Abstract
Background:
Vaccination is the most remarkable intervention in public health and is an effective strategy in controlling infectious diseases among infants.
Objectives:
The aim of this study was to compare the adverse events of Pentavalent vaccine and DPT vaccine in two- to six-month-old infants in Iran.
Methods:
This is an analytical cross-sectional study in which healthy infants aged two to six months, having received DPT vaccine in 2013 and Pentavalent vaccine in 2015, were studied for any experienced adverse events related to these vaccines. Percentage, mean, standard deviation and chi-square tests were used to describe and analyze the data (p < 0.05).
Findings:
The results showed that 10,464 and 17,561 adverse events, which were associated with DPT vaccine and Pentavalent vaccine respectively, were recorded in the infants who received these vaccines throughout Iran. Mazandaran, Qazvin and Golestan provinces reported the highest number of adverse events, respectively (15.74%, 11.25%, and 9.12%). Moreover, Pentavalent vaccine seemed to have more recorded adverse events compared to DPT, high fever had the highest record rate for DPT vaccine (47.4%) and mild localized complications was the highest for Pentavalent vaccines (31.68%). There was a significant relationship between the kind of vaccine and the type of reaction, adverse event categorization and the country that produced the vaccine (p < 0.05).
Conclusion:
Severe localized adverse events including high fever, vomiting, diarrhea and restlessness seemed to be less in Pentavalent vaccine compared to DPT vaccine. Therefore, substituting Pentavalent vaccine for DPT vaccine in infants seems to reduce the adverse events among them.
Background
The World Health Organization (WHO) considers infant vaccination the most influential health intervention for promoting a healthy society [1]. Infant vaccination programs have been merged into public health service networks from their starting point in Iran. With 98% of infants vaccinated, it has brought great success in eradicating, removing and controlling preventable diseases [2,3,4]. Although modern and new vaccines used throughout the country are supposed to be safe, there is no vaccine without adverse effects. Each vaccine has its own side effects that might appear following their use [5]. Based on the WHO and Iran’s care guide recommendations, regardless of any causal relationship, every side effect observed in the vaccinated person by physicians, family members or the person himself is known as an adverse event following immunization [6]. Immunization adverse events can be due to errors in the vaccination program, reactions due to the nature of the vaccine, reactions to injection or unknown factors. Sometimes there might be some adverse events temporarily assigned to vaccination because of their concurrency [5,7].
Furthermore, the Pentavalent vaccine immunization program, which is used to prevent hepatitis B, Diphtheria, anthrax, Tetanus and Haemophilus influenzae type b (Hib) flu and is injected in three different time intervals (2, 4 and 6 months), started in October 2014 in Iran [8]. This vaccine is used broadly in more than 100 countries for preventing Diphtheria, Tetanus, and Pertussis (DPT), Hepatitis B and Hib in recent years. A study in the USA showed that fever (25.8%), injection site sensitivity (15.8%) and injection site edema (10.8%) were the most remarkable adverse events of Pentavalent vaccine [9,10]. Some of the Pentavalent vaccine advantages include reducing the number of injections and syringes used, less pain and restlessness in infants, decreasing the complications of injection, ease of planning, increasing cost effectiveness and increasing the immunization coverage [11].
However, no national study has been done on the possible adverse events of this vaccine in Iran since its merging into the country’s national vaccination program on November 22, 2014 [10]. This study attempted to broadly compare the possible adverse events of Pentavalent vaccine as a new vaccine in comparison to DPT vaccine in two- to six-month-old infants in Iran in 2016.
Methods
This is an analytical cross-sectional study. All healthy infants (male and female of two to six months) who visited health centers to receive DPT vaccine from April 18 to March 25, 2013, as well as those who received Pentavalent vaccine from April 30 to March 25, 2015, and experienced adverse events following vaccination were studied. Those infants with allergic reactions or convulsions prior to vaccination and those who received immunosuppressive and neurological disorder medications were excluded from the study. Pentavalent vaccine and DPT vaccine used in this study had been produced in Iran, Korea, India, Indonesia, France and Belgium, and they are used broadly in the world. To perform vaccination, 0.5 mL was injected into the anterior part of the quadriceps muscle of the infants.
