Abstract
Objective.
The presence or relative proportion of progesterone nuclear receptors (PR) in different tissues may contribute to sexual dimorphism in these tissues. PR is expressed in chondrocytes, but its function is mostly unknown. We hypothesized that the PR may regulate chondrocyte metabolism and affect subchondral bone structure.
Methods.
We utilized genetic fate mapping and immunohistochemistry to elucidate PR expression in and effect on cartilage. To define sex-dependent and chondrocyte-specific effects of the PR on subchondral bone, we selectively deleted PR in osteochondrogenic progenitor cells marked by Prx1 (Prx1; PRcKO) and Collagen 2 (Col2; PRcKO), or in matured chondrocytes marked by aggrecan (Acan; PRcKO) and evaluated subchondral bone structure at 4 months of age. Chondrocyte aging was monitored by anti-senescence marker, p16INK4a, and MMP13, one of the Senescence-Associated Secretary Phenotype (SASP) components.
Results.
Compared to wild-type (WT) mice, the female Prx1; PRcKO and the Col2; PRcKO mice had greater total subchondral bone volume and greater subchondral cortical bone thickness, with increased estimated subchondral bone stiffness and failure load in both female and male Col2; PRcKO mice. Moreover, Col2; PRcKO mice from both sexes had greater bone formation and bone strength at the femurs. In contrast, we did not observe any subchondral bone changes in Acan; PRcKO mice other than higher work-to-failure observed in the male Acan; PRcKO mice. Despite no detected difference in articular cartilage between the WT and the PR; chondrocyte conditional deletion mice, there were greater numbers of senescent chondrocytes and increased MMP13 expression, especially in the male mutant mice.
Conclusion.
These findings suggest that selective inhibition of PR in osteoprogenitor cells, but not in terminally differentiated chondrocytes, induced an increased subchondral bone phenotype and high estimated subchondral bone strength, which might be associated with the development of osteoarthritis in older age.
Introduction
The presence or relative proportion of progesterone nuclear receptors (PR) in different tissues may contribute to sexual dimorphism in these tissues. The function of the PR in chondrocytes is mostly unknown. Over the past ten years, our research group and others have studied PR action on the skeleton. PR is expressed by cultured osteoblasts, osteoclasts [1–3], and chondrocytes [4] and is present in vivo in mouse bone [3, 5]. Both genetic fate mapping and immunohistochemistry have identified PR (esp. PR-B) expression in subchondral cartilage and the chondrocytes in the growth plate [5]. Additionally, conditional PR deletion in osteochondrogenic progenitor cells significantly suppressed immunomodulation pathways that might affect disease pathways involved in the development of osteoarthritis (OA) [6].
OA is characterized by degenerative changes in the whole joint leading to pain and ultimately physical disability. The risk of developing OA increases in post-menopausal women [7, 8], suggesting a link between OA and hormonal status, which has traditionally been attributed to changes in estrogen levels. Women with low circulating estradiol levels were nearly twice as likely to develop OA or experience joint pain as those with higher hormone levels, after adjusting for age, injury history, and body-mass index [9]. Long-term estrogen replacement therapy might provide a moderate level of protection against knee OA [8, 10–12] which might be due to attenuation of pro-inflammatory cytokines resulting from estrogen deficiency [8, 13]. Gene polymorphisms in the estrogen receptors (ERs) α & β have been found to be associated with knee and hand OA [14–20]. The association of ERγ with OA has been confirmed in animal studies using an ER gain-of-function approach [21]. PR is recognized to be more important than the ER in immunomodulation of female prevalence dominant diseases such as systemic lupus erythematosus, rheumatoid arthritis, and OA [6, 22, 23]. Progressive OA has been reported to sometimes develop during pregnancy and may result from increased hormone levels, including progesterone [24]. Samples of synovial fluid from OA patients demonstrates high levels of cytokines as well as MMPs [25–27]. Progesterone has consistently been found to have inhibitory effects on MMPs in the endometrium and synovial joints [25, 27, 28], suggesting a connection between the PR and OA. In this study, we aimed to evaluate the role of the PR in subchondral bone. Since both global and bone-cell specific PR knockout mice have higher bone mineral density (BMD) than their wild type (WT) controls and there are observed sex differences in this relationship [5, 6, 29], we hypothesized that the PR may regulate osteo-chondrocyte metabolism and might have a role in regulating subchondral bone structures, which is related to OA incidence and progression [30–36]. For this reason, we set out to use a Cre recombinase driven approach to examine the effects of tissue-specific inactivation of PR in osteochondral progenitor cells or terminally differentiated chondrocytes on subchondral bone architecture.
Materials and Methods
Mice
PR-flox mice were obtained from Baylor College of Medicine (Houston, TX, USA). A targeting vector designed to replace part of exon 2 of the PR gene with a selectable marker was employed to create a strain of mice carrying a conditional null PR allele [37]. Prx1-Cre, Col2a1-CreERT(Col2-Cre), Aggrecan-CreERT(Acan-Cre), PR-Cre, mT/mG, and Ai14D transgenic mice were obtained from the Jackson Laboratory (Farmington, CT, USA). The PR-Cre mice (B6.129S(Cg)-Pgrtm1.1(cre)Shah/AndJ) harbor an internal ribosome entry site and Cre recombinase gene downstream of the progesterone receptor transcriptional stop codon. As such, Cre expression was driven by the endogenous PR promoter/enhancer elements [38]. Ai14D mouse was bred with the PR-Cre mouse to track PR expression. Each Cre line was crossed with PR-flox mice to generate conditional PR knockout mice. Tamoxifen (Sigma-Aldrich, St Louis, MO, USA) was injected intraperitoneally at 5 mg/kg for three consecutive days to both the WT (Cre−) and the Cre+ mice when they were one month of age to activate the Cre and selectively remove PR from the targeted cells. We have previously determined that this dose did not significantly affect bone turnover [39]. Mice were euthanized at four months of age. Two fluorochrome labels were used, alizarin red (20 mg/kg) or calcein (10 mg/kg), which were given to the female WT mice at days −7 and −1 before euthanization. Since we found relatively weak alizarin red staining in the samples, we switched to double calcein labeling for female cKO mice and all the male mice.
PCR-based strategies were used for genotyping mouse genomic DNA. All animal work was done in compliance with the guiding principles of UC Davis’ “Care and Use of Animals.” Mice were housed in the animal facility under strictly controlled environmental conditions (12-hour light/dark cycle, room temperature 22°C), and fed ad libitum (food and water). The Institutional Animal Care and Use Committee of the University of California, Davis approved the animal protocol.
Chondrogenic differentiation of bone marrow stromal cells.
Mouse bone marrow stromal cells were extracted from PR-tdTomato mice and allowed to expand for 7-10 days. Cells were then placed at 1.25 x 105 density in 5 mL of the pre-warmed completed StemXVivo Chondrogenic Base Media with 0.5 mL of pre-warmed completed StemXVivo Chondrogenic Differentiation Media (R&D Systems, Minneapolis, MN, USA). Media was replaced every 2-3 days. Cell culture images were captured, and RNA was extracted from the cells at culture days 0, 8 and 14 to monitor the levels of PR expression.
MicroCT measurements
Right femurs were scanned and analyzed using VivaCT 40 (Scanco Medical, Bassersdorf, Switzerland) with a voxel resolution of 10 μm in all three spatial dimensions and a mono-energetic (70 Kev) X-ray source. We evaluated the entire epiphyseal area. A total metaphysis tissue volume of 3–4 mm3 for each scan was used to obtain the subchondral bone volume/total volume (BV/TV) ratio, subchondral trabecular and cortical bone thickness [6, 40,41].
We used a microCT-based finite-element model (FEA) to estimate the biomechanical properties of the subchondral bone. The same microCT images that we used for subchondral bone architectural evaluation were incorporated into the FEA model as previously described [42]. The 3D image voxels were converted to elements; each finite element model mesh had approximately 9-18 million elements. Each element segmented was assigned as bone using Young’s modulus of 18 GPa and a Poisson ratio of 0.3 [43]. Details of the numerical method were published elsewhere [44, 45]. The boundary conditions that defined the load platen-specimen interface were assumed to be frictionless. Subchondral bone stiffness and changes in the load-carrying capacity of the subchondral bone (estimated failure load) and apparent modulus were calculated from FEA analyses [46].
