Abstract
Background
Development of skeletal muscle is closely related to broiler production traits. The myocyte-specific enhancer binding factor (MEF) 2D gene (MEF2D) and its variant transcripts play important parts in myogenesis.
Methods
To identify the transcript variants of chicken MEF2D gene and their function, this study cloned chicken MEF2D gene and identified its transcript variants from different tissue samples. The expression levels of different transcripts of MEF2D gene in different tissues and different periods were measured, and their effects on myoblast proliferation and differentiation were investigated. Variations in MEF2D were identified and association analysis with chicken production traits carried out.
Results
Four novel transcript variants of MEF2D were obtained, all of which contained highly conserved sequences, including MADS-Box and MEF2-Domain functional regions. Transcript MEF2D-V4 was expressed specifically in muscle, and its expression was increased during embryonic muscle development. The MEF2D-V4 could promote differentiation of chicken myoblasts and its expression was regulated by RBFOX2. The single nucleotide polymorphism g.36186C > T generated a TAG stop codon, caused MEF2D-V4 to terminate translation early, and was associated with several growth traits, especially on early body weight.
Conclusion
We cloned the muscle-specific transcript of MEF2D and preliminarily revealed its role in embryonic muscle development.
Keywords: MEF2D, Chicken, Myoblast, Variant transcripts, SNP
Introduction
The myocyte-specific enhancer binding factor 2 (MEF2) family is widely present in muscle cells. It plays an important role in the development, growth and maintenance of organisms through interacting with various genes in the calcineurin signaling pathway (Potthoff & Olson, 2007). MEF2 is a major regulator of myogenic genes expression, which can activate expression of various myogenic related genes, and interact with members of myogenic regulatory factors to regulate myogenesis (Molkentin et al., 1995; Desjardins & Naya, 2016). In vertebrates, the MEF2 family has four members, including MEF2A, MEF2B, MEF2C and MEF2D genes. MEF2 belongs to the MADS-Box family of transcription regulators. The N-terminal of the four MEF2 proteins all contain the highly conserved MADS-box domain and MEF2 domain. The structural difference among them is due mainly to the difference in C-terminal transcriptionally active regions (Molkentin et al., 1996; Black & Olson, 1998). Breitbart et al. (1993) first cloned MEF2D in humans, and found that it plays a key role in muscle development. As a member of MEF2 family, MEF2D has been reported that plays a key role in myogenesis. In MEF2D knockout mice, the differentiation of muscle cells in each muscle tissue was found to be inhibited (Bour et al., 1995; Lilly et al., 1995). The MEF2D has also been found to be involved in skeletal myogenesis, cardiac hypertrophic growth and proliferation of vascular smooth muscle cells (Ogawa, Sakakibara & Kamemura, 2013; Hu et al., 2017; Li et al., 2017).
The chicken MEF2D gene has been cloned, but only one transcript has been reported (Caldwell et al., 2004). In humans and mice, multiple different transcripts of MEF2D gene have been found, and these transcripts can perform different functions (Ogawa, Sakakibara & Kamemura, 2013; Sebastian et al., 2013). In this study, we aim to identify the variant transcripts of chicken MEF2D gene from different tissue samples, measure expression levels of these transcripts in various tissues and at different periods, and to study their roles in skeletal myogenesis.
Materials and Methods
Animals
The fertilized eggs of Xinghua chicken in this experiment were purchased from a livestock farm of South China Agricultural University (Guangzhou, China). They were hatched in a full-automatic incubator. During the period from the 10th embryo age (E10) to the 1st day post-hatching (P1), the breast muscle and leg muscle tissues of 20 chickens were collected each day and stored at −80 °C. Five 7-weeks-old Xinghua female chickens were purchased from a livestock farm of South China Agricultural University. A total of 15 tissues (cerebrum, cerebellum, hypothalamus, pituitary, heart, liver, spleen, lung, kidney, breast muscle, leg muscle, subcutaneous fat, abdominal fat, muscular stomach and glandular stomach) of each chicken were collected and stored at −80 °C.
DNA samples
The DNA samples were obtained from an F2 resource population crossed from Xinghua and White Recessive Rock (XH & WRR) as described previously (Lei et al., 2005). The population consists of 17 full-sibling families, and 434 F2 individuals (221 male and 213 female chickens) with a detailed record of growth traits, carcass traits and meat quality traits. Weight (body, semi-eviscerated, eviscerated, breast muscle, leg muscle and abdominal fat pad) was measured in grams using an electronic scale. The shank length, head width, breast width, breast depth and body length were measured with vernier caliper. The shank diameter was measured in the middle of the shank with string and straightedge.
RNA isolation, cDNA synthesis and quantitative real time PCR
Total RNA of all tissues were isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA), following the recommended manufacturer’s protocol. The quality and quantity of RNA samples were assessed by gel electrophoresis and a spectrophotometer (NanoDrop 2000c; Thermo, Waltham, MA, USA). The cDNA synthesis was performed with 1 μg of RNA for each sample using a RevertAid™ First Strand cDNA Synthesis Kit (Fementas, Waltham, MA, USA) in a total reaction volume of 20 μl.
The mRNA level of MEF2D and its four variant transcripts, RBFOX2, MHC and MYOD were measured by qPCR. The qPCR was performed using SsoFast Eva Green Supermix (BIO-RAD, Hercules, CA, USA) in CFX9600 (BIO-RAD, Hercules, CA, USA). Each sample was assayed in triplicate under the following conditions: 95 °C for 2 min, followed by 40 cycles of 10 s at 95 °C, 30 s at the annealing temperature (58–62 °C), 30 s at 72 °C, a melt curve by 65–95 °C, and increments 0.5 °C for 5 s. Chicken GAPDH was used as the reference gene for tissue-samples of 7-weeks-old chickens and myoblasts, whereas 18S rRNA was used as the reference gene for embryonic muscle samples. The relative mRNA level in each sample was calculated using the comparative 2−ΔΔCt (CT is threshold cycle; ΔΔCt = ΔCttarget sample −ΔCtcontrol sample) method (Livak & Schmittgen, 2001).