The recorded information includes the name of the province and medical university in the area, the date of the report, the city, the patient’s sex, the type of report (urgent vs. non-urgent), hospitalization status (outpatient vs. inpatient), type of reporting health center (urban vs. rural), infant’s age, their birth weight, birth date, immunization date, type of reaction to vaccination, the vaccine name, the vaccine serial number, the date of receiving the vaccine, the name of the institute or factory producing the vaccine, the adverse event incidence date, the patient’s visit date, their mother’s age in the time of pregnancy, the treatment procedure (recovery, being treated, lasting adverse event, death, other), the final diagnosis and the adverse event categorization (vaccine reaction, error in the vaccination program, simultaneous injection response, unknown). Because the infant’s recording information form was used to gather the required data and the data analysis was done in groups, there was not any ethical problem in this study; moreover, the researchers considered themselves to be committed to research ethics.
Data Analysis
STATA version 12 was used to analyze the data. Percentage, mean, standard deviation and chi-square tests were used to describe the data and to investigate the studied variables. Significance level was considered to be p < 0.05.
Findings
The results showed 10,464 adverse events (about 0.3%) among 4,249,050 infants who received DPT vaccine in 2013 and 17,561 adverse events (about 0.3%) among 423,0870 infants who received Pentavalent vaccine in 2015. The results also showed that 53.36% of the infants who experienced DPT vaccine adverse events were male and about 61.53% lived in rural areas (Table 1). In addition, the results showed that the average birth weight of these infants were 3160 ± 487 g and 3202 ± 455.2 g and their average gestational age were 38.4 ± 1.51 and 38.5 ± 1.31 weeks for DPT vaccine and Pentavalent vaccine, respectively.
Table 1.
Demographic variables of infants with vaccine complications.
| Variable | Vaccine | ||
|---|---|---|---|
| DPT | Pentavalent | ||
| Sex | Male | 5584 (53.36) | 9137 (52.1) |
| Female | 4880 (46.64) | 8399 (47.9) | |
| Location | Urban | 4025 (38.47) | 8788 (50.04) |
| Rural | 64399 (61.53) | 8773 (49.96) | |
Moreover, the results showed that Mazandaran (15.74%), Ghazvin (11.25%), Golestan (9.12%) and Zanjan (8.44%) have reported the highest and Chaharmahal and Bakhtiari (0.08%), Hormozgan and Bushehr (0.21%), Kurdistan and South Khorasan (0.023%) have reported the fewest number of vaccination adverse events, respectively. The highest number of DPT adverse events were reported from Mazandaran (17.27%), Golestan (13.47%) and Qazvin (11.55%), and the fewest were reported from Chaharmahal and Bakhtiari (0.03%), South Khorasan (0.11%), Bushehr and Kurdistan (0.16%), respectively. Moreover, the highest number of Pentavalent vaccine adverse events were reported from Mazandaran (14.83%), Qazvin (11.05%) and Zanjan (8.01%), and the fewest were reported from Chaharmahal and Bakhtiari (0.11%), Hormozgan (0.22%), and Bushehr and Kurdistan (0.27%), respectively. This difference was not statistically significant (p > 0.05) (Table 2).
Table 2.
The frequency of adverse events DPT vaccine and Pentavalent vaccine in different provinces of Iran.