Bone and Knee Histology
The right distal femurs were collected, fixed in 4% formaldehyde with 10% sucrose (W/V) at 4°C for 2 days, further dehydrated in 30% sucrose overnight and embedded in Optimal Cutting Temperature (OCT) medium (Thermo Fisher, Waltham, MA, USA). Cryosections were prepared and imaged with a Keyence BZ-X9000 all-in-one Fluorescence Microscope (Itasca, IL, USA). Surface-based bone formation was quantitated using Bioquant (Bioquant Image Analysis Corporation, Nashville, TN, USA) [6]. Primary antibodies against p16NIK4a and MMP13 (Abeam, Cambridge, MA, USA) and Alexa-Fluor 594 or 488-conjugated secondary antibody were counterstained with DAPI. Positive stained cells were counted using the cell count software in Keyence BZ-X9000 all-in-one imaging system (Itasca, IL, USA).
The left knee joints were fixed in 10% phosphate-buffered saline formalin for 2 days, decalcified in 10% EDTA for 3 weeks, and embedded in paraffin. Sections were stained with Safranin-O – Fast green for measurement of articular cartilage thickness, subchondral bone plate thickness, subchondral trabecular bone number and diameter, and cartilage content using Bioquant Imaging software (Bioquant Imaging System, Nashville, TN, USA) [40, 41].
Whole bone strength measurements
Each right femur was loaded to failure along its long axis using an MTS 831 electro-servo-hydraulic testing system (MTS Systems Corp., Eden Prairie, MN, USA) at a displacement rate of 0.01 mm/s with a 90 N load cell. Sample loads and displacements were continuously recorded throughout each test. The maximum load was determined from the load-displacement curve, and the work-to-failure was calculated from the area under the load-displacement curve [3, 47–49].
Statistical analysis.
The results are expressed as mean ± standard deviation for bone structure, bone turnover, and bone strength variables. Two-way ANOVA was used to account for genotype and sex. If significant differences were observed, then a Tukey’s multiple comparisons test was used to assess pairwise comparisons. A value of p < 0.05 was considered statistically significant. Data were analyzed using the GraphPad Prism 7 software package (La Jolla, CA, USA).
Results
PR and Col2 expression in bone and joint
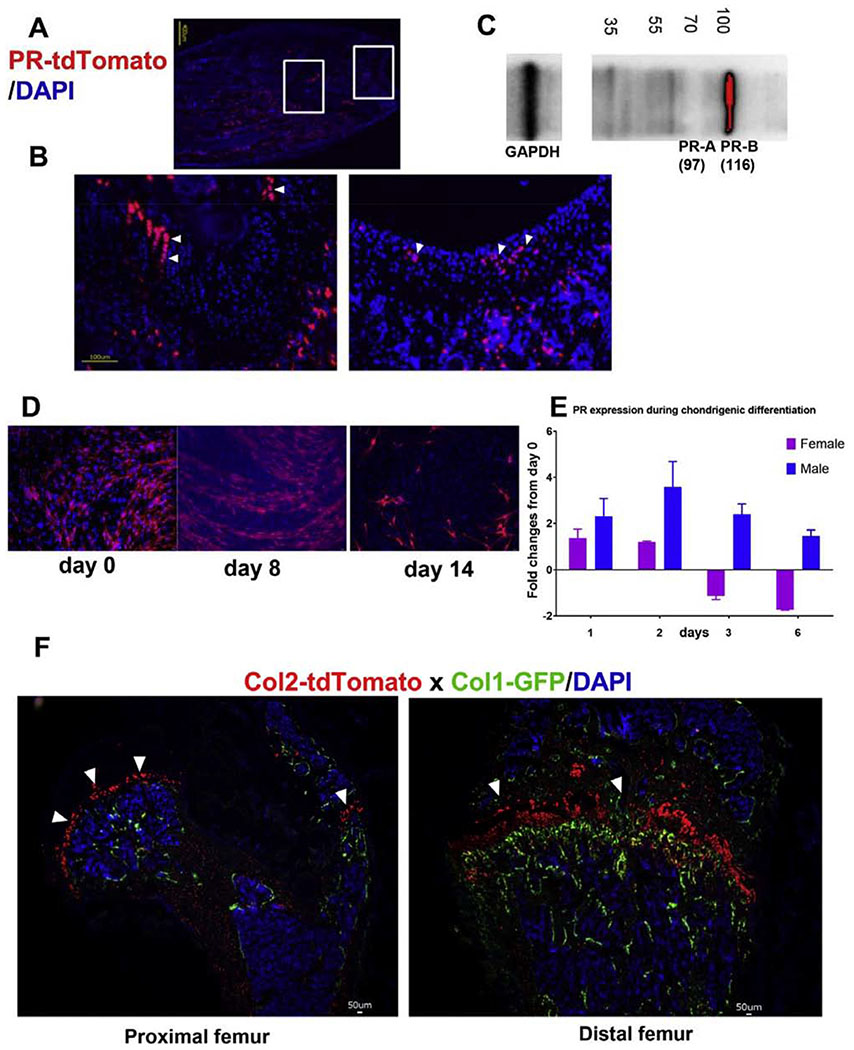
To characterize the pattern of PR expression in cartilage, we generated a reporter mouse strain PR-Cre; tdTomato, in which the Cre was driven by the endogenous PR promoter, and this activated the tdTomato expression. We collected the right distal femurs from PR-Cre; tdTomato mice at three weeks of age and created cryosections. Red fluorescence, corresponding to PR expression, was observed within some areas of the growth-plate cartilage as well as the subchondral cartilage area (Figures 1A,B). Western Blot confirmed PR expression on protein extracted from distal femurs, including the growth plate (Figure 1B). When we collected the bone marrow stromal cells (BMSCs) from 4-week old PR-Cre; tdTomato mice and cultured them in chondrogenic media for 14 days, we found decreased PR-RNA level when the BMSCs differentiated into chondrocytes, especially in the females (Figures 1D, E). Lineage tracing studies in vivo suggested Col2-tdTomato+ cells were observed in the articular chondrocytes and growth plate chondrocytes (Figure 1F, white arrowheads), while the Col1+ green cells were observed in the endosteal and periosteal bone surfaces (Figure 1F).
Figure 1.
Characterization of PR expression in joint and chondrocytes.
(A) PR-Cre mice were crossed with tdTomato, a red fluorescent protein. Fields on left and right showed PR expression in the growth plate subchondral bone and cartilage (white arrowheads). (B) Western blot performed on proteins extraction from the subchondral bone and growth plates regions from WT female mice, 1 month of age. (C) Enlarged area indicated in the rectancular boxes in (B) howing PR expressions in the growth plate and the cartilage. (D) Bone marrow stromal cells were collected from one-month-old PR-tdTomato female and male mice and were differentiated into chondrocytes in chondrogenic media for 14 days. Cell nuclei were stained blue with DAPI. (E) Quantitative RT-PCR was performed to detect PR expression in BMSC differentiating into chondrocytes at 1, 2, 3, and 6 days. The result was presented as fold-changes from day 0. (F) Col2-CreERT mice were first crossed to tdTomato mice and then to Col1-GFP mice resulting in Col2 + cells expressing red (white arrowheads) and Col1 expressing green fluorescence.