Gene cloning and sequences analysis
Referring to the MEF2D gene sequence in chicken (NM_001031600.3) reported by National Center for Biotechnology Information (NCBI), primers were designed to amplify MEF2D gene by PCR. Products of PCR were purified using an Agarose Gel DNA Extraction Kit (Takara, Osaka, Japan) and then cloned into the pMD-18T vector (Takara, Osaka, Japan) according to the manufacturer’s protocol. Positive clones were identified by PCR and then sequenced by Invitrogen Co. Ltd (Guangzhou, China).
The sequencing results were analyzed and compared with the chicken genome (Gallus_Gallus-5.0/Galgalgal5; http://genome.ucsc.edu/cgi-bin/hgBlat) and MEF2D sequence (NM_001031600.3). DNAStar software (DNASTAR, Madison, WI, USA) was used to analyze the homology of the amino acid (AA) sequence of MEF2D between different species and the conserved regions of the sequence. The AA sequences of MEF2D from the other species were obtained from GenBank (Table S1).
Plasmid construction, cell culture and transfection
The coding sequences of chicken RBFOX2 and MEF2D-V4 were amplified from cDNA of chicken leg muscle using PCR, and then cloned into the pEGFP-C1 vector (Invitrogen, Guangzhou, China) using the EcoRI and BamHI restriction sites.
Chicken primary myoblasts were isolated from the leg muscle of chickens at 10–11 embryo age as described previously (Luo et al., 2014). Cells were maintained in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 20% (v/v) fetal bovine serum (Gibco, Grand Island, NY, USA), and 100 μg/ml penicillin/streptomycin (Invitrogen, Guangzhou, China) at 37 °C with 5% CO2, humidified atmosphere. Cells were seeded in 12-well plates with one ml per well at 105 cells/ml. When the cells had grown to 70–80% confluence, they were transfected with plasmids (one μg/ml) of MEF2D or RBFOX2 or pEGFP-C1 vector control using lipofectamine 3,000 reagent (Invitrogen, Guangzhou, China) according to the manufacturer’s instructions.
Cell proliferation assay
After overexpressing RBFOX2 and MEF2D genes in myoblasts for 48 h, respectively, cells were collected and fixed with 70% ethanol overnight at −20 °C. The fixed cells were collected by centrifugation at 1,000×g, washed once with PBS, and stained with 0.5 ml propidium iodide (PI) dye solution (5 mg PI + 0.1 ml Triton X-100 + 3.7 mg EDTA +10 ml PBS), and then incubate for 30 min at 4 °C in the dark. After staining, cells were detected by BD FACSAriaII flow cytometer (BD, Franklin Lakes, NJ, USA). The results were analyzed by software ModFit Lt 4.1.
Western blotting
Proteins of transfected myoblasts were extracted using RIPA lysis buffer (Beyotime, Shanghai, China) and the concentration was determined by a bicinchoninic acid protein assay kit (Beyotime, Shanghai, China). The primary antibodies MYOG (1:500 dilution; Biorbyt, Cambridge, UK) and MHC (1:1,000 dilution; DSHB, Iowa, IA, USA) were using to measure the protein levels of MYOG and MHC respectively by Western blotting as described previously (Ouyang et al., 2018). GAPDH (1:1,000 dilution; Bioworld, St Louis Park, MN, USA) was used as the reference gene.
Primers
Primers were designed using primer premier 5 software (PREMIER Biosoft, Palo Alto, CA, USA) and synthesized by Bioengineering Co., Ltd. (Shanghai, China). Specific primer sequences are shown in Tables S2 and S3.
Identification and genotyping of SNPs
Variations in the coding sequences of chicken MEF2D were identified using PCR with primers PM1–PM9 in our F2 resource population (XH & WRR). The locations of primers were shown in Fig. S1. PCR was performed in 50 μl of a mixture containing 50 ng of chicken genomic DNA, 25 pmol of primers and 25 μl PCR Master Mix (Transgen, Beijing, China), and using the following protocol: 94 °C for 3 min, followed by 32 cycles of 30 s at 94 °C, 30 s at the annealing temperature (58–63 °C), 30 s at 72 °C and 72 °C for 5 min at last. Twenty DNA samples were selected randomly from the F2 resource population (XH & WRR) for PCR using primers PM1–PM9. PCR products were sequenced by Bioengineering Co., Ltd. (Shanghai, China) and the results were then blasted with each other to identify variations. The special Single-nucleotide polymorphisms (SNPs) we were interested were genotyped by PCR and sequencing in all DNA samples of the F2 resource population (XH & WRR).
Statistical analysis
Single-nucleotide polymorphism frequencies were calculated using the observed numbers of alleles for each SNP. SNP genotypes were tested for Hardy–Weinberg equilibrium with the Chi-square test. Association analysis of SNPs and fatness traits were performed using the General Linear Models Procedures of SAS 9.0 (SAS Institute Inc., Cary, NC, USA) using the following model:
Where Y = the traits phenotypic values; µ = the overall population mean; S = the effect of gender; G = the effect of genotype; H = the effect of incubation batch; F = the effect of family; e = the random residuals.
Data on gene expression were analyzed using SPSS 21.0 (IBM, Armonk, NY, USA). The ANOVA was used to compare expression levels among different groups. All values are presented as means ± standard error of mean (SEM). The threshold for significance was set at P < 0.05 and for high significance at P < 0.01.
Animal ethics
Animal experiments were handled in compliance and all efforts were made to minimize suffering. It was approved by the Animal Care Committee of South China Agricultural University (Guangzhou, People’s Republic of China) with approval number SCAU#0014.
Results
Sequence alignment and phylogeny analysis of MEF2D
According to the information from NCBI database, the chicken MEF2D gene cDNA sequence (NM_001031600.3) is 4,111 bp in length, the coding region is 715–2,271 nt, and it encodes 518 AAs (NP_001026771.3). Blast with the chicken genome (GRCg6a/galGal6), this gene is located on chicken chromosome 25 (2,742,900–2,782,225), the full length of the gene is 39,326 bp, and it contains 10 exons and nine introns.
