Abstract
Background/Objectives
The incidence of myeloma in older adults is increasing, yet little is known about geriatric impairments in these patients. We aimed to examine the prevalence of geriatric impairments in older adults with myeloma and association between geriatric assessment and autologous stem cell transplant eligibility.
Design
Prospective cohort study
Setting
Two academic medical centers
Participants
40 adults aged ≥65 years with newly diagnosed myeloma were enrolled.
Measurement
Participants completed a primarily self-administered geriatric assessment, including measures of functional status, comorbidities, polypharmacy, psychosocial status, social support, quality-of-life, cognition and physical performance. Outcomes were autologous stem cell transplant eligibility and receipt.
Results
Forty patients enrolled; mean age was 71. Geriatric impairments were common: 62% reported dependence in ≥1 instrumental activities of daily living (IADL), 76.9% had polypharmacy (≥4 medications), and 47.5% had ≥1 comorbidities. Median time on the Timed Up and Go was 13.3 ± 4.9 seconds. Those considered candidates for autologous stem cell transplant (N= 26) were younger, with fewer comorbidities, better performance status, and faster performance on the Timed Up and Go test. Factors independently associated with receiving autologous stem cell transplant (N=21) included age and IADL dependence.
Conclusion
Impairments in geriatric domains are common in this population, even among those considered to have a good performance status. Geriatric assessment domains are associated with both transplant eligibility and receipt.
Keywords: geriatric assessment, multiple myeloma, elderly, aged, hematopoietic stem cell transplantation
Introduction
With the aging of the population, within fifteen years, nearly 3 of every 4 patients diagnosed with myeloma will be aged 65–84.1 While advances in treatment over the past 2 decades have improved survival for patients with myeloma, these advances have disproportionately benefited younger patients.2 Autologous stem cell transplant (ASCT) is an aggressive myeloma treatment which is feasible in selected older patients and lengthens survival.3 Treatment algorithms for myeloma diverge based on the patient’s ASCT-eligibility, yet there is no established definition for ASCT-eligibility.
Geriatric assessment encompasses the complex and heterogeneous health of older adults, comprising measures of function, comorbidity, polypharmacy, depression and other geriatric syndromes.4 In general older cancer populations, geriatric assessment is predictive of chemotherapy toxicity5,6 and prognostic of survival.7 In myeloma, comorbidities and functional dependence are prognostic of survival, 8 but to date, other geriatric domains, including cognition, psychosocial status, and geriatric syndromes, have not been examined.
In this study, we examined the prevalence of geriatric impairments and identified associations between geriatric assessment and ASCT-eligibility, as well as whether the patient ultimately underwent this intensive treatment.
Methods
This pilot prospective cohort study was approved by the Human Subjects Committees at Washington University and Duke University. Patients aged ≥65 with newly diagnosed myeloma were enrolled within 3 months of diagnosis from two tertiary care institutions. Because of referral patterns to academic medical centers and the sometimes-urgent need to initiate therapy for myeloma, participants who had started antimyeloma therapy were eligible. Patients were excluded if they had a life expectancy <6 months, were not returning to the participating institutions for ongoing care or had concomitant amyloidosis.
Participants completed the Cancer and Aging Research Group Geriatric Assessment Tool,5 including functional status, comorbidities, polypharmacy, psychosocial status, social support, cognition, and physical performance. Self-administered measures included the Lawton instrumental activities of daily living (IADLs)9, Medical Outcomes Survey – Physical health,10 the Mental Health Inventory-17,10 and items on falls, weight loss, medications, and sensory impairments. A research coordinator administered the Timed Up and Go11 and Blessed Orientation-Memory-Concentration test.12 Laboratory data, International Staging System stage,13 cytogenetics, Charlson Comorbidity Index14 and clinician-rated Karnofsky Performance Status (KPS)15 were also documented.
Aside from Centers for Medicare & Medicaid Services guidance that individuals undergoing ASCT should have responsive myeloma and “adequate cardiac, renal, pulmonary & hepatic function,” there are no objective, universally applied criteria for transplant eligibility; ASCT-eligibility is at the discretion of the myeloma physician, incorporating their clinical gestalt of the risk of this intensive treatment approach. At enrollment, the treating hematologist/oncologist, blinded to the results of the geriatric assessment, was asked whether, based on their clinical assessment, the patient was ASCT-eligible.
Data were summarized using descriptive statistics as appropriate. Frequencies in unordered categorical variables were compared using Fisher’s Exact test, ordinal scales with an ordinal test for trend (Jonckheere’s test) and fully continuous but non-Gaussian variables with a Kruskal-Wallis test.
Single and multivariate logistic regression was performed to examine factors associated with whether the patient underwent ASCT and estimate the odds of undergoing ASCT with 95% confidence intervals. Variable selection was based on partial non-collinearity, information measures (e.g., Akaike’s information criterion) and, for comparison of nested models, likelihood ratio tests. P-values <0.05 were considered significant. Analyses were performed with SAS 9.4/SAS/STAT 13.2.
Results
Forty patients enrolled between 2012 and 2015. Twenty-three patients (57.5%) had started myeloma therapy at enrollment, and 17 (42.5%) had not. The median number of days between diagnosis of myeloma and assessment was 24.0 ± (standard deviation) 25.5. Among those who had not started treatment, the median time from diagnosis to enrollment was 10.0 ± 11.8 days. Among those who had started treatment, the median time from diagnosis to assessment and from initiation of treatment to assessment was 34.0 ± 25.2 days and 25.0 ± 23.7 days, respectively. Data on the exact number of cycles of treatment are not available, but 25 days would be about 1 cycle of therapy (a typical cycle length is 21 or 28 days).
Baseline characteristics are described in Table 1. Geriatric assessment revealed a high prevalence of functional limitations and other geriatric impairments (Table 2). Almost two-thirds of the participants (62.5%) required assistance in ≥1 IADLs. Over one-fourth (28.2%) reported one or more falls in the prior 6 months. Polypharmacy was present in almost all patients, with 92.5% taking ≥4 medications. No standard cut-points are published for the MHI-17, but to provide context, 7.5% scored 2 standard deviations below the mean score for euthymic individuals.16 The only geriatric domain that differed between patients who had versus had not started myeloma therapy was greater number of medications among patients who had started therapy (Supplementary Table 1).
Table 1.
Baseline characteristics of cohort of older patients with myeloma within 3-months of diagnosis
| Variable | Entire cohort (N=40) | Transplant ineligible (N=14) | Transplant eligible (N=26) |
|---|---|---|---|
| Age (Mean ± standard deviation) | 71.1 ± 5.1 | 74.0 ± 6.0 | 69.6 ± 3.8 |
| Male gender, No. (%) | 25 (62.5%) | 7/14 (50%) | 18/26 (69.2%) |
| Race, No. (%) | |||
| White | 31 (77.5%) | 11/14 (78.6%) | 20/26 (77.5%) |
| Black | 5 (12.5%) | 2/14 (14.3%) | 3/26 (11.5%) |
| Other | 4 (10%) | 1/14 (7.1%) | 3/26 (11.5%) |
| Marital status: married, No. (%) | 30 (75%) | 12/14 (85.7%) | 18/26 (69.2%) |
| International Staging System Stage, No. (%) * | |||
| Stage I | 11/35 (31.4%) | 3/13 (23.1%) | 8/22 (36.4%) |
| Stage II | 13/35 (37.1%) | 5/13 (38.5%) | 8/22 (36.4%) |
| Stage III | 11/35 (31.4%) | 5/13 (38.5%) | 6/22 (27.3%) |
| Chromosomal abnormalities, No. (%)* | |||
| T4;14 | 2/30 (6.7%) | 0/9 (0%) | 2/21 (9.5%) |
| T11;14 | 6/29 (20.7%) | 1/8 (12.5%) | 5/21 (23.8%) |
| Deletion 13 by FISH | 21/31 (67.7%) | 6/9 (66.7%) | 15/22 (68.2%) |
| Deletion 17p | 8/31 (25.8%) | 3/8 (37.5%) | 5/23 (21.7%) |
Denominator reflects staging/FISH not performed
Table 2.
Geriatric assessment measures in older adults with myeloma within 3-months of diagnosis (N=40)
| Variable | Entire cohort | Transplant ineligible (N=14) | Transplant eligible (N=26) | P value |
|---|---|---|---|---|
| Functional status | ||||
| Any IADL dependence, No. (%) | 25 (62.5%) | 10/14 (71.4%) | 15/26 (57.7%) | 0.50 |
| MOS: Patient limited a lot in vigorous activities, No. (%) | 27 (67.5%) | 11/14 (78.6%) | 16/26 (61.5%) | 0.44 |
| Karnofsky Performance Status (clinician-rated) (mean ± standard deviation) | 77.0 ± 13.2 | 70.7 ± 14.9 | 80.4 ± 11.1 | 0.026 |
| Report of one or more falls in prior 6 months, No. (%) | 11/39 (28.2%) | 4/14 (28.6%) | 7/25 (28.0%) | 1.00 |
| Timed Up and Go time (mean seconds ± standard deviation) | 13.3 ± 4.9 | 15.8 sec ± 6.1 | 11.9 ± 3.4 | 0.013 |
| Number of Medications (mean ± standard deviation) | 9.8 ± 5.6 | 10.2 ± 6.3 | 9.6 ± 5.3 | 0.75 |
| Charlson comorbidity index (mean ± standard deviation) | 1.0 ± 1.6 | 1.9± 2.1 | 0.6± 1.0 | 0.0065 |
| Cognition: Short Blessed Test score (mean ± standard deviation) | 4.2 ± 5.2 | 5.0 ± 6.2 | 3.7 ± 4.6 | 0.47 |
| Psychological status: Mental Health Inventory-17 score (mean ± standard deviation) | 73.2 ± 15.6 | 67.4 ± 19.6 | 76.2 ± 12.4 | 0.10 |
| Sensory impairments | ||||
| Self-reported vision: fair or poor, No. (%) | 12/40 (30%) | 5/14 (35.7%) | 7/26 (26.9%) | 0.41 |
| Self-reported hearing: fair or poor, No. (%) | 12/40 (30%) | 5/14 (35.7%) | 7/26 (26.9%) | 0.41 |
| Self-reported weight loss, No. (%) | 22/40 (55.0%) | 7/14 (50.0%) | 15/26 (57.7%) | 0.64 |
IADL, instrumental activity of daily living; MOS, Medical Outcomes Study
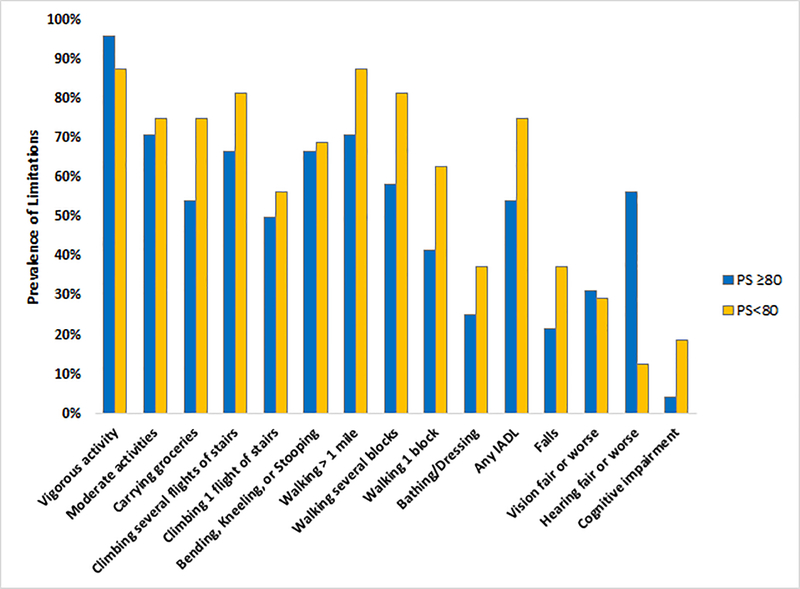
Even among those with a clinician-rated Karnofsky performance status ≥80, geriatric impairments were common. Prevalent limitations included limitation in vigorous activities (95.9%), in moderate activities (70.8%), in one or more IADLs (54.2%), in bathing or dressing (25%) and ≥1 falls (21.7%). Figure 1 demonstrates the frequency of limitations in additional domains, stratified by Karnofsky performance status.
Figure 1.
