Abstract
Rodents used in scientific research are typically housed in cages containing natural bedding materials. Despite extensive evidence of biological harm from inhaled particulate matter (PM), relatively little work has been performed to measure bedding-generated PM exposure in caged animals used in basic science research. We were interested in determining whether bedding-generated PM was present in significant concentrations in rodent cages and the main factors affecting the accumulation and attenuation of bedding-generated PM inside cages. Our objectives were to measure PM concentrations in cages containing common bedding materials (pine, aspen shavings, absorbent paper, and corncob) with filter top isolator absent or present on the cages. PM2.5 concentrations were monitored with rats inside cages as well as during artificial manipulation of the bedding (designed to simulate rodent activity). Upon rodent digging or mechanical/manual stirring, all four bedding materials produced significant increases in respirable PM concentrations (as much as 100–200 μg/m3 PM2.5, 50 to 100-fold higher than during periods of no rodent activity), and concentrations in cages fitted with filter tops were an order of magnitude higher than in cages without filter tops. Elevated concentrations were sustained for longer durations in cages with filter tops (5–10 minutes) compared to cages with only bar lids (0–2 minutes). These results indicate that standard laboratory housing conditions can expose rodents to substantial levels of PM. Bedding-generated PM has potential implications as an environmental agent in rodent studies.
Keywords: particulate matter (PM), PM2.5, filter top isolator, bar lids, bedding material, rodent cages, rodent bedding, microenvironment, endotoxin, cage ventilation
Introduction
Particulate matter (PM) exposure has been associated with adverse pulmonary, cardiovascular, immune, developmental, and neurobehavioral effects in rodent assays (Campbell et al., 2005; Gerlofs-Nijland et al., 2010; Guerra et al., 2013; Harkema et al., 2004; Kleinman et al., 2008). Rodents are housed in cages containing bedding (NAP, 2011) and despite extensive evidence of biological harm from inhaled particulate matter (PM), and some recognition of generation of PM by bedding material (Rosenbaum et al., 2010), relatively little work has been performed to measure bedding-generated PM exposure in caged animals used in basic science research. Cage micro-environment ventilation studies have primarily focused on how best to provide thermal comfort and adequate ventilation to the animals, protection from pathogens, and minimizing the release of rodent-generated waste materials (allergens, dander and ammonia) from micro- to macro-environment so as to minimize occupational exposure (Geertsema and Lindsell, 2015; Kacergis et al., 1996; Memarzadeh et al., 2004; NAP, 2011; NIOSH, 1998; Reeb et al., 1997; Schweitzer et al., 2003).
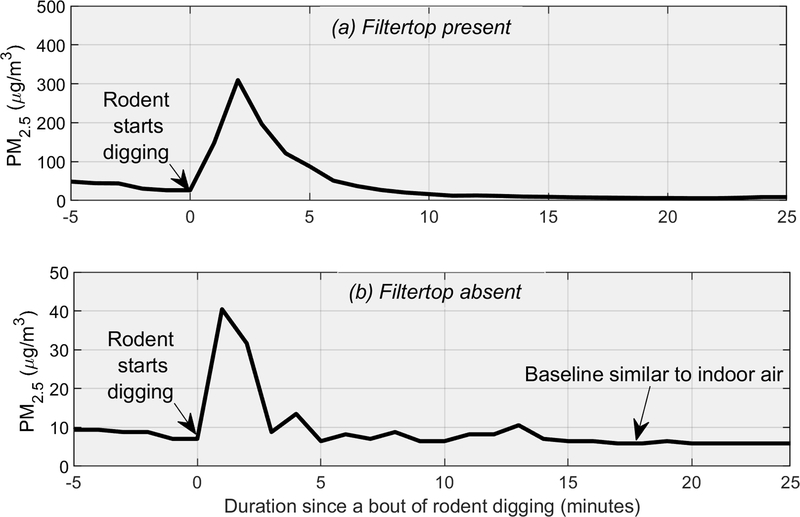
Inhalation exposures are not regularly carried out in chambers with bedding. Instead, often during PM inhalation trials, animals are placed into specialized chambers and exposed to airborne PM, but the animals may also return to their bedded cages in between exposure sessions (depending on the study design) and be exposed to bedding-generated PM. During a recent PM exposure study in our laboratory, we observed that despite being supplied with HEPA-filtered air, concentrations of PM2.5 (particles with an aerodynamic diameter of ≤2.5 microns) were elevated in control cages that housed pregnant rat dams with bedding material. The PM2.5 concentrations in such control cages averaged 14 ± 10 μg/m3 compared to 3–6 μg/m3 outside the cages in the animal-facility room. Temperature and relative humidity ranged from 70–72°F and 67–78% during these measurements. Figure 1 illustrates our observation; it shows that concentrations of PM2.5, in a control cage following several minutes of intense rodent digging, spiked as high as 300 μg/m3, i.e., more than 50-fold higher than background PM2.5 concentrations in the animal-facility room. [Insert Figure 1 near here.]