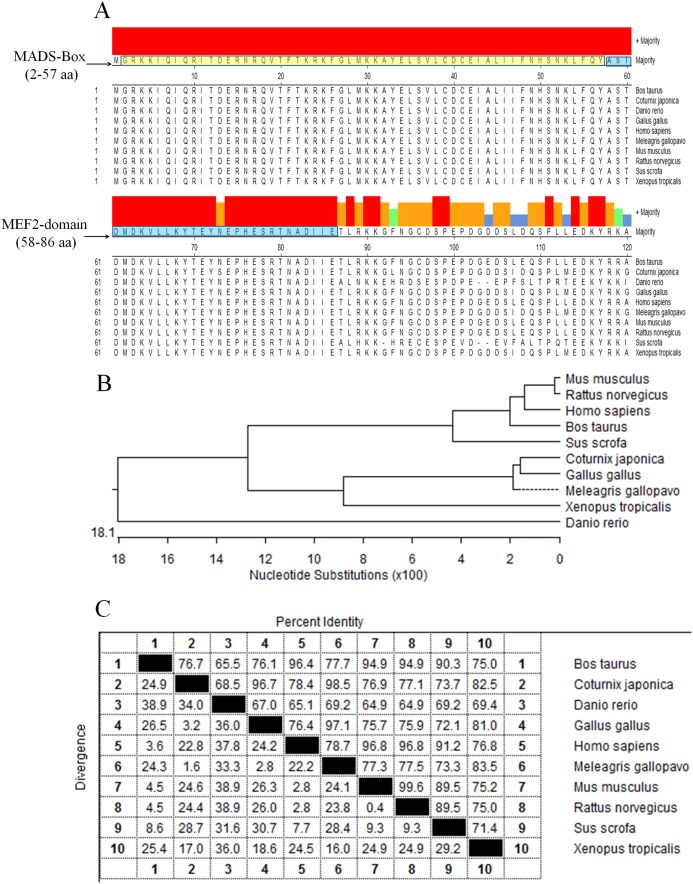
The protein sequences of MEF2D in 10 species (Gallus gallus, Meleagris gallopavo, Coturnix japonica, Homo sapiens, Mus musculus, Rattus norvegicus, Sus scrofa, Bos taurus, Danio rerio and Xenopus laevis) were compared and analyzed by homologous clustering. The results showed that the protein sequences of MEF2D were highly conserved, and had conserved domain of MADS-Box (2–57 AA) and MEF2-Domain (58–86 AA) in chicken and the other nine species tested (Fig. 1A). Phylogenetic tree clustering showed that 10 species were divided into four distinct groups: birds (Gallus gallus, Meleagris gallopavo and Coturnix japonica), mammals (Homo sapiens, Mus musculus, Rattus norvegicus, Sus scrofa and Bos taurus), Danio rerio and Xenopus laevis (Fig. 1B). Homology between chicken, turkey and quail was more than 96%. Homology among mammals (human, mouse, rat, pig and cow) was also very high, while the homology between zebrafish and frogs and other species was relatively low (Fig. 1C).
Figure 1. Analysis of MEF2D protein sequence.
(A) The highly conserved functional region of the MEF2D protein sequence. (B) Clustering analysis of MEF2D protein sequences in ten different species. (C) Homology analysis of MEF2D protein sequences in 10 different species.
Variant transcripts of chicken MEF2D
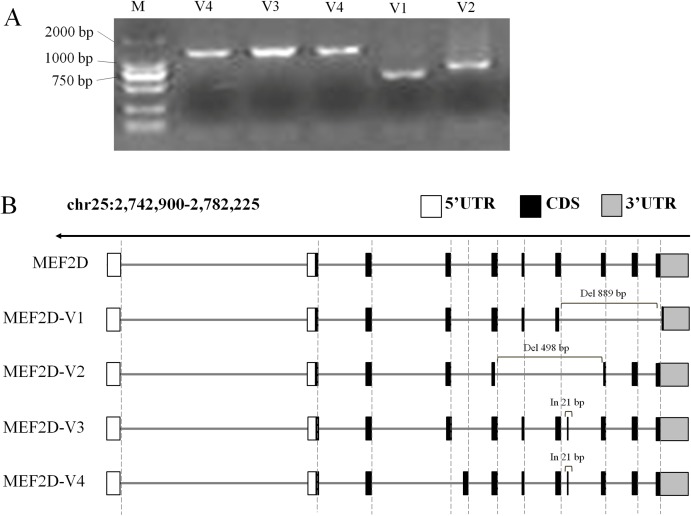
In this experiment, cDNA samples from liver, hypothalamus and muscle tissue at different stages were used as PCR templates to clone chicken MEF2D gene, and positively clone PCR products were detected by agarose gel electrophoresis (Fig. 2A). Sequencing analysis of PCR products identified four novel variant transcripts (V1–V4) of MEF2D (Fig. 2B). Compared with the transcript of MEF2D gene on NCBI, the transcript V1 (NCBI Accession Number: KY680649) was 3,222 bp in length, had a deletion of 889 bp (1,446–2,334 nt), and was predicted to encode 251 AA. The transcript V2 (KY680650) was 3,616 bp in length, had a deletion of 498 bp (1,139–1,636 nt) and was predicted to encode 353 AA. The transcript V3 (KY680651) was 4,135 bp in length, had an insertion of 21 bp after exon 8 (1,570 nt) and an AAC insertion at 1,813 nt, and was predicted to encode 526 AA. The full length of the transcript V4 (KY680652) was 4,132 bp, and a 21 bp is inserted after exon 8 (1,570 nt), and was predicted to encode 526 AA. The complete DNA and protein sequences of these four variants are shown in File S2.
Figure 2. Gene structures of various transcripts of chicken MEF2D.
(A) PCR amplification results of MEF2D gene cloning. (B) Gene structures of four novel transcripts. UTR, un-tranlated region; CDS, coding DNA sequence; In/Del, Insertion/deletion.
Comparative analysis of the AA sequences of the four novel MEF2D transcripts V1–V4 revealed that they contained conserved functional MADS-Box and MEF2-Domain. The position and sequence of exon 4 (87–132 AA) of the transcript V4 was different from that of the other transcripts. This was the same as the variant transcripts found in humans and mice, and they also mutated in the AA sequence 87–132 (Fig. S2).