Subchondral bone microarchitectural changes following PR conditional knockouts in osteochondrogenic progenitor cells with noted sex differences
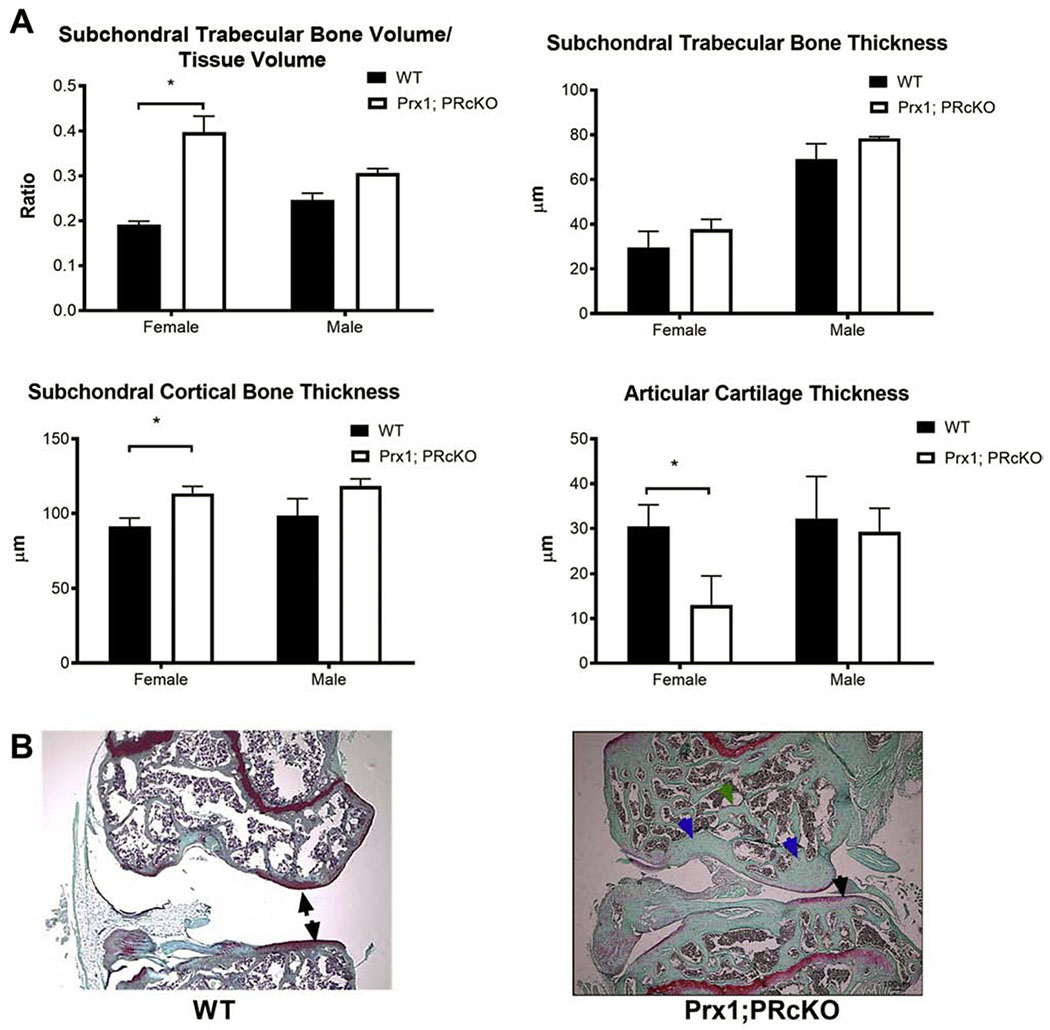
We have previously reported that global PRKO or PR conditional knockouts (PRcKO) in osteoprogenitor cells using Prx1-Cre resulted in high bone mass in long bones, especially in females [5, 6, 29]. Similarly, in the current analysis, we found that subchondral bone volume was significantly higher in the female Prx1;PRcKO mice, with significantly thicker subchondral cortical bone and thinner articular cartilage (Figure 2A). The subchondral cortical bone plate was thicker (blue arrowheads) than the WT mice with thinner articular cartilage (black arrowhead) at eight months of age in the female Prx1; PRcKO mice (Figure 2B).
Figure 2.
Subchondral bone microarchitecture in Prx1; PRcKO mice.
(A) Subchondral bone volume and microarchitectures were measured by microCT. (B) Representative histology images from WT or Prx1; PRcKO female mice at eight months of age. Arrows illustrated articular cartilage. N=5-8/group.
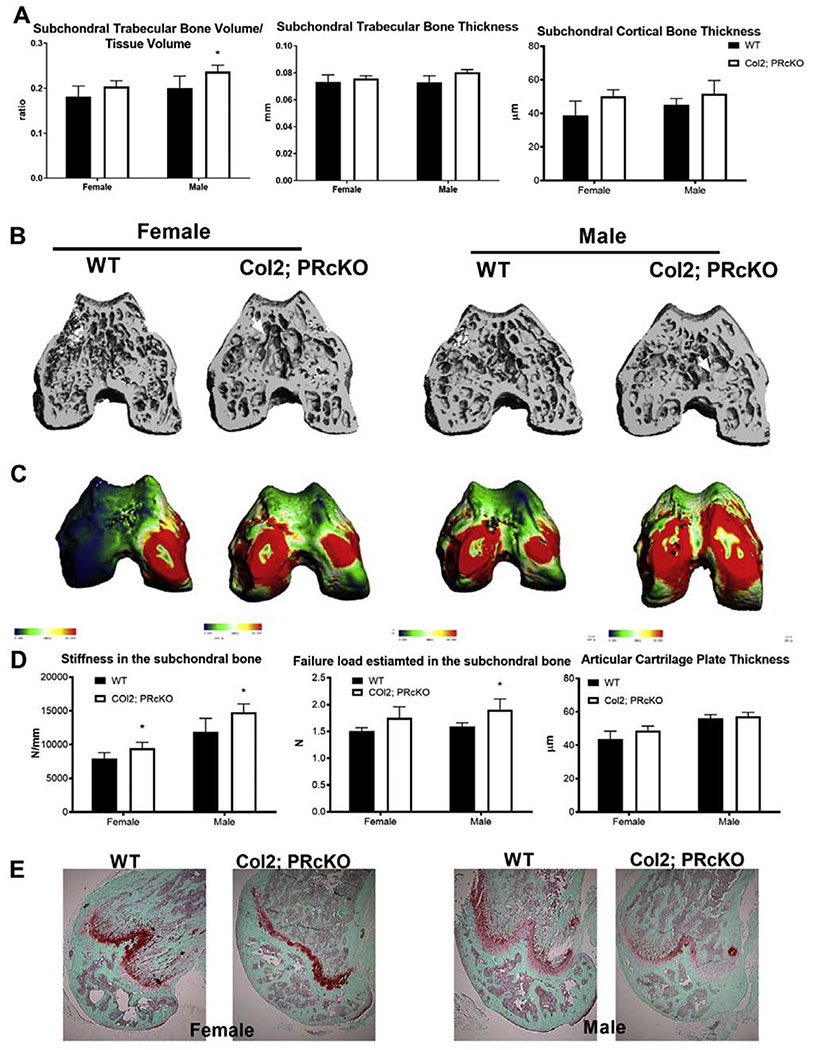
We next crossed Col2-CreERT or Acan-CreERT mice with PR-flox/flox mice to conditionally inactivate the PR gene in chondrocytes starting from one month of age, when tamoxifen was given to both wild-type (WT) and these PRcKO mice. Knee joints were collected from homozygous Col2; PRcKO and their WT littermates at four months of age. There were no differences in either body weights or femoral length between the WT and the Col2; PRcKO mice (data-on-file). Measurements of subchondral bone structures by microCT and histology yielded similar results. We chose to present results from microCT measurements in this report. Compared to the wild-type (WT) mice, only the male Col2; PRcKO mice had higher subchondral trabecular bone volume/tissue volume (Figure 3A, B) but had similar subchondral trabecular bone and cortical bone thickness (Figure 3 A, B). The estimated stiffness was higher in both female and male Col2; PRcKO mice and failure load carried by the subchondral bone was significantly higher in the male Col2; PRcKO mice compared to the WT (Figure 3 C, D). Articular cartilage thickness measured by Safranin-O staining at the femoral chondral and was not different between WT and the Col2; PRcKO mice in either sex (Figure 3E).
Figure 3.
Subchondral bone microarchitecture and estimated bone strength in Col2; PRcKO mice.
(A) Subchondral bone volume, trabecular bone thickness, and cortical bone thickness measured by microCT. (B) Representative microCT images of the subchondral bone from WT or Col2; PRcKO female and male mice. (C) Representative FEA maps of the distal femur subchondral bone from female and male WT andCol2; PRcKO mice. Blue to red indicated low to high stiffness in a continuous manner. (D) Stiffness and failure load were estimated by FEA analyses at the subchondral bone in 4-month-old WT and Col2; PRcKO mice. N=8-14/group.
