Abstract
Purpose:
The purpose is to investigate cardiac magnetic resonance and laboratory findings in patients with clinically suspected acute myocarditis and re-assess the evolution of findings in relation to clinical parameters and smoking habits.
Methods:
We prospectively analyzed 68 consecutive patients (4 females, 64 males, median age 25 years) at baseline and 51 patients 12 months later with regard to age, symptoms, and signs, smoking history, cardiac troponin I, erythrocyte sedimentation rate, c-reactive protein blood levels, electrocardiography changes, and cardiac magnetic resonance findings. Statistical analysis included group comparisons and linear regression between clinical parameters and the obtained data.
Results:
A statistically significant correlation was recorded between smoking and late gadolinium enhancement extent, both at baseline and follow-up study. Late gadolinium enhancement extent was positively associated with cardiac troponin I serum levels and c-reactive protein and negatively with left ventricular ejection fraction at baseline study. Myocardial segments 4 and 5 were most frequently involved. Late gadolinium enhancement persisted in 96% of patients with no significant extent change at 12-month follow-up, while improved.
Conclusions:
A strong correlation was recorded between smoking patients with acute myocarditis and extent both at baseline and follow-up cardiac magnetic resonance. Myocardial segments 4 and 5 involvement was most prevalent. Late gadolinium enhancement persisted at follow-up, its incidence was higher than that reported in other studies and did not have an impact on the patient's clinical status or cardiac function. However, longer-term follow-up is highly recommended in these patients.
Keywords: Acute myocarditis, cardiac magnetic resonance, cigarette smoking, follow-up, late gadolinium enhancement, troponin I
INTRODUCTION
Myocarditis is defined as an acquired cardiomyopathy that may be an acute or chronic inflammation of myocardial tissue in any cardiac chamber, either focal or diffuse.[1,2,3] In North America and Europe, viral infections constitute the most frequent causative agent of acute myocarditis and include among others coxsackievirus B3, Epstein–Barr virus, human herpesvirus 6, and cytomegalovirus.[4,5,6]
Clinical presentation may be vague ranging from completely asymptomatic and generalized malaise to hemodynamic compromise such as heart failure or even mimic a myocardial infarction, while sudden cardiac death that may occur in up to 8.6% of cases.[7,8,9]
Electrocardiographic and echocardiographic investigation, together with peripheral blood biomarkers analysis such as cardiac troponin and CK-MB represent the first-line diagnostic approach of acute myocarditis, but they lack sufficient accuracy.[10,11,12,13] Endomyocardial biopsy (EMB) traditionally remains the reference standard for acute myocarditis diagnosis but is an invasive procedure; therefore, it is considered in critically ill patients and carries the potential of a false-negative sampling error.[14,15]
Cardiac magnetic resonance (CMR) imaging has become an important and noninvasive diagnostic tool for the diagnosis of acute myocarditis by revealing areas of hyperemia, edema, and myocardial necrosis/fibrosis.[16,17,18] The important role of follow-up CMR in monitoring the evolution of the inflamed myocardium in patients with myocarditis has been underlined in various studies.[19,20,21,22,23,24]
The purpose of the current prospective study is to investigate CMR and laboratory findings in patients with clinically suspected acute myocarditis and reassess the evolution of findings in relation to smoking habits, clinical and laboratory parameters.
METHODS
Study design and patient selection
Between November 2015 and August 2018, we conducted a longitudinal prospective study enrolling consecutive patients presenting with suspected acute myocarditis. The vast majority of patients (85%) presented with a history of a recent viral infection within the preceding 2 weeks of the upper or lower respiratory tract (i.e., sore throat, fever, cough, and chest discomfort) or gastrointestinal tract (i.e., abdominal pain, diarrhea, and fever).
Patients were clinically examined and underwent chest X-ray, electrocardiography (ECG), echocardiography, and laboratory analysis including cardiac troponin I (cTnI), erythrocytes sedimentation rate (ESR), C-reactive protein (CRP). Serological tests focused on coxsackievirus B, Epstein–Barr virus, cytomegalovirus, influenza virus, HHV6, and parvovirus B 19.
In case two or more of the following diagnostic criteria consistent with acute myocarditis existed, further imaging investigation with CMR was performed: (a) at least one of the following symptoms: recent onset of chest discomfort, dyspnea at rest or under exercise, palpitations or/and fatigue; (b) ECG changes or arrhythmias such as ST-T abnormalities, conduction defects, abnormal Q waves, ventricular tachycardia, frequent ventricular premature beats, atrial fibrillation; (c) new-onset or otherwise unexplained at least mild regional or global impairment of left ventricular function as assessed by echocardiography; and (d) peripheral blood elevated cardiac troponin levels. In addition, patients with persisting ST-segment elevation >1 mm on ECG in at least two contiguous derivations, with increased serum levels of cardiac troponin underwent coronary angiography. Those with significant coronary artery stenosis were excluded from the study.
Patients with (a) preexisting congenital or acquired cardiovascular disease or (b) previously known renal failure, oncologic or other terminal stage illness, or (c) known contraindication to CMR were also excluded from the present study. Only those patients with CMR findings suggestive of myocarditis were included in the present follow-up study and re-evaluated by CMR and laboratory tests 1 year later.
The study was approved by the Local Institution Ethics Committee according to the principles of the Helsinki Declaration, and all patients who participated gave written consent.
Cardiac magnetic resonance protocol and image analysis
The CMR study was performed 3–7 days after patient admission to the hospital, using a 1.5 T system (Signa CV/i, GE Medical Systems, Milwaukee, Wisconsin, USA) with a 5-element cardiac phased-array coil.