Figure 1.
PM2.5 generated by a typical bout of rodent digging activity (and several preceding minutes). The cage housed two rodents with pine shaving bedding material. The one-rodent digging bout lasted approximately 1 minute.
Based on these observations, we hypothesized that the cage bedding was the source of the elevated PM2.5. Our objectives were to study the main factors affecting the accumulation and attenuation of bedding-generated PM. We studied commonly-used bedding materials (pine and aspen shavings, corncob, and paper) and ventilation configurations (specifically, filter top absence and presence at varying air exchange rates).
Methods
Animals and Facility
Ten adult male Royal College of Surgeons (RCS) hooded rats were used in this study and cared for in accordance with the guidelines of the committee of the Care and Use of Laboratory Animal Resources, National Research Council (NAP, 2011). Cage bedding was changed twice weekly based on protocols approved by the Tufts Institutional Animal Care and Use Committee. All animal experiments were conducted at the Cummings School of Veterinary Medicine, Tufts University. The ventilation in the animal facility rooms was maintained at 8–10 exchanges of room volume per hour of HEPA-filtered ambient air.
Cages and Bedding Material
During the experiments, the rats were housed in standard polycarbonate cages (Allentown, Inc., Allentown, NJ; 49 cm long, 28 cm wide, and 20.25 cm high, 28 L volume) fitted with a lid made of round stainless-steel wire. This configuration allows free exchange of air between the micro- and macro-environment of the cage. An additional filter top lid (49 cm long, 28 cm wide, and 8.25 cm high) was placed on the cage in a set of the experiments (39 L volume total for the filter top lid and cage combination). It contained paper filter (Reemay 2024 filter) which restricted the air exchange in the cage. The manufacturer (Allentown, Inc., Allentown, NJ) reports a 91% particle removal efficiency in the 8–10 μm range for the filter; we investigated particles <2.5 μm and no information was available from the manufacturer regarding filtration efficiency for that specific size range. Bedding materials used were: corncob (Bed-O-Cob, Anderson’s Lab Bedding, Maumee, OH), pine shavings (Hancock Lumber, ME), aspen shavings (Nepco, Warrensburg, NY), and paper (ALPHA-dri, Shepherd Specialty Papers, Framingham, MA).
Particulate Matter Monitors
Bedding dust has a wide size range. Rosenbaum et al. 2010 reported that mass and volume is dominated by larger (>10 μm) particles and number by smaller, as expected. We studied PM2.5 and ultrafine particles. We expect the findings to generally apply to a wider size range based on physical principles with attenuation rates being higher for larger particles. PM2.5 inside the cages was measured using a DustTrak DRX Aerosol Monitor 8533 (TSI Inc., MN, USA). The DRX contains a photometer, the voltage from which is proportional to PM concentration based on the factory calibration with Arizona road dust. The 8533 model also allows for collection of gravimetric samples on a serial in-line filter, which was used to calibrate the device to the sampled aerosol instead of the factory default, i.e., a factor specific to the optical and physical properties of the sampled aerosol. Calibration factors for bedding types ‒ 0.586, 0.556, and 2.074 for corncob, pine, and paper, respectively ‒ were obtained by following the method detailed in the Dusttrak DRX manual (TSI Inc., 2018), and the PM2.5 measurements reported by the Dusttrak were corrected by these factors. Calibration factor could not be obtained for aspen bedding so we have not made comparisons between absolute PM2.5 concentrations generated by that bedding. However, comparisons could be made across ventilation settings for the same bedding type without needing a custom calibration factor. PM2.5 concentrations were monitored continuously at a 10-second averaging time and aggregated to one minute for the analysis. In addition, the number concentrations of particles in the 7–1,000 nm size range were measured in the rate cages using a TSI condensation particle counter (CPC; Model 3783) at 1 second resolution.
Experimental Design
We conducted measurements of PM2.5 inside the cages with and without rats (i.e., without inter-rat variability in digging behavior). Two different lid configurations (filter top absent and present) and up to four different bedding materials (aspen shavings, pine shavings, paper and corncob) were tested. Aspen bedding was only included in the initial trials; further use was discontinued after it caused a strong allergic reaction in a researcher. The continuous air turnover configuration simulated the condition in individually ventilated cages (IVC) and was achieved by the suction flow of the measurement devices creating a negative pressure in the cage. In the filter top absent configuration, where the bar lid was the only restriction on the cage top, the air in the cage micro-environment mixes unrestricted with the macro-environment air. In the filter top present configuration, the filter top restricts the free exchange of air between the micro- and macro-environment but not completely, the cracks in the sides, the air holes in the filter top, and porous material of the filter allow for air exchange.