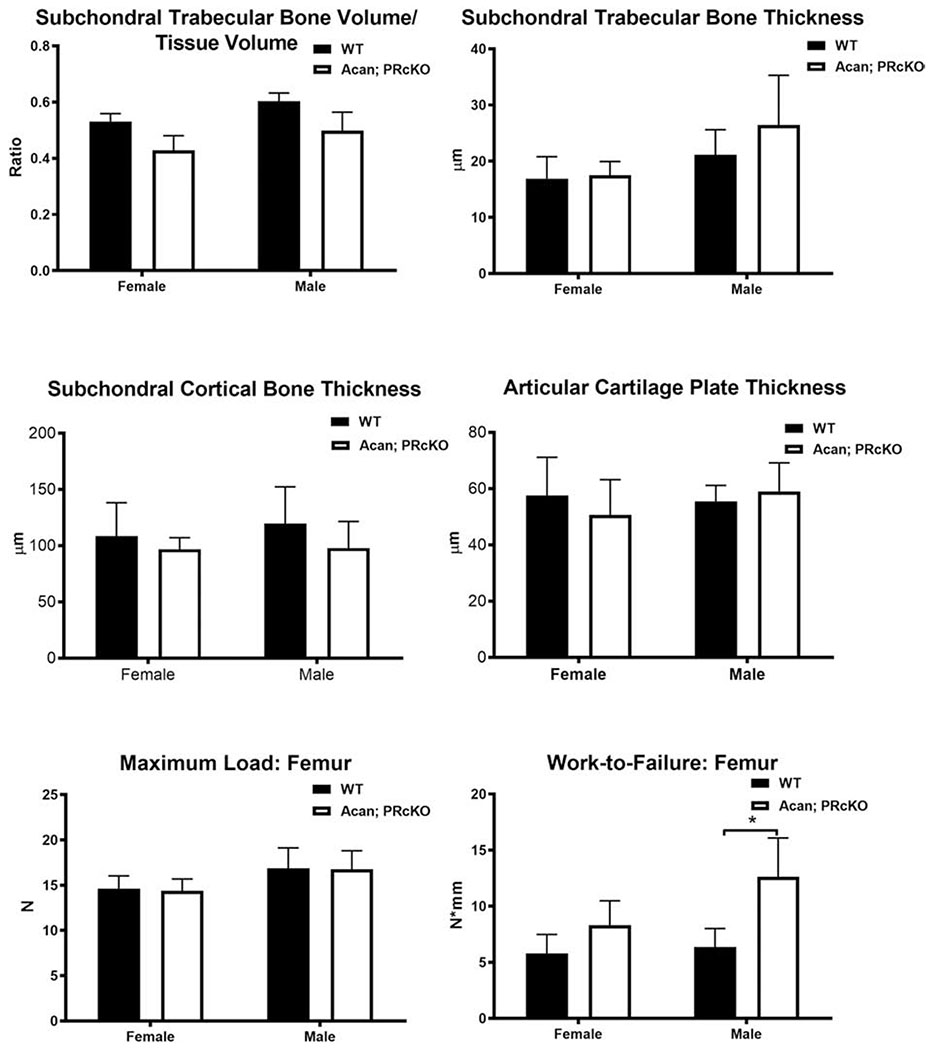
We failed to detect subchondral bone structures using Acan-Cre [50] to selectively removed PR from terminally differentiated chondrocytes. Although the subchondral bone structure and estimated load carried by subchondal bone area were not different between Acan; PRcKO mice and their WT littermates in both sexes (Figure 4), there was a 2-fold higher work-to-failure in the male Acan; PRcKO mice compared to their WTs (Figure 4).
Figure 4.
Subchondral bone microarchitecture and bone strength in Acan; PRcKo mice.
Subchondral bone volume, trabecular bone thickness, and cortical bone thickness were measured by microCT. Whole bone strength was measured at the femurs in 4-month-old WT and Acan; PRcKO mice. N=5-10/group.
These data suggested that PR inactivation in the osteochondral progenitor cells, but not in the terminally differentiated chondrocytes, altered subchondral bone architectures with noted sex differences. Articular cartilage plate thickness did not differ between the WT and mutant mice at four months of age.
PRcKO in chondrocytes marked by Col2 increased bone formation and bone strength
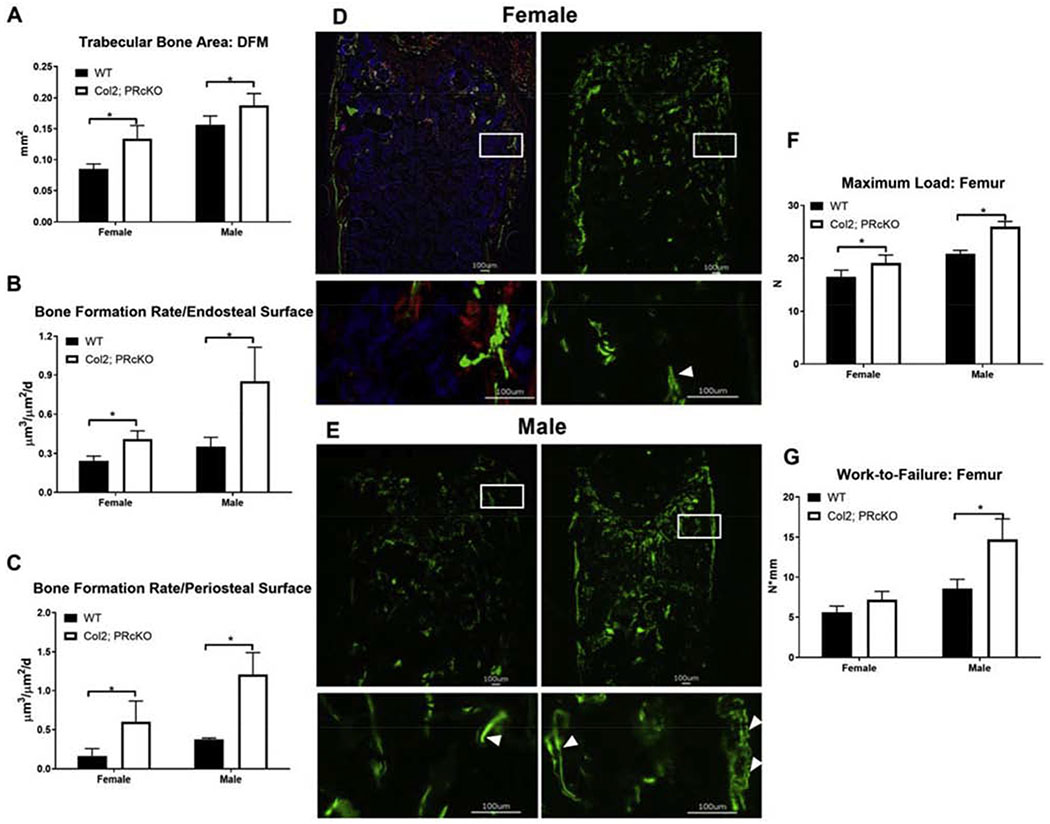
Consistent with the high subchondral bone mass phenotype in the epiphysis in the Col2; PRcKO mice, we also found the trabecular bone area to be higher at the distal femoral metaphysis (DFM) in Col2; PRcKO mice in both sexes (Figure 5A). Dynamic histomorphometric analysis of bone formation measured at the distal femoral metaphysis in 4-month-old Col2; PRcKO mice revealed that the female and male Col2; PRcKO mice had 60-90% higher surface-based bone formation rate at both the endosteal and the periosteal bone surfaces compared to their WT littermates (Figure 5 B–E). Selective removal of PR from Col2+ chondrocytes increased bone strength (maximum load and Work-to-Failure) by 15-25% in the male Col2; PRcKO mice (Figure 5 F–G) as compared to their WT littermates. These data suggested that PR inactivation in the Col2+ chondrocytes increased bone formation and whole bone strength, especially in the male Col2; PR conditional KO mice.
Figure 5.
Col2; PRcKO mice had higher bone formation rate and whole bone strength.
(A) Bone histomorphometry was performed at the distal femur metaphysis from wild type and Col2; PRcKO mice of both sexes at four months of age. Mice were given two fluorescent labels at −7 and −1-day(s) before sacrifice to calculate bone formation rate at the (B) endosteal and (C) periosteal bone surface. (D) Representative images of double fluorescence labeled distal femurs in the 4-month-old female or (E) male WT or Col2; PRcKO mice. Boxes represent the enlarged periosteal and endosteal surfaces. (F) Maximum load and (G) Work-to-Failure were obtained at the femurs by three-point bending. N=8-10/group.