Tissue specific expression of MEF2D
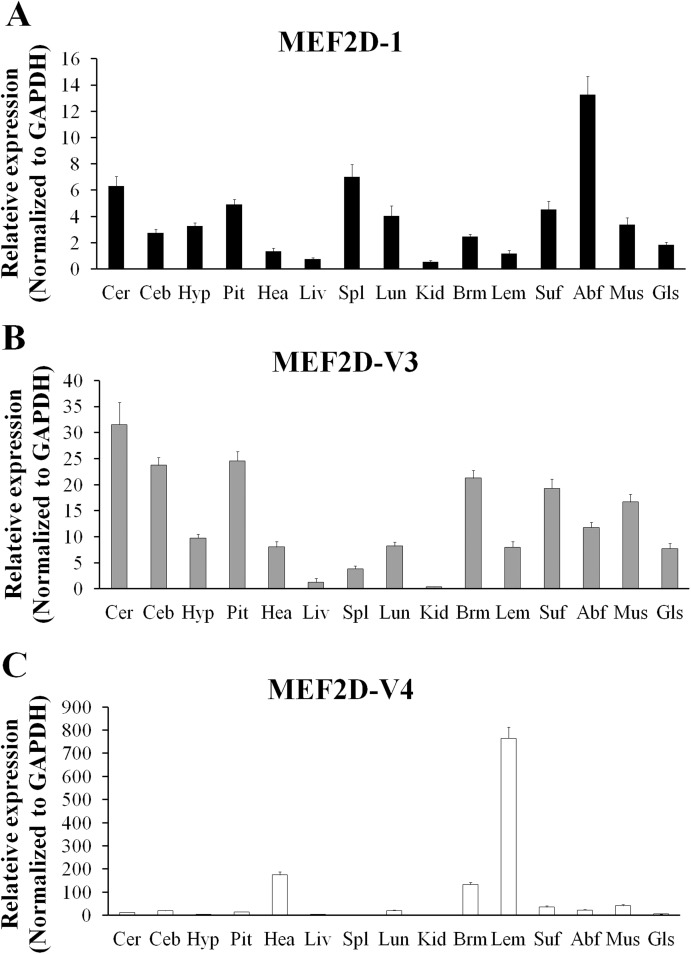
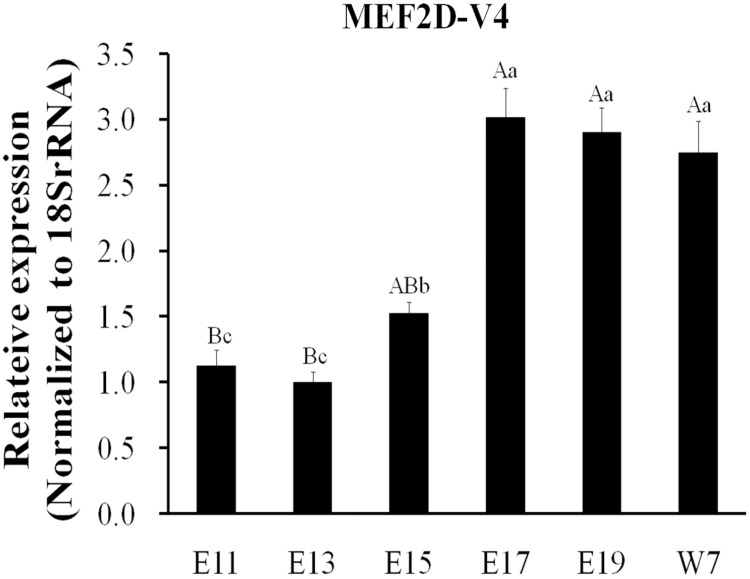
The expression of MEF2D transcripts in the different tissues of chickens was measured. Two deletion transcripts, MEF2D-V1 and MEF2D-V2 were barely expressed. The main transcripts expressed were MEF2D-1 (the same transcription as reported by NCBI), MEF2D-V3 and MEF2D-V4. MEF2D-1 and MEF2D-V3 were expressed widely in various tissues, and the relative expression levels in adipose tissue and brain tissue were higher, and in the liver and kidney were lower (Figs. 3A and 3B). The transcript MEF2D-V4 exhibited muscle-specific expression and was highly expressed in the heart, chest muscles and leg muscles, but its expression in other tissues was extremely low (Fig. 3C). We measured expression level of MEF2D-V4 in embryonic leg muscles, and found that the expression level of MEF2D-V4 increased from E11 to E19. The expression level of MEF2D-V4 increased significantly at E15 and E17, and it was stably expressed at E17 to E19 (Fig. 4).
Figure 3. The expression pattern of different MEF2D variants in various tissues of chicken.
(A) MEF2D-1. (B) MEF2D-V3. (C) MEF2D-V4. Cer, cerebrum; Ceb, cerebellum; Hyp, hypothalamus; Pit, pituitary; Hea, heart; Liv, liver; Spl, spleen; Lun, lung; Kid, kidney; Brm, breast muscle; Lem, leg muscle; Abf, abdominal fat; Suf, subcutaneous fat; Mus, muscular stomach; Gls, glandular stomach.
Figure 4. Expression patterns of MEF2D-V4 in leg muscle at different stage.
Different uppercase letters on the error bar indicated extremely significant differences (P < 0.01), different lowercase letters indicated significant differences (P < 0.05), while the same letters show no significant differences (P > 0.05).
Novel transcript MEF2D-V4 promotes myoblast differentiation in chicken
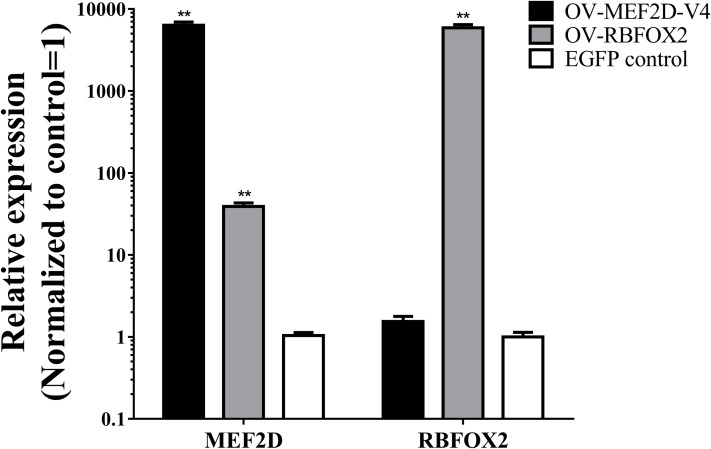
The sequence of MEF2D-V4 was similar to that of the human variant transcript Mef2Dα2, expression of which was regulated by RBFOX2 and was required for muscle differentiation (Singh et al., 2014; Runfola et al., 2015). Thus, to explore the effects of MEF2D-V4 and RBFOX2 on muscle differentiation, the eukaryotic overexpression vector of RBFOX2 and MEF2D-V4 were constructed, and transfected into chicken myoblasts respectively. After 48 h, the expression levels of RBFOX2 and MEF2D-V4 were measured by qPCR: both of these two vectors could induce overexpression of the corresponding genes effectively. Furthermore, overexpression of RBFOX2 gene could also increased the expression level of the MEF2D-V4 significantly (Fig. 5).