Measurements in Occupied Cages
PM concentrations were measured in occupied cages (two rats per cage) with the filter top absent and present with two types of clean bedding: pine shavings and corncob. At the start of each experiment, two randomly selected rats from a group of twelve were placed in a cage with fresh bedding, food, and water using a standard cage change protocol. The DRX inlet tube was placed 2–3 cm above the top of the bedding (typical height of the rat mouth and nose when they are active) in the middle of the cage along the longer side, and the top of the cage (bar lid only or bar lid with filter top) was then placed on the cage. Cage ventilation was provided by the continuous suction of air into the inlet of the DRX (3 L/min) but PM2.5 concentrations were only surveyed at 0, 5, 10, 15, 30, 45, and 60 minutes. Eight trials were conducted for each filter top/bedding-type combination over a four-week period, and a new cage was used in each trial. We did not record if food consumption by animals created PM spikes.
Measurement in Unoccupied Cages
To control the variation in bedding agitation that is a result of real-world rat digging behavior, we conducted a set of experiments in unoccupied cages where rat digging activity was mechanically simulated in two ways.
First, the bedding was mechanically stirred using a short-bristled brush wrapped around a metal rod that went from one end of the cage to the other and was rotated from outside the cage which was filled with ~3.5 cm of bedding material. Three types of bedding materials were tested: pine shavings, paper and corncob. The protocol involved rotating the rod for 30 seconds to simulate a robust digging event. Between each trial, the bedding was mixed by hand and spread evenly over the cage floor. The DRX inlet was placed 2–3 cm above the top of the bedding in holes that were drilled for this purpose. Data was recorded continuously even during the stirring event. Eight trials each were conducted at 6 L/min flow for both filter top absent and present configurations. To characterize the impact of ventilation rate on PM attenuation experiments were also conducted at 1.5 L/min (three trials) and 20 L/min (eight trials) with the filter top present but only for one type of bedding material (pine shavings ). A pump was added to provide the flow for the 20 L/min configuration.
Second, the bedding was manually manipulated. Clean cages were filled with ~3.5 cm of bedding and allowed to rest for 15 minutes with either the filter top absent or present depending on the configuration being tested. The bedding was then agitated by grabbing and releasing five successive handfuls over a span of five seconds to simulate a robust digging event. At the end of this manual manipulation, the bar lid or bar lid and filter top combination was immediately replaced with the PM recording tube placed in the center of the long side of the cage, 2.5 cm above the bedding. The first recording of PM concentration was made once the cage top was replaced and subsequently concentrations were surveyed at one-minute intervals for the next 5 minutes. Three types of bedding materials (aspen shavings, pine shavings and corncob) were tested. Eight trials were conducted for each filter top/bedding-type combination and fresh bedding was used in each set of eight trials.
Statistical Analysis
Data represent means ± standard error and since the data were not normally distributed, nonparametric statistics (Wilcoxon rank-sum test) was used to compare between bedding material or filter top lid presence and differences were considered significant when p ≤0.05.
Results and Discussion
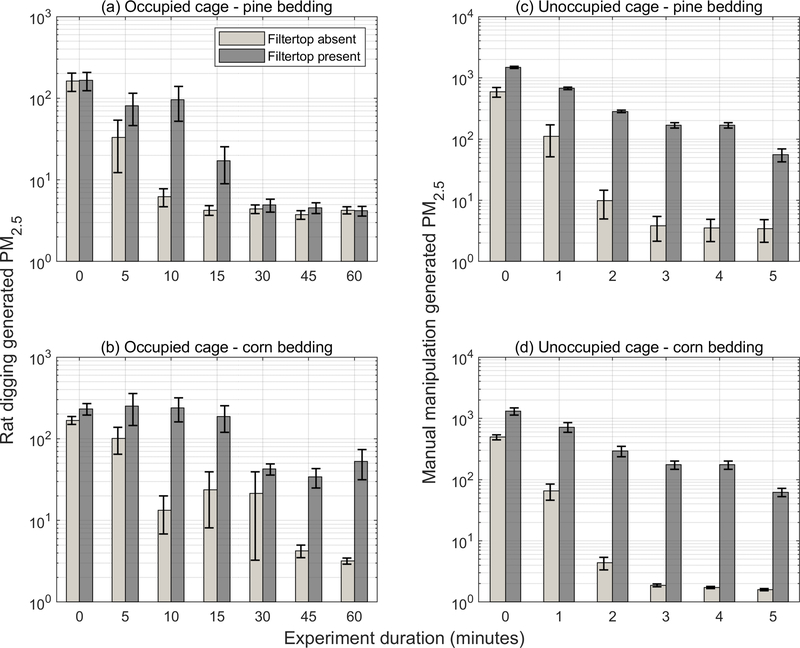
In all experiments, PM2.5 concentrations in the cages exceeded the animal-facility room or laboratory air concentrations by an order of magnitude or more irrespective of the bedding type or air flow rate through the cage. Furthermore, concentrations were elevated regardless of whether the filter top was absent or present. These results clearly indicate that PM2.5 was generated inside the cages. [Insert Figure 2 near here.]