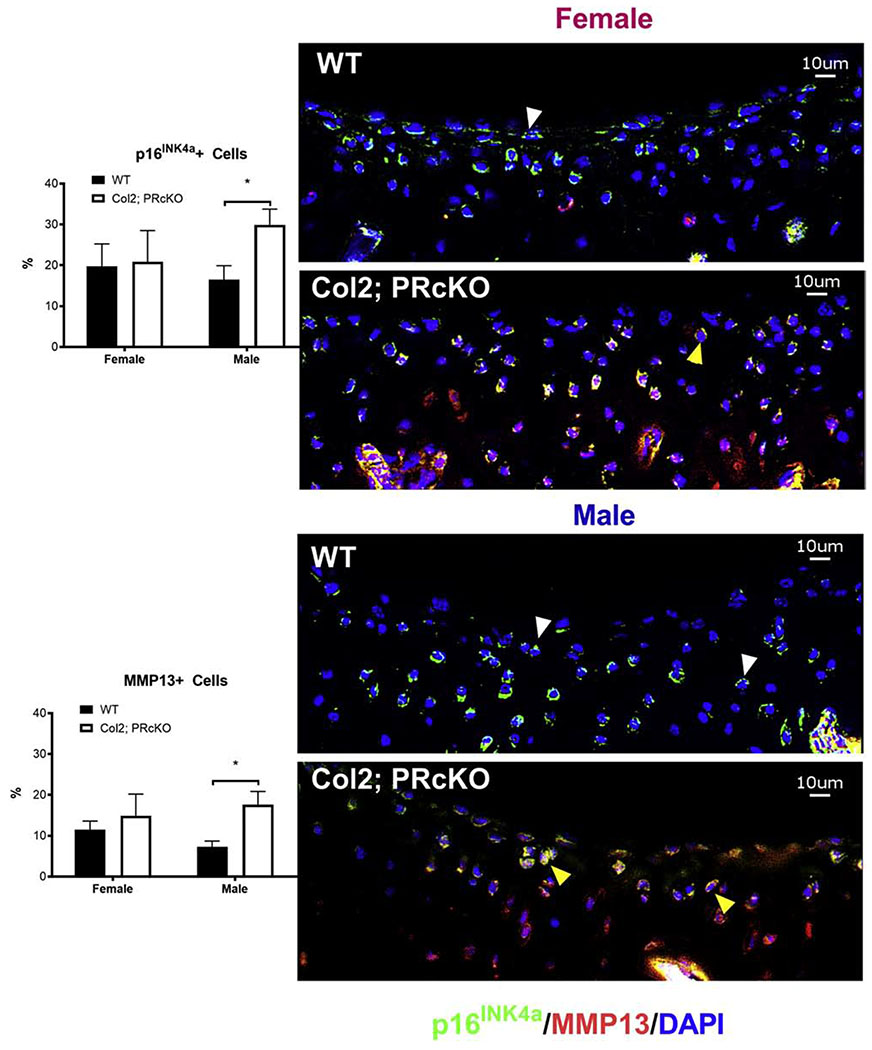
Lack of PR in the Col2+ chondrocytes accelerated chondrocyte aging
To explore the cellular mechanisms of PR on chondrocytes that might affect the age-related cartilage degradation and sex differences, we examined the subchondral cartilage of 4-month-old Col2; PRcKO mice from both sexes. p16INK4a was a marker for senescent cells [51, 52]. Using anti-p16INK4a staining as a marker for cell senescence, we observed approximately 18% subchondral cartilage and subchondral bone marrow cells were positive for p16INK4a in all the mice (Figures 6, white arrowheads). p16INK4a expression was higher particularly in subchondral cartilage as well as in subchondral bone marrow in the male Col2; PRcKO mice. The number of cells positive for a senescence-associated secretory component, MMP13, was expressed at relatively low levels in the cartilage in the WT mice, but its expression was higher at subchondral cartilage/bone marrow in male Col2; PRcKO mice as compared to the WT mice (Figure 6, red stained cells) . Some p16INK4a+ senescent cells co-expressed MMP13 in both the female and male Col2; PRcKO mice, (Figure 6, yellow heads). Taken together, these exploratory findings suggested that selective removal of PR from Col2+ chondrocytes was associated with higher levels of chondrocyte senescence and accompanied by higher MMP13 expressions in the male mutant mice, which might have accelerated cartilage degradation as they aged.
Figure 6.
Col2; PRcKO male mice had higher cellular senescence at the subchondral bone regions.
Distal femurs from both female and male 4-month-old WT and Col2; PRcKO mice were stained with anti-p16INK4a (stained in green, white arrowheads) and MMP13 (stained in red). Some of the chondrocytes expressed both p16INK4a and MMP13 (yellow arrowheads). Scale bar 10μm. N=5-8/group.
Discussion
Mice lacking PR in osteochondral progenitor cells displayed high subchondral trabecular bone volume and subchondral cortical bone plate thickness, which were associated with higher estimated stiffness and failure load carried by the subchondral bone. The femoral bone formation rate and bone strength were both significantly higher when PR was selectively removed from chondrocytes marked by Col2. Additionally, using Acan-cre [50, 53], which was more specific to mature articular chondrocytes, did not cause development of a significant bone or joint phenotype except for higher Work-to-Failure in the male mutant mice. Moreover, cellular senescence, marked by p16INK4a, and MMP13, a component for the senescence-associated secretory phenotype, were significantly higher in the subchondral cartilage and subchondral bone marrow regions in the 4-month-old male Col2; PRcKO mice. Based on our observations, we propose that PR inhibition in the osteochondral progenitor cells could result in subchondral bone sclerosis and increased OA incidence or progression.
Contribution of subchondral and whole bone architectural changes to OA
OA affects both bone and joint. Hip and knee OA, in particular, are characterized by loss of articular cartilage, sclerosis, and recurrent inflammation of joint tissues [30–36]. OA is more common in women and the risk of developing OA is increased in post-menopausal women [7, 8], suggesting a link between OA and hormonal status. Subchondral bone might be essential for maintaining knee joint stability by supporting the articular cartilage. Changes in the subchondral bone plate and subchondral trabecular bone have been reported to be associated with incident knee OA [35]. Loss of subchondral trabecular rods with thickening of the remaining trabecular plates have been identified in advanced knee OA surgical specimens [32, 54]. Subchondral bone changes are reported present even in early stages of OA, preceding cartilage lesions [30, 35]. Subchondral bone and cartilage might play complementary roles in load-bearing joints. Subchondral bone supports articular cartilage and distributes mechanical loads across joint surfaces. Architectural changes in the subchondral bone could alter the load on the overlying cartilage, leading to inconsistent load distribution, and creating localized structural load excess allowing for incident cartilage wear and degeneration. Alternatively, loss of cartilage could also transmit increased and uneven loads to the underlying subchondral bone and thereby induce sclerosis of the subchondral bone [36]. Given these types of potential interaction between bone and cartilage, the current study findings support the concept of the PR as a potentially important contributor to OA: PR had significant demonstrable effects on subchondral bone structures that preceded other changes in the joint and differed by sex in ways reminiscent of sex differences noted in OA. Decreased activity of inflammatory arthritis has been reported during pregnancy [55–59] while multiple pregnancies appear to increase the risk of developing OA [60]. Based on our findings together with these prior observations, we propose that a therapeutic intervention using PR signaling in the osteochondral progenitor cells could be beneficial for maintaining cartilage integrity and could potentially decrease OA incidence or progression. However, to date, PR inhibition has not been used in the prevention or the treatment of OA.
More carefully designed translational and human clinical trials are needed to evaluate the effects of the progesterone/PR signaling axis in the pathogenesis of OA, considering other factors of the genetic, cellular and physical environments during aging.
Aging, chondrocyte senescence, and PR
Increased levels of senescence markers, such as senescence-associated beta galactosidase (SA-βGal) activity, telomere shortening, and accumulation of cytokines and MMPs have been linked to cell senescence within joint tissues and might play a pathologic role in the etiogenesis of primary OA [61–63]. Using histological staining, SA-βGal has been observed in chondrocytes from elderly persons [61] and in chondrocytes from OA patients [64, 65]. Selective removal of p16INK4a positive cells, a marker for senescent cells, delayed the onset of several aging-related phenotypes in mice [66]. Since articular chondrocytes are terminally differentiated cells, it was unlikely that they would experience telomere shortening resulting from classical replicative senescence. Diekman et al. showed that the expression of p16INK4a itself might be associated with dysfunctional chondrocytes. OA phenotypes might arise in part due to the production of Senescence-Associated Secretary Phenotype (SASP) produced by the senescent chondrocytes [67]. Extrinsic factors, such as repetitive loading, stress, or local hormonal regulation, might also contribute to chondrocyte aging/senescence. Also, the lack of progenitor cells in joints limits the regenerative ability of the cartilage to respond to aging or injury/microfractures. Since chondrocytes under normal physiology do not readily enter cell division, the increased population of senescent chondrocytes would result in an altered ratio of active to senescent chondrocytes over time, as well as indirectly inducing the chronic inflammation associated with primary OA.
