Abstract
Rationale & Objective.
The intestinal microbiome may affect urinary stone disease (USD) by modulating the amount of oxalate absorbed from the intestine and subsequently excreted in the urine. This study sought to explore the association between antibiotics, which alter the intestinal microbiota, and the risk of USD.
Study design.
Prospective cohort study.
Setting & Participants.
5,010 women in the Nurses’ Health Study (NHS) I and II who had collected 24-hour urine samples.
Exposures.
Use of antibiotics during the age range of 40-49 (NHS II), 40-59 (NHS I) and 20-39 years (both cohorts).
Outcomes.
Incident, symptomatic USD; urine composition.
Analytical approach.
Cause-specific hazards regression adjusted for age, BMI, comorbidities, thiazide use, and dietary factors. Follow-up was censored at the time of asymptomatic kidney stones, cancer, or death.
Results.
Cumulative use of antibiotics for a total of 2 months or more during the age range of 40-49 years (NHS II) and 40-59 years (NHS II) was associated with a significantly higher risk of developing incident stones compared with no use (pooled HR 1.48; 95% CI, 1.12-1.96). Similar results were found for the period 20-39 years (pooled HR, 1.36; 95% CI, 1.00-1.84). Results remained unchanged after excluding participants who reported urinary tract infection with their stone event or as the most common reason for antibiotic use. Urine composition was generally similar across antibiotic groups except for marginally lower urine pH and citrate among those taking antibiotics for 2 months or more.
Limitations.
Observational design; lack of information on the type of antibiotic used; relatively large span of time between antibiotic use and urine collection.
Conclusions.
Use of antibiotics for >2 months in early adulthood and middle age is associated with higher risk of USD in later life.
Keywords: Antibiotics, Cohort studies, Urine chemistry, Urolithiasis, kidney stone, stone formation, intestinal microbiome, urine composition, cumulative antibiotic use, lithogenesis, women, antibacterial agents, drug effects, bacterial infection
Introduction
Kidney stones are increasingly common, with an estimated prevalence in the most recent NHANES survey of about 9%.1 The intestinal microbiome may play a role in the development of kidney stones by influencing the metabolism of substances involved in stone formation. For example, the normal intestinal microbiome hosts Oxalobacter formigenes, a specialist oxalotrophe that degrades oxalate in the intestine, thus reducing its absorption and subsequently its urinary excretion; it is also thought to stimulate intestinal secretion of oxalate.2 More generally, recent studies suggest that the intestinal microbiome of individuals affected with kidney stones might be different than those without a history of stones.3,4
Antibiotics are frequently used in the general population and have the potential to modify the intestinal microbiome. Given the purported role of intestinal bacteria in the development of kidney stones, it could be hypothesized that the prolonged use of antibiotics could increase the risk of forming stones; furthermore, it could also be speculated that antibiotics could have a direct effect on tubular handling of lithogenic substances.5 A recent longitudinal study reported associations between antibiotic use and higher risk of stones predominantly in children and young adults;6 however, that study did not account for potential differences in dietary intakes, and the results may have been due in part to higher rates of abdominal imaging in individuals taking more antibiotics as the study likely included asymptomatic, incidentally diagnosed kidney stones. We analyzed the independent, prospective association between duration of antibiotic use and the subsequent risk of a first symptomatic kidney stone in two large cohorts of adult women. We also evaluated the association between antibiotic use and 24h urine composition in a subsample of the cohorts with available 24h urine data.
Methods
Study population
The Nurses’ Health Study (NHS) I cohort was started in 1976 with the enrollment of 121,700 female nurses aged 30 to 55 years; the NHS II cohort was started in 1989 with the enrollment of 116,430 female nurses aged 25 to 42 years. For both cohorts, participants completed a detailed questionnaire with information on lifestyle, medical history and medications. The questionnaire was subsequently mailed every two years to update information. Follow-up of eligible person-time has exceeded 90% in each cohort. These studies were approved by the Partners Healthcare Institutional Review Board. Return of completed baseline and biennial questionnaires was accepted by the institutional review board as implied informed consent.
