Abstract
Embelin is a naturally occurring para-benzoquinone isolated from Embelia ribes (Burm. f.) of the Myrsinaceae family. It was first discovered to have potent inhibitory activity (IC50 = 4.2 μM) against α-glucosidase in this study. Then, four series of novel embelin derivatives were designed, prepared and evaluated in α-glucosidase inhibition assays. The results show that most of the embelin derivatives synthesised are effective α-glucosidase inhibitors, with IC50 values at the micromolar level, especially 10d, 12d, and 15d, the IC50 values of which are 1.8, 3.3, and 3.6 μM, respectively. Structure–activity relationship (SAR) studies suggest that hydroxyl groups in the 2/5-position of para-benzoquinone are very important, and long-chain substituents in the 3-position are highly preferred. Moreover, the inhibition mechanism and kinetics studies reveal that all of 10d, 12d, 15d, and embelin are reversible and mixed-type inhibitors. Furthermore, docking experiments were carried out to study the interactions between 10d and 15d with α-glucosidase.
Keywords: Embelin, α-glucosidase inhibitor, anti-diabetes, hypoglycaemic agent, benzoquinone
Introduction
Diabetes mellitus (DM) is a group of chronic metabolic disorders characterised by hyperglycaemia resulting from defects in insulin secretion, insulin action, or both1,2. According to WHO data, DM was the 7th cause of death worldwide in 2016, when it killed 1.6 million people3. There are two major forms of the disease: Type 1 and Type 2 diabetes. The latter accounts for 90 ∼ 95% of all cases, formerly called non-insulin-dependent diabetes mellitus (NIDDM) or adult-onset diabetes, and usually occurs after age 40, becoming more common with increasing age1,2,4,5.
The management of Type 2 diabetes includes hyperglycaemia treatment, diabetic comorbidity prevention, and metabolism adjustment6,7. One therapeutic approach is to retard the absorption of glucose via inhibition of enzymes, such as α-glucosidase and α-amylase, in the digestive organs8–11. α-Glucosidase is an exo-type carbohydrase widely distributed in microorganisms, plants, and animal tissues. Inhibiting α-glucosidase slows the elevation of blood sugar after meals8,12. α-Glucosidase inhibitors (AGIs) are a unique class of oral hypoglycaemic agents approved for the prevention and management of Type 2 diabetes. Nowadays, there are four AGIs, including acarbose, miglitol, voglibose, and emiglitate, used in the clinical treatment of Type 2 diabetes9,13. However, current approaches including AGIs have some shortcomings such as safety concerns, limited efficacy, failure in metabolism adjustment, and the prevention of diabetic complications6,9,14. Thus, developing new therapeutic drugs to treat Type 2 diabetes is necessary, and has received wide attention.
Embelin is a naturally occurring para-benzoquinone isolated from Embelia ribes (Burm. f.) of the Myrsinaceae family, and contains two carbonyl groups, an active methylene group and two hydroxyl groups15,16. Embelin and its derivatives have been reported to possess anti-cancer17,18, antimicrobial19,20, antioxidant21, analgesic22, anti-inflammatory22, anxiolytic23, antifertility24 activities, etc. Thanks to these diverse biological activities, embelin is considered as the “second solid gold of India” next to curcumin25. In the last decade, several studies have reported antidiabetic activity of embelin26–28. In 2016, Sharanbasappa et al.29 reviewed the antidiabetic activity of embelin and its derivatives. It was concluded from this review and meta-analysis that the E. ribes extract, embelin and its derivatives have positive effects on blood glucose, HbAlc, insulin, and lipid profiles. In addition, heart rate, systolic blood pressure, lactate dehydrogenase, creatinine kinase and oxidative stress markers in diabetic rats return to normal after treatment with E. ribes extract and embelin. Moreover, Dang et al.30,31 reported that the methanolic extract of E. ribes and several compounds isolated from the leaves of E. ribes have significant α-glucosidase inhibitory activity. These results inspire us to investigate α-glucosidase inhibition of embelin, which is the main constituent of E. ribes. In this study, four series of novel embelin derivatives were designed and prepared in 5- to 6-step chemical reactions. Their α-glucosidase inhibitory activity was evaluated, and the mode of action and SAR analysis were described by means of kinetic and molecular modelling evaluations.
Materials and methods
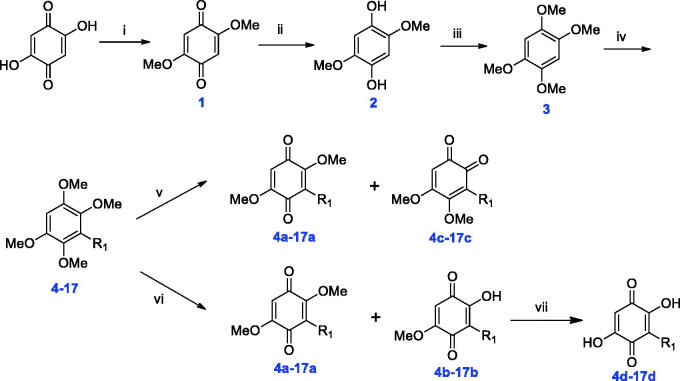
Procedure for the synthesis of 1,2,4,5-tetramethoxybenzene (compound 3)
2,5-Dihydroxycyclohexa-2,5-diene-1,4-dione (5 g, 3.6 mmol) was dissolved in methanol (200 ml), and HCl solution (12 M, 6 ml) was added slowly. The mixture was stirred overnight. The reaction was monitored by TLC. After reaction completion, the mixture was filtered to obtain 2,5-dimethoxycyclohexa-2,5-diene-1,4-dione 1 as a yellow solid. Compound 1 was dissolved in H2O (50 ml), and Na2S2O4 (10 g, 57.4 mmol) was added. The mixture was heated to reflux for 8 ∼ 15 min. Then the mixture was cooled to crystallise, and filtered to furnish 2,5-dimethoxybenzene-1,4-diol 2 as a white crystalline solid. Compound 2 (1.7 g, 10 mmol) was dissolved in DMSO (10 ml), and KOH (1.4 g, 25 mmol) was added. The mixture was stirred for 15 min, and then MeI (1.88 ml, 25 mmol) was added. The mixture was stirred overnight. After reaction completion, the pH value was adjusted to 5 ∼ 6 by saturated NH4Cl solution. The mixture was washed with saturated NaCl solution and H2O and dried over MgSO4. The solution was condensed under reduced pressure to give crude product, which was purified by silica gel column chromatography (PE/EtOAc = 10/1), yielding 1,2,4,5-tetramethoxybenzene 3 (1.05 g, 53%).