| ID | Province | Frequency of vaccination complications (%) | Total | |
|---|---|---|---|---|
| DPT | Pentavalent | |||
| 1 | Mazandaran | 1807 (17.27) | 2605 (14.83) | 4412 (15.74) |
| 2 | Qazvin | 1211 (11.57) | 1941 (11.05) | 3152 (11.25) |
| 3 | Golestan | 1409 (13.47) | 1146 (6.53) | 2555 (9.12) |
| 4 | Zanjan | 958 (9.16) | 1407 (8.01) | 2365 (8.44) |
| 5 | Khuzestan | 1019 (9.74) | 1183 (6.74) | 2202 (7.86) |
| 6 | Gilan | 84 (8.04) | 838 (4.77) | 1676 (5.99) |
| 7 | Fars | 469 (4.48) | 994 (5.66) | 1463 (5.22) |
| 8 | Isfahan | 580 (5.54) | 877 (4.99) | 1457 (5.2) |
| 9 | Alborz | 144 (1.38) | 1202 (6.84) | 1346 (4.8) |
| 10 | East Azerbaijan | 290 (2.77) | 819 (4.66) | 1109 (3.96) |
| 11 | Khorasan Razavi | 331 (3.16) | 756 (4.3) | 1087 (3.88) |
| 12 | Tehran | 255 (2.44) | 682 (3.88) | 937 (3.34) |
| 13 | Sistan & Baluchestan | 162 (1.55) | 423 (2.41) | 585 (2.09) |
| 14 | Lorestan | 99 (0.95) | 401 (2.28) | 500 (1.78) |
| 15 | West Azerbaijan | 162 (1.55) | 250 (1.42) | 412 (1.47) |
| 16 | Ardebil | 17 (0.16) | 379 (2.16) | 396 (1.41) |
| 17 | Ilam | 161 (1.54) | 221 (1.26) | 382 (1.36) |
| 18 | Yazd | 102 (0.97) | 193 (1.1) | 295 (1.05) |
| 19 | Hamedan | 93 (0.89) | 179 (1.02) | 272 (0.97) |
| 20 | Kermanshah | 48 (0.46) | 157 (0.89) | 205 (0.73) |
| 21 | North Khorasan | 39 (0.37) | 143 (0.81) | 182 (0.65) |
| 22 | Semnan | 46 (0.44) | 136 (0.77) | 182 (0.65) |
| 23 | Kerman | 53 (0.51) | 126 (0.72) | 179 (0.64) |
| 24 | Markazi | 44 (0.42) | 121 (0.69) | 165 (0.59) |
| 25 | Qom | 41 (0.39) | 83 (0.47) | 124 (0.44) |
| 26 | Kohgiluyeh & Boyer-Ahmad | 13 (0.12) | 95 (0.54) | 108 (0.39) |
| 27 | Bushehr | 17 (0.16) | 47 (0.27) | 64 (0.23) |
| 28 | Southern Khorasan | 12 (0.11) | 53 (0.3) | 64 (0.23) |
| 29 | Kurdistan | 17 (0.16) | 47 (0.27) | 64 (0.23) |
| 30 | Hormozgan | 21 (0.2) | 38 (0.22) | 59 (0.21) |
| 31 | Chaharmahal & Bakhtiari | 13 (0.12) | 95 (0.54) | 108 (0.39) |
| 32 | Total country | 10464 (100) | 1756 (100) | 28025 (100) |
The dispersion of the DPT vaccine and Pentavalent vaccine adverse events in different parts of the country is shown using geographical information system in Figures 1 and 2. Most of the complications were in the northern provinces of the country.
Figure 1.
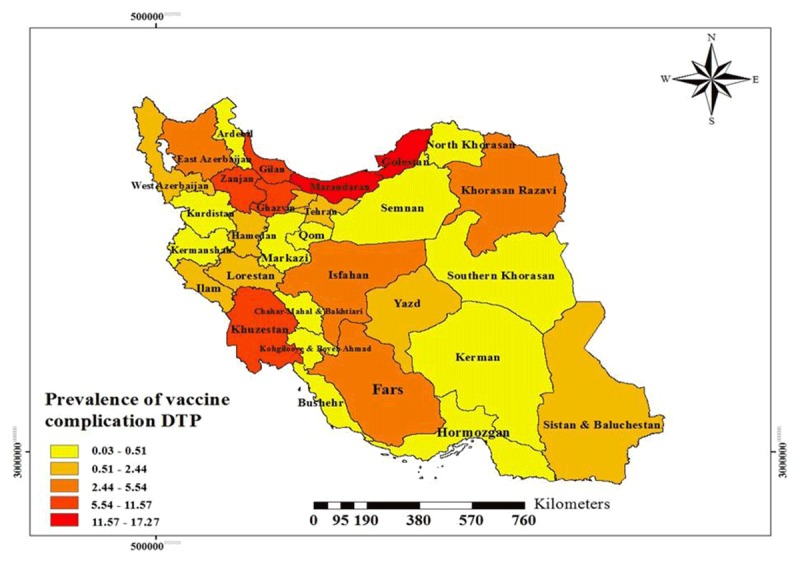
Prevalence of Adverse Events Following Immunization with DPT Vaccine.