Cine steady-state free precession sequences were performed in two-, three-, four-chamber, and short-axis view. Images were prescribed every 10 mm from base to apex (TR 3.5 ms, TE 1.7 ms, FA 55°, slice thickness 8 mm, and in-plane resolution 1.2 mm × 1.8 mm). Epicardial and endocardial contours were manually outlined on the short-axis cine images for calculating the left ventricle ejection fraction (LVEF), the left ventricle end-systolic, and left ventricle end-diastolic volume.
Triple inversion recovery black blood turbo spin echo pulse sequences (short-tau inversion-recovery [STIR]) were acquired for the detection of myocardial edema in apical, mid-cavity, and basal short-axis levels (sequence parameters: inversion time (TI) 180 ms, TSE factor 32, TR 2 RR intervals, and TE 100 ms). Endocardial and epicardial contours were manually drawn, and global edema was defined by a T2 signal intensity ratio (T2 ratio) of LV myocardium over skeletal muscle calculated in the same slice of at least 2.0. Regional edema was assessed by a semi-automatic computerized analysis of myocardial SI with color-coded map, normalized to SI of a nearby skeletal muscle. A region within myocardium of at least 10 conjoint pixels with a signal intensity ratio of at least 2.0 was considered positive for regional edema.[16,17,18,19,20]
T1-w black-blood fast spin-echo images were acquired in 3 identical slices before and within the first 3 min after intravenous (IV) administration of 0.2 mmol/kg of Gadobutrol Gd-CA (Gadovist®, Bayer-Schering Pharma, Berlin, Germany) in short axis view for detecting areas of early gadolinium enhancement (EGE) corresponding to hyperemia and capillary leak (sequence parameters: TR 252 ms, TE 26 ms, FOV 292 × 360 mm, matrix size 208 × 256, slice thickness 15 mm). For the EGE assessment, endocardial and epicardial contours were delineated before contrast administration, and the same contours were copied onto images acquired during the first 3 min after contrast injection. EGE ratio (EGEr) was calculated by dividing the enhancement of myocardium to the enhancement of the skeletal muscle. A ratio of at least 4.0 was linked to increased EGE uptake.[16,17,18,19,20]
Late gadolinium enhancement images (LGE) were acquired 8–15 min after IV Gd-CA administration using a typical phase-sensitive inversion-recovery gradient-echo sequence (sequence parameters: TR 4.7 ms, TE 2.0 ms, FA 15°, 2 signal averages) continuously adapting the TI values for the best nulling of normal myocardium and images were acquired in standardized apical, mid-cavity, and basal short-axis levels covering all AHA 17-segment model.
LGE images were analyzed after manually drawing the endocardial and epicardial contours on short-axis view. LGE areas were quantified using a signal intensity threshold of >3.0 standard deviations (SDs) above the mean signal intensity of normal myocardium while their distribution was visually classified as subepicardial, subendocardial, endocardial, or transmural. Both the presence and distribution of LGE areas were further confirmed on long-axis views. The spatial extent of LGE areas was estimated using a visual scoring method based on the standard 17-segment model of the left ventricle.[21]
The percentage of LGE for each cardiac segment was visually evaluated and scored as 0 (no enhancement), 1 (0%–25%), 2 (26%–50% enhancement), 3 (51%–75% enhancement), or 4 (76%–100% enhancement). The resulting global extent of LGE (LGE score) was calculated by the sum of the degrees attributed to each segment. LGE score was expressed as a percentage of the total maximum score (4 × 17 = 68) using the following formula: 100× (LGE score)/68 to calculate the LGE volume of the left ventricle.[17,18,19,20,25,26]
The presence of concomitant pericardial effusion was also recorded. CMR scan was indicative of active myocarditis when at least two out of three Lake Louise criteria (LLC) were positive.[17,18,19,20]
The same imaging protocol was performed in all patients, at baseline study and 12-month follow-up, and images were postprocessed using Signa® CV/i™ analysis software package (Signa® CV/i™; General Electric Medical Systems, USA) and evaluated by consensus by two experienced physicians (E. D and M. R) of at least 6 years of expertise in CMR, blinded to nonimaging data.
Smoking habits, clinical, laboratory, and smoking investigation
Depending on cigarette smoking history, patients were divided into smokers and nonsmokers. Smoking patients were further subdivided into light (<10 pack-years), and heavy smokers (>10 pack-years). Clinical charts and ECG findings were recorded for all patients fulfilling the inclusion criteria both at baseline and at 12 months. The laboratory investigation was focused on serum levels of cTnI, ESR, CRP, as well as serological tests including the detection of coxsackievirus B, Epstein–Barr and influenza virus, cytomegalovirus, HHV6, and parvovirus B19 antibodies.
Statistical analysis
The Kolmogorov–Smirnov test was used to determine whether the data obtained follow a normal distribution pattern. Group comparisons were made by analysis of variance, Student's t-test, Wilcoxon rank-sum test, or Chi-square testing as appropriate. Regression analysis was performed with nonparametric measure of rank correlation, using the Spearman's rank correlation coefficient. A P < 0.05 was considered statistically significant. Statistical calculations were performed on SPSS 20 statistical package (SPSS, Chicago, IL, USA).