Scheme 1.
Synthesis of novel embelin derivatives.
Reagents and conditions: (i) conc. HCl, MeOH, rt., overnight; (ii) Na2S2O4, H2O, reflux, 8 ∼ 15 min; (iii) KOH, MeI, DMSO, rt., overnight; (iv) n-BuLi, HMPA, THF, −40 °C to rt., overnight; (v) CAN, MeCN/H2O, −5 °C, 10 min; (vi) CAN, MeCN/H2O, −5 °C to rt., 2 h; (vii) NaOH, EtOH, 80 °C, 2 ∼ 3 h.
General procedure for the synthesis of 4–17
Hexamethylphosphoric triamide (HMPA, 353 μL, 2 mmol) was added to a solution of compound 3 (1 g, 5.1 mmol) in dry THF (60 ml) under N2 atmosphere. Next, n-BuLi (2.44 ml, 6.12 mmol) was added slowly to the mixture at −40 °C and stirred at this temperature for 10 min. Then the mixture was allowed to heat to −10 °C, and various halogenated hydrocarbons (5.5 mmol) were added dropwise. The mixture was stirred at room temperature overnight. THF in the reaction mixture was removed under reduced pressure, and the residue was redissolved in EtOAc. The organic solution was washed with 1 M HCl solution and saturated brine, then dried over MgSO4 and evaporated. The crude product was chromatographed (PE/EtOAc = 15/1 to 5/1) to supply pure compound 4–17 (20%∼60%).
General procedure for the synthesis of 4a–17a, 4b–17b, and 4c–17c
A solution of ceric ammonium nitrate (CAN, 1.4 mmol) in MeCN/H2O (7/3, 5 ml) was added to a solution of compounds 4–17 (0.55 mmol) in MeCN (4 ml) in a salt-ice bath.
Method A. The suspension was stirred for 10 min at −5 °C. The organic solvent was removed and the residue was redissolved in EtOAc (5 ml). The organic layer was washed with saturated brine, dried over MgSO4 and condensed. The crude product was purified by flash chromatography (PE/EtOAc = 10/1), furnishing first pure 4a–17a (32%∼40%). Then, the eluent was changed to PE/EtOAc = 5/1, giving pure 4c–17c (23%∼30%).
Method B. The suspension was stirred for 2 h at room temperature. The organic solvent was removed and the residue was redissolved in EtOAc (5 ml). The organic layer was washed with saturated brine, dried over MgSO4 and condensed. The crude product was purified by flash chromatography (PE/EtOAc = 10/1), furnishing first pure 4a–17a (35%∼43%). Then, the eluent was changed to PE/EtOAc = 3/1, giving pure 4b–17b (18%∼27%).
General procedure for the synthesis of 4d–17d
2 M NaOH solution (8 ml) was added to a solution of compounds 4a–17a or 4b–17b (0.35 mmol) in EtOH (2 ml). The mixture was heated to reflux for 2 ∼ 3 h. Next, the organic solvent was removed. The pH value of the aqueous solution was adjusted to 5 ∼ 6. Then, the solution was diluted with EtOAc (3 ml). The organic layer was washed with saturated brine, dried over MgSO4, and condensed. The crude product was then washed with a low-polar organic solvent to remove impurities, and filtered to obtain pure 4d–17d (20%∼45%).
α-Glucosidase inhibition
The α-glucosidase inhibitory activity was determined on a Thermo Scientific Multiskan GO Microplate Reader. All compounds were dissolved in DMSO. p-Nitrophenol glucoside (PNPG) as a substrate and α-glucosidase (from Saccharomyces cerevisiae) were used for the bioassay. First, 0.7 U/ml α-glucosidase solution and 1 mmol/L PNPG solution in 0.1 M PBS (pH 6.8) were prepared. Next, 35 µl of 0.1 M PBS (pH 6.8), 10 µl of 0.7 U/ml α-glucosidase solution (final concentration: 0.07 U/ml), and 5 µl of sample solution were added to the 96-well plate. The mixture was pre-incubated for 10 min at 37 °C. Then, 50 µl of 1 mM PNPG (final concentration: 0.5 mM) was rapidly added to initiate the reaction. Then the 96-well plate was quickly transferred into the microplate reader, incubated at 37 °C for 30 min (1 min shake + 9 min incubation, repeated 3 times). After that, 50 µl of 1 M of Na2CO3 solution was added to each well to stop the reaction. The 96-well plate was quickly transferred into the microplate reader and shaken for 30 s. In the blank reference group, 5 µl DMSO replaced the sample solution, other operations were the same. The OD value was measured at 405 nm. The inhibition rate was calculated by Equation (1).
| (1) |
where A0 is the OD of the control, and A1 is the OD of the sample.