Studies on the effects of PR on cell metabolism and viability have often focused on its impact in the reproductive system; for example, the PR controls cell fate decision for healthy mammary gland development [68]. PR expression levels in bone and joint were approximately 100-fold lower than in other PR-dominant tissues such as the ovary and uterus, but there is evidence of the importance of these signaling pathways in these structural tissues nonetheless [5]. Sex differences in the regulation of PR have been reported in bone [3, 37]. Earlier studies using mouse genetic fate-mapping approaches demonstrated that some PRs were expressed by cells at the articular and growth plate regions at 1-2 months-old [5]. PR expression was detected in early osteochondral progenitor cells, and its expression decreased as the osteochondral progenitor cells differentiated into either osteoblasts or chondrocytes. PR-B, the same isotype that we detected in the epiphyseal and metaphyseal regions [5], induced senescence in ovarian cancer cells [69] via a FOXO1 dependent mechanism and was ligand-dependent [70]. FOXO1 is a critical transcriptional factor for chondrocyte senescence. Downregulation of FOXO1 increases the susceptibility of human-derived chondrocytes to oxidative stress [71]. Selectively knocking out FOXO 1/2/4 completely or FOXO1 from the chondrocytes marked by Col2 and Acan decreased gene expression associated with cell viability and antioxidant defense and made mice more susceptible to developing post-traumatic OA [72]. The current study confirmed chondrocyte senescence was detected as early as four months with the lack of PR, and that PR expression in osteochondral cells might be protective against chondrocyte aging and prevent OA-like changes as the mice aged. Combining these various pieces of information creates a powerful argument that PRs are important in bone and in the development of OA, and that this influence may start early in the lifespan but may act in part through processes related to senescence throughout the lifespan.
Limitations and strengths
A primary limitation of the study is that we did not follow the WT or the mutant mice to more advanced ages when OA-like phenotypes may be more prevalent and more directly comparable to typical age-related primary OA. Further studies to evaluate age-related OA effects, as well as post-traumatic injuries would provide valuable information to determine if the presence of PR inhibition in osteochondral cells could serve as a therapeutic target to prevent the progression of OA or explain the sex difference in OA incidence.
We used a lineage tracing method in combination with immunohistochemistry staining to monitor PR expression in the chondrocytes [5], but we did not use molecular markers such as Col10a, sox9 or ihh to validate expression of the Col2-reporter; nor did we use histological sections to determine subpopulations or stages of chondrocyte maturation to identify chondrocytes in bone. These could be considered for future studies.
Aggrecan is the most abundant protein in cartilage and has been reported to have numerous functional roles from the articular cartilage to the growth plate zones as well as contributing to biomechanical properties [50, 73, 74]. We we used two low dose tamoxifen injections at 5mg/kg to activate Cre expression, dued to a concern for tamoxifen doses affecting bone turnover and bone mass [39]. Prx1 and Col2-Cre targeted greater cell populations than Acan-Cre [50, 75, 76], which might explain the greater changes that we observed in Prx1; PRcKO and Col2; PRcKO mice compared to the Acan; PRcKO mice. The expression of PR in chondrocytes quickly decreased as the osteochondral progenitors differentiated into the chondrocytes, especially in the females, which could explain the sex difference we observed in the study. Selective deletion of PR from the osteoprogenitors using Prx1-cre or Col2-Cre resulted in high bone mass and bone strength, suggesting inhibitory effects of PR on bone mass acquisition [5]. Although PR selective deletion from Acan+ cells did not affect the subchondral bone structures, it did induce higher Work-to-Failure measured at the femur, suggesting that lack of PR signaling in the chondrocytes might impact the material bone strength.
The effects of estrogen (E2) or estrogen receptors (ER) might counter-balance some of our findings in the female mice [77]. We did not monitor the levels of E2 and ER expressions in the current studies. It has been reported that double ERα and β estrogen receptor knockout mice developed OA features such as osteophytosis formation as early as at six months of age, while no cartilage damage was observed [78]. Female Col2; ERαcKO mice had less bone erosion and cartilage degradation in a model of inflammatory arthritis, suggesting that ERα is important for maintaining joint integrity under inflammatory conditions. There is a lack of studies on how hormonal receptors regulate chondrocyte aging and in the pathogenesis of primary OA.
Conclusion
Loss of function of PR in osteochondral progenitors resulted in a high subchondral bone phenotype and high estimated load carried by the subchondral bone that might precede cartilage degradation, an important component of late stage OA. The lack of PR on osteoprogenitor cells might cause an increased risk of OA as mice age. An aging study to define the role of PR in primary osteoarthritis is warranted.
Highlights for the reviewers:
Selectively deleted progesterone receptor (PR) from osteoprogenitor cells using PRx1-Cre or in the chondrocytes using Col2-Cre-ERT and Acan-Cre-ERT in both sexes.
Focus on subchondral bone structures, which were related to osteoarthritis incidence and progression.
We found that selective inhibition of PR in osteoprogenitor cells, but not in terminally differentiated chondrocytes, increased subchondral bone mass with sex difference. This study might also lead to the identification of a mechanism explaining sex-differences in the primary OA and that the lack of PR in osteoporosgenitor cells increased the risk of developing OA as they aged.
Acknowledgments:
SCOR supported these studies: NIH/NIAMS 1P50AR063043 and NIH/NIAMS R01AR061366 (WY). PR-flox mouse was obtained from Dr. John Lydon at Baylor College of Medicine. We thank the SCOR External Advisory Board Member Dr. Mark Johnson, and the Internal Advisory Board Members Dr. Robert Nissenson and Dr. Edward Hsiao for their consultation, technical support, and editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
Data and materials availability: Data is archived at the University of California, Davis
References:
- [1].Wei LL, Leach MW, Miner RS, Demers LM, Evidence for progesterone receptors in human osteoblast-like cells, Biochem Biophys Res Commun 195(2) (1993) 525–32. [DOI] [PubMed] [Google Scholar]
- [2].MacNamara P, O’Shaughnessy C, Manduca P, Loughrey HC, Progesterone receptors are expressed in human osteoblast-like cell lines and in primary human osteoblast cultures, Calcif Tissue Int 57(6) (1995) 436–41. [DOI] [PubMed] [Google Scholar]
- [3].Yao W, Dai W, Shahnazari M, Pham A, Chen Z, Chen H, Guan M, Lane NE, Inhibition of the progesterone nuclear receptor during the bone linear growth phase increases peak bone mass in female mice, PloS one 5(7) (2010) e11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang W, Hayami T, Kapila S, Female hormone receptors are differentially expressed in mouse fibrocartilages, Osteoarthritis Cartilage 17(5) (2009) 646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhong ZA, Kot A, Lay YE, Zhang H, Jia J, Lane NE, Yao W, Sex-Dependent, Osteoblast Stage-Specific Effects of Progesterone Receptor on Bone Acquisition, J Bone Miner Res 32(9) (2017) 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kot A, Zhong ZA, Zhang H, Lay YE, Lane NE, Yao W, Sex dimorphic regulation of osteoprogenitor progesterone in bone stromal cells, J Mol Endocrinol 59(4) (2017) 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Parazzini F, Progretto G Menopausa Italia Study, Menopausal status, hormone replacement therapy use and risk of self-reported physician-diagnosed osteoarthritis in women attending menopause clinics in Italy, Maturitas 46(3) (2003) 207–12. [DOI] [PubMed] [Google Scholar]
