Abstract
Objective:
This study aimed to examine programmed death protein 1 (PD-1) and programmed death lig-and 1 (PD-L1) expression on leukocytes from chronic apical periodontitis, and to determine the levels of cytokines in the apical periodontitis lesions.
Methods:
Leukocytes from healthy gingival tissue (n=16) and chronic apical periodontitis (n=10) were eval-uated using flow cytometry. The PD-1 and PDL-1 expressions were evaluated using flow cytometry. The cy-tokine levels were evaluated by enzyme-linked immunosorbent assay. Data were analyzed using one-way ANOVA. The statistical significance level was set at P<0.05.
Results:
Results showed that the apical periodontitis lesions are more infiltrated by PD-1+ and PDL1+ lym-phocytes than the control samples. In addition, the PDL-1 expression was detected on macrophages in the apical periodontitis lesions, and was significantly higher compared to leukocytes from healthy gingival tis-sue. The IFN-γ, TGF-β, IL-10, and TNF-α levels were significantly higher in the apical periodontitis lesions com-pared to control samples.
Conclusion:
The PD-1, PD-L1, and CTLA-4 molecules are evident in apical periodontitis, and can be an impor-tant immune checkpoint in chronic periapical periodontitis.
Keywords: Chronic periapical periodontitis, cytokines, lymphocytes, PD-1, PDL-1
HIGHLIGHTS.
Chronic inflammatory immune response leads to exhaustion or depletion of effector T cells.
Recent studies have suggested that engagement of PD-1 with their ligands, PD-L1 and PD-L2, im-pairs effector T-cell function resulting in an exhaus-tion phenotype.
The correlation between co-inhibitory receptors and T-cell exhaustion in chronic apical periodonti-tis has not been described.
PD-1 and PD-L1 are expressed at higher levels by CD4+ T cells during chronic apical periodontitis.
PD-1 signals may have an inhibitory effect on T-cell activation during chronic apical periodontitis.
INTRODUCTION
Root canal infection occurs be-cause of tissue necrosis caused by the inflammatory response ofthe pulp to invading bacteria fromcaries or dental trauma (1). Infec-tion is controlled by adaptive im-mune response, and unbalanced immune response is described as an important determinant in the disease outcome (2). Chronic in-fections in general are difficult to overcome because long inflam-matory immune response leads to exhaustion or depletion of effector T cells (3). Failure to eliminatethe pathogen results in loss of effector functions by T cells, such as reduced proliferative potential and cytokine secretion [i.e. interleukin (IL) 2, tumor-necrosis factor (TNF), and interferon gamma (IFN-γ)] (4). T-cell exhaustion has been described in many experimental models of disease and in humans with chronic infections or cancer (4). The most well defined feature of exhausted T cells is their expression of multiple co-inhibitory receptors, which in turn highly correlates with their degree of unresponsiveness (5). Co-inhibitory molecules associated with exhausted T cells include programmed death protein 1 (PD-1), TIM3, CTLA-4, BTLA, CD160, LAG3, and 2B4 (5).
PD-1 is a negative regulator of T-cell activation (6), and is a characteristic marker of exhausted T cells during chronic in-fections (4, 7). Blockade of PD-1 has been suggested as an ap-proach to enhance T-cell responses and control viral infections (8, 9). PD-1 is expressed on CD4+ T cells, CD8+ T cells, natural killer T cells, B cells, and activated monocytes. It negatively regulates T-cell receptor signals (10). PD-1 interacts with the B7H1 (programmed death ligand 1, PD-L1) and B7-DC (programmed death ligand 2, PD-L2) (3, 5, 10). PD-L1 is expressed by APCs, and its expression is upregulated by IFN-γ, IL-12, GM-CSF, and IL-4 (3, 5, 10). Upon engagement by its ligand (PD-L1 or PDL-2), the PD-1 signals lead to the inhibition of T-cell proliferation and downregulation of cytokine production (3, 5, 10). The PD-1 pathways play an important role in T-cell exhaustion during HIV infection (9). Blocking the PD-1 signaling during animal models improves survival (11). The PD-1 signaling has a critical role in limiting the effectiveness of antigen-specific T cells during other persisting infections and cancer (12). However, the correlation between PD-1 signals and T-cell exhaustion in lesions from patients with chronic apical periodontitis has not been described.