RESULTS
Population characteristics
Overall, 82 patients were initially recruited to participate. Fourteen of them were excluded (6 due to claustrophobia, 2 due to orthopedic metallic implants at the level of the thoracic spine, and 6 due to inability to comply with breath-hold requirements). Thus, 68 patients were finally included and were mostly men (n = 64, 94%) with a median age of 25 (15–56) years. Patients were divided into two different groups with regard to smoking habits, i.e., smokers and nonsmokers. Thirty-eight patients (56%) were smokers, among them, 17 were light and 22 were heavy smokers. The patient's characteristics are given in Table 1.
Table 1.
Study of population characteristics
| Baseline | 12 month-follow-up | |
|---|---|---|
| Number of patients | 68 | 51 |
| Age (years) | 24.94±7.68 | 25.92±8.45 |
| Sex | ||
| Men | 64 | 51 |
| Women | 4 | |
| Smoking habits, n (%) | ||
| Nonsmokers | 30 (44) | 14 (27) |
| Light smokers <10 pack-years | 16 (24) | 15 (30) |
| Heavy smokers >10 pack-years | 22 (32) | 22 (43) |
Baseline study results
Clinical, laboratory, and electrocardiography
During the acute phase, patients presented with malaise (96%), dyspnea (70%), chest pain, and discomfort (57%) [Table 2]. Most patients (85%) reported a viral infection of the upper respiratory tract (generalized malaise and prodromal symptoms, sore throat, dry cough, and fever, 80%) or the gastrointestinal tract (acute gastroenteritis with abdominal discomfort, diarrhea, and fever), 5–15 days before the onset of cardiac symptoms. Specific viral antibody titers were isolated in 36 patients (53%), including parvovirus B19 in 31, HHV6 in 3, and coxsackievirus Group B in 2 patients.
Table 2.
Clinical, laboratory, and electrocardiography findings
| Baseline | 12-month follow-up | P | |
|---|---|---|---|
| Symptoms and signs related to recent viral infection, number patients (%) | 58 (85) | - | - |
| Specific serum viral antibodies (for Coxsackie virus, PVB 19, and HHV6) | 36 (53) | Below detection value | - |
| Chest pain/discomfort, number patients (%) | 39 (57) | - | - |
| Malaise number patients (%) | 65 (96) | - | - |
| ECG abnormalities number patients (%) | 65 (96) | Normal findings | - |
| Erythrocytes sedimentation rate, mm/hr | 17.44±12.51 | 2.75±2.01 | <0.0001 |
| C-reactive protein, mg/L | 16.82±13.16 | 0.46±0.71 | <0.0001 |
| Cardiac Troponin I, ng/mL | 2.038±1.334 | 0.003±0.005 | <0.0001 |
ECG: Electrocardiography, PVB: Premature ventricular beat, HHV6: Human herpes virus-6, ESR: Erythrocyte sedimentation rate, CRP: C-reactive protein, cTnI: Cardiac troponin I
ECG work-up revealed ST-segment changes in 65 patients (96%), frequent ventricular premature beats in 13 patients (19.5%), ventricular tachycardia in 4 (6%), and an atrioventricular block in 4 (6%). Within the first 1–2 days of hospital admission, laboratory tests revealed abnormally high serum levels of cTnI (52 patients, 76% with values > 0.10 ng/mL), ESR (36 patients, 53%), and CRP (38 patients, 56%) [Table 2].
Due to persistent elevated serum levels of cTnI and ST-segment changes at ECG, eight patients underwent coronary artery angiography that was negative for severe coronary artery disease (>50% coronary artery stenosis).
Cardiac magnetic resonance findings
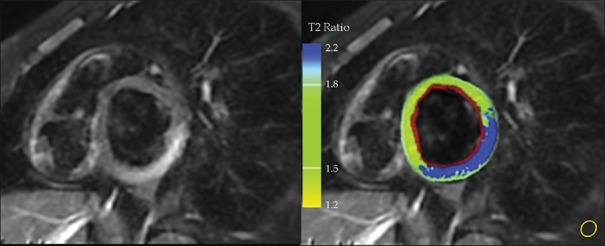
Thirty-eight patients (56%) fulfilled all three LLC for acute myocarditis, 13 patients (19%) presented edema on STIR images and LGE without EGE fulfilling two LLC criteria, while 17 patients (25%) were LLC negative. Fifty-one patients (75%) showed edema on STIR images, among them global edema was found in 6, regional edema in 42, and both regional and global edema in 3 patients. The T2 ratio was 2.6 ± 0.4 (mean value ± SD) [Figure 1].
Figure 1.
Regional myocardial edema assessment in a patient with acute myocarditis. Left, T2w-Short-tau inversion-recovery image with an increased signal intensity region mostly affecting segment 4 segments 5 of the left ventricular wall with a subepicardial to mid-wall distribution. Right, computer-aided signal intensity analysis of the T2-weighted image with color-coded display of relative signal intensity, normalized to skeletal muscle (region of interest, lower right part). Blue color: signal intensity ratio of myocardial/skeletal muscle of >2.0, indicating edema along 4 and 5 segments, green color: normal signal intensity
EGE occurred in 38 patients (56%), and EGEr measured 4.8 ± 2.8 (mean value ± SD). Myocardial LGE was detected in 51 patients (75%), who exhibited the following scores: twenty-nine scored 1 (LGE extent 0%–25%) and 22 scored 2 (LGE extent 26%–50%). The median LGE extent in all 51 patients was 18% (11%–42%) of the total LV mass [Table 3].
Table 3.