Figure 5. Overexpression of MEF2D and RBFOX2 in chicken myoblast.
OV-MEF2D-V4 indicates overexpression vector of MEF2D-V4, OV-RBFOX2 indicates overexpression vector of RBFOX2, EGFP control indicates control vector of pEGFP-C1. **P < 0.01.
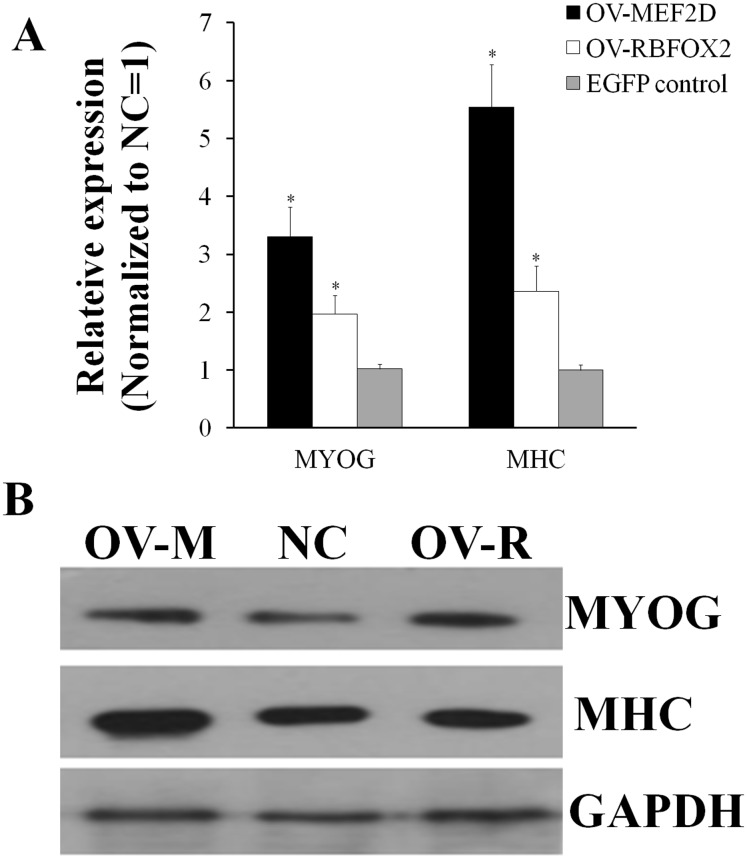
After overexpressing MEF2D-V4 and RBFOX2 in chicken primary myoblasts, the cell cycle was detected by flow cytometry. Compared with the control group, the number of S phase cells was increased in overexpressed MEF2D-V4 or RBFOX2 group, but did not reach significant levels (P > 0.05; Fig. S3). After overexpressing MEF2D-V4 and RBFOX2 for 48 h in myoblast differentiation, the mRNA level of MYOG and MHC was both increased (P < 0.05) in cells overexpressing MEF2D-V4 or RBFOX2 (Fig. 6A). The expression of MYOG and MHC was also detected by Western blot. Protein levels of MYOG and MHC were also increased, in accordance with mRNA levels (Fig. 6B).
Figure 6. Chicken MEF2D promotes primary myoblast differentiation.
(A) The expression of MYOG and MHC was determined by qPCR in primary myoblast after overexpressed MEF2D and RBFOX2. (B) The expression of MYOG and MHC was determined by Western blotting in primary myoblast after overexpressed MEF2D and RBFOX2. OV-MEF2D-V4 or OV-M indicates overexpression vector of MEF2D-V4, OV-RBFOX2 or OV-R indicates overexpression vector of RBFOX2, EGFP control or NC indicates control vector of pEGFP-C1. *P < 0.05.
SNPs identification and its association analysis with production traits
In the F2 resource population (XH & WRR), 31 SNPs were identified in the full length chicken MEF2D DNA through PCR sequencing (Table 1), including one insertion/deletion, 14 synonymous mutations and 16 missense mutations. Interestingly, there was a T–C mutation at exon 9, g.36186C > T, generate a TAG stop codon, resulting in a change in the coding sequence and termination of translation in both MEF2D-1 and MEF2D-V4. Therefore, we genotyped this SNP by PCR amplification and sequencing on exon 9, and carried out association analysis in the F2 resource population (XH & WRR). Several growth traits were associated significantly with this SNP g.36186C > T, including first days, 7, 14, 21, 28 and 63 days of body weight, 42, 77 and 88 days of shank length, 42 and 56 days of shank diameter, and 0–4 weeks of average weight gain (Table 2). The dominant genotype of SNP g.36195C > T was the CC type, and the average early body weight of TT type individuals was lower than that of CC type individuals.