The inhibition mechanism of 10d, 12d, and 15d was determined by the following method: A series of diluted inhibitor solutions was prepared, at constant PNPG concentration (1 mM). The inhibition rates were measured by the above method with different concentrations of α-glucosidase (0.00, 0.35, 0.7, and 1.05 U/ml).
The inhibition type of the enzyme was assayed by Lineweaver–Burk plots. A series of diluted inhibitor solutions was prepared, at constant α-glucosidase concentration (0.7 U/ml). The inhibition rates were measured by the above method with different concentrations of PNPG (1, 0.5, 0.4, 0.3, 0.25, and 0.2 mM).
Molecular docking
The molecular modelling and docking simulation were implemented by using Sybyl 2.0. The 3D structure of α-glucosidase (PDB code: 1UOK) was obtained from RCSB Protein Data Bank and the two most potent embelin derivatives (10d and 15d) were built by molecular modelling package in Sybyl. All the water molecules were removed and hydrogen atoms were added to make protein and ligand protonation. Tripos force field was adopted to make the energy of protein and ligand to be minimised. Ligand–protein docking simulations were processed by the Surflex-Dock within Sybyl. The catalytic pocket of acarbose docking with α-glucosidase was defined as active site. Other settings were kept default.
Results and discussion
Compound design and synthesis
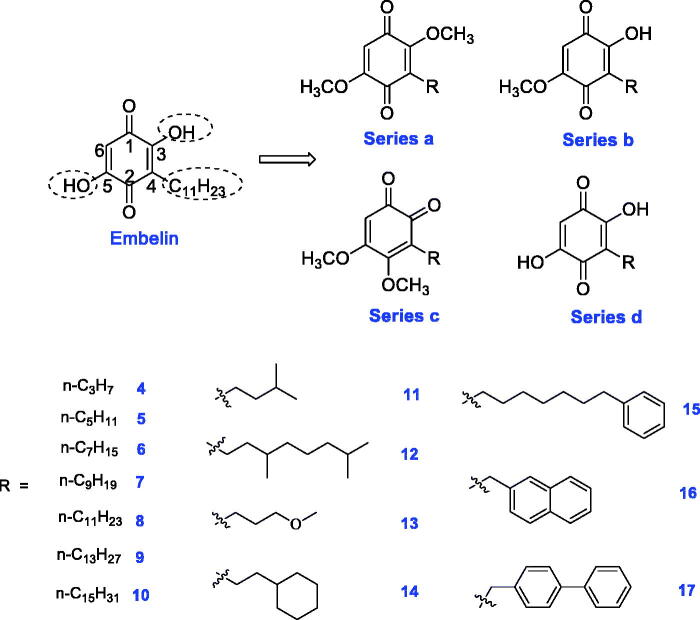
In our previous study, embelin displayed potent α-glucosidase inhibitory activity with an IC50 value of 4.20 μM. In order to investigate structure–activity relationships (SAR) and obtain more active AGIs, four series (a, b, c, and d) of novel embelin derivatives were designed (Figure 1). First, in order to study the function of the hydroxyl groups in the 2/5-position, they were monomethylated or dimethylated, giving series a and b. Second, the core structure was varied from para-benzoquinone to ortho-benzoquinone, giving series c. Third, because the long C11H23 tail in the 3-position results in the poor bioavailability of embelin, new groups were introduced at this position, giving series d. The structures of R groups are shown in Figure 1. In order to study the length of the substituents, C3- to C15-n-alkyl groups were introduced (Compounds 4 ∼ 10). In addition to the straight-chain groups, branched alkyl groups or straight-chain groups containing a heteroatom were introduced. Moreover, aromatic rings or cycloalkanes were introduced in the terminal group.
Figure 1.
Design of novel embelin derivatives.
The target compounds were prepared according to a published method32 with slight modification. 2,5-Dihydroxycyclohexa-2,5-diene-1,4-dione was used as starting material: first, the two hydroxyl groups were protected by methylation to yield compound 1. Then the benzoquinone core structure was reduced by Na2S2O4, giving compound 2. Further methylation produced 1,2,4,5-tetramethoxybenzene 3. Next, n-BuLi was used to abstract the hydrogen atom from the benzene ring, and then various halogenated hydrocarbons were added to furnish 4 ∼ 17. The yield of this step depended on the steric hindrance of the halogenated hydrocarbon. For example, we tried to introduce α-naphthyl into the core structure but failed due to its large steric hindrance. Although β-naphthyl and biphenyl were introduced successfully, the synthetic yields of 16 and 17 were low. The oxidation of 4 ∼ 17 by CAN produced different oxidised products depending on the reaction time. At the beginning of the oxidation reaction, a mixture of 4a∼17a and 4b∼17b was obtained. As the reaction proceeded, mixtures of 4a∼17a and 4c∼17c were obtained as the main products. Finally, the demethylation of 4a∼17a and 4b∼17b under basic conditions gave 4d∼17d. The final products 4a∼17a, 4b∼17b and 4c∼17c were purified by flash chromatography, and 4d∼17d were purified by washing with petroleum ether or dichloromethane.