- [8].Roman-Blas JA, Castaneda S, Largo R, Herrero-Beaumont G, Osteoarthritis associated with estrogen deficiency, Arthritis Res Ther 11(5) (2009) 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sowers MR, McConnell D, Jannausch M, Buyuktur AG, Hochberg M, Jamadar DA, Estradiol and its metabolites and their association with knee osteoarthritis, Arthritis Rheum 54(8) (2006) 2481–7. [DOI] [PubMed] [Google Scholar]
- [10].Gokhale JA, Frenkel SR, Dicesare PE, Estrogen and osteoarthritis, Am J Orthop (Belle Mead NJ) 33(2) (2004) 71–80. [PubMed] [Google Scholar]
- [11].Ham KD, Loeser RF, Lindgren BR, Carlson CS, Effects of long-term estrogen replacement therapy on osteoarthritis severity in cynomolgus monkeys, Arthritis Rheum 46(7) (2002) 1956–64. [DOI] [PubMed] [Google Scholar]
- [12].Chlebowski RT, Cirillo DJ, Eaton CB, Stefanick ML, Pettinger M, Carbone LD, Johnson KC, Simon MS, Woods NF, Wactawski-Wende J, Estrogen alone and joint symptoms in the Women’s Health Initiative randomized trial, Menopause 20(6) (2013) 600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Martin-Millan M, Castaneda S, Estrogens, osteoarthritis and inflammation, Joint Bone Spine 80(4) (2013) 368–73. [DOI] [PubMed] [Google Scholar]
- [14].Fytili P, Giannatou E, Papanikolaou V, Stripeli F, Karachalios T, Malizos K, Tsezou A, Association of repeat polymorphisms in the estrogen receptors alpha, beta, and androgen receptor genes with knee osteoarthritis, Clin Genet 68(3) (2005) 268–77. [DOI] [PubMed] [Google Scholar]
- [15].Ushiyama T, Ueyama H, Inoue K, Nishioka J, Ohkubo I, Hukuda S, Estrogen receptor gene polymorphism and generalized osteoarthritis, J Rheumatol 25(1) (1998) 134–7. [PubMed] [Google Scholar]
- [16].Wise BL, Demissie S, Cupples LA, Felson DT, Yang M, Shearman AM, Aliabadi P, Hunter DJ, The relationship of estrogen receptor-alpha and -beta genes with osteoarthritis of the hand, J Rheumatol 36(12) (2009) 2772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bergink AP, van Meurs JB, Loughlin J, Arp PP, Fang Y, Hofman A, van Leeuwen JP, van Duijn CM, Uitterlinden AG, Pols HA, Estrogen receptor alpha gene haplotype is associated with radiographic osteoarthritis of the knee in elderly men and women, Arthritis Rheum 48(7) (2003) 1913–22. [DOI] [PubMed] [Google Scholar]
- [18].Lee SW, Song JH, Choi WS, Yoon JH, Kim O, Park YG, Nam SW, Lee JY, Park WS, The single nucleotide polymorphism (SNP) of the estrogen receptor-beta gene, rs1256049, is associated with knee osteoarthritis in Korean population, Knee 21(1) (2014) 242–6. [DOI] [PubMed] [Google Scholar]
- [19].Riancho JA, Garcia-Ibarbia C, Gravani A, Raine EV, Rodriguez-Fontenla C, Soto-Hermida A, Rego-Perez I, Dodd AW, Gomez-Reino JJ, Zarrabeitia MT, Garces CM, Carr A, Blanco F, Gonzalez A, Loughlin J, Common variations in estrogen-related genes are associated with severe large-joint osteoarthritis: a multicenter genetic and functional study, Osteoarthritis Cartilage 18(7) (2010) 927–33. [DOI] [PubMed] [Google Scholar]
- [20].Kerkhof HJ, Meulenbelt I, Carr A, Gonzalez A, Hart D, Hofman A, Kloppenburg M, Lane NE, Loughlin J, Nevitt MC, Pols HA, Rivadeneira F, Slagboom EP, Spector TD, Stolk L, Tsezou A, Uitterlinden AG, Valdes AM, van Meurs JB, Common genetic variation in the Estrogen Receptor Beta (ESR2) gene and osteoarthritis: results of a meta-analysis, BMC Med Genet 11 (2010) 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Son YO, Chun JS, Estrogen-related receptor gamma is a novel catabolic regulator of osteoarthritis pathogenesis, BMB Rep 51(4) (2018) 165–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hughes GC, Progesterone and autoimmune disease, Autoimmun Rev 11(6–7) (2012) A502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yates MA, Li Y, Chlebeck P, Proctor T, Vandenbark AA, Offner H, Progesterone treatment reduces disease severity and increases IL-10 in experimental autoimmune encephalomyelitis, J Neuroimmunol 220(1–2) (2010) 136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jones DL, Philippi MT, Maak TG, Aoki SK, Progressive osteoarthritis during pregnancy several years following hip arthroscopy for femoroacetabular impingement, J Orthop 15(2) (2018) 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kapila S, Wang W, Uston K, Matrix metalloproteinase induction by relaxin causes cartilage matrix degradation in target synovial joints, Ann N Y Acad Sci 1160 (2009) 322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Malemud CJ, Matrix Metalloproteinases and Synovial Joint Pathology, Prog Mol Biol Transl Sci 148 (2017) 305–325. [DOI] [PubMed] [Google Scholar]
- [27].Schroen DJ, Brinckerhoff CE, Nuclear hormone receptors inhibit matrix metalloproteinase (MMP) gene expression through diverse mechanisms, Gene Expr 6(4) (1996) 197–207. [PMC free article] [PubMed] [Google Scholar]
- [28].Powell BS, Dhaher YY, Szleifer IG, Review of the Multiscale Effects of Female Sex Hormones on Matrix Metalloproteinase-Mediated Collagen Degradation, Crit Rev Biomed Eng 43(5–6) (2015) 401–28. [DOI] [PubMed] [Google Scholar]
- [29].Yu HJ, Fei J, Chen XS, Cai QY, Liu HL, Liu GD, Yao ZX, Progesterone attenuates neurological behavioral deficits of experimental autoimmune encephalomyelitis through remyelination with nucleus-sublocalized Olig1 protein, Neuroscience letters 476(1) (2010) 42–5. [DOI] [PubMed] [Google Scholar]
- [30].MacKay JW, Kapoor G, Driban JB, Lo GH, McAlindon TE, Toms AP, McCaskie AW, Gilbert FJ, Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: data from the Osteoarthritis Initiative Bone Ancillary Study, Eur Radiol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fang H, Huang L, Welch I, Norley C, Holdsworth DW, Beier F, Cai D, Early Changes of Articular Cartilage and Subchondral Bone in The DMM Mouse Model of Osteoarthritis, Sci Rep 8(1) (2018) 2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen Y, Hu Y, Yu YE, Zhang X, Watts T, Zhou B, Wang J, Wang T, Zhao W, Chiu KY, Leung FK, Cao X, Macaulay W, Nishiyama KK, Shane E, Lu WW, Guo XE, Subchondral Trabecular Rod Loss and Plate Thickening in the Development of Osteoarthritis, J Bone Miner Res 33(2) (2018) 316–327. [DOI] [PubMed] [Google Scholar]
- [33].Huang H, Skelly JD, Ayers DC, Song J, Age-dependent Changes in the Articular Cartilage and Subchondral Bone of C57BL/6 Mice after Surgical Destabilization of Medial Meniscus, Sci Rep 7 (2017) 42294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhao W, Wang T, Luo Q, Chen Y, Leung VY, Wen C, Shah MF, Pan H, Chiu K, Cao X, Lu WW, Cartilage degeneration and excessive subchondral bone formation in spontaneous osteoarthritis involves altered TGF-beta signaling, J Orthop Res 34(5) (2016) 763–70. [DOI] [PubMed] [Google Scholar]
- [35].Funck-Brentano T, Cohen-Solal M, Subchondral bone and osteoarthritis, Curr Opin Rheumatol 27(4) (2015) 420–6. [DOI] [PubMed] [Google Scholar]
- [36].Yamada K, Healey R, Amiel D, Lotz M, Coutts R, Subchondral bone of the human knee joint in aging and osteoarthritis, Osteoarthritis Cartilage 10(5) (2002) 360–9. [DOI] [PubMed] [Google Scholar]
- [37].Fernandez-Valdivia R, Jeong J, Mukherjee A, Soyal SM, Li J, Ying Y, Demayo FJ, Lydon JP, A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland, Genesis 48(2) (2010) 106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM, Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males, Cell 153(4) (2013) 896–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhong ZA, Sun W, Chen H, Zhang H, Lay YE, Lane NE, Yao W, Optimizing tamoxifen-inducible Cre/loxp system to reduce tamoxifen effect on bone turnover in long bones of young mice, Bone 81 (2015) 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, Carrino JA, Cosgarea A, Artemov D, Chen Q, Zhao Z, Zhou X, Riley L, Sponseller P, Wan M, Lu WW, Cao X, Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis, Nat Med 19(6) (2013) 704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cui Z, Crane J, Xie H, Jin X, Zhen G, Li C, Xie L, Wang L, Bian Q, Qiu T, Wan M, Xie M, Ding S, Yu B, Cao X, Halofuginone attenuates osteoarthritis by inhibition of TGF-beta activity and H-type vessel formation in subchondral bone, Ann Rheum Dis 75(9) (2016) 1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ladd AJ, Kinney JH, Haupt DL, Goldstein SA, Finite-element modeling of trabecular bone: comparison with mechanical testing and determination of tissue modulus, Journal of orthopaedic research : official publication of the Orthopaedic Research Society 16(5) (1998) 622–8. [DOI] [PubMed] [Google Scholar]