Prevalence of functional limitations in older adults with myeloma
IADLs, instrumental activities of daily living; PS, Karnofsky performance status
Almost 2/3 of the cohort (26/40, 65%) were deemed ASCT-candidates by their myeloma physician, who was blinded to the results of the geriatric assessment. Aside from age, demographics and disease-characteristics were similar between those who were ASCT-eligible and ineligible. ASCT-eligible and ineligible patients differed in their clinician-rated Karnofsky Performance Status, number of comorbidities and the Timed Up and Go test (Table 2). Of the 26 ASCT-candidates, 21 did undergo ASCT. Reasons for not proceeding to up-front ASCT included progression (N=1), failed mobilization (N=1), patient preference (N=2) and unknown (N=1). Factors associated with receipt of ASCT are detailed in Supplementary Table 2.
Discussion
In this pilot prospective study, we demonstrated that 1) impairments in geriatric domains are highly prevalent in older adults with myeloma (both ASCT-eligible and ineligible, and those with KPS ≥ 80), and 2) geriatric assessment domains were associated with ASCT-eligibility and with the participant undergoing ASCT.
We demonstrated a high prevalence of geriatric impairments. Almost 2/3 (62.5%) of patients required assistance with one or more of their IADLs, which is slightly higher than the 40–50% noted in other studies of older adults with hematologic malignancies.17 IADL dependence is predictive of adverse outcomes in older adults with cancer, including chemotherapy toxicity, functional decline, and falls.6,18 Over one-quarter (28%) of the patients in our cohort reported one or more falls in the prior 6 months. This was remarkably similar to the fall-rate in over 400 patients with newly diagnosed myeloma (26%), which was higher than matched controls, suggesting that people with newly diagnosed myeloma are at increased risk for falls.19 Polypharmacy was also extremely prevalent in our cohort with the median number of medications being 9; polypharmacy in older adults with cancer is associated with increased risk for use of potentially inappropriate medications, falls, and drug interactions. Patients who completed the geriatric assessment after initiation of myeloma therapy tended to be taking more medications, likely reflecting new prescriptions for supportive care.
Even among those considered to have a “good” performance status (Karnofsky performance status ≥ 80), geriatric impairments were quite prevalent in our cohort. The ability of geriatric assessment to detect limitations in patients with good performance status has been shown in patients with acute myeloid leukemia,20 patients undergoing allogeneic stem cell transplantation,21 and solid tumors.22,23 The fact that many of these factors have prognostic significance or predict chemotherapy toxicity in other malignancies supports the premise that geriatric assessment adds information beyond traditional oncologic assessment and may aid in risk-stratification and shared decision-making in older adults with myeloma.5–8,24
Geriatric assessment components are associated with older adults with myeloma being considered eligible for and actually undergoing ASCT. ASCT utilization is increasing among older patients, with similar outcomes between younger and selected older adults.3 Yet it is not clearly defined which older adults are ASCT-eligible. In our study, as would be expected, performance status and comorbidities are associated with the patient being considered ASCT-eligible. Interestingly, speed on the Timed Up and Go test is also associated with ASCT-eligibility, suggesting that clinicians may observe slower physical performance to some degree in their standard clinical assessment. Geriatric assessment may allow development of objective criteria to correspond to the currently subjective assessment of an older patient’s ASCT-eligibility.
Another important finding is that, independent of age, dependence in IADLs was associated with lower odds of undergoing ASCT, while Karnofsky performance status was not. Two prior observational studies comparing survival in older adults who did versus did not undergo ASCT have shown a survival benefit with ASCT, with 40–50% lower hazard for mortality.25,26 Both of these studies controlled for comorbidities, and one controlled for performance status. Yet, neither of these retrospective studies included data on IADL dependence. Thus, because IADL dependence is associated with mortality in the general geriatric population and in myeloma, residual confounding could be present.8 Comparative-effectiveness studies comparing ASCT and non-ASCT strategies need to account for differences in patient population beyond just comorbidities and performance status to yield valid conclusions.
Strengths of our study include the assessment of a multiple geriatric domains, extending beyond the functional status and comorbidities incorporated into prior studies in older adults with myeloma.8,27 We have demonstrated highly prevalent geriatric vulnerabilities, including falls, cognitive impairment, sensory impairments and polypharmacy, which have not been demonstrated previously. Importantly, in general oncology populations, falls and hearing impairment are independently associated with toxicity of chemotherapy.5 Cognitive impairment and impaired physical performance are associated with survival in both acute myeloid leukemia and chronic lymphocytic leukemia.24,28 The impact of these impairments in other hematologic malignancies underscores the need for assessment of domains beyond comorbidity and functional status.
Our study has several limitations. First, our population was derived from two tertiary care centers and may not be generalizable to the population of older adults who are patients not referred to a tertiary care center. Some patients had started on treatment at the time of enrollment, which may have impacted their geriatric assessment results, though we found no significant differences between those who had versus had not initiated treatment, aside from number of medications. While some have may have had decline in their function due to toxicity of treatment,18 those who were highly symptomatic of their myeloma may have had improvements with response to therapy. In addition, the limited number of myeloma providers at the enrolling sites may not reflect the heterogeneity in the clinical ascertainment of ASCT-eligibility across hematology providers.
Another limitation is our sample size. Our small study was powered to detect rather large differences in the two groups; it was originally powered to detect a 47% difference in the prevalence of dependence in IADLs between the groups, assuming that, overall, 38% of patients were dependent in one or more IADLs. The actual rate of dependence in the whole cohort was higher than expected at 62%, and the observed difference was 14%. Thus, the sample size may have limited our ability to detect smaller but clinically meaningful differences in the individual geriatric parameters. Our limited sample size also did not allow for inclusion of all potential predictors in the multivariate model to avoid overfitting the model; indeed, some would argue that including 3 variables in our multivariate model could result in overfitting, while others have argued that as few as 5 events per predictor is acceptable. 29 Our study was not powered to determine whether geriatric assessment components are associated with outcomes such as survival or toxicity in this population. Finally, we are unable to compare our findings with the International Myeloma Working Group (IMWG) frailty model8 or the Revised Myeloma Comorbidity Index30 which were published after our study was developed, as the geriatric assessment tool utilized in our study does not capture all of the variables necessary to calculate each.
In conclusion, we have demonstrated that impairments in geriatric domains are prevalent in this population. ASCT-eligible patients were younger, had fewer comorbidities, better performance status and faster time on the Timed Up and Go test than transplant-ineligible patients. Factors independently associated with whether the patient ultimately underwent transplant were age and IADL dependence. Future study is needed to define whether these prevalent geriatric impairments predict adverse outcomes and shorter survival in older adults with myeloma and how geriatric assessment may aid in decision-making and risk-stratification among older adults with myeloma.
Supplementary Material
Supplementary Table 1. Comparison of baseline geriatric assessment variables by whether myeloma treatment had been initiated at the time of enrollment.
Supplementary Table 2. Univariate and multivariate analysis of association between demographic, stage and geriatric assessment variables with whether participant underwent high dose therapy and autologous stem cell transplantation.
Impact statement.
We certify that this work is novel clinical research. This research adds estimates of the prevalence of geriatric impairments in older adults with multiple myeloma and gives insight into which older adults with multiple myeloma are considered eligible for high dose therapy and autologous stem cell transplantation.
Acknowledgements
This publication was made possible by Grant Number K12CA167540 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH), Grant Number R03 AG042374 through the National Institute of Aging and Grant Number UL1 TR000448 through the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCI, NCATS or NIH.
Sponsor’s Role: The grant sponsors had no role in study design, methods, subject recruitment, data collection, analysis or preparation of the manuscript.
Conflicts:
T.W. Research funding: Janssen. S.T: Speakers’ bureau: Celgene; Research funding: Celgene, Takeda, J&J, Janssen, Prothena H.K. has nothing to disclose. J.M. has nothing to disclose. K.T. has nothing to disclose. K.S.G. has nothing to disclose. R.V. has nothing to disclose. G.C. has nothing to disclose.
REFERENCES
- 1.Rosenberg PS, Barker KA, Anderson WF. Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood. 2015;125(2):410–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa LJ, Brill IK, Omel J, et al. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Advances. 2017;1(4):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auner HW, Szydlo R, Hoek J, et al. Trends in autologous hematopoietic cell transplantation for multiple myeloma in Europe: increased use and improved outcomes in elderly patients in recent years. Bone Marrow Transplantation. 2015;50(2):209–215. [DOI] [PubMed] [Google Scholar]
- 4.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. Journal of Clinical Oncology. 2014;32(24):2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurria A, Togawa K, Mohile SG, et al. Predicting Chemotherapy Toxicity in Older Adults With Cancer: A Prospective Multicenter Study. Journal of Clinical Oncology. 2011;29(25):3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–3386. [DOI] [PubMed] [Google Scholar]
- 7.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. Journal of Clinical Oncology. 2012;30(15):1829–1834. [DOI] [PubMed] [Google Scholar]
- 8.Palumbo A, Bringhen S, Mateos M-V, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawton M, Brody E. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–1986. [PubMed] [Google Scholar]
- 10.Stewart A, Ware J. Measuring Functioning and Well-Being. Durham and London: Duke University Press; 1992. [Google Scholar]
- 11.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 12.Kawas C, Karagiozis H, Resau L, Corrada M, Brookmeyer R. Reliability of the Blessed Telephone Information-Memory-Concentration Test. J Geriatr Psychiatry Neurol. 1995;8(4):238–242. [DOI] [PubMed] [Google Scholar]
- 13.Greipp PR. International Staging System for Multiple Myeloma. Journal of Clinical Oncology. 2005;23(15):3412–3420. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 15.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J. Clin. Oncol 1984;2(3):187–193. [DOI] [PubMed] [Google Scholar]
- 16.Leidy NK, Palmer C, Murray M, Robb J, Revicki DA. Health-related quality of life assessment in euthymic and depressed patients with bipolar disorder. Psychometric performance of four self-report measures. J Affect Disord. 1998;48(2–3):207–214. [DOI] [PubMed] [Google Scholar]
- 17.Hamaker ME, Schiphorst AH, Bokkel Huinink ten D, Schaar C, van Munster BC. The effect of a geriatric evaluation on treatment decisions for older cancer patients--a systematic review. Acta Oncol. 2014;53(3):289–296. [DOI] [PubMed] [Google Scholar]
- 18.Hoppe S, Rainfray M, Fonck M, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. Journal of Clinical Oncology. 2013;31(31):3877–3882. [DOI] [PubMed] [Google Scholar]
- 19.Wildes TM, Fiala MA. Falls in older adults with multiple myeloma. European Journal of Haematology. 2018;100(3):273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klepin HD, Geiger AM, Tooze JA, et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. Journal of the American Geriatrics Society. 2011;59(10):1837–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muffly LS, Boulukos M, Swanson K, et al. Pilot Study of Comprehensive Geriatric Assessment (CGA) in Allogeneic Transplant: CGA Captures a High Prevalence of Vulnerabilities in Older Transplant Recipients. Biol. Blood Marrow Transplant 2013;19(3):429–434. [DOI] [PubMed] [Google Scholar]
- 22.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J. Clin. Oncol 2002;20(2):494–502. [DOI] [PubMed] [Google Scholar]
- 23.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J. Clin. Oncol 1998;16(4):1582–1587. [DOI] [PubMed] [Google Scholar]
- 24.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winn AN, Shah GL, Cohen JT, Lin PJ, Parsons SK. The Real World Effectiveness of Hematopoietic Transplant Among Elderly Individuals With Multiple Myeloma. JNCI Journal of the National Cancer Institute. 2015;107(8):djv139–djv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wildes TM, Finney JD, Fiala M, et al. High-dose therapy and autologous stem cell transplant in older adults with multiple myeloma. 2015;50(8):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engelhardt M, Dold SM, Ihorst G, et al. Geriatric assessment in multiple myeloma patients: validation of the International Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica. 2016;101(9):1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goede V, Bahlo J, Chataline V, et al. Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: Results of the CLL9 trial of the German CLL study group. Leuk. Lymphoma 2016;57(4):789–796. [DOI] [PubMed] [Google Scholar]
- 29.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. American Journal of Epidemiology. 2007;165(6):710–718. [DOI] [PubMed] [Google Scholar]
- 30.Engelhardt M, Domm A-S, Dold SM, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102(5):910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Comparison of baseline geriatric assessment variables by whether myeloma treatment had been initiated at the time of enrollment.
Supplementary Table 2. Univariate and multivariate analysis of association between demographic, stage and geriatric assessment variables with whether participant underwent high dose therapy and autologous stem cell transplantation.