Figure 2.
PM2.5 concentrations inside occupied cages (two rats) and inside unoccupied cages during manual manipulation of bedding to generate PM. Error bars represent standard error (n = 8).
Bedding Material
Agitation of all bedding materials tested resulted in dust spikes; however, only slight differences were observed based on bedding type. For the manual manipulation of bedding in unoccupied cages, neither the peak nor the average concentrations were significantly different between pine and corn bedding. In occupied cages, where concentrations are impacted by both the bedding material and uncontrolled rodent behavior, peak concentrations were higher in cages with corncob bedding compared to pine but not statistically significantly either in the filter top present configuration (417±87 vs. 214±43 μg/m3; p = 0.13) or in the filter top absent configuration (195±22 vs. 162±41 μg/m3; p = 0.65). However, time-weighted average concentrations were significantly higher for corncob bedding than pine bedding in the filter top present configuration (113±18 μg/m3 vs. 27±7 μg/m3; p < 0.001) but differences were not significant when the filter top was absent (29±10 μg/m3 vs. 13±3 μg/m3; p = 0.12).
Filter top Lids
The presence of a filter top lid on the cages was the foremost determinant of PM2.5 concentrations inside the cages. Results from manual manipulation experiments in unoccupied cages (Figure 2 (c) and (d)), indicated that the concentrations were statistically significantly higher when filter top was present for both pine bedding (p <0.001) and corncob bedding (p = 0.002). Figure 2 (a) and (b) compare data from occupied cages for these two bedding materials (pine shavings and corncob) for the filter top absent and present configurations. (Data for aspen shaving bedding is shown in Figure S2 of the Supporting Information.) Elevated concentrations were more frequently observed at the beginning of the one-hour experiments, i.e., when the rats were settling in to their new cages and nesting with determination. Concentrations were not statistically significantly different for the filter top absent versus present scenario for pine bedding (p = 0.10) but were so (lower when filter top was absent) for corncob bedding (p = 0.001).
Ventilation Rate
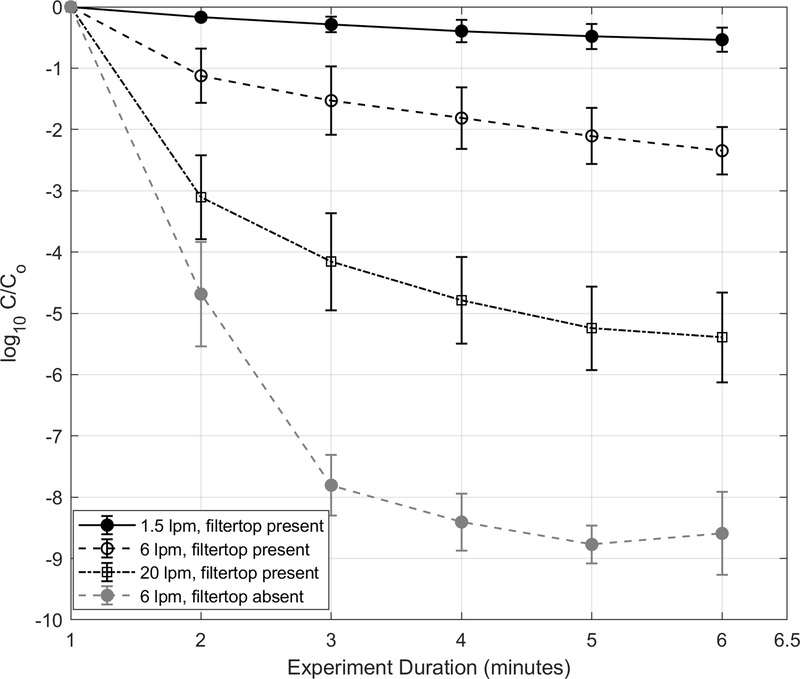
The ventilation rate resulting from air turnover inside the cages was also a significant determinant of the PM2.5 concentrations. [Insert Figure 3 near here.] Higher peak and average concentrations and slower attenuation rates were observed at lower ventilation rates. Data from mechanical stirring of the bedding at different flow and filter top lid configurations are summarized in Figure 3 (bedding material for these experiments was pine shavings). Trial-to-trial the peak concentration (Co) varied despite adherence to study protocol of 30 s of mechanical stirring for these characterization tests. Nonetheless, concentrations were lower at higher ventilation rates. Average peak PM2.5 concentrations with filter top present were 2900±270 μg/m3 and 1800±390 μg/m3 during low (6 L/min) and high (20 L/min) air turnover in the cage. They were further lower when filter top was absent; peak PM2.5 concentrations with no filter top were 1900±180 μg/m3 and 970±240 μg/m3 during low and high air turnover in the cage. Attenuation is expected to be faster with no filter top because there are no restrictions on air exchange between the cage’s micro- and macro-environment, which rapidly diluted the spikes. Peak concentrations decayed by 90% (i.e., C/Co = 0.1) within 10.3±1, 4.8±1, <1 and <1 (t90) minutes after the was mechanically stirred at flow rate-lid scenarios of 1.5 L/min-filter top present (air exchange rate ~ 2.25), 6 L/min-filter top present (air exchange rate ~ 9), 20 L/min-filter top present (air exchange rate ~ 31) and 6 L/min-filter top absent scenarios, respectively, i.e., slower at lower ventilation rates.