Considering apical periodontitis as a long-lasting inflamma-tory disease, it seems reasonable to hypothesize the mecha-nisms involved with occurrence of exhausted T lymphocytes at such lesions. The purpose of this study was to analyze the potential participation of PD-1 in the development of human chronic apical periodontitis. Our hypothesis was that bacterial persistence and chronic pulp infection is facilitated by exhaus-tion of T cells that express the inhibitory receptor PD-1.
MATERIALS AND METHODS
Patients with apical periodontitis and healthy volunteers
This study was approved by the Institutional Review Boards at the University of São Paulo (School of Dentistry of Bauru). Written informed consent regarding the use of specimens was given by all volunteers. All studies were performed in accor-dance with the relevant guidelines and regulations. Sixteen patients with chronic apical periodontitis (six men and ten women; age range 41-69 years, mean age=58.42±2.25 years) participated in the study. Radiographic examination was per-formed to evaluate the presence of periapical pathology (13). Samples were collected from patients referred to the Endodon-tics Clinic of School of Dentistry of Bauru (FOB/USP) and Post-graduate Endodontics Clinic of CPO Uningá Bauru/Brazil for root canal therapy. Medically compromised patients (i.e. those using systemic antibiotics, anti-inflammatory drugs, and hor-mone therapy) and patients with preexisting conditions such as periodontal disease, and pregnant or lactating women were excluded from the study. At the time of surgery, one part of the tissue sample was sent to histopathological analysis, and the other part was sent to isolation of leukocytes and super-natant collection. The samples from 16 lesions were diagnosed as cysts (n=4) and granulomas (n=12). Samples of healthy periodontal tissues were used as control samples and taken from patients undergoing procedures unrelated to periodontal dis-ease, such as the extraction of premolars for orthodontic rea-sons (n=10, five men and five women; age range 27-60 years).
Isolation of leukocytes
To characterize the leukocytes present in the lesion site, the samples of periodontal tissue collected from patients were incubated with 50 μg/mL collagenase (Boehringer Ingelheim Chemicals, São Paulo, Brazil) at 37°C for 60 min. One cycle of cellular dissociation were performed for 4 min using a Medi-machine (BD Biosciences, CA, USA). The tissue homogenates were briefly centrifuged, and the cell suspension was passed through a 30 μm cell strainer using the plunger from a 2 ml syringe (BD Bioscience). All cell counts were determined using a Neubauer chamber. Dead cells were excluded on the basis of trypan blue staining (14).
Flow cytometry analysis
Single cell suspensions isolated from human periodontal tissue were used for flow cytometry analysis. Cells were stained with surface antibodies (eBiosciences, San Diego, CA, USA) as previously described (14). Fixed cell suspensions were collected using FACSCalibur flow cytometer (BD Immunocytometry Systems, Franklin Lakes, NJ). Data were analyzed using CellQuest software (BD Biosciences).
ELISA
IFN-γ, TGF-β, and IL-10 were determined in whole periodon-tal tissue homogenates using a standardized sandwich ELISA technique, according to the manufacturer’s instructions (BD Pharmingen Corp., San Diego, CA).
Statistical analysis
All results were expressed as mean±SEM. One-way ANOVA was used for statistical analysis of all experiments to deter-mine the difference between the apical periodontitis lesions and healthy samples (controls). Data analysis was performed using the GraphPad Prism 5 software (GraphPad Software, Inc, La Jolla, CA, USA). All values were considered significantly dif-ferent at P<0.05.