Cardiac magnetic resonance findings at baseline and at 12 months’ follow-up
| AHA segments involved 4 and 5 only | P | AHA segments involved 4, 5 ,6 ,7 ,8 ,9 ,10 ,11 ,12* | P | Total | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | ||||
| Number of patients (n) | 39 | 37 | NS | 12 | 12 | NS | 51 | 49 | NS |
| LVEF (%) | 54±8 | 62±6 | 0.0022 | 45±9 | 55±6 | 0.0026 | 45±9 | 62±6 | 0.0026 |
| LVEDV (ml) | 148±16 | 128±22 | 0.0072 | 158±22 | 132±25 | 0.0072 | 158±25 | 132±25 | 0.0072 |
| LVESV (ml) | 39±4 | 34±7 | 0.0045 | 42±6 | 34±5 | 0.0045 | 42±6 | 34±7 | 0.0045 |
| Segmental hypokinesia, n (%) | 39 (57) | - | <0.0001 | - | - | - | 39 (57) | - | <0.0001 |
| Global hypokinesia, n (%) | - | - | - | 12 (18) | 12 (18) | 0.001 | 12 (18) | 12 (18) | NS |
| Visible myocardial oedema, n (%) | 39 (57) | - | <0.0001 | 12 (18) | - | <0.0001 | 51 (75) | - | <0.0001 |
| T2 ratio | 2.4±0.2 | 0.7±0.4 | <0.0001 | 2.6±0.6 | 0.8±0.2 | <0.0001 | 2.6±0.4 | 0.8±0.2 | <0.0001 |
| EGE, n (%) | 32 (47) | - | <0.0001 | 6 (9) | - | <0.0001 | 38 (56) | - | <0.0001 |
| EGEr (range) | 3.9±1.2 | 1.6±1.1 | <0.0001 | 4.2±2.2 | 1.8±1.2 | <0.0001 | 4.2±2.2 | 1.6±1.4 | <0.0001 |
| LGE, n (%) | 42 (62) | 40 (60) | NS | 9 (13) | 9 (13) | NS | 51 (75) | 49 (72) | NS |
| LGE total extent (%) | 16±11 | 15±9 | NS | 18±9 | 17±10 | NS | 18±12 | 17±11 | NS |
| Pericardial effusion, n (%) | 2 (3%) | - | <0.0001 | 7 (10) | 1 (1) | <0.0001 | 9 (13) | 1 (1) | <0.0001 |
*P < 0.05 considered statistically significant. AHA: American Heart Association, LV: Left ventricular, LVEF: LV ejection fraction, LVEDV: LV end-diastolic volume, LVESV: LV end-systolic volume, EGE: Early gadolinium enhancement, EGEr: EGE ratio, LGE: Late gadolinium enhancement, NS: Not significance
Of all 867 segments (17 segments/patient × 68 patients), LGE was noted in 110 segments (13%). Interestingly, all 22 patients belonging to the subgroup of heavy smokers had an LGE extent ranged from 26% to 50% (Score 2).
Edema, EGE, and LGE areas had a subepicardial only, or a subepicardial to mid-wall distribution, with sparing of endocardium and subendocardium in all patients. LV wall basal inferior and basal inferolateral segments (AHA segments 4 and 5) were most commonly affected and always involved simultaneously (r2 =0.1934, r = +0.9060, P = 0.0142) [Figure 2 and Table 3].
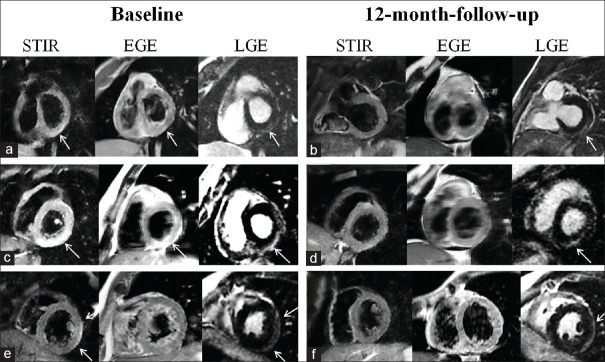
Figure 2.
Cardiac magnetic resonance findings in three different patients, at baseline (a, c and e) and at 12-month follow-up (b, d and f). Short-tau inversion-recovery images in a, c and e patients reveal hyperintense areas of myocardial edema (arrows). Early gadolinium enhancement images in a and c patients, showing early gadolinium enhancement areas (arrows, a, c, and e). At 12-month follow-up, a complete resolution of edema and hyperemia was recorded in all three patients (b, d and f) while areas of late gadolinium enhancement persisted (arrows). Cardiac magnetic resonance findings had a subepicardial to mid-wall distribution. STIR: Short-tau inversion-recovery, EGE: Early gadolinium enhancement, LGE: Late gadolinium enhancement
Of all 68 patients, 54 (80%) showed a preserved LVEF (range between 50% and 54%), 9 patients (13%) a moderate decrease of LVEF (range between 40% and 49%) and five patients (7%) had severe decrease of LVEF (<40%), with concomitant symptoms of NYHA class III heart failure. Segmental hypokinesia was found in 50 (74%) and global hypokinesia in 15 patients (22%). A mild pericardial effusion was detected in 9 patients (13%).
All five patients with severely decreased LVEF underwent EMB, and all were diagnosed with acute myocarditis by immunohistology. Viral genomes regarding coxsackievirus were detected in 2 patients, parvovirus B19 in 1, Epstein–Barr virus in 1 patient, and cytomegalovirus in other 1 patient.