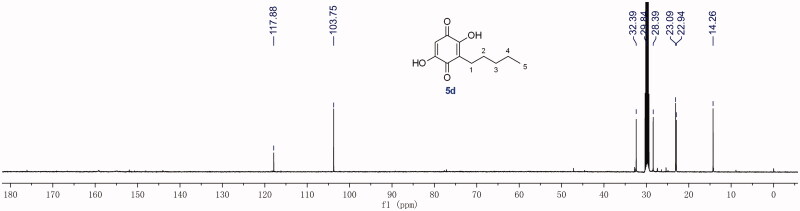
All final compounds were characterised by 1H-NMR, 13C-NMR, and HRMS. It is noteworthy that there is an interesting phenomenon in the 13C-NMR spectra of compounds 4d∼17d. Taking 5d as an example (Figure 2), six different carbons exist in the para-benzoquinone ring. However, there are only two carbon signals at >90 ppm, which belong to the carbons in the 3/6-position. This may be due to keto-enol tautomerism of para-benzoquinone (Figure 3). This rapid transfer between two tautomers makes it difficult to record the corresponding carbon signals. Once one of the hydroxyl groups is methylated, the 13C-NMR signals of the core structure return to be normal.
Figure 2.
13C-NMR spectrum of compound 5d.
Figure 3.
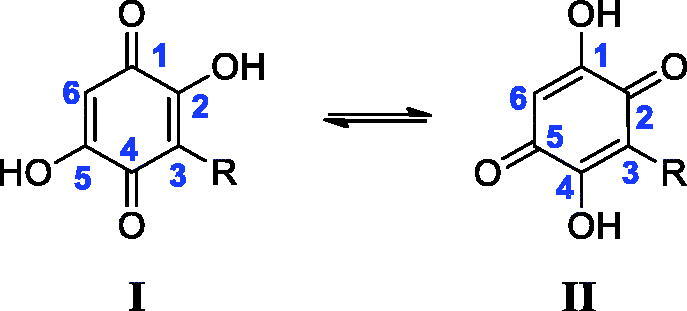
Tautomerism between two forms of para-benzoquinone.
α-Glucosidase inhibitory activity and SAR analyses
The inhibitory activities of embelin and its derivatives on α-glucosidase from baker’s yeast were determined by the reported method6,11 with slight modification using acarbose and ursolic acid as the positive reference compounds. As depicted in Table 1, the inhibitory activities of all compounds were screened at the concentration of 250 μM. Most compounds showed an obvious inhibitory effect on α-glucosidase. Among the four series, compounds from series d showed much better inhibitory activity than those from the other three series, which indicates that the hydroxyl groups in the 2/5-positions are very important for the α-glucosidase inhibitory activity. Moreover, the modification of the core structure from para-benzoquinone to ortho-benzoquinone resulted in a significant reduction of activity.
Table 1.
Inhibition rate of all compounds at 250 μM against α-glucosidase.
| Comp. | Inhibition rate | Comp. | Inhibition rate | Comp. | Inhibition rate | Comp. | Inhibition rate |
|---|---|---|---|---|---|---|---|
| 4a | 9.0% | 4b | 10.4% | 4c | 14.9% | 4d | 18.1% |
| 5a | 11.5% | 5b | 14.9% | 5c | 16.4% | 5d | 19.0% |
| 6a | 1.5% | 6b | 5.4% | 6c | 16.1% | 6d | 80.8% |
| 7a | 13.5% | 7b | 20.9% | 7c | 33.2% | 7d | 99.4% |
| 8a | 12.5% | 8b | 12.0% | 8c | 0.6% | Embelin | 100% |
| 9a | nt | 9b | nt | 9c | nt | 9d | 100% |
| 10a | nt | 10b | nt | 10c | nt | 10d | 100% |
| 11a | 1.5% | 11b | 2.6% | 11c | 3.3% | 11d | 37.6% |
| 12a | nt | 12b | nt | 12c | nt | 12d | 100% |
| 13a | 0% | 13b | 0% | 13c | 0% | 13d | 0% |
| 14a | 70.1% | 14b | 71.1% | 14c | 74.7% | 14d | 85.2% |
| 15a | nt | 15b | nt | 15c | nt | 15d | 100% |
| 16a | 6.0% | 16b | 4.3% | 16c | 37.2% | 16d | 81.4% |
| 17a | 5.1% | 17b | 14.3% | 17c | 59.7% | 17d | 99.5% |
nt: not tested.
Then, the inhibition rates of the compounds which inhibited more than 80% of α-glucosidase in the initial screening were further evaluated at more than eight concentrations. The IC50 values were calculated by GraphPad Prism 7.0 software. The results are summarised in Table 2 and the inhibition curves are shown in Figure 4. The length of the chain in the 3-position is a key factor affecting the activity. The compounds with longer chains display better activity. For example, compounds 4d and 5d have a very low inhibitory effect, while 6d∼10d have potent activity, which suggests the length of the chain should be longer than n-C7H15. Moreover, when the length was similar, branched groups slightly enhance the activity. For example, the lengths of the chain in the 3-position of compounds 5d and 11d, 7d, and 12d were similar, but 11d exhibited better inhibitory activity than 5d, and 12d showed slightly better activity than 7d. However, the introduction of an oxygen atom in the chain results in the loss of activity (compounds 13a∼13d). In addition, the introduction of large hydrophobic groups such as cyclohexyl, phenyl, β-naphthyl, and 4-biphenyl (compounds 14d, 15d, 16d, and 17d) in the terminal, seems to have positive influence on the activity. Meanwhile, the length of these large hydrophobic groups is closely related to the activity.
Table 2.
IC50 values of selected compounds against α-glucosidase.