Figure 3.
PM2.5 concentration decay curves of mechanically generated dust from pine bedding material inside the cages during filter top absent and filter top present (at four different ventilation rates) conditions. Error bars represent standard deviation.
Attenuation Rates
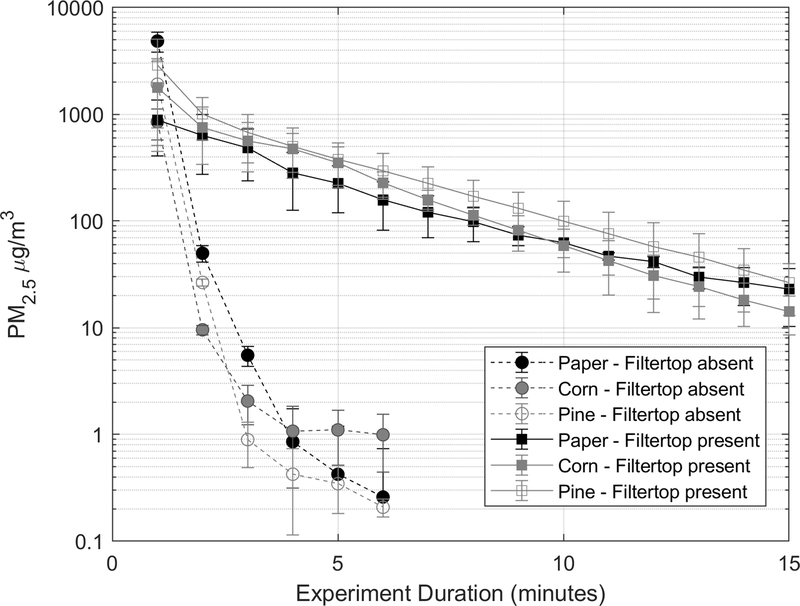
Figure 4 further contrasts the attenuation rates of mechanical stirring generated PM spikes for three bedding types (pine shavings, paper and corncob) for the filter top absent and present scenarios at flow rate of 6 L/min through the cage (about 9 air changes per hour; lower end of the range employed in individually ventilated cages). [Insert Figure 4 near here.] When filter top was absent, PM2.5 concentrations attenuated by over 90% within a minute (t90) while when the cage was lidded with filter top 90% attenuation took longer (five to eight minutes; see Table 1). This is expected because filter top restricts turnover of cage air and thus entraps PM or other materials like ammonia or dander generated inside the cage. First degree decay curves fitted to this data indicated that overall attenuation rates (resulting from the combined effects of air turnover causing dilution, gravitational settling, and losses to the cage walls) for the same forced airflow of 6 L/min through the cage were approximately 5–10 times higher when filter top was absent compared to it being present.
Figure 4.
PM2.5 concentration decay curves of mechanically generated dust from pine bedding material inside the cages during filter top lid present and absent conditions. Error bars represent standard deviation. The flow rate through the cage was 6 L/min. See Table 1 for corresponding decay constants.
Table 1:
PM2.5 attenuation rates (based on eight trials) and T90 (time for 90% attenuation).
| Bedding Material |
Filter top | Flowrate through the cage (L/min) |
Air exchange rate per hour |
Decay/Attenuation rate per hour* |
T90 (minutes)** |
|---|---|---|---|---|---|
| Pine | present | 6 | 9 | 51±27 | 4.8 ± 1.7 |
| Pine | present | 20 | 31 | 76±11 | < 1 |
| Pine | absent | 6 | unconfined | 141±11 | < 1 |
| Paper | present | 6 | 9 | 20±4 | 7.6 ± 2.1 |
| Paper | absent | 6 | unconfined | 129±20 | < 1 |
| Corn | present | 6 | 9 | 34±14 | 4.8 ± 1.3 |
| Corn | absent | 6 | unconfined | 196±28 | < 1 |
Average of fits for individual trials ± standard deviation; n = 8 for 6 L/min and n = 3 for 20 L/min. Based on exponential fit on the first fifteen minutes of data for filtertop lid present and first five minutes for filter top absent configuration.
Average of T90 values rounded to the nearest minute for individual trials
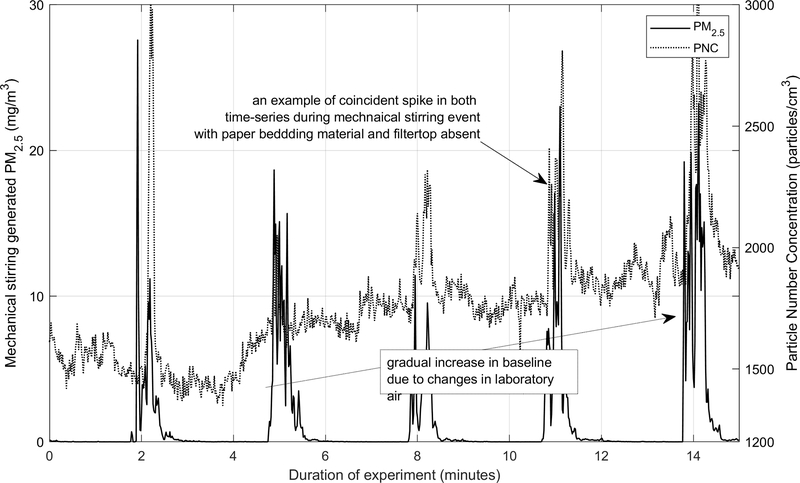
Bedding-generated ultrafine particle number concentrations
Particle number concentrations also spiked concurrently with PM2.5 suggesting a common source. [Insert Figure 5 near here.] Figure 5 shows that PNC spiked repeatedly from mechanical stirring of paper bedding in the filter top absent configuration and it also shows the comparatively gradual changes in the laboratory air PNC (also, see Figure S2 in the Supporting Information for corncob bedding in the filtertop present configuration). Spikes in PNC attenuated rapidly, almost instantly after the 30-second stirring event, to the laboratory background. Although we observed that bedding also generated PNC spikes, the change in concentrations was modest, limited to 500 – 1000 particles/cm3, well below the indoor concentration expected in the animal facilities or even the ambient.
Figure 5.
Concurrent PM2.5 and particle number concentrations generated via mechanical stirring of paper bedding material during filter top absent configuration.
Limitations
Our study had several limitations. First, we under report bedding-generated PM concentrations because we only measure the fine fraction, PM2.5, of the total bedding-generated PM; the total airborne or inhalable PM is expected to be higher. Second, reported intra-cage PM2.5 concentrations were likely at the lower end of the expected range because of low animal-facility/macro-environment concentrations. Both occupied and activity simulation trials were conducted in environments with good airflow, low ambient PM, good air filtration, and only a few cages in the room. Macro-environments with high cage rack and cage densities and/or poor air flow will have higher PM levels and slower decay rates. Most rodent facilities, especially considering the popularity of main colony rooms, are going to have substantially high cage densities. Third, the assessment of rat-activity generated PM was performed during the daytime, when rats are less active. Fourth, we did conduct measurements inside nests where concentrations would likely be higher. Because rats are nocturnal, digging and locomotor activity and associated PM levels would likely be greater at night and inside nests. Fifth, we did not assess the impact of varying humidity on PM concentration and attenuation rates. The recommended range is 30–70% (NAP, 2011); both animal exposure and laboratory manipulation experiments were conducted at the higher end of that range.
Further, we tested air exchange rates that are rather at the lower end of those employed in individually ventilated cages where air exchange rates range from 20–125 times per hour (Langham et al., 2006). Increasing ventilation or air exchange rates will lower bedding-generated PM exposures for rodents but the drafts caused by higher air velocities in individually ventilated cages may also have detrimental effects on animals (Baumans et al., 2002). Conversely, standard non-ventilated cages or static isolation cages, especially those with filter top lids, have much lower air exchange rates than we studied. Because our results indicate that increased ventilation leads to faster decay rates and lower intra-cage PM concentrations, it follows that bedding-generated PM attenuates slower in such cages.
Implications
Our results showed that bedding-generated dust elevates PM concentrations inside rat cages and that elevated PM concentrations persist substantially longer with the use of filter tops. These findings were consistent across four common types of bedding (pine shavings, corncob, paper, and aspen shavings). These results are broadly relevant because the cage configurations in the present study are widely used in rodent based research. Individually ventilated cages are widely used but are not adequately detailed in scientific studies (Perkins and Lipman, 2017). For example, PM inhalation studies such as Campbell et al., 2005 and Guerra et al., 2013 employed individually ventilated cages as housing for rodents, but bedding materials and resulting bedding-generated PM exposures were not mentioned.