Figure 2.
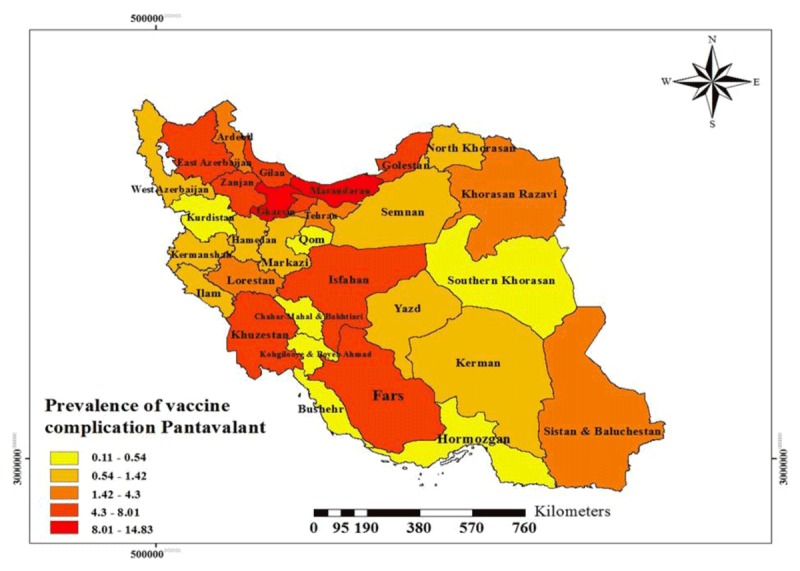
Prevalence of Adverse Events Following Immunization with Pentavalent Vaccine.
The results also showed that high fever (47.4%) and mild localized complications (31.68%) were reported to be the most frequent events for DPT vaccine and Pentavalent vaccine, respectively. Therefore, although more adverse events were reported for Pentavalent vaccine (17,561 vs. 10,464 cases) compared to DPT vaccine, most of the adverse events reported with Pentavalent vaccine were mild; whereas, adverse events reported with DPT vaccine were mostly high fever (p < 0.05) (Figure 3).
Figure 3.
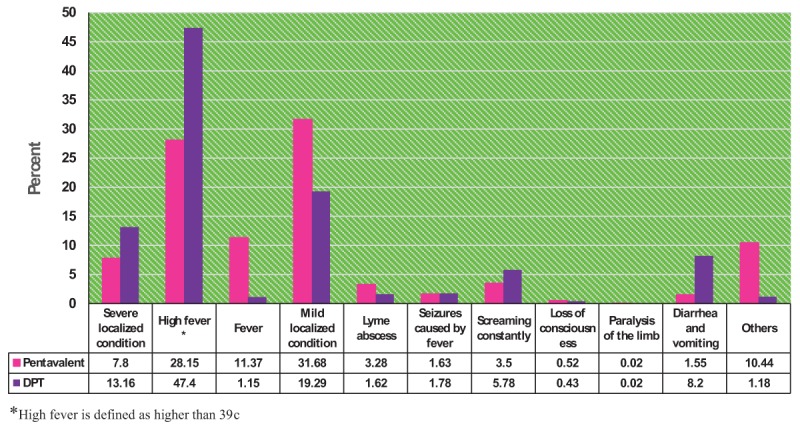
Comparison of Reported Adverse Events in DPT and Pentavalent Vaccines.
According to the results, only 3% of the adverse events in both vaccines led to hospitalization. Vaccine adverse event categorization in both DPT vaccine and Pentavalent vaccine showed that the largest category belonged to vaccine reaction (about 95%). The results also indicated that there was a statistically significant relationship between the kind of vaccine and the type of reaction, adverse event categorization and the infant’s place of residence (p < 0.05). However, there was no statistically significant relationship between the kind of vaccine and the infant’s sex or their hospitalization status (p < 0.05) (Table 3).
Table 3.
The relationship between DPT and Pentavalent vaccine characteristics and their adverse events.
| Variable | Type Vaccine | Chi-2 | P-value | ||
|---|---|---|---|---|---|
| DPT | Pentavalent | ||||
| Type of reaction | Severe localized condition | 1375 (7.83) | 1377 (13.16) | 3.8 | 0.0001 |
| High fever* | 4944 (28.15) | 4960 (47.4) | |||
| Fever | 1997 (11.37) | 120 (1.15) | |||
| Mild localized condition | 5564 (31.68) | 2018 (19.29) | |||
| Lyme abscess | 576 (3.28) | 170 (1.62) | |||
| Seizures caused by fever | 287 (1.63) | 186 (1.78) | |||
| Screaming constantly | 614 (3.5) | 605 (5.78) | |||
| Loss of consciousness | 91 (0.52) | 45 (0.43) | |||
| Paralysis of the limb | 3 (0.02) | 2 (0.02) | |||
| Diarrhea and vomiting | 277 (1.58) | 858 (8.2) | |||
| Others | 1833 (10.44) | 123 (1.18) | |||
| Complaint classification | Programming error | 718 (4.4) | 298 (2.85) | 263.1 | 0.0001 |
| Reactions to vaccines | 15099 (92.47) | 10110 (96.63) | |||
| Reaction to the injection | 287 (1.76) | 489(0.46) | |||
| Concurrency | 139 (0.85) | 4 (0.04) | |||
| Unknown | 85 (0.52) | 3 (0.03) | |||
| Hospitalization | Yes | 157 (1.5) | 420 (2.39) | 0.11 | 0.7 |
| No | 10307 (98.5) | 17141 (61.97) | |||
| Report type | Immediate | 472 (4.51) | 966 (5.5) | 13.19 | 0.0001 |
| Non-Immediate | 9992 (95.49) | 16595 (94.5) | |||
| Gender | Male | 5584 (53.36) | 9137 (52.1) | 4.2 | 0.05 |
| Female | 4880 (46.64) | 8399 (52.1) | |||
| Location | Urban | 4025 (38.47) | 8788 (47.9) | 354 | 0.0001 |
| Rural | 6439 (61.53) | 8773 (49.96) | |||
* High fever is defined as higher than 39c.
Discussion
Although vaccines used in the country’s immunization program are safe and very effective, no vaccine is completely safe and there might be some adverse events following immunization [12]. On the whole, the number of adverse events following immunization are low in Iran, and most are reported to be mild and temporary and mostly resolved without any medical treatment [13]. The results showed that the general incidence of adverse events in DPT vaccine and Pentavalent vaccine were 0.2% and 0.3%, respectively (p > 0.05). Among other adverse events, high fever and mild localized complication were reported to have the highest incidence in DPT vaccine (47.7%) and Pentavalent vaccines (31.68%).
The increase in adverse events reported with the Pentavalent vaccine in 2015 compared to adverse events reported with the DPT vaccine in 2013 could be explained by the general increase in the number of adverse events reported throughout the country and by the increased ability of the care and health system in identifying and recording more vaccine adverse events in 2015 compared to 2013.
In fact, at least one out of four infants having received the vaccines showed some kind of adverse event, which were mostly associated with fever [14]. Fever could generally be produced after receiving all kinds of vaccines. This study showed that high fever was the most common adverse event of DPT vaccine (47.4%); whereas, it was 28.15% for the Pentavalent vaccine.
Similarly, in studies by Sharifi et al. [9] and Ayatollahi et al. [15], high fever was reported to be the most frequent adverse event of the DPT vaccine. Mansour et al. in New Zealand reported longtime crying among infants who had received the DPT vaccine [16]. Al-Jadiryinin Iraq reported pain and inflammation as the most common events associated with the DPT vaccine [17]. Barkin et al. reported that 54% of infants who received the vaccine showed fever as the most common event [18]. In a study by Suser et al., localized pain, redness, fever and edema were reported to be the most common adverse events of DPT vaccine [19]. The benefits of childhood vaccines far outweigh any potential risks. Significant global data on vaccination showed that lack of Hib vaccination caused a significant amount of disease and mortality among infants. Therefore, WHO strongly recommends global Hib Vaccination [20]. Vaccination with the Pentavalent vaccine automatically increased the coverage of Hepatitis B and Hib immunization [11].
In addition, the results of different studies show that the Pentavalent vaccine was safe and tolerable and possessed a high level of immunizing for all molecular antigens and some biological reactions [21,22,23,24]. This vaccine was tested for 10 years in some Asian countries, from 2002 (when its use started in Ghana) to 2012 (when its use started in India) [24]. The results of these studies show that the most common reported adverse event of the Pentavalent vaccine was localized mild events. A study by the Indian Institute of Serology shows that local reactions to the Pentavalent vaccine included pain, redness and edema in the injection site, and its general systematic reactions were reported to be fever, unusual crying and irritability [25]. Hatami et al. in Tehran reported fever as the most common adverse event of the Pentavalent vaccine (71.2%) [13]. In a study in the United States of America, high fever was reported to be the most common adverse event of the Pentavalent vaccine [26]; whereas, Cunha et al. reported hypo-tony as the most common adverse event for the DPT vaccine and the Pentavalent vaccine [27].
Finally, it should be noted that most parents were aware of the fever following vaccination and they preventively gave Acetaminophen to their infants, which could decrease their fever and prevent high fever; therefore, the incidence rate of this event might be changed due to the parents’ interventions [14]. Categorizing the adverse events showed that most of the reported events were associated with the reaction to the vaccine, which was in line with Raisi et al. in Shahre Kurd [28] and Parisay et al. in Kohkiloye va Boir Ahmad [29]. In addition, the findings of the study showed that there was not any significant relationship between the infants’ genders and the DPT vaccine and Pentavalent vaccine adverse events. However, the possible complications of the vaccines seemed to be more common among males compared to females. This finding was in line with the findings of Reisi et al. in which there wasn’t a significant relationship between the gender of the infants and the vaccines’ adverse events [28]. Moreover, in studies by Parisay et al. [29] and Ayatollahi et al. [30], although there was not a significant relationship between the infants’ genders and the vaccines’ adverse events, the possible adverse events were reported to be more common among males compared to females, which was in line with the findings of this study. However, Nabavi et al. reported that the vaccines’ adverse events seemed to be more common among females than males, which is in contradiction to the findings of this study [31].
However, severe cases of adverse events led to hospitalization for a small percentage of the vaccinated infants. The findings of the study show that only 1.5% of all reported complications in infants receiving the DPT vaccine and the Pentavalent vaccine led to their hospitalization and the number hospitalized were almost the same for both vaccines. In fact, many hospitalizations can be due to other factors contemporarily existing with vaccination.
Conclusion
In conclusion, the Pentavalent vaccine demonstrated fewer adverse events and reduced the number of injections and the required injection equipment, showing that this vaccine is better than the DTP vaccine.
Acknowledgements
This study was a research project in Kurdistan University of Medical Sciences with the ethics code of IR.MUK.REC.1395.307. We would like to thank our dear colleagues for providing this data, as well as all those who were involved in the project and helped us to collect the data throughout the country.
Competing Interests
The authors have no competing interests to declare.
Author Contributions
SMZ, MMG led data collection. DR, ZKH and analyzed data, and GM, ZKH, EG and DR interpreted data. ZKH, GM, EG and DR drafted the initial manuscript. MMG, SMZ, ZKH and FY were major contributors in writing the manuscript, with critical revisions from ZKH, GM and EG. All authors read and reviewed the final manuscript.
References
- 1.Pearson ML, Bridges CB, Harper SA. Influenza vaccination of health-care personnel. CDC, Mortality and Morbidity Weekly Report. 2006; 55: 1–16. DOI: 10.4161/hv.28154 [DOI] [PubMed] [Google Scholar]
- 2.Control CfD, Prevention. Vaccine preventable deaths and the Global Immunization Vision and Strategy, 2006–2015. Morbidity and Mortality Weekly Report. 2006; 55: 511. PMID: 16691182. [PubMed] [Google Scholar]
- 3.Khazaei S, Rezaeian S, Razani M, et al. Adverse events following immunization (AEFI) in children under 7 years of age during 2014 in Hamedan Province, Iran. International Journal of Pediatrics. 2016; 4: 1697–703. http://eprints.lums.ac.ir/id/eprint/650. [Google Scholar]
- 4.Ayatallahi A, Rajaeefard A, Namakin K. Epidemiologic study of infant mortality and its risk factors in Birjand health houses in rural areas are covered. Journal of Babol University of Medical Sciences. 2013; 10: 9–15. [Google Scholar]
- 5.Agergaard J, Nante E, Poulstrup G, et al. Diphtheria–tetanus–pertussis vaccine administered simultaneously with measles vaccine is associated with increased morbidity and poor growth in girls. A randomised trial from Guinea-Bissau. Vaccine. 2011; 29: 487–500. DOI: 10.1016/j.vaccine.2010.10.071 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO vaccine-preventable diseases: Monitoring system: 2009 global summary. Geneva: World Health Organization; 2009. [Google Scholar]
- 7.Uprety P, Poudel A. Earthquake risk perception among citizens in Kathmandu, Nepal. Australasian Journal of Disaster and Trauma Studies. 2012; 2012: 3–10. https://www.massey.ac.nz/~trauma/issues/2012-1/AJDTS_2012-1_Uprety.pdf. [Google Scholar]
- 8.Watson JC, LeBaron CW, Hutchins SS, et al. General Recommendations on Immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report: Recommendations and Reports. 1994; 1–38. [PubMed] [Google Scholar]
- 9.Sharafi R, Mortazavi J, Heidarzadeh A. Comparison of complications of pentavalent and DTP vaccination in infants aged 2–6 months in Anzali, Iran. Iranian Journal of Neonatology. 2016; 7: 1–6. http://eprints.mums.ac.ir/id/eprint/2055. [Google Scholar]
- 10.Kliegman R, Stanton BM, Geme JS. Nelson tratado de pediatría. Brasil: Elsevier; 2014. [Google Scholar]
- 11.Sreedhar S, Antony A, Poulose N. Study on the effectiveness and impact of pentavalent vaccination program in India and other south Asian countries. Human Vaccines & Immunotherapeutics. 2014; 10: 2062–5. DOI: 10.4161/hv.28785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira CR, Baltimore RS. Complications of Vaccination With Bacille Calmette-Guérin. Clinical Pediatrics. 2014; 53: 914–6. DOI: 10.1177/0009922814533414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatami H, Arshi S, Sarikhani R. The relationship between side effects of the Pentavalent vaccine and mother’s education level in areas under the auspices of Shahid Beheshti University of Medical Sciences in 2015. Journal of Health in the Field. 2016; 3. [Google Scholar]
- 14.Karami M, Ameri P, Bathaei J, et al. Adverse events following immunization with pentavalent vaccine: Experiences of newly introduced vaccine in Iran. BMC Immunology. 2017; 18: 42 DOI: 10.1186/s12865-017-0226-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayatollahi J, Zare A. Short Course Adverse Events following DTP vaccination in Yazd during 2005. Iran J Pediatr. 2006; 16: 332–4. [Google Scholar]
- 16.Mansoor O, Pillans P. Vaccine adverse events reported in New Zealand 1990–5. The New Zealand Medical Journal. 1997; 110: 270–2. PMID: 9269289. [PubMed] [Google Scholar]
- 17.Al-Jadiryinin. Childhood Immunization. J Lecture. 2012; 21: 311–5. [Google Scholar]
- 18.Barkin RM, Pichichero ME. Diphtheria-pertussis-tetanus vaccine: Reactogenicity of commercial products. Pediatrics. 1979; 63: 256–60. http://www.pediatrics.org. [PubMed] [Google Scholar]
- 19.Key S. Whooping Cough: A Study in Immunization. The American Journal of the Medical Sciences. 1933; 185: 586 DOI: 10.1001/jama.1933.02740040007003 [DOI] [Google Scholar]
- 20.Malik A. Pentavalent Vaccine and Adverse Events Following Immunization—Untangling the Misinterpretations. The Indian Journal of Pediatrics. 2014; 81: 1353–7. DOI: 10.1007/s12098-013-1322-2 [DOI] [PubMed] [Google Scholar]
- 21.Eskola J, Käyhty H, Gordon LK, et al. Simultaneous administration of Haemophilus influenzae type b capsular polysaccharide-diphtheria toxoid conjugate vaccine with routine diphtheria-tetanus-pertussis and inactivated poliovirus vaccinations of childhood. The Pediatric Infectious Disease Journal. 1988; 7: 480–4. DOI: 10.1097/00006454-198807000-00006 [DOI] [PubMed] [Google Scholar]
- 22.Guerra FA, Blatter MM, Greenberg DP, et al. Safety and immunogenicity of a pentavalent vaccine compared with separate administration of licensed equivalent vaccines in US infants and toddlers and persistence of antibodies before a preschool booster dose: A randomized, clinical trial. Pediatrics. 2009; 123: 301–12. DOI: 10.1542/peds.2007-3317 [DOI] [PubMed] [Google Scholar]
- 23.Ferreccio C, Clemens J, Avendano A, et al. The clinical and immunologic response of Chilean infants to Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine coadministered in the same syringe with diphtheria-tetanus toxoids-pertussis vaccine at two, four and six months of age. The Pediatric Infectious Disease Journal. 1991; 10: 764–71. DOI: 10.1097/00006454-199110000-00009 [DOI] [PubMed] [Google Scholar]
- 24.Folb PI, Bernatowska E, Chen R, et al. A global perspective on vaccine safety and public health: The Global Advisory Committee on Vaccine Safety. American Journal of Public Health. 2004; 94: 1926–31. DOI: 10.2105/AJPH.94.11.1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma HJ, Yadav S, Lalwani SK, et al. Immunogenicity and safety of an indigenously manufactured reconstituted pentavalent (DTwP-HBV+Hib) vaccine in comparison with a foreign competitor following primary and booster immunization in Indian children. Human Vaccines. 2011; 7: 451–7. DOI: 10.4161/hv.7.4.14208 [DOI] [PubMed] [Google Scholar]
- 26.Faingezicht I, Avila-Aguerro ML, Cervantes Y, et al. Primary and booster vaccination with DTPw-HB/Hib pentavalent vaccine in Costa Rican children who had received a birth dose of hepatitis B vaccine. Revista Panamericana de Salud Pública. 2002; 12: 247–57. DOI: 10.1590/S1020-49892002001000005 [DOI] [PubMed] [Google Scholar]
- 27.Cunha MP, Dórea JG, Marques RC, et al. Vaccine adverse events reported during the first ten years (1998–2008) after introduction in the state of Rondonia, Brazil. BioMed Research International. 2013; 85: 6 DOI: 10.1155/2013/853083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raeeisi a MM, Karimi F, Saberinejad F. Assessment of Diphteria, Tetanus and Pertusis Vaccine-associated Complications in Infants under 7 Years Old in Shahrekord in 2010–2012. Journal of Ilam University of Medical Sciences. 2014; 22(10): 321. [Google Scholar]
- 29.Parisaee Z, Esteghamati A, Ghashghaei KZ, et al. Adverse Effects of DPT Vaccine in Children below 7 Years of Age in Rural and Urban Areas of Kohgiloye and Boyerahmad Province In 2006. Armaghane Danesh Bimonthly Journal. 2008; 13: 105–14. [Google Scholar]
- 30.Ayatollahi J, Zare A. Evaluation of the side effects of triple vaccine in Yazd in 2005. Iranian Journal of Pediatrics. 2006; 16: 332–6. http://hdl.handle.net/1807/58309. [Google Scholar]
- 31.Nabavi M, Jandaghi J, Ghorbani R, Khaleghi Hashemian M, et al. The incidence of complications of vaccination in infants and infants of Semnan, Iran. Koomesh. 2010; 11: 245–54. [Google Scholar]