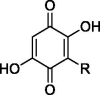 | ||
|---|---|---|
| Compound | R | IC50 (μM) |
| 6d | n-C7H15 | 126.8 |
| 7d | n-C9H19 | 5.7 |
| embelin | n-C11H23 | 4.2 |
| 9d | n-C13H27 | 2.3 |
| 10d | n-C15H31 | 1.8 |
| 12d | 3.3 | |
| 14d | 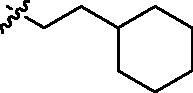 |
45.3 |
| 15d | 3.6 | |
| 16d | 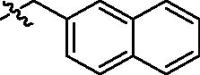 |
74.8 |
| 17d | 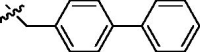 |
11.2 |
| Acarbose | 584.0 | |
| Ursolic acid | 4.3 | |
Figure 4.
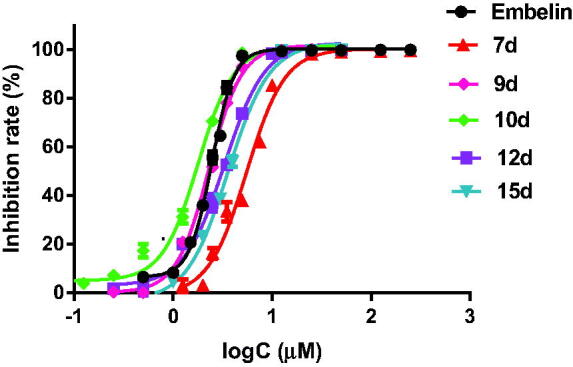
Inhibition curves of embelin and its derivatives.
Inhibition mechanism
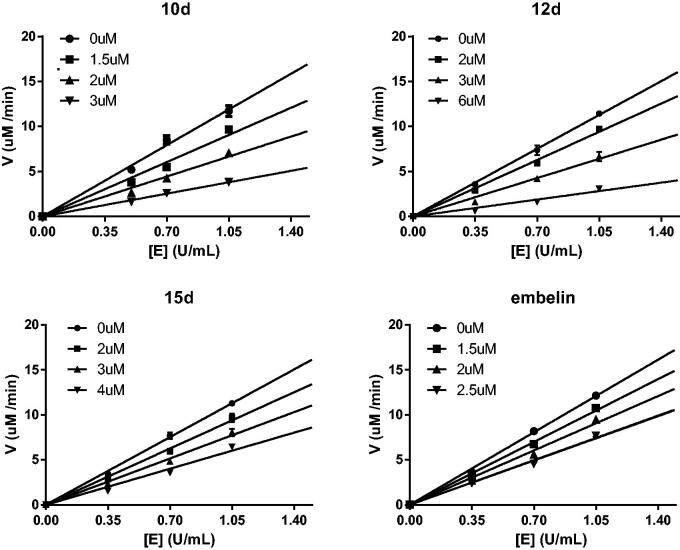
Compounds 9d, 10d, 12d, and 15d displayed the most potent activity in the α-glucosidase inhibitory assays. Because the chemical structures of compounds 9d and 10d are very similar, compound 10d was selected as a representative in the next mechanism studies. The inhibition mechanism of the selected compounds 10d, 12d, 15d, and embelin on α-glucosidase was investigated using PNPG as the substrate. The relationship between enzyme activity and concentration in the presence of different concentrations of selected compounds was studied. As shown in Figure 5, the plots of enzyme activity versus enzyme concentration give a set of straight lines, which all pass through the origin. The increase in the inhibitor concentration resulted in the reduction of the slope of the line, which suggests that the inhibitors reduce the activity of α-glucosidase. Therefore, the inhibition of compounds 10d, 12d, 15d, and embelin against α-glucosidase is reversible.
Figure 5.
Determination of the mechanism of the inhibition of α-glucosidase by 10d, 12d, 15d, and embelin.
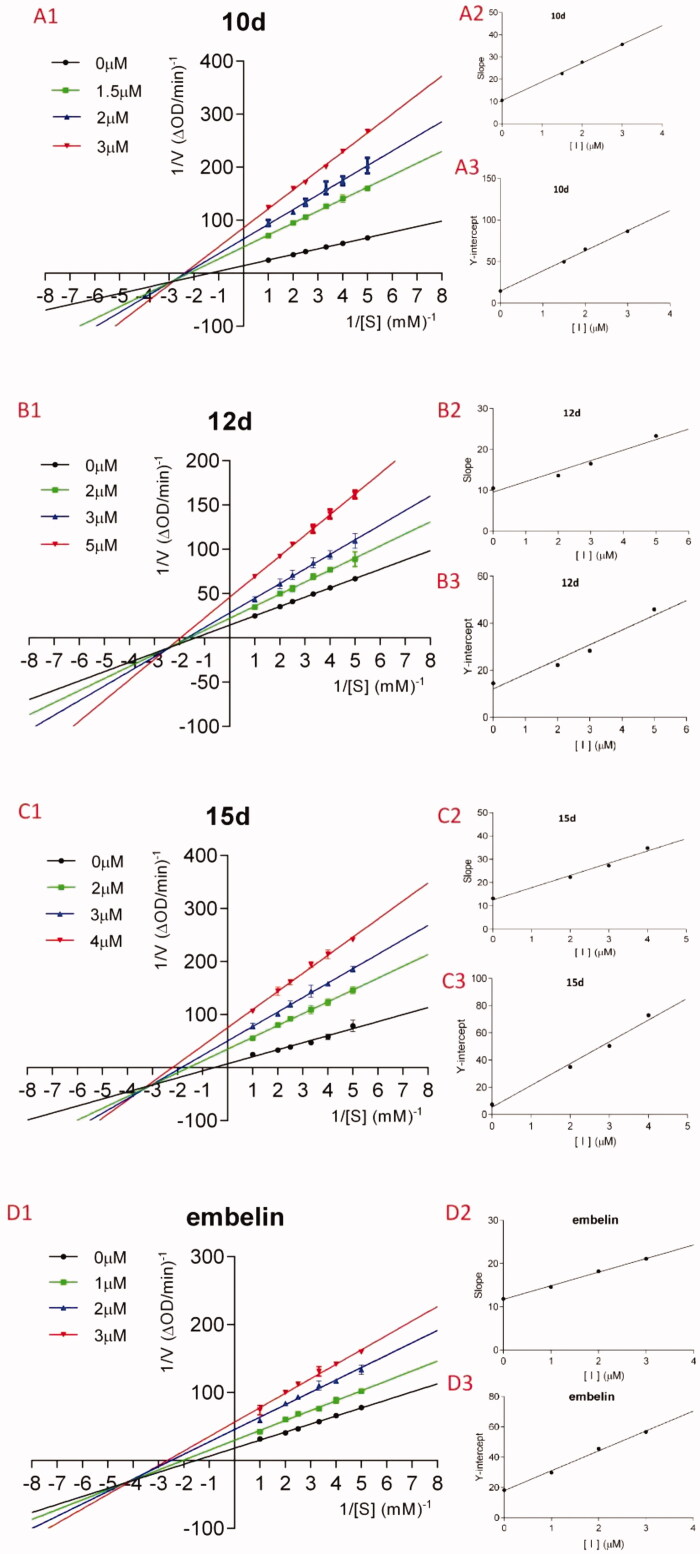
In order to elucidate the action mode, the most potent compounds 10d, 12d, 15d, and embelin were selected for enzyme kinetic studies. The kinetic data were expressed by Lineweaver–Burk double-reciprocal plots. As shown in Figure 6, the plots of 1/v versus 1/[S] give a group of straight lines with different slopes that intersect at the third quadrant (Figure 6(A1–D1)), suggesting that all of them are mixed-type inhibitors. Thus, these compounds bind not only with the free α-glucosidase but also with the α-glucosidase-PNPG complex. In other words, they inhibit the function of α-glucosidase by not only directly binding to free enzyme (EI), but also by interfering with the formation of the α-glucosidase-PNPG (ES) intermediate through producing an α-glucosidase-PNPG-inhibitor (ESI) complex in a non-competitive manner33. The inhibition constant for the inhibitor binding with free enzyme (Ki) was determined from a plot of the slope (Km/Vm) versus the inhibitor concentration (Figure 6(A2–D2)), and the inhibition constant for the inhibitor binding with enzyme–substrate complex (Kis) was obtained from the vertical intercept (1/Vm) versus the inhibitor concentration (Figure 6(A3–D3))34. The results are collected in Table 3: the Kis values of all of them are smaller than their Ki values, which mean that they have higher affinity with the enzyme-substrate complex than with the free enzyme.
Figure 6.
A1–D1: Lineweaver–Burk double-reciprocal plots; A2–D2: Plots of slope versus concentration of inhibitors for the determination of the inhibition constant Ki; A3–D3: Plots of Y-intercept versus concentration of inhibitors for the determination of the inhibition constant Kis.
Table 3.
Ki, Kis, and inhibition type of selected compounds against α-glucosidase.
| Compound | Ki value (μM) | Kis value (μM) | Inhibition type |
|---|---|---|---|
| Embelin | 3.72 | 1.37 | Mixed-type |
| 10d | 1.24 | 0.60 | Mixed-type |
| 12d | 3.71 | 1.93 | Mixed-type |
| 15d | 2.40 | 0.33 | Mixed-type |
Molecular docking
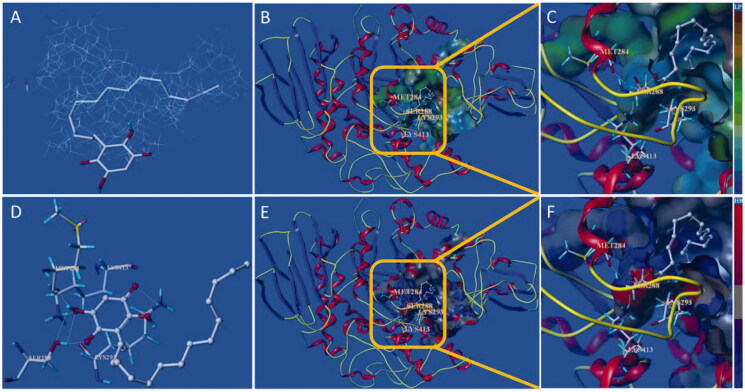
Compound 10d and 15d have the strongest inhibition against free α-glucosidase (Table 3), and molecular docking was introduced to confirm the binding mode of the two compounds with the active site of α-glucosidase. The protomol of the active site integrate with 10d is as shown in Figure 7(A), which reveals that compound 10d can be effectively inserted into the protomol. The results show that the long-chain n-C15H31 group can be inserted into the hydrophobic region of the active site selectively (Figure 7(B,C)). The hydrophobic pocket is long and relatively narrow, which explain why long-chain substituents in the 3-position are highly preferred. As depicted in Figure 7(D–F), the two carbonyl groups and two hydroxyl groups from para-benzoquinone core structure interact with several amino acid residues including MET284, LYS413, SER288, and LYS293, which locate in the entrance of the active pocket. These hydrophilic interactions reduce the binding free energy of inhibitor and target protein, resulting in the increase of inhibitory function. Thus, the hydroxyl groups of embelin and its derivatives are more helpful to improve the inhibitory activity against α-glucosidase, which also is in accordance with the in vitro assay.
Figure 7.