Assessment of antibiotic use
In 2004 (for NHS I) and 2005 (NHS II) participants were asked to report the cumulative amount of time they used antibiotics between age 20 to 39 years and between 40 to 59 years (NHS I) or 40 to 49 years (NHS II), excluding skin creams, mouthwash, or isoniazid. To ensure that the assessment of antibiotic use in our longitudinal study occurred before an incident kidney stone, the baseline of our analysis was 2004 for NHS I and 2005 for NHS II. Categories for total time of antibiotic use during these age periods were “none”, “less than 15 days”, “15 days to 2 months”, “2-4 months”, “4 months to 2 years”, “2-3 years”, “3-5 years”, “more than 5 years”. Thus, we could not determine whether longer periods of reported previous antibiotic use represented a single episode of exposure, or the sum of multiple, shorter episodes. Participants also were asked about the most common reason for their antibiotic use (“respiratory infection”, “Urinary tract infection” [UTI], “Acne/Rosacea”, “Chronic bronchitis”, “Dental”, or “Other”).
Assessment of kidney stones
The study outcome was an incident, symptomatic kidney stone. Participants reporting a kidney stone were asked to complete a supplementary questionnaire with information about date of occurrence and accompanying symptoms. The supplementary questionnaire also queried presence or absence of urinary tract infection during or preceding the kidney stone event. Self-reported kidney stone diagnosis was found to be reliable by medical record review of a sample of 1,634 participants (confirmed in ≥95% who completed the supplementary questionnaire).7 In a subsample of the study population with stone composition reports, the stone type was predominantly calcium oxalate (>50%) in 77% of participants in the NHS I, and 79% of participants in the NHS II cohort.7
Assessment of other covariates
Starting in 1986 (for NHS I) and 1991 (for NHS II), participants completed a food-frequency questionnaire (FFQ) with information on average use of more than 130 foods and more than 20 beverages in the previous year. Intake of individual nutrients was calculated from the frequency of consumption of foods and from data on the content of the relevant nutrients obtained from the US Department of Agriculture, except for oxalate intake which was directly measured in foods by capillary electrophoresis.8 The FFQ has been sent every 4 years and also queries the use of multivitamins as well as individual supplements such as calcium and vitamin D. Information on nutrients obtained with the FFQ was validated in the cohorts.9,10
Information about age, BMI, history of diabetes, and use of thiazides was obtained from the biennial questionnaires.
Assessment of urine chemistries
Urine samples were collected in three separate cycles. In the first, which spanned from 1994 to 1999, eligible participants were ≤65 years of age in the NHS I with no history of cancer or cardiovascular disease. In the second, performed in 2003, eligible participants were ≤75 years of age and had no history of cancer (other than nonmelanoma skin cancer). The third cycle was performed in 2009 only in the NHS II cohort, and eligible participants were ≤55 years old, white race, with no history of hypertension, coronary heart disease, or cancer. Urine samples collected in the first two cycles were analyzed by Mission Pharmacal (San Antonio, TX), whereas the samples collected in the third cycle were analyzed by the Litholink Corporation (Chicago, IL). Participants with possible over- or undercollections (defined as urinary creatinine excretion in the top or bottom 1% of the nonstone-formers distribution) were removed from the analysis. For participants who provided more than one collection, the first sample was analyzed.