RESULTS
Phenotypic characterization of leukocytes in the gingival tissue from patients with chronic apical periodontitis
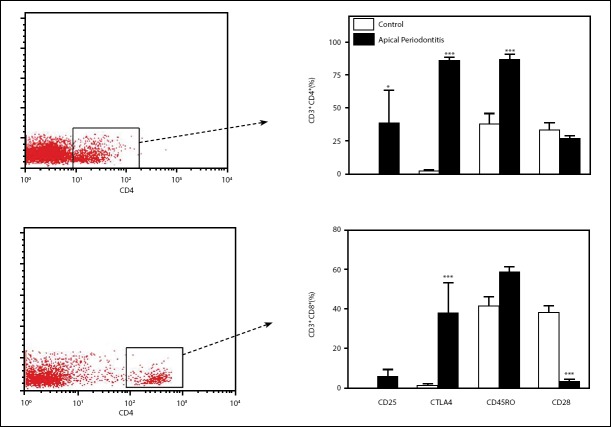
Flow cytometry examination showed higher leukocytes num-bers isolated from the apical periodontitis lesions when com-pared with healthy control gingival tissue (Fig. 1a). Analysis of apical periodontitis tissue confirmed that most leukocyte cells (75.3%) were CD3+ T cells. Among CD3+ cells, the proportion of CD4+ T cells was higher in the apical periodontitis lesions compared to control samples (Fig. 1b). Results showed similar frequencies of CD8+ T cells, B cells (CD19+), and macrophages (CD14+) in the lesions of apical periodontitis and control sam-ples (Fig. 1b). Frequencies of CD4+ T cells expressing CD45RO, CD25, and CTLA-4 were significantly higher in the apical peri-odontitis lesions than in cells from the control samples (Fig. 2a). In addition, only CD8+ CTLA-4 frequencies were significantly higher in the chronic apical periodontitis lesions compared to cells from control samples (Fig. 2b). Interestingly, the CD28 expression was lower in CD8+ T cells from chronic apical peri-odontitis. Our data indicate that apical periodontitis leads to higher amounts of infiltrating leukocytes in tissue, especially
Figure 1.
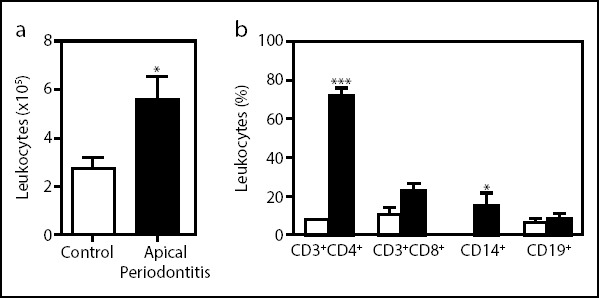
Apical periodontitisis associated within creased immune cell infiltration in lesions. The frequencies of total leukocytes, macrophages, and B and T cells were determined using immunostaining and FACS analysis. (a) The absolute number of leukocytes isolated of the control tissue (n=10, open bar) and apical periodontal lesions (n=10, closed bar). (b) Bars show the frequencies of T cells (CD3+ CD4+ and CD3+ CD8+), B lymphocytes (CD19+), or macrophages (CD14+) isolated of apical lesions. Data are mean±SEM. *P<0.05, ***P<0.001
Figure 2.
Phenotypic characterization of lymphocytes in the gingival tissue from patients with chronic apical periodontitis.(a) Bar graph shows expression of CD25, CTLA-4, CD45RO, and CD28 in CD4+ T lymphocytes, and histogram shows gated CD4+ T cells isolated of apical lesions. (b) Bar graph shows expression of CD25, CTLA-4, CD45RO, and CD28 in CD8+ T lymphocytes, and histogram shows gated CD8+T cells isolated of apical lesions. Data are mean±SEM. *P<0.05, **P<0.01; ***P<0.001
CD4+ T cells, compared to healthy tissues. Isolated lympho-cytes from apical periodontitis displayed activated phenotype in the collected samples.