Table 1. SNPs in the chicken MEF2D gene.
| SNP name | Site in the gene | Mutation type | Note |
|---|---|---|---|
| g.26427T > G | exon5 | Missense | Thr/Pro |
| g.26446G > T | exon5 | Missense | Pro/Asn |
| g.26501G > A | exon5 | Synonymous | |
| g.26561T > G | exon5 | Synonymous | |
| g.26564C > G | exon5 | Missense | Gln/His |
| g.26590A > C | exon5 | Missense | Val/Gly |
| g.26608T > G | exon5 | Missense | Gln/Pro |
| g.26621A > C | exon5 | Missense | Ser/Arg |
| g.28390C > T | exon6 | Synonymous | |
| g.28405G > A | exon6 | Synonymous | |
| g.28423T > C | exon6 | Synonymous | |
| g.30792A > G | exon7 | Missense | Ser/Pro |
| g.30808G > A | exon7 | Missense | Pro/Leu |
| g.30852A > T | exon7 | Missense | Ser/Thr |
| g.30857A > G | exon7 | Synonymous | |
| g.30860C > G | exon7 | Synonymous | |
| g.30866T > G | exon7 | Synonymous | |
| g.30888A > G | exon7 | Missense | Ser/Pro |
| g.30892T > G | exon7 | Missense | Asn/Thr |
| g.30921T > G | exon7 | Missense | Thr/Pro |
| g.33959C > G | exon8 | Missense | Ala/Pro |
| g.36092A > G | exon9 | Synonymous | |
| g.36094CAGIns/Del | exon9 | Insert/Delete | |
| g.36137A > G | exon9 | Synonymous | |
| g.36176A > G | exon9 | Synonymous | |
| g.36179A > G | exon9 | Synonymous | |
| g.36186C > T | exon9 | Missense | Gln/stop |
| g.37162T > C | exon10 | Synonymous | |
| g.37187T > G | exon10 | Missense | Thr/Pro |
| g.37270T > G | exon10 | Synonymous | |
| g.37287T > G | exon10 | Missense | His/Pro |
Table 2. SNP g.36186C > T associated with growth traits in chicken.
| Traits | P-value | Least-mean-squares ± s.e.m | ||
|---|---|---|---|---|
| BW1 (g) | 0.0001 | 26.59 ± 0.64C (TT, 15) |
28.84 ± 5.05B (TC, 56) |
29.91 ± 0.16A (CC, 251) |
| BW7 (g) | 0.0004 | 54.90 ± 2.48AB (TT, 15) |
55.08 ± 1.17B (TC, 56) |
59.85 ± 0.54A (CC, 251) |
| BW14 (g) | 0.0001 | 116.62 ± 4.61AB (TT, 15) |
113.80 ± 2.39B (TC, 56) |
125.44 ± 1.13A (CC, 251) |
| BW21 (g) | 0.0007 | 193.15 ± 8.25AB,b (TT, 15) |
195.66 ± 4.35B,b (TC, 56) |
221.01 ± 2.05A,a (CC, 251) |
| BW28 (g) | 0.0064 | 294.51 ± 11.95AB (TT, 15) |
291.62 ± 6.19B (TC, 56) |
312.22 ± 2.94A (CC, 251) |
| BW63 (g) | 0.0264 | 949.07 ± 40.02ab (TT, 15) |
965.01 ± 23.14b (TC, 56) |
1023.99 ± 11.65a (CC,251) |
| SL42 (mm) | 0.0159 | 59.38 ± 1.07AB (TT, 15) |
59.20 ± 0.58B (TC, 52) |
60.90 ± 0.26A (CC, 248) |
| SL77 (mm) | 0.0417 | 84.23 ± 1.67b (TT, 9) |
88.34 ± 1.06a (TC, 23) |
88.80 ± 0.60a (CC, 69) |
| SL84 (mm) | 0.0465 | 86.95 ± 1.81ab (TT, 9) |
90.96 ± 0.95a (TC, 33) |
88.63 ± 0.43b (CC,157) |
| SD42 (mm) | 0.0069 | 7.75 ± 0.17AB (TT, 15) |
7.56 ± 0.093B (TC, 52) |
7.89 ± 0.043A (CC, 248) |
| SD56 (mm) | 0.014 | 8.63 ± 0.20AB (TT, 15) |
8.47 ± 0.10B (TC, 56) |
8.79 ± 0.049A (CC, 248) |
| 0-4 Wks ADG (g/w) | 0.0149 | 9.57 ± 0.42AB (TT, 15) |
9.38 ± 0.22B (TC, 56) |
10.07 ± 0.10A (CC, 251) |
Notes:
BW, body weight; SL, shank length; SD, shank diameter; 0–4 WKs ADG (g/w), 0–4 weeks of average weight gain (g/week).
Letters and numbers in bracket refer to genotype and number of chickens with that genotype.
P < 0.05.
P < 0.01.
In addition, g.36094CAGIns/Del (another SNP site of exon 9) was associated with carcass traits in chickens (Table 3), including eviscerated weight (EW), leg muscle weight (LMW), abdominal fat pad weight (AFW) and small intestine length (SIL). The dominant genotype of g.36094CAGIns/Del was the Del/del type. EW and LMW of the Del/del type were lower than that of the Ins/ins type.
Table 3. SNP g.36094CAGIns/Del associated with carcass traits in chicken.
| Traits | P-value | Least-mean-squares ± s.e.m | ||
|---|---|---|---|---|
| EW (g) | 0.0042 | 1093.2 ± 107.3A (Ins/ins, 62) |
1075.5 ± 114.2AB (Ins/del, 80) |
1024.9 ± 102.5B (Del/del, 160) |
| LMW (g) | 0.013 | 119.5 ± 13.2a (Ins/ins, 62) |
117.4 ± 14.3a (Ins/del, 80) |
112.6 ± 10.8b (Del/del, 160) |
| AFW (g) | 0.021 | 24.33 ± 2.54b (Ins/ins, 54) |
28.92 ± 4.24a (Ins/del, 69) |
26.91 ± 4.16ab (Del/del, 142) |
| SIL (mm) | 0.028 | 133.6 ± 15.4ab (Ins/ins, 50) |
149.5 ± 11.5a (Ins/del, 62) |
136.2 ± 10.2b (Del/del, 137) |
Notes:
EW, eviscerated weight; LMW, leg muscle weight; AFW, abdominal fat pad weight; SIL, small intestine length.
Letters and numbers in bracket refer to genotype and number of chickens with that genotype.
P < 0.05.
P < 0.01.
Discussion
MEF2D gene is a member of the MEF2 family and plays a key role in myogenesis (Du et al., 2008; Nebbioso et al., 2009; Della et al., 2012). MEF2D gene has several transcripts in humans and mice, and among them, there are specific transcripts that can have different functions (Ogawa, Sakakibara & Kamemura, 2013; Sebastian et al., 2013), but only one transcript sequence has been reported in chicken. Therefore, we cloned the variant transcript of the MEF2D gene from several different tissues of chicken, and obtained four novel transcripts. Blast analyses of their AA sequences revealed that they all contained the conserved functional regions MADS-Box and MEF2-Domain, which conformed to the structural characteristics of the MEF2 family.
