, majority of patients in the older age groups present with some degree of periodontitis and it is desirable to include this factor where age estimation is concerned, in the absence of debilitating conditions such as infections or systemic diseases.
Patients with collagen vascular disorders and autoimmune disorders were excluded from the study Alteration in structure and function of microcirculation has been described in patients with cardiovascular disorders by Gates et al.[30] Catanzaro et al.[31] In diabetics, evidenced deposition of atheromatous material in the lumen of pulpal blood vessels and decreased collagen content in pulp.
Tangjit et al.[32] have demonstrated decreased percentage MVA in occlusal hypofunction conditions and Popescu et al.[33] concluded that teeth subjected to occlusal trauma may present with fibrosis, inflammation and calcifications. Furthermore, Santamaria et al.[34] have reported increased blood vessel density and fibrosis in orthodontically displaced teeth. Similar results were described by Kayhan et al.[35] after rapid palatal expansion technique. Inability to exclude these confounding factors from the study sample was experienced, as most of the teeth obtained from the younger age group were indicated for extraction due to impaction or as part of the orthodontic treatment plan.
Assessment of the number of odontoblasts/mm of pulp-dentinal border was carried out in accordance with the procedure described by Murray et al.[17] and Vavpotic et al.[18] These authors have used a micro ocular grid for manual counting of the number of odontoblasts, while in the present study photomicrography followed by the use of ImageJ software (Open source software developed by National Institute of Health, USA) for counting of cells were employed. A significant gradual decline in odontoblast numbers was noted with age and some teeth in the 71–80 years age group demonstrated total absence of odontoblasts with intense fibrosis of the pulp chamber. Another noteworthy observation was change in the morphology of odontoblasts, with tall columnar cells in the younger age group and cuboidal to flattened cells in older age groups. However, owing to cellular damage due to the decalcification procedure, the intracellular and nuclear changes of odontoblast cells were not well appreciated. It may be advisable to avoid decalcification and split the tooth in the sagittal plane and extract pulp followed by fixation and subsequent histological processing, as described by Lovschall et al.[36] and Hillman and Geurtsen,[11] if the analysis of individual cell morphology is desired. However, for quantitative analysis of number of cells as required in this study, the decalcification procedure provides adequate results.
Reduction in odontoblast density was correlated with age of patients, and the results were strongly significant. These findings are in agreement with those described by Murray et al.,[2] who, in 51–59 years age group have recorded decrease of 15.6% in crown odontoblasts and 40% in root odontoblasts compared to younger patients 10–30 years of age. The authors have also remarked that irrespective of age group, odontoblast density was higher in the crown compared to root with a range of values from 316 to 350 cells/mm of pulp-dentinal border in crown and 226–284/mm of pulp-dentinal border in root. These values approximate the numbers recorded in the current study, although for standardization purposes observations were limited to the middle one-third of the pulp chamber in the current study and not divided into coronal or radicular segments. Based on the data collected, an equation was derived for age estimation, with linear regression, yielding an accuracy of 58.7% and a standard error of ± 11.022 years.
Several authors have described the advantages of the PSR staining method for the characterization of collagen fibers. The advantage, being that even very thin fibers show birefringence under polarized light. The thickness of collagen fibers was found to increase gradually from youngest to oldest age group. A steady increase in the thickness of collagen fiber bundles was perceived across age groups, ranging from 4.61 ± 0.61 μm in the third decade to 14.06 ± 2.19 μm in the eighth decade of life. Correlation between collagen fiber thickness and age was strongly significant with P < 0.001 (ANOVA test). Regression formula derived for age estimation based on collagen fiber thickness had an accuracy of 85.7%, and standard error of ±6.483 years, the most precise estimate for any of the individual parameters assessed. Thus, increasing collagen fiber thickness appears to be an important and consistent factor in age estimation with good correlation between estimated biological age and chronological age.
The evaluation of MVA and MVD was carried out according to the protocol described by Tomaszewska et al.[19] All endothelium lined channels were considered as blood vessels, since according to the immunohistochemical study conducted by Gerli et al.,[37] lymphatic vessels are absent in the DP. A steady decrease in MVA and MVD was noted with increasing age, consistent with diminishing vascularity. Significant correlation between reduction in MVA and MVD was seen with increasing age, with P < 0.001 (ANOVA test). Age changes in the microcirculation, such as decreased peripheral blood flow, fibrosis of vessel walls, and narrowing of lumen were described by Ogrin et al.[38] and Yoshida and Ohshima,[39] which are consistent with our findings. Regression formula for age estimation based on percentage MVA also showed a linear regression model with an accuracy of 66.1% and standard error of ±10.018 years. However, regression model based on MVD showed a low accuracy of 10.9% with a standard error of ±16.239 years, showing poor correlation between estimated biological age and chronological age.
The age estimation model derived after factoring in all the measured variables, displayed linear regression pattern with good accuracy of 90.9% and a standard error of ±5.293 years. Thus, incorporation of all quantified parameters in a single regression model provided a better approximation between estimated age and chronological age compared to that estimated with individual factors. Rösing and Kvaal have stated that “methods with a standard error of regression of more than 5 or 7 years are not suitable for routine forensic application.”[40] Based on this criterion, the evaluation of collagen fiber thickness would be the sole parameter suitable for age estimation with a standard error of 6.483 years. However, for forensic age estimation, the use of the regression formula incorporating all the parameters analyzed in this study would be best since it has an acceptable standard error of 5.293 years.
It would be ideal if confounding factors such as periodontitis, occlusal hypofunction and trauma as well as the effect of orthodontic manipulation can be eliminated from the study sample. Differences in morphometrics between various races and ethnicities also need to be studied and age estimation formulae must be customized according to those results. This study has attempted to conform the sample to physiologically sound subjects as much as possible. Further studies may be required to tailor the age estimation formulae for adults with common age-related infirmities such as hypertension, diabetes mellitus and other degenerative disorders. Experimental application of this technique in a postmortem situation, factoring in the varying decomposition and degeneration of tissues may be helpful in further defining the circumstances where this method can be used.
Conclusion
Based on the results and observations made in our study, following conclusions were drawn:
DP showed quantifiable changes with aging, which can be used for age estimation
Decrease in odontoblast numbers was seen with increasing age, with significant difference between each decade of life
Collagen fiber thickness increased with advancing age and can be qualitatively assessed with change in fibrillar hue under polarized light as well as quantitatively with increasing thickness of collagen fiber bundles. If a single pulpal parameter for age estimation is desired, collagen fiber thickness would be an ideal candidate for providing an accuracy of 85.7%
Mean blood vessel area and MVD also showed significant reduction with advancing age
Factoring all these four parameters in a regression model was more accurate than the assessment of individual parameters.
The role of dental hard tissues in age estimation has been well established. This study was an attempt to define the role of DP in age estimation. The results obtained have demonstrated that histological assessment of DP can be an effective addition to existing age estimation techniques. Further studies with larger samples are required for the validation of these findings and also may be helpful in realizing the objective of forensic age estimation, i.e., a more accurate correlation between estimated biological age and chronological age of the subject.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge the contributions of Ms. Dorothy Anitha, Histotechnician and Ms. Hayat Moosa, Statistician.
References
- 1.Guiglia R, Musciotto A, Compilato D, Procaccini M, Russo L, Ciavarella D, et al. Aging and oral health: Effects in hard and soft tissues. Curr Pharm Des. 2010;16:619–30. doi: 10.2174/138161210790883813. [DOI] [PubMed] [Google Scholar]
- 2.Murray PE, Stanley HR, Matthews JB, Sloan AJ, Smith AJ. Age-related odontometric changes of human teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:474–82. doi: 10.1067/moe.2002.120974. [DOI] [PubMed] [Google Scholar]
- 3.An G. Normal ageing of teeth. Geriatr Aging. 2009;12:513–17. [Google Scholar]
- 4.Ten Cate AR, Nanci A. Tencate's Oral Histology Development, Structure and Function. 8th ed. India: Elsevier; 2013. [Google Scholar]
- 5.Yu C, Abbott PV. An overview of the dental pulp: Its functions and responses to injury. Aust Dent J. 2007;52:S4–16. doi: 10.1111/j.1834-7819.2007.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 6.Avery JK, Chiego Jr DL. 3rd ed. Missouri: Mosby Elsevier; 2008. Essentials of Oral Histology and Embryology: A Clinical Approach. [Google Scholar]
- 7.Mckenna G, Burke FM. Age-related oral changes. Dent Update. 2010;37:519–23. doi: 10.12968/denu.2010.37.8.519. [DOI] [PubMed] [Google Scholar]
- 8.Hamet P, Tremblay J. Genes of aging. Metabolism. 2003;52:5–9. doi: 10.1016/s0026-0495(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 9.Kumar GS, Bhasker SN, editors. Orban's Oral Histology and Embryology. 13th ed. India: Elsevier; 2014. [Google Scholar]
- 10.Sivapathasundaram B, editor. Shafer's Textbook of Oral Pathology. 8th ed. India: Reed Elsevier; 2015. [Google Scholar]
- 11.Hillmann G, Geurtsen W. Light-microscopical investigation of the distribution of extracellular matrix molecules and calcifications in human dental pulps of various ages. Cell Tissue Res. 1997;289:145–54. doi: 10.1007/s004410050860. [DOI] [PubMed] [Google Scholar]
- 12.Suvarna SK, Layton C, Bancroft JD. 7th ed. England: Churchill Livingstone Elsevier; 2013. Bancroft's Theory and Practice of Histological Techniques. [Google Scholar]
- 13.Culling CF, Allison RT, Barr WT. 4th ed. London, UK: Butterworths; 1985. Cellular pathology technique. [Google Scholar]
- 14.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 15.Deroulers C, Ameisen D, Badoual M, Gerin C, Granier A, Lartaud M. Analyzing huge pathology images with open source software. Diagn Pathol. 2013;8:92. doi: 10.1186/1746-1596-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadopulos F, Spinelli M, Valente S, Foroni L, Orrico C, Alviano F, et al. Common tasks in microscopic and ultrastructural image analysis using ImageJ. Ultrastruct Pathol. 2007;31:401–7. doi: 10.1080/01913120701719189. [DOI] [PubMed] [Google Scholar]
- 17.Murray PE, About I, Lumley PJ, Franquin JC, Remusat M, Smith AJ. Human odontoblast cell numbers after dental injury. J Dent. 2000;28:277–85. doi: 10.1016/s0300-5712(99)00078-0. [DOI] [PubMed] [Google Scholar]
- 18.Vavpotic M, Turk T, Martincic DS, Balazic J. Characteristics of the number of odontoblasts in human dental pulp post-mortem. Forensic Sci Int. 2009;193:122–6. doi: 10.1016/j.forsciint.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Tomaszewska JM, Miskowiak B, Matthews-Brzozowska T, Wierzbicki P. Characteristics of dental pulp in human upper first premolar teeth based on immunohistochemical and morphometric examinations. Folia Histochem Cytobiol. 2013;51:149–55. doi: 10.5603/FHC.2013.0023. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu K, Mosekilde L, Viidik A, Chiba M. Polarized light microscopic analyses of collagen fibers in the rat incisor periodontal ligament in relation to areas, regions, and ages. Anat Rec. 2002;268:381–7. doi: 10.1002/ar.10179. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Chaudhary AK, Pandya S, Debnath S, Singh M, Singh PA, et al. Morphometric analysis in potentially malignant head and neck lesions: Oral submucous fibrosis. Asian Pac J Cancer Prev. 2010;11:257–60. [PubMed] [Google Scholar]
- 22.Sanders AE. Shifting the focus of aging research into earlier decades of life. Oral Dis. 2016;22:166–8. doi: 10.1111/odi.12431. [DOI] [PubMed] [Google Scholar]