We found that in occupied cages (Figure 2) time-weighted PM2.5 concentrations for pine bedding were 2–28 μg/m3 with filter tops absent and 4–47 μg/m3 with filter tops present. For corncob bedding, corresponding values were 8–89 μg/m3 and 56–194 μg/m3, respectively. These concentrations were comparable to those associated with adverse health effects in animal models. For example, Batalha et al. (2002) found that exposure to ambient PM at concentrations of 75 μg/m3 or above (median: 183 μg/m3) induced vasoconstriction in rodent models and Kleinman et al. (2005) found evidence of immune activation in mice brains following exposure to 30.4 μg/m3 of ambient PM.
Our results suggest that bedding generated PM exposures are not negligible and inclusion of information on bedding and housing conditions and measures taken to minimize such exposures in study reports may be pertinent to interpretation of PM exposures and study outcomes, in particular, low dose PM exposures. Furthermore, literature is sparse on investigations of health effects of exposure to bedding-generated PM in rodents and if there are any implications for baseline health of animals. Further study is needed to address this question.
Additionally, the following questions could be investigated. What are the physical and chemical attributes of bedding-generated PM and how do they affect experiments involving ambient PM, which may have very different physical and chemical properties? Are the effects of bedding-generated PM different depending on the age, gender, and species of animals being tested? Are the effects of bedding-generated PM different depending on whether the bedding is soiled with animal waste? (Animal bedding is often contaminated with mycobacteria, fungi, bacteria, and endotoxins which are picked up by bedding-generated particulate matter (Kaliste et al., 2004). Exposure to endotoxins, which are ubiquitous in the cage micro-environment (Whiteside et al., 2010), is associated with adverse respiratory outcomes (Alexis et al., 2006; Harper and Andrew, 2006; Yeatts et al., 2007) and can cause inflammatory changes both in the respiratory tract and systemically (Rylander, 2002).) In experiments where PM exposure is administer while animals are housed with bedding material, how do bedding-generated and experimental-exposure PM interact?
In conclusion, bedding-generated PM exposure needs to be better characterized to ensure that the laboratory rodent housing micro-environment does not impact the baseline animal health and hence the outcomes of exposure experiments, for example, pre-exposure or co-exposure with PM from bedding may exacerbate the effect of exposure under investigation, which may not be accounted for by the control group.
Supplementary Material
Acknowledgements
We are grateful to Jack Bitney and Richard Gilland, undergraduate seniors in Department of Civil and Environmental Engineering at Tufts University, for assistance with data collection. This work was supported by the NIH under grant R01ES026980 and Tufts Provost Office via an award to BCN, DB, and JLD to support collaborative inflammation research. Authors also acknowledge the helpful commentary provided by the reviewers that improved and clarified this manuscript.
Footnotes
Disclosure Statement
The authors declare no conflict of interest.
References
- Alexis N, Lay J, Zeman K, Bennett W, Peden D, Soukup J, Devlin R, Becker S, 2006. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J. Allergy Clin. Immunol 117, 1396–1403. 10.1016/j.jaci.2006.02.030 [DOI] [PubMed] [Google Scholar]
- Batalha JRF, Saldiva PHN, Clarke RW, Coull BA, Stearns RC, Lawrence J, Murthy GGK, Koutrakis P, Godleski JJ, 2002. Concentrated ambient air particles induce vasoconstriction of small pulmonary arteries in rats. Environ. Health Perspect 110, 1191–7. 10.1289/ehp.021101191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumans V, Schlingmann F, Vonck M, van Lith HA, 2002. Individually ventilated cages: beneficial for mice and men? Contemp. Top. Lab. Anim. Sci 41, 13–9. [PubMed] [Google Scholar]
- Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, Misra C, Mendez LB, Kleinman M, 2005. Particulate Matter in Polluted Air May Increase Biomarkers of Inflammation in Mouse Brain. Neurotoxicology 26, 133–140. 10.1016/J.NEURO.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Geertsema RS, Lindsell CE, 2015. Effect of Room Ventilation Rates in Rodent Rooms with Direct-Exhaust IVC Systems. J. Am. Assoc. Lab. Anim. Sci 54, 521–6. [PMC free article] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, Van Berlo D, Cassee FR, Schins RP, Wang K, Campbell A, 2010. Open Access RESEARCH Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain, Particle and Fibre Toxicology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra R, Vera-Aguilar E, Uribe-Ramirez M, Gookin G, Camacho J, Osornio-Vargas AR, Mugica-Alvarez V, Angulo-Olais R, Campbell A, Froines J, Kleinman TM, De Vizcaya-Ruiz A, 2013. Exposure to inhaled particulate matter activates early markers of oxidative stress, inflammation and unfolded protein response in rat striatum. Toxicol. Lett 222, 146–154. 10.1016/J.TOXLET.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema JR, Keeler G, Wagner J, Morishita M, Timm E, Hotchkiss J, Marsik F, Dvonch T, Kaminski N, Barr E, 2004. Effects of concentrated ambient particles on normal and hypersecretory airways in rats. Res. Rep. Health. Eff. Inst 1–68 discussion 69–79. [PubMed] [Google Scholar]
- Harper M, Andrew ME, 2006. Airborne endotoxin in woodworking (joinery) shops. J. Environ. Monit 8, 73–8. 10.1039/b508065g [DOI] [PubMed] [Google Scholar]
- Kacergis JB, Jones RB, Reeb CK, Turner WA, Ohman JL, Ardman MR, Paigen B, 1996. Air Quality in an Animal Facility: Particulates, Ammonia, and Volatile Organic Compounds. Am. Ind. Hyg. Assoc. J 57, 634–640. 10.1080/15428119691014693 [DOI] [PubMed] [Google Scholar]
- Kaliste E, Linnainmaa M, Meklin T, Torvinen E, Nevalainen A, 2004. The bedding of laboratory animals as a source of airborne contaminants. Lab. Anim 38, 25–37. 10.1258/00236770460734362 [DOI] [PubMed] [Google Scholar]
- Kleinman MT, Araujo JA, Nel A, Sioutas C, Campbell A, Cong PQ, Lia H, Bondy SC, 2008. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol Lett 178, 127–130. 10.1016/j.toxlet.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman MT, Hamade A, Meacher D, Oldham M, Sioutas C, Chakrabarti B, Stram D, Froines JR, Cho AK, 2005. Inhalation of Concentrated Ambient Particulate Matter near a Heavily Trafficked Road Stimulates Antigen-Induced Airway Responses in Mice. J. Air Waste Manage. Assoc 55, 1277–1288. 10.1080/10473289.2005.10464727 [DOI] [PubMed] [Google Scholar]
- Langham GL, Hoyt RF, Johnson TE, 2006. Particulate matter in animal rooms housing mice in microisolation caging. J. Am. Assoc. Lab. Anim. Sci 45, 44–8. [PubMed] [Google Scholar]
- Memarzadeh F, Harrison PC, Riskowski GL, Henze T, 2004. Comparison of environment and mice in static and mechanically ventilated isolator cages with different air velocities and ventilation designs. Contemp. Top. Lab. Anim. Sci 43, 14–20. [PubMed] [Google Scholar]
- NAP, 2011. Guide for the care and use of laboratory animals 8th Edition Committee for the Update of the Guide for the Care and Use of Laboratory Animals Institute for Laboratory Animal Research Division on Earth and Life Studies. [Google Scholar]
- NIOSH, 1998. NIOSH alert: preventing asthma in animal handlers. 10.26616/NIOSHPUB97116 [DOI]
- Perkins SE, Lipman NS, 2017. Properly Describing Individually Ventilated Caging Systems in Scientific Manuscripts. J. Am. Assoc. Lab. Anim. Sci 56, 488. [PMC free article] [PubMed] [Google Scholar]
- Reeb CK, Jones RB, Bearg DW, Bedigian H, Paigen B, 1997. Impact of Room Ventilation Rates on Mouse Cage Ventilation and Microenvironment. Contemp. Top. Lab. Anim. Sci 36, 74–79. [PubMed] [Google Scholar]
- Rosenbaum MD, Vandewoude S, Volckens J, Johnson TE, 2010. Disparities in Ammonia , Temperature , Humidity , and Airborne Particulate Matter between the Micro-and Macroenvironments of Mice in Individually Ventilated Caging 49, 177–183. [PMC free article] [PubMed] [Google Scholar]
- Rylander R, 2002. Endotoxin in the environment – exposure and effects. J. Endotoxin Res 8, 241–252. 10.1179/096805102125000452 [DOI] [PubMed] [Google Scholar]
- Schweitzer IB, Smith E, Harrison DJ, Myers DD, Eggleston PA, Stockwell JD, Paigen B, Smith AL, 2003. Reducing exposure to laboratory animal allergens. Comp. Med 53, 487–92. [PubMed] [Google Scholar]
- TSI Inc., 2018. DUSTTRAKTM DRX aerosol monitor model 8533/8534/8533 operation and service manual. [Google Scholar]
- Whiteside TE, Thigpen JE, Kissling GE, Grant MG, Forsythe D, 2010. Endotoxin, coliform, and dust levels in various types of rodent bedding. J. Am. Assoc. Lab. Anim. Sci 49, 184–9. [PMC free article] [PubMed] [Google Scholar]
- Yeatts K, Svendsen E, Creason J, Alexis N, Herbst M, Scott J, Kupper L, Williams R, Neas L, Cascio W, Devlin RB, Peden DB, 2007. Coarse particulate matter (PM2.5–10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ. Health Perspect 115, 709–14. 10.1289/ehp.9499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.