PD-1 expression in leukocytes from patients with apical periodontitis
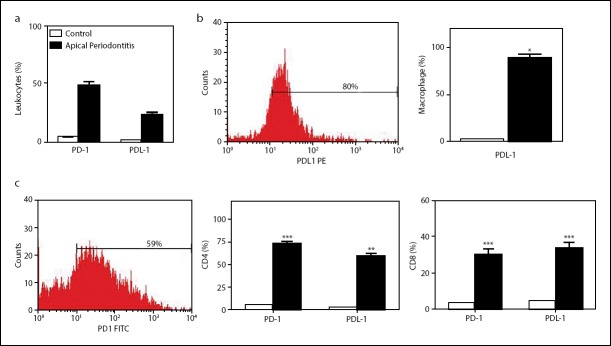
To investigate the possible participation of costimulatory molecules in T-cell exhaustion, we evaluated the expression of PD-1 in leukocytes isolated from healthy and chronic api-cal periodontitis tissues. In freshly isolated CD4+ T cells, the expression of PD-1 was significantly higher (P<0.01) than in leukocytes from healthy gingival tissue (Fig. 3c). The percent-age of CD8+PD-1+ T cells was higher in apical lesions than in healthy tissues (Fig. 3c). PD-L1 is a major co-inhibitory mole-cule identified on stromal cells of lymphoid and non-lymphoid organs (3). When we analyzed the PDL-1 expression, the re-sults show higher frequencies of T cells and macrophages ex-pressed PDL-1 in apical periodontitis samples (Fig. 3d). More-over, macrophages showed higher PDL-1 expression than other immune cells analyzed.
Figure 3.
Apical periodontitisis associated with increased PD-1 expression in CD4+ lymphocytes. (a) Bar graph shows expression of PD-1 and PD-L1 in total leukocytes isolated from lesions. (b) Bar graph shows expression of PD-L1 in macrophages, and histogram shows PD-L1 expression in gated macrophages isolated from chronic apical periodontitis. Data are mean±SEM. P<0.05, **P<0.01; ***P<0.001
Cytokines were increased in chronic apical periodontitis
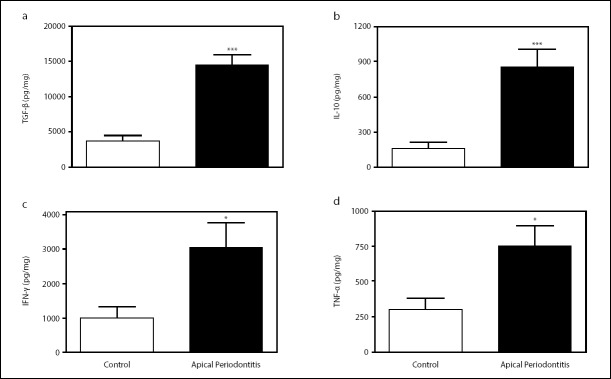
Chronic infections support the induction of terminally differentiated T cells that shows reduced capacity to produce cy-tokine (15). The identification of cytokines profiles in chronic periapical periodontitis could provide clues that specific mediators are involved in the development of this chronic infection. Thus, we chose to determine the levels of cytokines in the apical periodontitis lesions. IL-10, IFN-γ, TNF-α, and TGF-β were detectable in the chronic apical periodontitis lesions, and the amount of TGF-β was significantly higher compared to the control (Fig. 4).
Figure 4.
Cytokinesin chronic apical periodontitis. The IFN-γ, TNF-α, IL-10, and TGF-β levels were measured in supernatants of gingival samples using the ELISA assay. Data are mean±SEM. P<0.05, **P<0.01; ***P<0.001
DISCUSSION
Apical periodontitis is an immune response to the bacterial con-tent of infected root canals (1, 16, 17). These inflammatory le-sions are infiltrates of lymphocytes, monocytes, macrophages, plasma cells, and mast cells. Among the mononuclear cells found in these tissues, lymphocytes are known as the prevail-ing cell type (16), especially T cells (17). It has been suggested that most T lymphocytes found in apical lesions are resting (18), which could be a result of the chronicity of persistent in-fection as apical periodontitis. The protective effector function of T cells may be compromised by inhibitory receptors such as CTLA-4 and PD-L1 (3, 5). Here, this study demonstrated that PD-1 and PD-L1 are expressed at higher levels by CD4+ T cells during chronic apical periodontitis.