Docking binding model of 10d with yeast α-glucosidase. (A): Binding mode of 10d docked with the prototype molecular of the active site. (B) and (C): Active site MOLCAD surface representation of lipophilic potential. (D): The interaction of 10d with the surrounding amino acids. (E) and (F): Active site MOLCAD surface representation of hydrogen bonding.
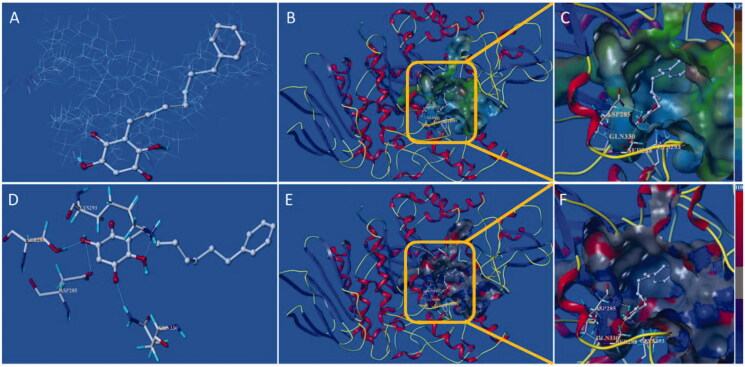
The docking binding mode of 15d with α-glucosidase is shown in Figure 8. The results suggest that 10d and 15d have similar binding mode with the active site. However, the flexibility of the substituent (n-heptylphenyl) in the 3-position of 15d is less than that of 10d (n-pentadecyl), thus the hydrophobic portion of 15d only interact with a part of the active hydrophobic pocket, while 10d is more effective of interaction with the active site. This difference may be contributed to 10d possessing more potent inhibitory activity.
Figure 8.
Docking binding model of 15d with yeast α-glucosidase. (A): Binding mode of 15d docked with the prototype molecular of the active site. (B) and (C): Active site MOLCAD surface representation of lipophilic potential. (D): The interaction of 15d with the surrounding amino acids. (E) and (F): Active site MOLCAD surface representation of hydrogen bonding.
Conclusions
Embelin was first discovered to have potent inhibitory activity against α-glucosidase in this study. Based on the structure of embelin, four series of embelin derivatives were designed and prepared. All final compounds were characterised by 1H-NMR, 13C-NMR, and HRMS. Enzyme inhibition bioassay results show that most of these embelin derivatives have potent inhibitory activity. The SAR study suggests that the two hydroxyl groups and long-chain substituents are the key factors affected the inhibitory activity. The most active derivatives 10d, 12d, 15d, and embelin were selected for the further mechanism and kinetic study. The results reveal all of them are reversible and mixed-type inhibitors. Molecular docking study was introduced to insight into the binding mode of 10d and 15d with α-glucosidase. The docking results suggest that the formation of hydrogen bonds between the hydrophilic groups and several amino acids at the active site, as well as the hydrophobic interaction between the hydrophobic substituents in the 3-position of para-benzoquinone and hydrophobic pocket, are important mechanism of inhibition. This study provides several leading structures for designing and developing novel AGIs. These findings encourage us to continue our efforts towards the optimisation of the pharmacological profile of these embelin derivatives.
Acknowledgements
The authors thank Dr. John S. Lomas from the University Paris Diderot for his help in correcting the English of this article.
Funding Statement
This work was supported by the Natural Science Foundation of Guangdong Province [No. 2016A030310441], National Natural Science Foundation of China [No. 81803390], the Special Fund Project of Science and Technology Innovation Strategy in Guangdong Province [No. Jiangke(2018)352], Jiangmen Science and Technology Project of Basic and Theoretical Science Research [No. 2019030102100008866], Science Foundation for Young Teachers of Wuyi University [No. 2016td01], the Foundation of Department of Education of Guangdong Province [Nos. 2017KQNCX200 and 2017KSYS010] and Innovation and Entrepreneurship Training Program for Undergraduates of Guangdong Province.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27:S5–S10. [DOI] [PubMed] [Google Scholar]
- 2.Kharroubi AT, Darwish HM.. Diabetes mellitus: the epidemic of the century. World J Diabetes 2015;6:850–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . The top 10 causes of death. Available from: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death [last accessed 6 Sep 2019].
- 4.Chen L, Magliano DJ, Zimmet PZ.. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 2012;8:228–36. [DOI] [PubMed] [Google Scholar]
- 5.Zaccardi F, Webb DR, Yates T, et al. . Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J 2016;92:63–9. [DOI] [PubMed] [Google Scholar]
- 6.Wu PP, Zhang BJ, Cui XP, et al. . Synthesis and biological evaluation of novel ursolic acid analogues as potential alpha-glucosidase inhibitors. Sci Rep 2017;7:45578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyenwe EA, Jerkins TW, Umpierrez GE, et al. . Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism 2011;60:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Narwal S, Kumar V, et al. . Alpha-glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev 2011;5:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santos CMM, Freitas M, Fernandes E.. A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur J Med Chem 2018;157:1460–79. [DOI] [PubMed] [Google Scholar]
- 10.Esmaeili S, Azizian S, Shahmoradi B, et al. . Dipyridamole inhibits α-amylase/α-glucosidase at sub-micromolar concentrations: in-vitro, in-vivo and theoretical studies. Bioorg Chem 2019;88:102972. [DOI] [PubMed] [Google Scholar]
- 11.Zhong YY, Chen HS, Wu PP, et al. . Synthesis and biological evaluation of novel oleanolic acid analogues as potential α-glucosidase inhibitors. Eur J Med Chem 2019;164:706–16. [DOI] [PubMed] [Google Scholar]
- 12.Van de Laar FA, Lucassen PL, Akkermans RP, et al. . α-Glucosidase inhibitors for patients with type 2 diabetes. Diabetes Care 2005;28:154–63. [DOI] [PubMed] [Google Scholar]
- 13.Joshi SR, Standl E, Tong N, et al. . Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin Pharmacother 2015;16:1959–81. [DOI] [PubMed] [Google Scholar]
- 14.Bonora E. Antidiabetic medications in overweight/obese patients with type 2 diabetes: drawbacks of current drugs and potential advantages of incretin-based treatment on body weight. Int J Clin Pract Suppl 2007;61:19–28. [DOI] [PubMed] [Google Scholar]
- 15.Poojari R. Embelin – a drug of antiquity: shifting the paradigm towards modern medicine. Expert Opin Investig Drugs 2014;23:427–44. [DOI] [PubMed] [Google Scholar]
- 16.Sheng Z, Ge S, Gao M, et al. . Synthesis and biological activity of embelin and its derivatives: an overview. Mini Rev Med Chem 2019. E-pub ahead of print. doi: 10.2174/1389557519666191015202723 [DOI] [PubMed] [Google Scholar]
- 17.Prabhu KS, Achkar IW, Kuttikrishnan S, et al. . Embelin: a benzoquinone possesses therapeutic potential for the treatment of human cancer. Future Med Chem 2018;10:961–76. [DOI] [PubMed] [Google Scholar]
- 18.Ko JH, Lee SG, Yang W, et al. . The application of embelin for cancer prevention and therapy. Molecules 2018;23:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitra M, Shyamala Devi CS, Sukumar E.. Antibacterial activity of embelin. Fitoterapia 2003;74:401–3. [DOI] [PubMed] [Google Scholar]
- 20.Feresin GE, Tapia A, Sortino M, et al. . Bioactive alkyl phenols and embelin from Oxalis erythrorhiza. J Ethnopharmacol 2003;88:241–7. [DOI] [PubMed] [Google Scholar]
- 21.Joshi R, Kamat JP, Mukherjee T.. Free radical scavenging reactions and antioxidant activity of embelin: biochemical and pulse radiolytic studies. Chem-Biol Interact 2007;167:125–34. [DOI] [PubMed] [Google Scholar]
- 22.Mahendran S, Badami S, Ravi S, et al. . Synthesis and evaluation of analgesic and anti-inflammatory activities of most active free radical scavenging derivatives of embelin – a structure-activity relationship. Chem Pharm Bull 2011;59:913–9. [DOI] [PubMed] [Google Scholar]
- 23.Afzal M, Gupta G, Kazmi I, et al. . Evaluation of anxiolytic activity of embelin isolated from Embelia ribes. Biomed Aging Pathol 2012;2:45–7. [Google Scholar]
- 24.Singh IP, Bharate SB, Singh A, et al. . Fate of embelin in Pippalyadi Yoga, an ayurvedic oral contraceptive: structure of embelin-borax complex and evaluation of anti-fertility activity. Indian J Chem 2007;46B:320–5. [Google Scholar]
- 25.Radhakrishnan N, Gnanamani A.. 2,5-dihydroxy-3-undecyl-1,4-benzoquinone (embelin) – a second solid gold of India – a review. Int J Pharm Pharmacol Sci 2014;6:23–30. [Google Scholar]
- 26.Naik SR, Niture NT, Ansari AA, et al. . Anti-diabetic activity of embelin: involvement of cellular inflammatory mediators, oxidative stress and other biomarkers. Phytomedicine 2013;20:797–804. [DOI] [PubMed] [Google Scholar]
- 27.Mahendran S, Badami S, Maithili V.. Evaluation of antidiabetic effect of embelin from Embelia ribes in alloxan induced diabetes in rats. Biomed Prev Nutr 2011;1:25–31. [DOI] [PubMed] [Google Scholar]
- 28.Alam MS, Ahad A, Abidin L, et al. . Embelin-loaded oral niosomes ameliorate streptozotocin-induced diabetes in Wistar rats. Biomed Pharmacother 2018;97:1514–20. [DOI] [PubMed] [Google Scholar]
- 29.Durg S, Veerapur VP, Neelima S, et al. . Antidiabetic activity of Embelia ribes, embelin and its derivatives: a systematic review and meta-analysis. Biomed Pharmacother 2017;86:195–204. [DOI] [PubMed] [Google Scholar]
- 30.Dang PH, Nguyen HX, Nguyen NT, et al. . α-Glucosidase inhibitors from the stems of Embelia ribes. Phytother Res 2014;28:1632–6. [DOI] [PubMed] [Google Scholar]
- 31.Dang PH, Nguyen NT, Nguyen HX, et al. . α-Glucosidase inhibitors from the leaves of Embelia ribes. Fitoterapia 2015;100:201–7. [DOI] [PubMed] [Google Scholar]
- 32.Filosa R, Peduto A, Schaible AM, et al. . Novel series of benzoquinones with high potency against 5-lipoxygenase in human polymorphonuclear leukocytes. Eur J Med Chem 2015;94:132–9. [DOI] [PubMed] [Google Scholar]
- 33.Wu PP, Zhang K, Lu YJ, et al. . In vitro and in vivo evaluation of the antidiabetic activity of ursolic acid derivatives. Eur J Med Chem 2014;80:502–8. [DOI] [PubMed] [Google Scholar]
- 34.Sheng Z, Ge S, Xu X, et al. . Design, synthesis and evaluation of cinnamic acid ester derivatives as mushroom tyrosinase inhibitors. MedChemComm 2018;9:897. [DOI] [PMC free article] [PubMed] [Google Scholar]