- [43].van Rietbergen B, Weinans H, Huiskes R, Odgaard A, A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models, Journal of biomechanics 28(1) (1995) 69–81. [DOI] [PubMed] [Google Scholar]
- [44].Eswaran SK, Gupta A, Adams MF, Keaveny TM, Cortical and trabecular load sharing in the human vertebral body, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 21(2) (2006) 307–14. [DOI] [PubMed] [Google Scholar]
- [45].Ulrich D, Rietbergen B, Laib A, Ruegsegger P, Mechanical analysis of bone and its microarchitecture based on in vivo voxel images, Technology and health care : official journal of the European Society for Engineering and Medicine 6(5-6) (1998) 421–7. [PubMed] [Google Scholar]
- [46].Amugongo SK, Yao W, Jia J, Lay YA, Dai W, Jiang L, Walsh D, Li CS, Dave NK, Olivera D, Panganiban B, Ritchie RO, Lane NE, Effects of sequential osteoporosis treatments on trabecular bone in adult rats with low bone mass, Osteoporos Int 25(6) (2014) 1735–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Turner CH, Burr DB, Basic biomechanical measurements of bone: a tutorial, Bone 14(4) (1993) 595–608. [DOI] [PubMed] [Google Scholar]
- [48].Yao W, Cheng Z, Shahnazari M, Dai W, Johnson ML, Lane NE, Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects, J Bone Miner Res 25(2) (2010) 190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yao W, Guan M, Jia J, Dai W, Lay YA, Amugongo S, Liu R, Olivos D, Saunders M, Lam KS, Nolta J, Olvera D, Ritchie RO, Lane NE, Reversing bone loss by directing mesenchymal stem cells to bone, Stem cells 31(9) (2013) 2003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Henry SP, Jang CW, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B, Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage, Genesis 47(12) (2009) 805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang X, Wu X, Tang W, Luo Y, Loss of p16(Ink4a) function rescues cellular senescence induced by telomere dysfunction, Int J Mol Sci 13(5) (2012) 5866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. , A biomarker that identifies senescent human cells in culture and in aging skin in vivo, Proc Natl Acad Sci U S A 92(20) (1995) 9363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M, Endochondral ossification: how cartilage is converted into bone in the developing skeleton, Int J Biochem Cell Biol 40(1) (2008) 46–62. [DOI] [PubMed] [Google Scholar]
- [54].Liu XS, Zhang XH, Guo XE, Contributions of trabecular rods of various orientations in determining the elastic properties of human vertebral trabecular bone, Bone 45(2) (2009) 158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Harris N, Eudy A, Clowse M, Patient-Reported Disease Activity and Adverse Pregnancy Outcomes in Systemic Lupus Erythematosus and Rheumatoid Arthritis, Arthritis Care Res (Hoboken) 71(3) (2019) 390–397. [DOI] [PubMed] [Google Scholar]
- [56].Zbinden A, van den Brandt S, Ostensen M, Villiger PM, Forger F, Risk for adverse pregnancy outcome in axial spondyloarthritis and rheumatoid arthritis: disease activity matters, Rheumatology (Oxford) (2018). [DOI] [PubMed] [Google Scholar]
- [57].de Man YA, Dolhain RJ, Hazes JM, Disease activity or remission of rheumatoid arthritis before, during and following pregnancy, Curr Opin Rheumatol 26(3) (2014) 329–33. [DOI] [PubMed] [Google Scholar]
- [58].Hazes JM, Coulie PG, Geenen V, Vermeire S, Carbonnel F, Louis E, Masson P, De Keyser F, Rheumatoid arthritis and pregnancy: evolution of disease activity and pathophysiological considerations for drug use, Rheumatology (Oxford) 50(11) (2011) 1955–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM, Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study, Arthritis Rheum 59(9) (2008) 1241–8. [DOI] [PubMed] [Google Scholar]
- [60].Wise BL, Niu J, Zhang Y, Felson DT, Bradley LA, Segal N, Keysor J, Nevitt M, Lane NE, The association of parity with osteoarthritis and knee replacement in the multicenter osteoarthritis study, Osteoarthritis Cartilage 21(12) (2013) 1849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Martin JA, Buckwalter JA, Aging, articular cartilage chondrocyte senescence and osteoarthritis, Biogerontology 3(5) (2002) 257–64. [DOI] [PubMed] [Google Scholar]
- [62].Loeser RF, Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix, Osteoarthritis Cartilage 17(8) (2009) 971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].McCulloch K, Litherland GJ, Rai TS, Cellular senescence in osteoarthritis pathology, Aging Cell 16(2) (2017) 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Martin JA, Buckwalter JA, The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair, J Bone Joint Surg Am 85-A Suppl 2 (2003) 106–10. [DOI] [PubMed] [Google Scholar]
- [65].Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, Clark IM, The role of chondrocyte senescence in osteoarthritis, Aging Cell 1(1) (2002) 57–65. [DOI] [PubMed] [Google Scholar]
- [66].Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM, Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan, Nature 530(7589) (2016) 184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Diekman BO, Sessions GA, Collins JA, Knecht AK, Strum SL, Mitin NK, Carlson CS, Loeser RF, Sharpless NE, Expression of p16(INK)(4a) is a biomarker of chondrocyte aging but does not cause osteoarthritis, Aging Cell (2018) e12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shyamala G, Yang X, Cardiff RD, Dale E, Impact of progesterone receptor on cell-fate decisions during mammary gland development, Proc Natl Acad Sci U S A 97(7) (2000) 3044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Takahashi A, Kato K, Kuboyama A, Inoue T, Tanaka Y, Kuhara A, Kinoshita K, Takeda S, Wake N, Induction of senescence by progesterone receptor-B activation in response to cAMP in ovarian cancer cells, Gynecol Oncol 113(2) (2009) 270–6. [DOI] [PubMed] [Google Scholar]
- [70].Diep CH, Charles NJ, Gilks CB, Kalloger SE, Argenta PA, Lange CA, Progesterone receptors induce FOXO1-dependent senescence in ovarian cancer cells, Cell Cycle 12(9) (2013) 1433–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Akasaki Y, Alvarez-Garcia O, Saito M, Carames B, Iwamoto Y, Lotz MK, FoxO transcription factors support oxidative stress resistance in human chondrocytes, Arthritis Rheumatol 66(12) (2014) 3349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Matsuzaki T, Alvarez-Garcia O, Mokuda S, Nagira K, Olmer M, Gamini R, Miyata K, Akasaki Y, Su AI, Asahara H, Lotz MK, FoxO transcription factors modulate autophagy and proteoglycan 4 in cartilage homeostasis and osteoarthritis, Sci Transl Med 10(428) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hodax JK, Quintos JB, Gruppuso PA, Chen Q, Desai S, Jayasuriya CT, Aggrecan is required for chondrocyte differentiation in ATDC5 chondroprogenitor cells, PloS one 14(6) (2019) e0218399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Feng L, Balakir R, Precht P, Horton WE Jr., Bcl-2 regulates chondrocyte morphology and aggrecan gene expression independent of caspase activation and full apoptosis, J Cell Biochem 74(4) (1999) 576–86. [PubMed] [Google Scholar]
- [75].Sakamoto A, Chen M, Kobayashi T, Kronenberg HM, Weinstein LS, Chondrocyte-specific knockout of the G protein G(s)alpha leads to epiphyseal and growth plate abnormalities and ectopic chondrocyte formation, J Bone Miner Res 20(4) (2005) 663–71. [DOI] [PubMed] [Google Scholar]
- [76].Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ, Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer, Genesis 33(2) (2002) 77–80. [DOI] [PubMed] [Google Scholar]
- [77].Karsdal MA, Bay-Jensen AC, Henriksen K, Christiansen C, The pathogenesis of osteoarthritis involves bone, cartilage and synovial inflammation: may estrogen be a magic bullet?, Menopause Int 18(4) (2012) 139–46. [DOI] [PubMed] [Google Scholar]
- [78].Sniekers YH, van Osch GJ, Ederveen AG, Inzunza J, Gustafsson JA, van Leeuwen JP, Weinans H, Development of osteoarthritic features in estrogen receptor knockout mice, Osteoarthritis Cartilage 17(10) (2009) 1356–61. [DOI] [PubMed] [Google Scholar]