Alterations within the T-cell repertoire are present during the apical periodontitis progression (19). The absolute CD4+ and CD8+ T cell numbers are increased during disease progression (20), although the relatively higher increase in the CD4+ sub-set may be observed (17). In accordance with these findings, we reported here the preferential accumulation of T CD4+ in the apical periodontitis lesions. CD4 T cells play critical roles in mediating adaptive immunity to a variety of pathogens (21). However, higher numbers of CD4+ T cells can result in exacer-bated inflammation during chronic infections, suggesting that enhanced CD4+ T cell responses should be sought carefully (22). Because the presence of T cells has been associated with the apical periodontitis lesions progression and chronicity (23), our data suggest the major infiltration of these cells in the apical periodontitis lesions may be related to the exacerbated inflammation and chronicity.
T-cell exhaustion is a state of dysfunction that commonly oc-curs during chronic infections due to the persistence of inflam-mation (6). Based on the current view, the exhausted pheno-type of T cells in human apical lesions was investigated. Almost half of the leukocytes found on apical lesions expressed PD-1, whereas PD-L1 was detected on one-fifth of cells. However, not only T lymphocytes but also macrophages express PD-L1. In line with our results, in patients with chronic periodonti-tis, T cells expressed significantly higher levels of PD-1 either upon isolation or after culture with antigens (24). Recent studies revealed that the engagement of PD-1 with their lig-ands, PD-L1 and PD-L2, impairs effector T-cell function such as proliferation, cytotoxic activity, and cytokine production, resulting in an exhaustion phenotype that has been observed in different chronic infections, including cancer and HIV infec-tion (9, 25, 26). This study demonstrated that PD-1 and PD-L1 are expressed at higher levels by CD4+ T cells during chronic apical periodontitis. In the context of inhibitory molecules, a range of additional molecules such as CD25 and CTLA-4 are also upregulated on T cells in apical periodontitis, and may contribute to the phenomenon. When CTLA4 is upregulated, CD28 expression is subsequently downregulated by endocy- tosis (5). In fact, our results showed that higher frequencies of CD8+ T cells do not express CD28. Similar phenotypes can be observed in T cells upon long-term exposure to tumor antigen (5). However, the mechanisms involved with the upregulation of inhibitory receptors by effector T cells during chronic api-cal infections have yet to be investigated. Cytokines play a key role in the pathogenesis periapical lesions. Cytokines from T helper 1 profile (IFN-γ and TNF-α) have been associated with lesion progression, whereas T helper 2 cytokines (IL-4, IL-10, and TGF-β) are described to attenuate the tissue damage (27). In this study, lesion supernatant showed increased levels of ei-ther anti-inflammatory TGF-β and IL-10 and modest amounts of proinflammatory IFN- γ and TNF-α. Although we did not ob-serve a direct effect of the PD-1–PD-L1 pathway in this study, it is notable that IFN-γ, as a proinflammatory cytokine, could be induced even in the presence of higher PD-L1 expression by lymphocytes in chronic apical periodontitis. IFN-γ can also upregulate molecules that impair T-cell responses by interfering with metabolic pathways (28). Studies have suggested the ex-istence of a correlation between the PD-1–PD-L1 pathway and the production of IL-10 (29). CTLA-4 binding may lead to TGF-β production (30). These data might explain the high levels of TGF-β in apical granulomas and radicular cysts (31). The pres-ence of exhausted CTLA4+ lymphocytes might be due to the upregulation of TGF-β apical periodontitis. PD1/PDL1 signals induced high levels of IL-10 production that in turn inhibited the function of CD4+ T cells (32). The IL-10-mediated inhibition of CD4+ T cell effector function was shown in chronic LCMV in-fection in mice (29). It should also be considered that in this context, not only lymphocytes but other leucocytes such as plasma cells and macrophages as well as resident cells such as endothelial cells and fibroblasts would be involved in cytokine production, which in turn proliferate because of chronic inflammatory processes (33, 34). However, because the anti-genic source remains in contact with living tissues during apical periodontitis, it is reasonable to suggest that proinflam-matory cytokine production will remain high because of the continuous exposure to bacterial products along the existence of the apical disease.