The position and sequence of exon 4 of the transcript MEF2D-V4 was different from that of the original transcript MEF2D-1, that is, the AA sequence 87–132 was different. The mutation region of this new transcript was similar to the variant transcript found in humans and mice, and they were both mutated in the AA sequence 87–132 region (Edmondson et al., 1994; Ornatsky & McDermott, 1996; Nagar et al., 2017). The human variant transcript Mef2Dα2 is expressed specifically in muscle, and it can avoid inhibitory phosphorylation, recruit Ash2L to activate muscle-related genes, and promote muscle cell differentiation (Sebastian et al., 2013). Splice variations were also found in mice, producing two transcripts, Mef2D1a and Mef2D1b. Mef2D1a can promote the expression of MYOG gene by binding to its promoter, and such binding is regulated by glycosylation (Ogawa, Sakakibara & Kamemura, 2013). We examined the expression patterns of these four novel transcripts, and found that MEF2D-V4 was also expressed specifically in muscle of the heart, breast and leg. The function of muscle-specific genes is often related to muscle development and growth. During embryonic development, the expression level of MEF2D-V4 in leg muscle was increased significantly in the late stage embryos, indicating that MEF2D-V4 may play an important role in embryonic muscle development and growth. Therefore, we studied further the function of MEF2D-V4 in chicken primary myoblasts. It has been reported in mice that RBFOX2 regulates alternative splicing of the MEF2D gene (Singh et al., 2014; Runfola et al., 2015). We also found that chicken RBFOX2 promotes the expression of MEF2D-V4. Overexpression of RBFOX2 and MEF2D-V4 promoted the differentiation of chicken myoblasts.
Studies have shown that SNPs of MEF2D gene could affect the production performance of livestock and poultry animals. The MEF2D variants have been found to be highly correlated with MEF2D mRNA and protein levels in the longissimus dorsi muscle of cattle (Juszczuk-Kubiak et al., 2012). In duck, a CAG repeat polymorphism has been found in MEF2D gene. This CAG repeat can generate significantly longer transcription products and positive correlations with five muscle-related traits (Wang et al., 2016). We also found that a CAG insertion/deletion in MEF2D gene was associated with EW and LMW of chicken. Furthermore, g.36186C > T was found to be associated with body weight at 1, 7, 14, 21 and 28 days. This mutation generated a TAG stop codon, caused MEF2D-V4 to terminate translation early, resulting in TT type individuals not being able to produce normal MEF2D-V4 protein products. The average early body weight of TT type individuals was lower than that of CC type individuals, which indicated that MEF2D V4 may be positively correlated with chicken growth traits and promote early growth of chickens.
Conclusions
In summary, the MEF2D gene can produce the muscle-specific transcript MEF2D-V4, which is positively regulated by RBFOX2 and can promote the differentiation of chicken myoblasts. The chicken MEF2D gene could regulate the embryonic development and early growth of skeletal muscle by alternative splicing.
Supplemental Information
Flow Cytometry raw data of cell cycle analysis for myoblast transfected with EGFP-Control (A) or OV-RBFOX2 (B) or OV-MEF2D-V4 (C). (D) Statistical results of cell population. Bars represent S.E.M (n = 4).
Funding Statement
This work was supported by the grants from the Science and Technology Planning Project of Guangzhou City (201504010017) and Guangdong Province (2018B020203001), and the Ten-Thousand Talents Program of China (W03020593). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Hongjia Ouyang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Jiao Yu performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Xiaolan Chen performed the experiments, prepared figures and/or tables, and approved the final draft.
Zhijun Wang performed the experiments, prepared figures and/or tables, and approved the final draft.
Qinghua Nie conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Animal experiments were approved by the Animal Care Committee of South China Agricultural University (Guangzhou, People’s Republic of China) with approval number SCAU#0014.
DNA Deposition
Data Availability
References
- Black & Olson (1998).Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annual Review of Cell and Developmental Biology. 1998;14(1):167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- Bour et al. (1995).Bour BA, O’Brien MA, Lockwood WL, Goldstein ES, Bodmer R, Taghert PH, Abmayr SM, Nguyen HT. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes & Development. 1995;9(6):730–741. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- Breitbart et al. (1993).Breitbart RE, Liang CS, Smoot LB, Laheru DA, Mahdavi V, Nadal-Ginard B. A fourth human MEF2 transcription factor, hMEF2D, is an early marker of the myogenic lineage. Development (Cambridge, England) 1993;118:1095–1106. doi: 10.1242/dev.118.4.1095. [DOI] [PubMed] [Google Scholar]
- Caldwell et al. (2004).Caldwell RB, Kierzek AM, Arakawa H, Bezzubov Y, Zaim J, Fiedler P, Kutter S, Blagodatski A, Kostovska D, Koter M, Plachy J, Carninci P, Hayashizaki Y, Buerstedde J-M. Full-length cDNAs from chicken bursal lymphocytes to facilitate gene function analysis. Genome Biology. 2004;6(1):R6. doi: 10.1186/gb-2004-6-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della et al. (2012).Della GB, Armand A-S, Lecolle S, Charbonnier F, Chanoine C. Mef2d acts upstream of muscle identity genes and couples lateral myogenesis to dermomyotome formation in Xenopus laevis. PLOS ONE. 2012;7(12):e52359. doi: 10.1371/journal.pone.0052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins & Naya (2016).Desjardins CA, Naya FJ. The function of the MEF2 family of transcription factors in cardiac development, cardiogenomics, and direct reprogramming. Journal of Cardiovascular Development and Disease. 