Statistical analysis
The study design was prospective; information on antibiotic use was collected before the incident kidney stone. Total time of antibiotic use during the two age periods in each cohort was categorized into “no use”, “less than 2 months” and “2 months or more” for statistical analysis. These categories were selected to examine clinically significant differences in previous antibiotic use while preserving adequate case numbers in each category. Time at risk started from the date of return of the 2004 (NHS I) or 2005 (NHS II) questionnaire and participants were followed until the development of a symptomatic kidney stone, an asymptomatic kidney stone, cancer, death, or end of follow-up (2012 for NHS I, 2011 for NHS II), whichever occurred first. While the end of follow-up was a censoring event, an asymptomatic kidney stone, cancer, and death were competing events. Participants with a history of cancer (except non-melanoma skin cancer) or a history of kidney stones at baseline were excluded from the study. Cause-specific hazards models, treating competing events as censoring, were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs), using the “no use” as the referent category. Models were adjusted for age, BMI, history of diabetes, use of thiazides, use of calcium supplements, intake of alcohol, dietary intakes of calcium, magnesium, oxalate, potassium, sodium, animal protein, vitamin D and total fluid intake. Further adjustment was performed for bisphosphonate use and (for NHS II) menopausal status. Effect modification by dietary oxalate and calcium intake (above or below the cohort-specific medians) was investigated. All the models were repeated after exclusion of participants reporting concurrent urinary tract infection at the time of stone diagnosis or as the main reason for antibiotic use. Results from the two cohorts were pooled by random-effects meta-analysis using the DerSimonian and Laird method.11
The association between antibiotic use and urine composition was assessed in a cross-sectional manner with general linear models adjusted for age, kidney stone status, BMI, use of alcohol, dietary intake of oxalate, calcium, magnesium, potassium, animal protein, total fluid, supplemental use of calcium, urinary creatinine and sodium. Information was obtained from the questionnaires closest in time to the date of urine collection.
Analyses were performed with SAS version 9.4. The research protocol for this study was reviewed and approved by the Brigham and Women’s Hospital institutional review board.
Results
A total of 112,324 participants remained in the study group (46,336 from NHS I and 65,988 for NHS II) as reported in the flow diagram (Figure 1). Baseline characteristics of the study population are reported in Tables 1 and 2 and Tables S1 and S2. Compared with those taking no antibiotics at age 40-49/59 years, those taking antibiotics for a total of 2 months or more during this age period were younger in NHS I, had higher mean BMI and fluid intake and a greater prevalence of hypertension and diabetes and greater use of thiazides. The main reasons for antibiotic use were respiratory infections (52% in NHS I, 53% in NHS II), urinary tract infections (11% in NHS I, 15% in NHS II), and dental infections (10% in NHS I, 7% in NHS II).
Figure 1.
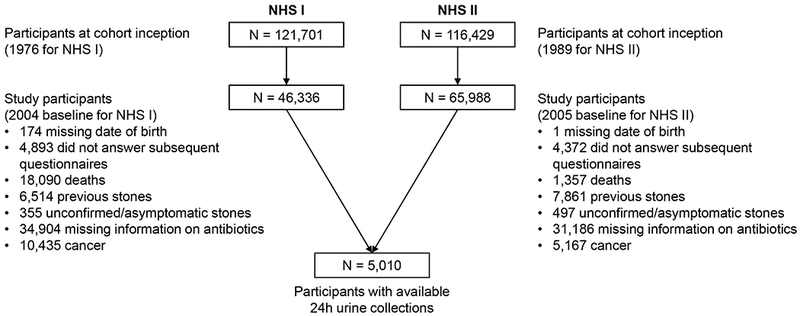
Flowchart of the study
Table 1.