Limitations of these data include the small sample size of 16 patients. Furthermore, while data regarding costimulatory molecules expression were obtained from the apical periodontitis lesions, and this allowed us to compare correlation with T cells unresponsiveness, we could not observe a direct effect of the PD-1–PD-L1 pathway. Even with the above-mentioned limitation, our results demonstrate that PD-1 and CTLA-4, which are associated with T-cell exhaustion, are expressed in higher levels in chronic periapical periodontitis. This confirms our hypothesis that exhausted T lymphocytes are present in the apical periodontitis lesions.
CONCLUSION
This study showed that PD-1, PD-L1, and CTLA-4 molecules are detected in the apical periodontitis lesions and can be an important immune checkpoint in chronic periapical peri-odontitis. The contribution of costimulatory molecules to the immunopathogenesis of chronic apical periodontitis needs to be be investigated further.
Footnotes
Disclosures
Conflict of interest: The authors declare that they have no conflicts of interest.
Ethics Committee Approval: CAAE number: #02084712.7.0000.5417
Financial Disclosure: This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [grant number 2008/09973-3]; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, scholarship to R.J.R., and C.R.P.), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, scholarship to J.S.S., G.P.G, and A.P.C.).
Authorship contributions: Concept – A.P.C., J.S.S., G.P.G.; Design – A.P.C., G.P.G, I.G.M., R.B.G., C.M.B., N.B., C.K.N., S.A.T.; Supervision – A.P.C.; Fundings - A.P.C., G.P.G, J.S.S., I.G.M., R.B.G., C.M.B., N.B., C.K.N., S.A.T.; Materials - A.P.C., J.S.S., I.G.M., R.B.G., C.M.B., N.B., C.K.N., S.A.T.; Data collection &/or processing – R.J.R.D., C.R.P., T.H.G.; Analysis and/or interpretation – R.J.R.D., C.R.P., T.H.G., C.R.S.; Literature search – R.J.R.D., C.R.P., C.R.S.; Writing – R.J.R.D., C.R.P., T.H.G., C.R.S.; Critical Review – R.J.R.D., C.R.P., C.R.S., A.P.C.
REFERENCES
- 1.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 2.Cotti E, Schirru E, Acquas E, Usai P. An overview on biologic medications and their possible role in apical periodontitis. J Endod. 2014;40(12):1902–11. doi: 10.1016/j.joen.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–76. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and coinhibition. Nat Rev Immunol. 2013;13(4):227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion:an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56(5):739–45. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 8.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 10.Lin DY, Tanaka Y, Iwasaki M, Gittis AG, Su HP, Mikami B, et al. The PD-1/PDL1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci U S A. 2008;105(8):3011–6. doi: 10.1073/pnas.0712278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–10. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12(10):2575–87. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bornstein MM, Lauber R, Sendi P, von Arx T. Comparison of periapical radiography and limited cone-beam computed tomography in mandibular molars for analysis of anatomical landmarks before apical surgery. J Endod. 2011;37(2):151–7. doi: 10.1016/j.joen.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Gasparoto TH, de Souza Malaspina TS, Benevides L, de Melo EJ Jr, Costa MR, Damante JH, et al. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother. 2010;59(6):819–28. doi: 10.1007/s00262-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speiser DE, Utzschneider DT, Oberle SG, Münz C, Romero P, Zehn D. T cell differentiation in chronic infection and cancer:functional adaptation or exhaustion? Nat Rev Immunol. 2014;14(11):768–74. doi: 10.1038/nri3740. [DOI] [PubMed] [Google Scholar]