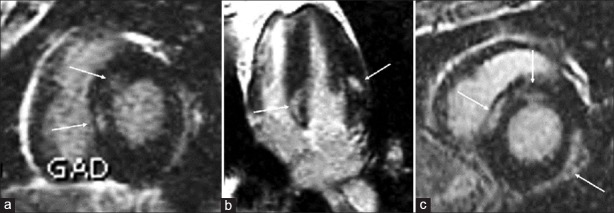
Regarding LGE distribution, basal inferior and basal inferolateral segments were simultaneously involved in all cases where specific serum antibodies for PVB19 were detected [Figure 2]. In three cases with more extended LGE areas, apart from lateral wall segments, also septal and anteroseptal segments were affected, and specific serum antibodies for HHV6 were isolated. All three patients were heavy smokers [Figure 3].
Figure 3.
(a-c) Three cases with more extended areas of late gadolinium enhancement, involving septal and anteroseptal segments together with inferolateral segments, where HHV6 antibodies were detected. All three cases regarded heavy smoking patients
All patients with CMR findings compatible with acute myocarditis received prompt treatment according to the guidelines, based on β-blocker alone or combined with an ACE inhibitor and were clinically and echocardiographically followed-up at 1, 3, 6, and 12 months.[27]
Follow-up
At 12-month re-evaluation serum levels of cTnI, CRP, and ESR returned to normal values, specific viral antibody titers decreased below detection levels, while ECG work-up was normal in all patients [Table 2 and Figure 4].
Figure 4.
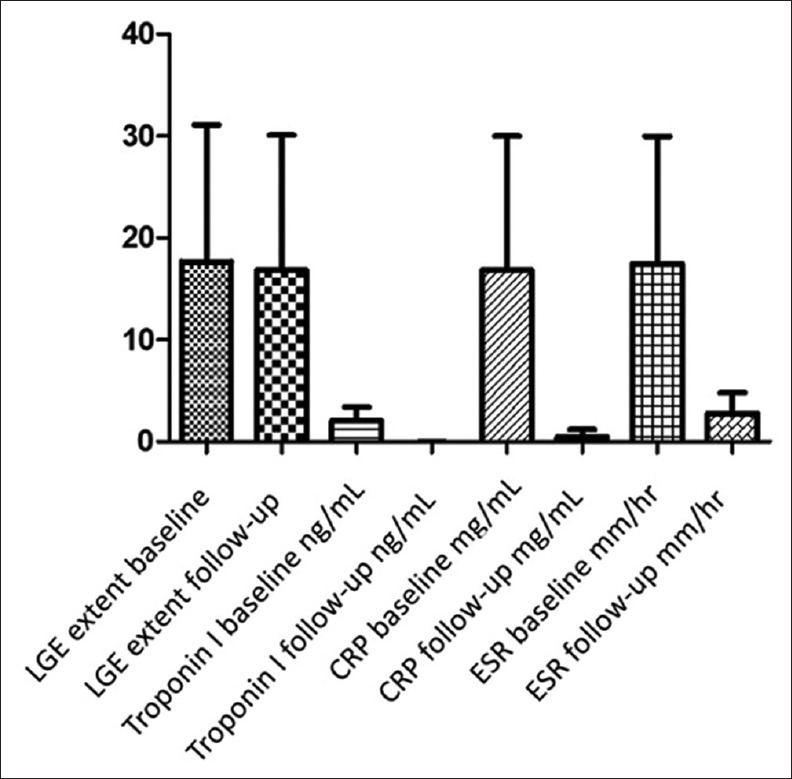
Bar chart indicating late gadolinium enhancement extent, Troponin I, c-reactive protein and erythrocyte sedimentation rate levels at baseline (left bar) and at 12-month (right bar) follow-up study
No deaths or severe cardiovascular events occurred during this 12-month period. Fifty-one patients with suggestive CMR findings of myocarditis at baseline were re-evaluated with CMR. No patient showed a deterioration of LV dysfunction or CMR features of dilated cardiomyopathy. LVEF significantly improved (58 ± 9% vs. 62 ± 6, P = 0.0026) while 46 patients became completely asymptomatic with an LVEF >55%. The remaining five patients were those with initial presentation of NYHA III heart failure and showed clinical and functional improvement with increase of LVEF (45%–55% at 12 months vs. <40% on admission).
All patients had a complete resolution of both regional/global edema on STIR images and EGE with normalization of both T2 ratio (P < 0.001) and EGEr (P < 0.001). Complete resolution of LGE occurred in 2 out of 51 patients, and both of them were no smokers. The remaining 49 patients, among them 37 (96%) were smokers, showed the persistence of LGE with the same distribution as at baseline but with insignificant extent decrease (mean value 18%, vs. 17%, P = 0.037). At both baseline and follow-up studies, AHA segments 4 and 5 were the most affected and were always involved simultaneously (r = +0.3566, P = 0.0451) [Table 3 and Figure 2].
A complete resolution of pericardial effusion was detected in 8 patients and persisted in one patient but of comparatively limited extent.
Statistical correlation between clinical, laboratory, cardiac magnetic resonance findings, and smoking
At baseline study, LGE extent positively correlated with the presence of chest pain (r = +0.755, P < 0.0001), with serum levels of cTnI (r = +0.949, P < 0.0001) and CRP (r = +0.975, P < 0.0001) [Figure 5a]. LGE extent at baseline study was inversely associated with LVEF (r = −0.4571, P = 0.0015).
Figure 5.
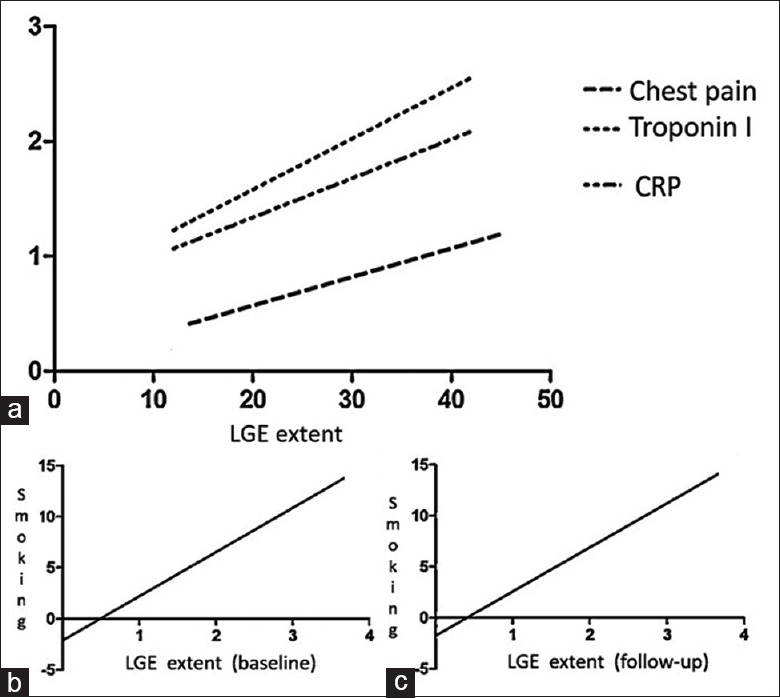
A statistically significant correlation was depicted at baseline study between late gadolinium enhancement extent, presence of chest pain, serum levels of troponin I and c-reactive protein, respectively (a). Both at baseline (b) and follow-up study (c) a significant association was found between heavy smoking and late gadolinium enhancement extent
A significant correlation was found between LGE extent and smoking in heavy smoking patients, both at baseline (r = +0.917, P < 0.0001) and at 12-month follow-up study (r = +0.926, P < 0.0001) [Figure 5b and c].
DISCUSSION
In this prospective study, we evaluated findings of CMR at diagnosis and at 12-month follow-up in patients with acute myocarditis, with regard to smoking habits, clinical and laboratory findings and assessed the longitudinal evolution of myocardial inflammation.
In our series, a significant association was revealed by regression analysis between smoking and LGE extent, both at baseline and follow-up study. Smokers exhibited more extensive multi-segmental LGE compared to nonsmokers. The deleterious cardiovascular effects of tobacco exposure have been previously studied.[28,29] Leone et al. suggest that chronic prolonged exposure to tobacco smoke may lead to myocardial cell necrosis and smoke cardiomyopathy, with hypoxia being the basic underlying mechanism.[28] In another, another study conducted by Bae et al. preexposure to tobacco significantly exacerbated the severity of viral myocarditis, probably by increasing the viral load and cardiomyocyte cell death.[29]
Consequently, our clinical study supports the hypothesis that smokers are more vulnerable to viral myocarditis due to underlying hypoxic smoke cardiomyopathy and potentially increased the viral load.
LGE extent positively correlated with the presence of chest pain, troponin, and CRP serum levels and compromised LVEF during the acute phase. All of our patients that experienced chest discomfort showed more extensive LGE areas (>10%) at first CMR. This probably occurs because the inflammation of the LGE area which corresponds to myocardial injury, is initially linked to increased concentrations of bradykinin, substance P and K+, production of lactate, prostaglandins, and finally cytokines, notably Interleukin 1 and tumor necrosis factor that probably contribute to the activation of nociceptive fibers, which are related to chest pain.[30] Patients with more extensive LGE (>10%) had more elevated troponin and CRP serum levels during the acute phase of the disease. These associations may be related to myocardial inflammatory response.[25,31,32,33]
Patients with more extensive LGE in the acute phase of myocarditis showed a more compromised LVEF (<50%), and this negative correlation proved to be statistically significant, as Tani et al. have also demonstrated.[34] Consequently, it appears that the degree of myocardial damage, quantified by CMR, paralleled the severity of clinical and laboratory findings.[25,27]
The most commonly involved LV wall segments in myocarditis, are those of the free lateral wall and those of the interventricular septum.[17,35] In our study, the most frequently involved myocardial segment was the basal inferior segment which was always involved together with the basal inferolateral segment. In the few cases where specific serum antibodies for PVB19 were identified the above two segments were always involved, while in contrast, in the few multifocal cases where anteroseptal segments were involved, specific serum antibodies for HHV6 were isolated.
These predilections of PVB19 infection for the LV lateral free wall and of HHV6 myocarditis for interventricular septum segments have been previously reported.[21,36,37] On the other hand, LGE involving the midwall of the anteroseptal segments has been associated with a worse prognosis and major adverse cardiovascular events which did not occur in our study.[37,38]
At 12-month follow-up, a complete resolution of myocardial edema and hyperemia was recorded with normalization of T2 ratio and EGEr, respectively. Normalization of the above CMR parameters together with normalization of inflammatory indexes including ESR and CRP were associated with LVEF improvement, LV function recovery, and a good prognosis. Consequently, edema and EGE may represent predictors of improvement of LV systolic function.[16,20,21,22]
In available follow-up, CMR studies of myocarditis in the literature, a decrease in the extent or complete resolution of LGE areas is recorded over several weeks or months, due to resolution of edema, scar contraction, and myocardium remodeling.[5,19,39] However, LGE areas may persist over time and this finding may be related to chronic myocardial inflammation and/or reinfection that may trigger dilated cardiomyopathy, heart failure, cardiac arrhythmias, or even sudden cardiac death.[23,40,41]
In our study, despite the high prevalence of LGE at follow-up and septal and anteroseptal segment involvement which traditionally have worse prognosis, none of our patients developed any major adverse cardiovascular event, dilated cardiomyopathy or deterioration of cardiac function 1 year later.[37] Consequently, LGE in our series may represent fibrotic tissue development, known for its late enhancement.
Limitations
Limitations of this study include the relatively small number of patients and lack of application of myocardial T1 and T2 mapping with the potential of subsequent analysis of T1 and T2 relaxation times.[42] However, this application would probably not affect the statistical significance of our results. Complete resolution of myocardial edema at follow-up and the concomitant LV functional improvement, suggests that no residual edema existed at follow-up, reducing the need for T2 maps and relaxation times.
Histological analysis through EMB would possibly provide us with more information on the persistence of LGE areas, but since most of our patients had no severe cardiac function compromise biopsy was not indicated. Despite normalization of peripheral blood biomarkers, we feel that a longer follow-up, of at least 2 years with individualized clinical, echocardiographic, and CMR studies might prove useful to all those patients, especially smokers with persisting LGE areas at 1-year follow-up, to confirm the fibrotic nature of persisting LGE areas and exclude latent inflammation.
CONCLUSIONS
Heavy smokers with myocarditis appear to have more extended LGE areas in CMR compared to nonsmoking patients. At baseline, LGE extent paralleled cTnI and CRP serum levels. In contrast to other studies, the persistence of LGE at follow-up did not appear to have an impact on the patient's clinical status or cardiac function. Early diagnosis of myocarditis by CMR and prompt initiation of treatment seems to reduce the likelihood of major adverse cardiovascular events.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 2.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ., Jr Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 3.Klingel K, Sauter M, Bock CT, Szalay G, Schnorr JJ, Kandolf R. Molecular pathology of inflammatory cardiomyopathy. Med Microbiol Immunol. 2004;193:101–7. doi: 10.1007/s00430-003-0190-1. [DOI] [PubMed] [Google Scholar]
- 4.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–55. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 5.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–90. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 6.Caforio AL, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–33. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 7.Dec GW, Waldman H, Southern J, Fallon JT, Hutter AM, Jr, Palacios I. Viral myocarditis mimicking acute myocardial infarction. J Am Coll Cardiol. 1992;20:85–9. doi: 10.1016/0735-1097(92)90141-9. [DOI] [PubMed] [Google Scholar]
- 8.Fabre A, Sheppard MN. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart. 2006;92:316–20. doi: 10.1136/hrt.2004.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hufnagel G, Pankuweit S, Richter A, Schönian U, Maisch B. The European study of epidemiology and treatment of cardiac inflammatory diseases. First epidemiological results. Herz. 2000;25:279–85. doi: 10.1007/s000590050021. [DOI] [PubMed] [Google Scholar]
- 10.Schultz JC, Hilliard AA, Cooper LT, Jr, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. 2009;84:1001–9. doi: 10.1016/S0025-6196(11)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanguineti F, Garot P, Mana M, O'h-Ici D, Hovasse T, Unterseeh T, et al. Cardiovascular magnetic resonance predictors of clinical outcome in patients with suspected acute myocarditis. J Cardiovasc Magn Reson. 2015;17:78. doi: 10.1186/s12968-015-0185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–32. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 13.Lauer B, Niederau C, Kühl U, Schannwell M, Pauschinger M, Strauer BE, et al. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol. 1997;30:1354–9. doi: 10.1016/s0735-1097(97)00317-3. [DOI] [PubMed] [Google Scholar]
- 14.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–33. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 15.Spartalis M, Tzatzaki E, Spartalis E, Damaskos C, Mavrogeni S, Voudris V. Parvovirus B19 myocarditis of fulminant evolution. Cardiol Res. 2017;8:172–5. doi: 10.14740/cr580w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermes E, Childs H, Faris P, Friedrich MG. Predictive value of CMR criteria for LV functional improvement in patients with acute myocarditis. Eur Heart J Cardiovasc Imaging. 2014;15:1140–4. doi: 10.1093/ehjci/jeu099. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: A JACC white paper. J Am Coll Cardiol. 2009;53:1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6:833–9. doi: 10.1161/CIRCIMAGING.113.000416. [DOI] [PubMed] [Google Scholar]
- 19.Stensaeth KH, Hoffmann P, Fossum E, Mangschau A, Sandvik L, Klow NE. Cardiac magnetic resonance visualizes acute and chronic myocardial injuries in myocarditis. Int J Cardiovasc Imaging. 2012;28:327–35. doi: 10.1007/s10554-011-9812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luetkens JA, Homsi R, Dabir D, Kuetting DL, Marx C, Doerner J, et al. Comprehensive cardiac magnetic resonance for short-term follow-up in acute myocarditis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003603. pii: e003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavrogeni S, Spargias C, Bratis C, Kolovou G, Markussis V, Papadopoulou E, et al. Myocarditis as a precipitating factor for heart failure: Evaluation and 1-year follow-up using cardiovascular magnetic resonance and endomyocardial biopsy. Eur J Heart Fail. 2011;13:830–7. doi: 10.1093/eurjhf/hfr052. [DOI] [PubMed] [Google Scholar]
- 22.Wagner A, Schulz-Menger J, Dietz R, Friedrich MG. Long-term follow-up of patients paragraph sign with acute myocarditis by magnetic paragraph sign resonance imaging. MAGMA. 2003;16:17–20. doi: 10.1007/s10334-003-0007-7. [DOI] [PubMed] [Google Scholar]
- 23.Bustos García de Castro A, Cabeza Martínez B, Ferreirós Domínguez J, García Villafañe C, Fernández-Golfín C. Myocarditis: Magnetic resonance imaging diagnosis and follow-up. Radiologia. 2013;55:294–304. doi: 10.1016/j.rx.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Bohnen S, Radunski UK, Lund GK, Ojeda F, Looft Y, Senel M, et al. Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation in healing myocarditis. Eur Heart J Cardiovasc Imaging. 2017;18:744–51. doi: 10.1093/ehjci/jex007. [DOI] [PubMed] [Google Scholar]
- 25.Goitein O, Sabag A, Koperstein R, Hamdan A, Di Segni E, Konen E, et al. Role of C reactive protein in evaluating the extent of myocardial inflammation in acute myocarditis. J Cardiovasc Magn Reson. 2015;17(Suppl 1):P291. [Google Scholar]
- 26.Satoh H, Sano M, Suwa K, Saitoh T, Nobuhara M, Saotome M, et al. Distribution of late gadolinium enhancement in various types of cardiomyopathies: Significance in differential diagnosis, clinical features and prognosis. World J Cardiol. 2014;6:585–601. doi: 10.4330/wjc.v6.i7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mewton N, Dernis A, Bresson D, Zouaghi O, Croisille P, Flocard E, et al. Myocardial biomarkers and delayed enhanced cardiac magnetic resonance relationship in clinically suspected myocarditis and insight on clinical outcome. J Cardiovasc Med (Hagerstown) 2015;16:696–703. doi: 10.2459/JCM.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 28.Bae S, Ke Q, Koh YY, Lee W, Choi JH, Kang PM, et al. Exacerbation of acute viral myocarditis by tobacco smoke is associated with increased viral load and cardiac apoptosis. Can J Physiol Pharmacol. 2010;88:568–75. doi: 10.1139/y10-003. [DOI] [PubMed] [Google Scholar]
- 29.Leone A, Landini L, Jr, Biadi O, Balbarini A. Smoking and cardiovascular system: Cellular features of the damage. Curr Pharm Des. 2008;14:1771–7. doi: 10.2174/138161208784746699. [DOI] [PubMed] [Google Scholar]
- 30.Zareifopoulos N. Is there such as a thing as non-ischaemic cardiac pain? Heart Asia. 2016;8:54–5. doi: 10.1136/heartasia-2016-010820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lydell CP, Vermes E, Childs HC, Carbone I, Aljizeeri A, Friedrich M, et al. Relationship of troponin T to cardiac MRI criteria for acute myocarditis. J Cardiovasc Magn Reson. 2011;13(Suppl 1):P271. [Google Scholar]
- 32.Schäufele TG, Rösch S, Wenzelburger IK, Sechtem UP, Yilmaz A. Diagnostic capability of CMR for the diagnosis of acute myocarditis in young patients is determined by the presence of elevated cardiac enzymes. J Cardiovasc Magn Reson. 2012;14(Suppl 1):P183. [Google Scholar]
- 33.Florian A, Schäufele T, Ludwig A, Rösch S, Wenzelburger I, Yildiz H, et al. Diagnostic value of CMR in young patients with clinically suspected acute myocarditis is determined by cardiac enzymes. Clin Res Cardiol. 2015;104:154–63. doi: 10.1007/s00392-014-0770-7. [DOI] [PubMed] [Google Scholar]
- 34.Tani H, Amano Y, Tachi M, Machida T, Mizuno K, Kumita S, et al. T2-weighted and delayed enhancement MRI of eosinophilic myocarditis: Relationship with clinical phases and global cardiac function. Jpn J Radiol. 2012;30:824–31. doi: 10.1007/s11604-012-0130-3. [DOI] [PubMed] [Google Scholar]
- 35.Camastra GS, Cacciotti L, Marconi F, Sbarbati S, Pironi B, Ansalone G. Late enhancement detected by cardiac magnetic resonance imaging in acute myocarditis mimicking acute myocardial infarction: Location patterns and lack of correlation with systolic function. J Cardiovasc Med (Hagerstown) 2007;8:1029–33. doi: 10.2459/JCM.0b013e3281053a83. [DOI] [PubMed] [Google Scholar]
- 36.Olimulder MA, van Es J, Galjee MA. The importance of cardiac MRI as a diagnostic tool in viral myocarditis-induced cardiomyopathy. Neth Heart J. 2009;17:481–6. doi: 10.1007/BF03086308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol. 2017;70:1977–87. doi: 10.1016/j.jacc.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 38.Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70:1964–76. doi: 10.1016/j.jacc.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Cobelli F, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R, et al. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol. 2006;47:1649–54. doi: 10.1016/j.jacc.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 40.Lurz P, Eitel I, Adam J, Steiner J, Grothoff M, Desch S, et al. Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc Imaging. 2012;5:513–24. doi: 10.1016/j.jcmg.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–48. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]
- 42.Pan JA, Lee YJ, Salerno M. Diagnostic performance of extracellular volume, native T1, and T2 mapping versus lake Louise criteria by cardiac magnetic resonance for detection of acute myocarditis: A meta-analysis. Circ Cardiovasc Imaging. 2018;11:e007598. doi: 10.1161/CIRCIMAGING.118.007598. [DOI] [PMC free article] [PubMed] [Google Scholar]