Baseline characteristics by antibiotic use at ages 40-59 – Nurses’ Health Study I
| No antibiotic use (n = 3,817) | Duration of Antibiotic Use | ||
|---|---|---|---|
| <2 months (n = 38,001) | >=2 months (n = 4,518) | ||
| Age, years* | 70.8 (6.9) | 68.6 (6.7) | 66.8 (6.6) |
| BMI, kg/m2 | 25.7 (4.7) | 26.6 (5.3) | 27.2 (5.9) |
| History of hypertension, % | 50.2 | 56.9 | 61.8 |
| Thiazide diuretic use, % | 13.7 | 18.2 | 22.0 |
| History of diabetes, % | 7.4 | 9.7 | 12.3 |
| Daily intake | |||
| Total fluid, mL | 1,694 (712) | 1,752 (689) | 1,815 (704) |
| Alcohol, g | 6.4 (11.1) | 6.2 (10.8) | 5.5 (10.3) |
| Dietary calcium, mg | 862 (336) | 858 (319) | 861 (316) |
| Supplemental calcium, mg | 583 (532) | 634 (524) | 698 (536) |
| Animal protein, g | 44.7 (14.4) | 46.3 (13.6) | 47.1 (13.5) |
| Potassium, mg | 3,080 (625) | 3,082 (591) | 3,071 (595) |
| Sodium, mg | 1,959 (434) | 1,996 (427) | 2,015 (419) |
| Oxalate, mg | 178 (107) | 175 (100) | 172 (93) |
| Magnesium, mg | 369 (126) | 374 (123) | 386 (128) |
| Vitamin D, IU | 542 (343) | 572 (336) | 589 (337) |
| Vitamin C, mg | 346 (382) | 374 (396) | 418 (411) |
| Sucrose, g | 38.1 (16.6) | 37.9 (15.7) | 38.0 (15.3) |
BMI, body mass index. Values are mean +/− SD or percentages and are standardized to the age distribution of the study population.
Value is not age adjusted
Table 2.
Baseline characteristics by antibiotic use at ages 40-49 – Nurses’ Health Study II
| No antibiotic use (n = 6,631) | Duration of Antibiotic Use | ||
|---|---|---|---|
| <2 months (n = 51,795) | >=2 months (n = 7,562) | ||
| Age, years* | 48.6 (5.2) | 50.5 (4.6) | 50.4 (4.2) |
| BMI, kg/m2 | 25.8 (5.4) | 27.2 (6.3) | 28.4 (7.2) |
| History of hypertension, % | 18.3 | 24.7 | 32.9 |
| Thiazide diuretic use, % | 5.3 | 8.3 | 12.2 |
| History of diabetes, % | 2.3 | 3.9 | 6.6 |
| Daily intake | |||
| Total fluid, mL | 1,748 (888) | 1,768 (906) | 1,849 (910) |
| Alcohol, g | 6.0 (9.7) | 5.8 (9.5) | 5.0 (9.6) |
| Dietary calcium, mg | 954 (343) | 937 (328) | 936 (329) |
| Supplemental calcium, mg | 468 (497) | 488 (504) | 532 (521) |
| Animal protein, g | 54.0 (16.9) | 54.9 (15.9) | 55.5 (15.9) |
| Potassium, mg | 3,220 (640) | 3,194 (601) | 3,160 (615) |
| Sodium, mg | 2,228 (492) | 2,267 (472) | 2,291 (473) |
| Oxalate, mg | 197 (125) | 194 (124) | 189 (108) |
| Magnesium, mg | 375 (124) | 374 (121) | 378 (126) |
| Vitamin D, IU | 494 (336) | 498 (334) | 516 (342) |
| Vitamin C, mg | 300 (372) | 305 (370) | 337 (409) |
| Sucrose, g | 40.0 (17.5) | 40.2 (17.4) | 40.7 (18.4) |
BMI, body mass index. Values are mean +/− SD or percentages and are standardized to the age distribution of the study population.
Value is not age adjusted
For the 40-49/59 years group analysis, there were 1,059 incident kidney stones during a cumulative follow-up of 694,194 person-years. The association between antibiotic use and risk of incident kidney stones is reported in Table 3. There was a significantly higher risk of incident kidney stones with antibiotic use of 2 months or more (pooled multivariable-adjusted HR, 1.48; 95% CI, 1.12-1.96).
Table 3.