- 16.Nilsen R, Johannessen AC, Skaug N, Matre R. In situ characterization of mononuclear cells in human dental periapical inflammatory lesions using monoclonal antibodies. Oral Surg Oral Med Oral Pathol. 1984;58(2):160–5. doi: 10.1016/0030-4220(84)90131-2. [DOI] [PubMed] [Google Scholar]
- 17.Stashenko P, Yu SM, Wang CY. Kinetics of immune cell and bone resorptive responses to endodontic infections. J Endod. 1992;18(9):422–6. doi: 10.1016/S0099-2399(06)80841-1. [DOI] [PubMed] [Google Scholar]
- 18.Piattelli A, Artese L, Rosini S, Quaranta M, Musiani P. Immune cells in periapical granuloma:morphological and immunohistochemical characterization. J Endod. 1991;17(1):26–9. doi: 10.1016/S0099-2399(07)80157-9. [DOI] [PubMed] [Google Scholar]
- 19.Sol MA, Tkaczuk J, Voigt JJ, Durand M, Sixou M, Maurette A, et al. Characterization of lymphocyte subpopulations in periapical lesions by flow cytometry. Oral Microbiol Immunol. 1998;13(4):253–8. doi: 10.1111/j.1399-302x.1998.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 20.Kawashima N, Okiji T, Kosaka T, Suda H. Kinetics of macrophages and lymphoid cells during the development of experimentally induced periapical lesions in rat molars:a quantitative immunohistochemical study. J Endod. 1996;22(6):311–6. doi: 10.1016/S0099-2399(96)80266-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207(11):2479–91. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tani-Ishii N, Wang CY, Stashenko P. Immunolocalization of bone-resorptive cytokines in rat pulp and periapical lesions following surgical pulp exposure. Oral Microbiol Immunol. 1995;10(4):213–9. doi: 10.1111/j.1399-302x.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 24.Belai EB, de Oliveira CE, Gasparoto TH, Ramos RN, Torres SA, Garlet GP, et al. PD-1 blockage delays murine squamous cell carcinoma development. Carcinogenesis. 2014;35(2):424–31. doi: 10.1093/carcin/bgt305. [DOI] [PubMed] [Google Scholar]
- 25.Malaspina TS, Gasparoto TH, Costa MR, de Melo EF, Jr, Ikoma MR, Damante JH, et al. Enhanced programmed death 1 (PD-1) and PD-1 ligand (PD-L1) expression in patients with actinic cheilitis and oral squamous cell carcinoma. Cancer Immunol Immunother. 2011;60(7):965–74. doi: 10.1007/s00262-011-1007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueira EA, de Rezende ML, Torres SA, Garlet GP, Lara VS, Santos CF, et al. Inhibitory signals mediated by programmed death-1 are involved with Tcell function in chronic periodontitis. J Periodontol. 2009;80(11):1833–44. doi: 10.1902/jop.2009.090057. [DOI] [PubMed] [Google Scholar]
- 27.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22(2):223–30. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garlet GP. Destructive and protective roles of cytokines in periodontitis:are-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89(12):1349–63. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 29.Dubey C, Croft M, Swain SL. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J Immunol. 1996;157(8):3280–9. [PubMed] [Google Scholar]
- 30.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12(11):1301–9. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGFbeta) production by murine CD4(+) T cells. J Exp Med. 1998;188(10):1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrade AL, Nonaka CF, Gordón-Núñez MA, Freitas Rde A, Galvão HC. Immunoexpression of interleukin 17, transforming growth factor β1, and forkhead box P3 in periapical granulomas, radicular cysts, and residual radicular cysts. J Endod. 2013;39(8):990–4. doi: 10.1016/j.joen.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 33.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 34.Trowbridge HO. Immunological aspects of chronic inflammation and repair. J Endod. 1990;16(2):54–61. doi: 10.1016/S0099-2399(06)81564-5. [DOI] [PubMed] [Google Scholar]