- 23.Gawande M, Chaudhary M, Das A. Estimation of the time of death by evaluating histological changes in the pulp. Indian J Forensic Med Toxicol. 2012;6:80–2. [Google Scholar]
- 24.Sandoval C, Nuñez M, Roa I, Sandoval C, Nuñez M, Roa I. Dental pulp fibroblast and sex determination in controlled burial conditions. Int J Morphol. 2014;32:537–41. [Google Scholar]
- 25.Viţalariu A, Căruntu ID, Bolintineanu S. Morphological changes in dental pulp after the teeth preparation procedure. Rom J Morphol Embryol. 2005;46:131–6. [PubMed] [Google Scholar]
- 26.Sentut T, Kirzioglu Z, Gockcimen A, Aslan H, Erdogan Y. Quantitative analysis of odontoblast cells in fluorotic and non-fluorotic primary tooth pulp. Turkish J Med Sci. 2012;42:351–7. [Google Scholar]
- 27.Zuza EP, Carrareto AL, Lia RC, Pires JR, de Toledo BE. Histopathological features of dental pulp in teeth with different levels of chronic periodontitis severity. ISRN Dent 2012. 2012:271350. doi: 10.5402/2012/271350. [Epub 2012 Apr 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caraivan O, Manolea H, Corlan Puşcu D, Fronie A, Bunget A, Mogoantă L. Microscopic aspects of pulpal changes in patients with chronic marginal periodontitis. Rom J Morphol Embryol. 2012;53:725–9. [PubMed] [Google Scholar]
- 29.Wan L, Lu HB, Xuan DY, Yan YX, Zhang JC. Histological changes within dental pulps in teeth with moderate-to-severe chronic periodontitis. Int Endod J. 2015;48:95–102. doi: 10.1111/iej.12282. [DOI] [PubMed] [Google Scholar]
- 30.Gates PE, Strain WD, Shore AC. Human endothelial function and microvascular ageing. Exp Physiol. 2009;94:311–6. doi: 10.1113/expphysiol.2008.043349. [DOI] [PubMed] [Google Scholar]
- 31.Catanzaro O, Dziubecki D, Lauria LC, Ceron CM, Rodriguez RR. Diabetes and its effects on dental pulp. J Oral Sci. 2006;48:195–9. doi: 10.2334/josnusd.48.195. [DOI] [PubMed] [Google Scholar]
- 32.Tangjit N, Kusakabe T, Iida J. Microvasculature of dental pulp in a rat molar in an occlusal hypofunctional condition. Hokkaido J Dent Sci. 2013;33:62–71. [Google Scholar]
- 33.Popescu MR, Deva V, Dragomir LP, Searpe M, Vătu M, Stefârţă A, et al. Study on the histopathological modifications of the dental pulp in occlusal trauma. Rom J Morphol Embryol. 2011;52:425–30. [PubMed] [Google Scholar]
- 34.Santamaria M, Jr, Milagres D, Stuani AS, Stuani MB, Ruellas AC. Initial changes in pulpal microvasculature during orthodontic tooth movement: A stereological study. Eur J Orthod. 2006;28:217–20. doi: 10.1093/ejo/cji117. [DOI] [PubMed] [Google Scholar]
- 35.Kayhan F, Küçükkeleş N, Demirel D. A histologic and histomorphometric evaluation of pulpal reactions following rapid palatal expansion. Am J Orthod Dentofacial Orthop. 2000;117:465–73. doi: 10.1016/s0889-5406(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 36.Lovschall H, Fejerskov O, Josephsen K. Age-related and site-specific changes in the pulpodentinal morphology of rat molars. Arch Oral Biol. 2002;47:361–7. doi: 10.1016/s0003-9969(02)00018-3. [DOI] [PubMed] [Google Scholar]
- 37.Gerli R, Secciani I, Sozio F, Rossi A, Weber E, Lorenzini G. Absence of lymphatic vessels in human dental pulp: A morphological study. Eur J Oral Sci. 2010;118:110–7. doi: 10.1111/j.1600-0722.2010.00717.x. [DOI] [PubMed] [Google Scholar]
- 38.Ogrin R, Darzins P, Khalil Z. Age-related changes in microvascular blood flow and transcutaneous oxygen tension under Basal and stimulated conditions. J Gerontol A Biol Sci Med Sci. 2005;60:200–6. doi: 10.1093/gerona/60.2.200. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida S, Ohshima H. Distribution and organization of peripheral capillaries in dental pulp and their relationship to odontoblasts. Anat Rec. 1996;245:313–26. doi: 10.1002/(SICI)1097-0185(199606)245:2<313::AID-AR14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.Ritz-Timme S, Cattaneo C, Collins MJ, Waite ER, Schütz HW, Kaatsch HJ, et al. Age estimation: The state of the art in relation to the specific demands of forensic practise. Int J Legal Med. 2000;113:129–36. doi: 10.1007/s004140050283. [DOI] [PubMed] [Google Scholar]