Association between antibiotic use and risk of incident kidney stones
| No antibiotic use | Duration of Antibiotic Use | ||
|---|---|---|---|
| <2 months | >=2 months | ||
| 40 to 49/59 years | |||
| Nurses Health Study I | |||
| - Cases | 20 | 285 | 44 |
| - Person-years | 26,555 | 271,480 | 32,155 |
| - Age-adjusted HR | 1.00 (reference) | 1.28 (0.81,2.01) | 1.57 (0.92, 2.67) |
| - MV-adjusted HR | 1.00 (reference) | 1.26 (0.80, 1.99) | 1.51 (0.88, 2.58) |
| Nurses Health Study II | |||
| - Cases | 58 | 549 | 103 |
| - Person-years | 36,791 | 285,664 | 41,549 |
| - Age-adjusted HR | 1.00 (reference) | 1.23 (0.93, 1.61) | 1.59 (1.15, 2.20) |
| - MV-adjusted HR | 1.00 (reference) | 1.18 (0.89, 1.55) | 1.47 (1.06, 2.04) |
| Pooled cohorts | |||
| - Age-adjusted HR | 1.00 (reference) | 1.24 (0.98, 1.57) | 1.58 (1.20, 2.09) |
| - MV-adjusted HR | 1.00 (reference) | 1.20 (0.95, 1.52) | 1.48 (1.12, 1.96) |
| 20 to 39 years | |||
| Nurses Health Study I | |||
| - Cases | 39 | 272 | 38 |
| - Person-years | 48614 | 255,481 | 26,094 |
| - Age-adjusted HR | 1.00 (reference) | 1.13 (0.81, 1.60) | 1.43 (0.91,2.26) |
| - MV-adjusted HR | 1.00 (reference) | 1.14 (0.81, 1.60) | 1.44 (0.91,2.28) |
| Nurses Health Study II | |||
| - Cases | 28 | 518 | 164 |
| - Person-years | 15,604 | 278,045 | 70,355 |
| - Age-adjusted HR | 1.00 (reference) | 1.05 (0.72, 1.54) | 1.33 (0.89, 1.99) |
| - MV-adjusted HR | 1.00 (reference) | 1.04 (0.71, 1.94) | 1.29 (0.86, 1.94) |
| Pooled cohorts | |||
| - Age-adjusted HR | 1.00 (reference) | 1.10 (0.85, 1.42) | 1.37 (1.02, 1.86) |
| - MV-adiusted HR | 1.00 (reference) | 1.09 (0.85, 1.41) | 1.36 (1.00, 1.84) |
Multivariate models adjusted for age, BMI, history of diabetes, use of thiazides, use of calcium supplements, intake of alcohol, dietary intakes of calcium, magnesium, oxalate, potassium, sodium, animal protein, vitamin D and total fluid intake
Similarly, for the 20-39 years group analysis the pooled multivariable-adjusted HR was 1.36 (95% CI, 1.00-1.84). The estimates were similar after exclusion of participants reporting urinary tract infection at the time of stone diagnosis or as the main reason for antibiotic use, as well as after further adjustment for bisphosphonate use and menopausal status. There was no significant effect modification by dietary intake of oxalate or calcium.
The association between antibiotic use and urine composition was studied in a subsample of 5,010 participants with at least one 24h urine collection available for analysis (Table 4). Urine composition was generally similar across groups except for marginally lower urine pH, calcium, and citrate among those taking antibiotics for 2 months or more.
Table 4.
Adjusted mean values of urine chemistries by antibiotic use
| No antibiotic use | Duration of Antibiotic Use | ||
|---|---|---|---|
| <2 months | >=2 months | ||
| 40 to 49/59 years | |||
| Nurses’ Health Study I | |||
| - Calcium (mg/d) | 197 | 187 | 178 |
| - Oxalate (mg/d) | 28 | 29 | 29 |
| - Citrate (mg/d) | 642 | 627 | 609 |
| - Volume (L/d) | 1.7 | 1.8 | 1.8 |
| - pH | 6.05 | 6.03 | 5.91* |
| Nurses’ Health Study II | |||
| - Calcium (mg/d) | 205 | 205 | 193 |
| - Oxalate (mg/d) | 30 | 30 | 30 |
| - Citrate (mg/d) | 816 | 782* | 754* |
| - Volume (L/d) | 2.0 | 2.0 | 2.0 |
| - pH | 6.17 | 6.14 | 6.15 |
| 20 to 39 years | |||
| Nurses’ Health Study I | |||
| - Calcium (mg/d) | 185 | 189 | 174 |
| - Oxalate (mg/d) | 29 | 29 | 30 |
| - Citrate (mg/d) | 629 | 629 | 624 |
| - Volume (L/d) | 1.7 | 1.8 | 1.8 |
| - pH | 6.09 | 6.02 | 5.95* |
| Nurses’ Health Study II | |||
| - Calcium (mg/d) | 201 | 205 | 199 |
| - Oxalate (mg/d) | 30 | 30 | 30 |
| - Citrate (mg/d) | 819 | 783 | 778 |
| - Volume (L/d) | 1.9 | 2.0 | 2.0 |
| - pH | 6.18 | 6.15 | 6.16 |
Values adjusted for age, kidney stone status, BMI, use of alcohol, dietary intake of oxalate, calcium, magnesium, potassium, animal protein, total fluid, supplemental use of calcium, urinary creatinine and sodium
p<0.05
Discussion
We found a higher risk of developing a first symptomatic kidney stone for users of antibiotics in two prospective cohorts of women. The association was statistically significant after adjusting for potential confounders. We speculate that this association may be due to changes in the intestinal microbiome composition induced by antibiotic use. The intestinal microbiome has been suggested to modulate the intestinal handling of calcium and oxalate;12 for example, the presence and relative abundance of Oxalobacter formigenes, a specialized oxalate-degrading microorganism, has been correlated with a history of kidney stones,13 although the oral administration of Oxalobacter formigenes has shown inconsistent results in randomized controlled trials.14–16 More generally, stone formers have been shown to have systematically different intestinal microbiome composition compared with non-stone formers,3,4 suggesting that alterations in the intestinal flora might predispose to stone formation. The intestinal microbiome has also been implicated in the intestinal handling of calcium through direct effects on the mechanisms of calcium absorption.12 Some antibiotics could also directly precipitate and form stones due to their low solubility in urine.17
Another explanation for the potential lithogenic effects of antibiotics is through the induction of changes in tubular handling of lithogenic substances described for certain antibiotics such as cotrimoxazole and ceftazidime, which have been shown to increase urinary excretion of phosphate and calcium, thus increasing the urinary supersaturation for calcium salts.5
In our own data, we found a tendency to lower urine citrate and pH among those taking antibiotics for more than 2 months in the past but we did not find a difference in urine oxalate or calcium. However, the temporal relationship between antibiotic use and urine collection in the cohorts was not standardized, i.e. about 2,500 urine collections in NHS II were obtained after the antibiotic exposure question, whereas for NHS I part of the urine collections were obtained during the time period of interest.
In the literature, there is a scarcity of data regarding the association between use of antibiotics and risk of kidney stones, except for a recent report6 in which participants with kidney stones were identified through administrative codes. Recognizing the known tendency of kidney stones to recur, the use of administrative codes would not allow to easily distinguish between a first stone event, a recurrence, and the expulsion of a previous stone. Furthermore, that study lacked information on dietary intakes (although our own data do not suggest a large impact of diet on the association between exposure to antibiotics and risk of stones) and likely included asymptomatic, incidentally diagnosed kidney stones that may have been detected as a result of more frequent imaging in individuals taking antibiotics.
Our study has limitations, including the observational design, the relatively large span of time between antibiotic use and urine collection, and the enrollment of only women in NHS. In addition, antibiotic use was not validated, and we did not have information on the types of antibiotics used.
In conclusion, use of antibiotics for a total of two months or more during early to middle adulthood is independently associated with a higher subsequent risk of developing kidney stones in later life. Our data provide an additional reason to minimize the unnecessary use of antibiotics.
Supplementary Material
Table S1. Baseline characteristics by antibiotic use at ages 20-39: Nurses’ Health Study I.
Table S2. Baseline characteristics by antibiotic use at ages 20-39: Nurses’ Health Study II.
Acknowledgments
Support: Supported by National Institutes of Health research grants DK094910, DK91417, CA186107, CA176726, and CA167552.
Financial Disclosure: GCC reports having served as a consultant for Allena Pharmaceuticals, Shire, AstraZeneca, and Decibel Therapeutics; as well as receiving royalties from UpToDate (author and Section Editor). The other authors declare that they have no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scales CD, Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–165. doi: 10.1016/j.eururo.2012.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatch M Gut microbiota and oxalate homeostasis. Ann Transl Med. 2017;5(2):36. doi: 10.21037/atm.2016.12.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern JM, Moazami S, Qiu Y, et al. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis. 2016;44(5):399–407. doi: 10.1007/s00240-016-0882-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang R, Jiang Y, Tan A, et al. 16S rRNA gene sequencing reveals altered composition of gut microbiota in individuals with kidney stones. Urolithiasis. 2018;46(6):503–514. doi: 10.1007/s00240-018-1037-y [DOI] [PubMed] [Google Scholar]
- 5.Böhles H, Gebhardt B, Beeg T, Sewell AC, Solem E, Posselt G. Antibiotic treatment-induced tubular dysfunction as a risk factor for renal stone formation in cystic fibrosis. J Pediatr. 2002;140(1):103–109. doi: 10.1067/mpd.2002.120694 [DOI] [PubMed] [Google Scholar]
- 6.Tasian GE, Jemielita T, Goldfarb DS, et al. Oral Antibiotic Exposure and Kidney Stone Disease. J Am Soc Nephrol JASN. 2018;29(6):1731–1740. doi: 10.1681/ASN.2017111213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol JASN. 2009;20(10):2253–2259. doi: 10.1681/ASN.2009030276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes RP, Kennedy M. Estimation of the oxalate content of foods and daily oxalate intake. Kidney Int. 2000;57(4):1662–7. doi: 10.1046/j.1523-1755.2000.00010.x [DOI] [PubMed] [Google Scholar]
- 9.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 10.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol. 2017;185(7):570–584. doi: 10.1093/aje/kww104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 12.Weaver CM. Diet, Gut Microbiome, and Bone Health. Curr Osteoporos Rep. 2015;13(2):125–130. doi: 10.1007/s11914-015-0257-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman DW, Kelly JP, Curhan GC, et al. Oxalobacter formigenes May Reduce the Risk of Calcium Oxalate Kidney Stones. J Am Soc Nephrol. 2008;19(6): 1197–1203. doi: 10.1681/ASN.2007101058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoppe B, Beck B, Gatter N, et al. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int. 2006;70(7):1305–1311. doi: 10.1038/sj.ki.5001707 [DOI] [PubMed] [Google Scholar]
- 15.Hoppe B, Groothoff JW, Hulton S-A, et al. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transplant. 2011. ;26(11):3609–3615. doi: 10.1093/ndt/gfr107 [DOI] [PubMed] [Google Scholar]
- 16.Milliner D, Hoppe B, Groothoff J. A randomised Phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis. 2018;46(4):313–323. doi: 10.1007/s00240-017-0998-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daudon M, Jungers P. Drug-induced renal calculi: epidemiology, prevention and management. Drugs. 2004;64(3):245–275. doi: 10.2165/00003495-200464030-00003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics by antibiotic use at ages 20-39: Nurses’ Health Study I.
Table S2. Baseline characteristics by antibiotic use at ages 20-39: Nurses’ Health Study II.