2016;3(3):26. doi: 10.3390/jcdd3030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du et al. (2008).Du M, Perry RLS, Nowacki NB, Gordon JW, Salma J, Zhao J, Aziz A, Chan J, Siu KWM, McDermott JC. Protein kinase A represses skeletal myogenesis by targeting myocyte enhancer factor 2D. Molecular and Cellular Biology. 2008;28(9):2952–2970. doi: 10.1128/MCB.00248-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson et al. (1994).Edmondson DG, Lyons GE, Martin JF, Olson EN. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120:1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2017).Hu ZQ, Luo JF, Yu XJ, Zhu JN, Huang L, Yang J, Fu YH, Li T, Xue YM, Feng YQ, Shan ZX. Targeting myocyte-specific enhancer factor 2D contributes to the suppression of cardiac hypertrophic growth by miR-92b-3p in mice. Oncotarget. 2017;8:92079–92089. doi: 10.18632/oncotarget.20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszczuk-Kubiak et al. (2012).Juszczuk-Kubiak E, Starzynski RR, Sakowski T, Wicińska K, Flisikowski K. Effects of new polymorphisms in the bovine myocyte enhancer factor 2D (MEF2D) gene on the expression rates of the Longissimus Dorsi muscle. Molecular Biology Reports. 2012;39(8):8387–8393. doi: 10.1007/s11033-012-1689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei et al. (2005).Lei MM, Nie QH, Peng X, Zhang DX, Zhang XQ. Single nucleotide polymorphisms of the chicken insulin-like factor binding protein 2 gene associated with chicken growth and carcass traits. Poultry Science. 2005;84:1191–1198. doi: 10.1093/ps/84.8.1191. [DOI] [PubMed] [Google Scholar]
- Li et al. (2017).Li K, Pan J, Wang J, Liu F, Wang L. MiR-665 regulates VSMCs proliferation via targeting FGF9 and MEF2D and modulating activities of Wnt/beta-catenin signaling. American Journal of Translational Research. 2017;9:4402–4414. [PMC free article] [PubMed] [Google Scholar]
- Lilly et al. (1995).Lilly B, Zhao B, Ranganayakulu G, Paterson BM, Schulz RA, Olson EN. Requirement of MADS domain transcription Factor D-MEF2 for muscle formation in drosophila. Science. 1995;267(5198):688–693. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2014).Luo W, Wu H, Ye Y, Li Z, Hao S, Kong L, Zheng X, Lin S, Nie Q, Zhang X. The transient expression of miR-203 and its inhibiting effects on skeletal muscle cell proliferation and differentiation. Cell Death & Disease. 2014;5(7):e1347. doi: 10.1038/cddis.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin et al. (1995).Molkentin JD, Black BL, Martin JF, Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83(7):1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- Molkentin et al. (1996).Molkentin JD, Black BL, Martin JF, Olson EN. Mutational analysis of the DNA binding, dimerization, and transcriptional activation domains of MEF2C. Molecular and Cellular Biology. 1996;16(6):2627–2636. doi: 10.1128/MCB.16.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar et al. (2017).Nagar S, Trudler D, McKercher SR, Pina-Crespo J, Nakanishi N, Okamoto S-I, Lipton SA. Molecular Pathway to protection from age-dependent photoreceptor degeneration in MEF2 deficiency. Investigative Opthalmology & Visual Science. 2017;58(9):3741–3749. doi: 10.1167/iovs.17-21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbioso et al. (2009).Nebbioso A, Manzo F, Miceli M, Conte M, Manente L, Baldi A, De Luca A, Rotili D, Valente S, Mai A, Usiello A, Gronemeyer H, Altucci L. Selective class II HDAC inhibitors impair myogenesis by modulating the stability and activity of HDAC-MEF2 complexes. EMBO Reports. 2009;10(7):776–782. doi: 10.1038/embor.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, Sakakibara & Kamemura (2013).Ogawa M, Sakakibara Y, Kamemura K. Requirement of decreased O-GlcNAc glycosylation of Mef2D for its recruitment to the myogenin promoter. Biochemical and Biophysical Research Communications. 2013;433(4):558–562. doi: 10.1016/j.bbrc.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Ornatsky & McDermott (1996).Ornatsky OI, McDermott JC. MEF2 protein expression, DNA binding specificity and complex composition, and transcriptional activity in muscle and non-muscle cells. Journal of Biological Chemistry. 1996;271(40):24927–24933. doi: 10.1074/jbc.271.40.24927. [DOI] [PubMed] [Google Scholar]
- Ouyang et al. (2018).Ouyang H, Chen X, Li W, Li Z, Nie Q, Zhang X. Circular RNA circSVIL promotes myoblast proliferation and differentiation by sponging miR-203 in chicken. Frontiers in Genetics. 2018;9:172. doi: 10.3389/fgene.2018.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff & Olson (2007).Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134(23):4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- Runfola et al. (2015).Runfola V, Sebastian S, Dilworth FJ, Gabellini D. Rbfox proteins regulate tissue-specific alternative splicing of Mef2D required for muscle differentiation. Journal of Cell Science. 2015;128(4):631–637. doi: 10.1242/jcs.161059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian et al. (2013).Sebastian S, Faralli H, Yao Z, Rakopoulos P, Palii C, Cao Y, Singh K, Liu Q-C, Chu A, Aziz A, Brand M, Tapscott SJ, Dilworth FJ. Tissue-specific splicing of a ubiquitously expressed transcription factor is essential for muscle differentiation. Genes & Development. 2013;27(11):1247–1259. doi: 10.1101/gad.215400.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh et al. (2014).Singh RK, Xia Z, Bland CS, Kalsotra A, Scavuzzo MA, Curk T, Ule J, Li W, Cooper TA. Rbfox2-coordinated alternative splicing of Mef2d and Rock2 controls myoblast fusion during myogenesis. Molecular Cell. 2014;55(4):592–603. doi: 10.1016/j.molcel.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2016).Wang Y, Wang J, Liu H, Zhang R, Zhang T, Gan X, Huang H, Chen D, Li L. Discovery, characterization, and functional study of a novel MEF2D CAG repeat in duck (Anas platyrhynchos) DNA and Cell Biology. 2016;35(8):398–409. doi: 10.1089/dna.2016.3222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow Cytometry raw data of cell cycle analysis for myoblast transfected with EGFP-Control (A) or OV-RBFOX2 (B) or OV-MEF2D-V4 (C). (D) Statistical results of cell population. Bars represent S.E.M (n = 4).
Data Availability Statement
The following information was supplied regarding data availability: