Abstract
Background and Objective: Patients with metastatically compromised vertebra can experience pathologic fracture with relevant neurological complications. Vertebroplasty is a low cost procedure and it can potentially prevent neurologic impairment if performed at an early stage. The aim of this study is to evaluate the effects of prophylactic vertebroplasty on stability of the metastatic spine and analyze load distribution at adjacent vertebrae.
Setting: A 3D finite element model of two spinal motion segments (L3-L5) was developed. A central core of elements was selected in L4 vertebral body and material properties of a lytic metastasis and successively PMMA were assigned. The model was settled in order to simulate a non-osteoporotic spine and an osteoporotic spine.
Outcome Measures: Vertebral stability was assessed by the measurement of vertebral bulge (VB) and vertebral height (VH) on L4. Load transfer on adjacent vertebrae was evaluated by observing the distribution of the von Mises stress on L3 and L5 endplates.
Results: The metastasis increased VB by 424% and VH by 626%, while prophylactic vertebroplasty decreased VB and VH by 99% and 95%, respectively, when compared to the normal/non-metastatic model. Prophylactic vertebroplasty increased the average von Mises stress of L3 lower endplate by 1.33% in the non-osteoporotic spine, while it increased to 16% in the osteoporotic model.
Conclusions: Prophylactic vertebroplasty could represent an interesting option to improve vertebral strength of metastatically compromised spine without excessively increasing the stresses on adjacent vertebrae in non-osteoporotic spine.
Keywords: Vertebroplasty, Metastasis, Spine, Vertebrae, Stress, Fracture, Stability
Introduction
The spine is the most common site of malignant disease of the skeletal system. Patients with metastatically compromised vertebra can experience pathologic fractures with related neurological complications. Prophylactic treatment is critical to prevent this catastrophic event. Conventional surgical options include decompression and stabilization with instrumentation either from an anterior, posterior, or a combined approach. However, morbidity and mortality associated with mayor surgery is significant.1
Vertebroplasty is a minimally invasive technique that involves the percutaneous injection of bone cement into the vertebral defect. The biomechanical objective of vertebroplasty is to restore vertebral body stability, so that the weight-bearing capability in the spine during regular daily activities is sufficiently supported. Vertebroplasty is an attractive option because it is less costly than major surgery and it can potentially prevent neurologic impairment if performed at an early stage. Ahn et al.6 quantified the ability of vertebroplasty to stabilize metastatically involved vertebrae against the risk of burst fracture initiation with a standardized model of vertebral metastases. They demonstrated that location of cement after injection into the vertebral body relative to the tumor is important in determining whether or not vertebral wall motion is reduced or increased with percutaneous vertebroplasty. Other studies examining the effects of vertebroplasty have focused mainly on osteoporotic fractured vertebral bodies.2–4 Cadaveric studies demonstrated that vertebroplasty restores biomechanical integrity of compressed osteoporotic vertebral bodies.5,7 A finite element study showed that a modest amount of cement can restore or increase the stiffness of a fractured vertebral body.8 However, cement augmentation procedures alter the biomechanics of the fractured segment, modifying the biomechanical responses of levels above and below the treated vertebra.9
The consequences of vertebroplasty on non-fractured vertebrae are largely unknown. Studies mainly focused on preventive reinforcement of vertebrae adjacent to a post-fractured augmented vertebra and results reported vary.10
Limited research has been conducted evaluating the effects of prophylactic vertebroplasty in metastatic vertebrae.11,12 Prophylactic vertebroplasty has a number of hypothetical advantages including the retention of the natural vertebral height, and thus spinal alignment,. However, evidence to support prophylactic vertebroplasty in metastatic vertebrae is not sufficient. Moreover, the application of procedures with preventive intents needs to be carefully considered to avoid unwanted complications and risks. The risk of cement leakage
is controversial in metastatic vertebrae. Some authors reported a lower risk of extravasation in a metastatic vertebra when compared with a vertebral body compromised by fracture.10 Others described a higher risk in patients with spinal malignancy compared with patients with osteoporotic vertebrae.13–15 Longer-term studies on osteoporotic patients treated with vertebroplasty suggested a possible accelerated failure rate in the adjacent vertebral body.16–18 The mechanism of adjacent vertebral failure can be explained by a stiffness mismatch between the treated and adjacent vertebrae.9,19 However, the risk of adjacent vertebral fracture in the setting of a metastatic spine needs to be better investigated. In a prospective cohort study, percutaneous selective vertebroplasty as first-line treatment option was clinically and radiologically evaluated in patients with well-confined metastatic vertebral lesions.20 According to Elnoamany et al.,20 there were no adjacent vertebral fractures at 6-14 months follow-up.
The aim of this study was to evaluate the effects of prophylactic vertebroplasty on the biomechanical stability of a metastatic vertebra21,22 and to analyze load distribution on adjacent vertebrae. Our purpose was to understand how cement augmentation influences the biomechanics of a metastatic vertebra and to assess the potential risk of adjacent vertebral fracture, thus providing an insight into the rationale and feasibility of prophylactic vertebroplasty. We hypothesized that 1) vertebroplasty has the potential to restore vertebral stability without excessively increasing stress on adjacent vertebrae in a non-osteoporotic spine; and 2) vertebroplasty can lead to inadequately high stresses on adjacent vertebrae in an osteoporotic spine.
Methods
Finite element model
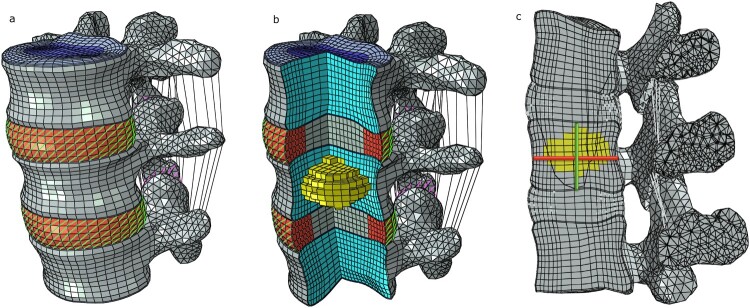
Two spinal motion segments (L3-L5) were selected from a previously developed and validated three-dimensional, nonlinear, ligamentous L3-Sacrum model that was described in detail in a prior publication23 (Fig. 1a). Briefly, a high-resolution computer tomography (CT) scan of a fresh-frozen human cadaveric spine specimen (male, 52 yrs. old) was obtained on a Siemens 79 Helical CT Scanner (Siemens Corp., Munich, Germany) and reconstructed with a pixel size of 0.293 mm and 0.4 mm slice thickness. Segmentation was performed using Mimics image processing and editing software (Materialise US, Plymouth, MI USA). Discs were generated using the wrap function in 3-matic (Materialise, Ann Arbor, MI). Meshes were created using Hypermesh (v10.0 Altair Engineering, Inc., Troy, MI, USA).
Figure 1.
(a) Finite element model of two motion segments of the lumbar spine (L3-L5). (b) A central core of elements was selected in L4 vertebral body and material properties of a lytic metastasis and PMMA were successively allocated. (c) Vertebral bulge (VB) is the horizontal line and vertebral height (VH) is the vertical line. VB was calculated by measuring the distance between two standard nodes at the mid-height of the vertebra on the sagittal plane. VH was calculated by measuring the distance between two standard nodes at the center of the inferior and superior endplates on the sagittal plane.
The model included vertebral bodies consisting of a cortical shell, cancellous core, endplates and posterior elements. Intervertebral discs consisted of a nucleus pulposus, annulus ground substances, and annulus fibrosus (seven layers). Ligaments (anterior longitudinal, posterior longitudinal, intertransverse, ligamentum flavum, interspinous, supraspinous and capsular) were also included in the model. Material properties of all model components are summarized in Table 1.
Table 1. Finite element model material properties.
| Elastic Modulus (MPa) | Poisson’s Ratio | |
|---|---|---|
| Vertebra | ||
| Cancellous Bone | 100 | 0.2 |
| Cortical Bone | 12000 | 0.3 |
| Vertebral Bony Endplate | 4000 | 0.3 |
| Cartilage Endplate | 5 | 0.17 |
| Posterior Bone | 3500 | 0.25 |
| Intervertebral Disc | ||
| Nucleus Polposus | 1 | 0.49 |
| Annular Fibers | Neo-Hooke | |
| Annular Layers | Neo-Hooke | |
| Joint | ||
| Facet Joints | 3500 | |
| Ligaments | ||
| Anterior Longitudinal | 15.6 - 20.0 | 0.3 |
| Posterior Longitudinal | 10.0 - 20.0 | 0.3 |
| Intertransverse | 12 - 58.7 | 0.3 |
| Ligamentum Flavum | 13.0 - 19.5 | 0.3 |
| Interspinous | 9.8 -12.0 | 0.3 |
| Supraspinous | 8.8 - 15.0 | 0.3 |
| Capsular | 7.5 - 33.0 | 0.3 |
Boundary conditions and loads
Nodes on the lower endplate of the L5 vertebra were encastred and constrained in all three axes of rotation and translations. A kinematic coupling was created by selecting surface nodes from L3 and a reference node placed 10 mm above the vertebra. This constraint limited the motion of aforementioned nodes to the motion applied to the reference node.24 An axial compressive load of 1,200 N was applied to the superior reference node atop the L3 vertebra, corresponding to a compressive force on the lumbar spine for an individual standing upright holding an 8.3 kg mass with outstretched arms.25
Parametric analyses
A metastatic lesion was first represented in the model, encompassing a central core of elements, covering about 30% of the vertebral volume26 in the L4 vertebral body. Material properties of a lytic metastasis were assigned to these elements (Elastic Modulus 0.01 MPa, Poisson’s Ratio 0.4995).27 Then, the model was modified to simulate prophylactic vertebroplasty assigning polymethylmethacrylate (PMMA) material properties to the same core of elements in the L4 vertebral body (Elastic Modulus 3000 MPa, Poisson’s Ratio 0.41)19 (Fig. 1b).
Since the risk of adjacent vertebral failure has been described in osteoporotic patients,28 the prophylactic vertebroplasty model was modified to simulate osteoporosis as well. Osteoporosis was defined by a 66% reduction in the elastic moduli of all bony structures for the cancellous bone and a 33% reduction modulus for the cortical shell, endplates and posterior elements.19 Soft tissue structures were left unchanged.
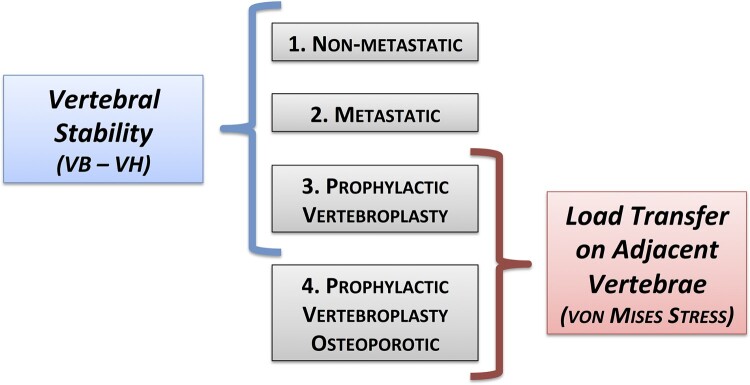
A total of four models were developed: non-metastatic model, metastatic model, prophylactic vertebroplasty model, prophylactic vertebroplasty-osteoporotic model (Fig. 2).
Figure 2.
Four models were obtained: non-metastatic model, metastatic model, prophylactic vertebroplasty model, prophylactic vertebroplasty in osteoporotic model. Vertebral stability was analyzed and compared between the non metastatic model, the metastatic model and the prophylactic vertebroplasty model. The von Mises stress was analyzed and compared between the prophylactic vertebroplasty model and the prophylactic vertebroplasty in osteoporotic model.
Outcomes variables
Vertebral stability was assessed by the measurement of vertebral bulge (VB) and vertebral height (VH).29 Vertebral bulge is defined as the maximum radial bulge of the vertebral body under load which has been correlated with load-induced spinal canal narrowing, vertebral cortex tensile hoop strains and bone marrow pressurization.30 Vertebral height represents the maximum axial displacement of the vertebral body under load and it characterizes the risk of endplate failure leading to subsequent burst fracture.30 VB and VH were measured on the L4 vertebra when the axial compressive load of 1,200 N was applied. VB was calculated by measuring the distance between two standard nodes at the mid-height of the vertebra on the sagittal plane. VH was calculated by measuring the distance between two standard nodes at the center of the inferior and superior endplates on the sagittal plane (Fig. 1c). Since the mesh was identical in all models, node numbering and position allowed for the identification of standard nodes in each model. Vertebral body stability was analyzed and compared between the non-metastatic model, the metastatic model and the prophylactic vertebroplasty model (Fig. 2).
The influence of cement augmentation on load transfer on adjacent vertebrae was evaluated by observing the distribution of the von Mises stress on L3 and L5 endplates. The von Mises stress combines multiaxial stresses into one equivalent uniaxial stress value and has been proposed as a failure criteria for bone.19 The average, maximum and minimum von Mises stress were evaluated on the endplates above and below the augmented vertebra (inferior of L3 and superior of L5). The distribution of von Mises stress on L3 lower endplate and L5 upper endplate was also evaluated. The percentage area above and below the average von Mises stress of the non-metastatic non-osteoporotic model was calculated for each condition. The von Mises stress was analyzed and compared between the prophylactic vertebroplasty model and the prophylactic vertebroplasty-osteoporotic model (Fig. 2).
Results
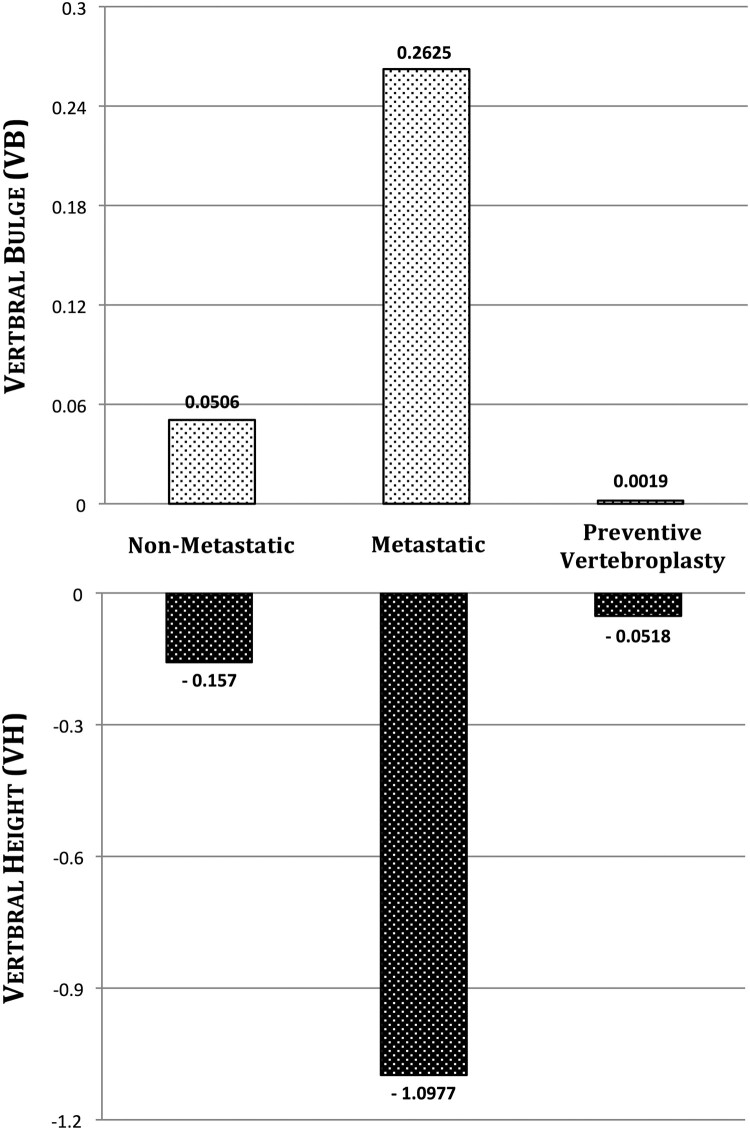
In the non-metastatic model, VB and VH were 0.05 mm and -0.15 mm respectively. The metastasis increased VB by 424% (0.262 mm) and VH by 626% (-1.09 mm) compared to the non-metastatic model. When prophylactic vertebroplasty was simulated, VB decreased by 99% (0.001 mm) and VH decreased by 95% (-0.051 mm) compared to the metastatic model. Comparing prophylactic vertebroplasty with the non-metastatic model, VB decreased by 98% and VH decreased by 66% (Fig. 3).
Figure 3.
VB and VH in the non-metastatic, metastatic and prophylactic vertebroplasty models.
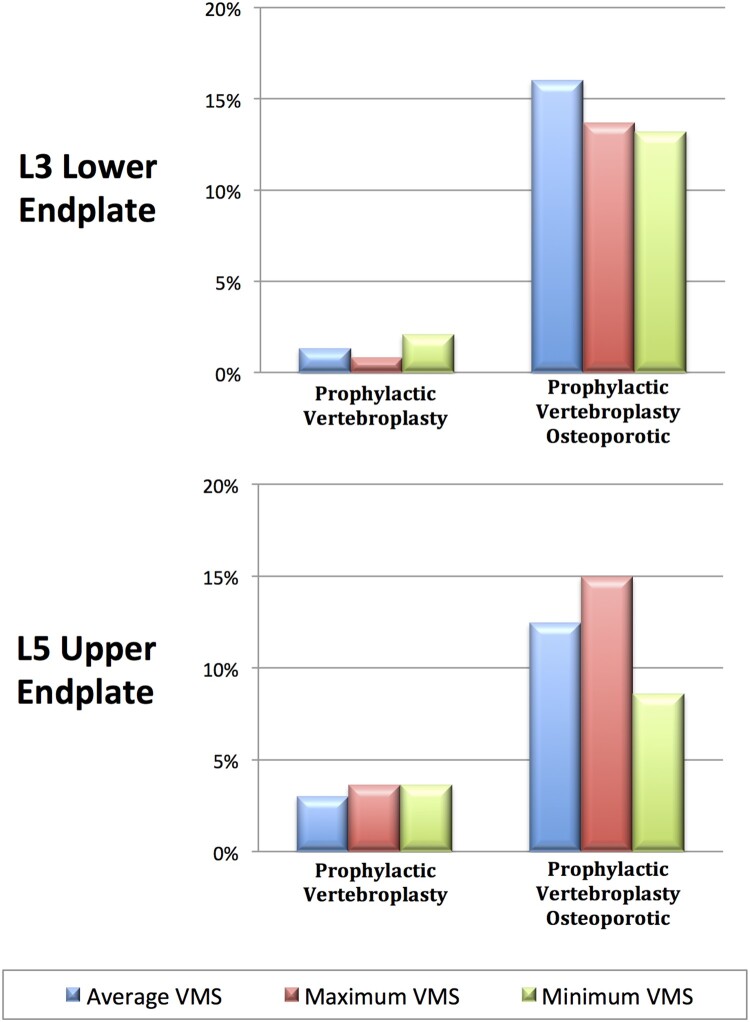
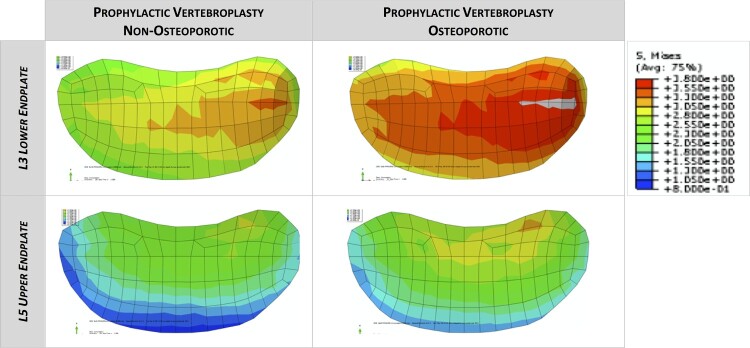
The average, maximum and minimum von Mises stress on adjacent vertebrae in the prophylactic vertebroplasty model were compared to those of the non-metastatic model. Prophylactic vertebroplasty increased the average von Mises stress of L3 lower endplate by 1.33%, the maximum value by 0.82% and the minimum value by 2.09%. The L3 lower endplate percentage area above the average von Mises stress increased by 8.9%. A similar trend was observed in L5 upper endplate. When prophylactic vertebroplasty was simulated in the osteoporotic model, the average von Mises stress of L3 lower endplate increased by 16.01%, the maximum value increased by 13.7% and the minimum value increased by 13.19%. The L3 lower endplate percentage area above the average von Mises stress increased by 102%. A similar trend was observed in L5 upper endplate (Figs. 4 and 5). The von Mises stress on the L3 lower endplate and L5 upper endplate showed an asymmetric distribution due to anatomical characteristics of the CT derived-finite element model.
Figure 4.
The percentage increment of average, maximum and minimum von Mises stress (VMS) on L3 lower endplate and L5 upper endplate in the prophylactic vertebroplasty model and prophylactic vertebroplasty-osteoporotic model. Data are normalized to the non-metastatic model.
Figure 5.
The distribution of von Mises stress on L3 lower endplate and L5 upper endplate in the prophylactic vertebroplasty model and prophylactic vertebroplasty-osteoporotic model.
Discussion
This finite element model demonstrated that VB and VH increase when a metastatic lesion affects the vertebral body and decrease when cement is added. These results suggest the positive influence of vertebroplasty on vertebral stability. However, this procedure could also induce unwanted effects above the non-pathologic condition. We hypothesized that prophylactic vertebroplasty would have had a minor effect on the stresses on adjacent vertebrae in a non-osteoporotic spine. The results confirmed our hypothesis by showing lower average von Mises stress with small distribution on adjacent endplates of a non-osteoporotic spine. In contrast, there is an increase in stresses with a wider distribution on adjacent endplates when prophylactic vertebroplasty is performed in an osteoporotic model. This agrees with previous studies demonstrating a shift in loads in adjacent vertebrae, with a subsequent increase in the occurrence of adjacent vertebral failure in osteoporotic patients.9,19,28 This is the first study to investigate stress distribution on adjacent vertebrae when prophylactic vertebroplasty is performed in the metastatic non-osteoporotic spine. Our findings suggest that, in treating the metastatic spine, prophylactic vertebroplasty does not lead to an increased load transfer on adjacent vertebrae. However, further experimental testing is required to recommend prophylactic vertebroplasty in the clinical setting. Moreover, complication risks, including cement leakage leading to neurologic or vascular damage and embolism, should be carefully considered before applying the technique with a preventive intent. Rather, our model aims to highlight trends in the behavior of metastatic spine following prophylactic vertebroplasty.
This study has several limitations. First, vertebroplasty was represented as a core of elements in L4 vertebral body whose material properties where augmented with those of the PMMA. It is an extreme simplification of cement injection into metastatically involved vertebra that does not consider cement and tumor distribution patterns. Despite this limitation, the model allowed comparison of different scenarios to describe the relative effects on vertebral stability. Mizrahi et al.31 developed a three-dimensional finite element model of a lumbar vertebral body to study the effects of geometry, material properties and loading conditions on stresses in the presence of a metastatic lesion. They found that the location of a defect which did not penetrate the cortex had a minor influence on the peak displacement and stresses, as did the presence of lesions occupying less than 40% of the volume of the vertebral centrum. The most severe case involved a defect penetrating the anterior cortex, osteoporotic bone properties and anteriorly eccentric loading. Second, the ideal cement volume required to restore vertebral stability was not investigated. Cement volume corresponding to 30% of vertebral volume was used in this study since it is the recommended amount of cement injected for vertebroplasty, corresponding to 4–8 cm.3,26 Third, only one type of cement has been considered. It is plausible that cements with lower modulus of elasticity could theoretically reduce the risk of adjacent segment fracture.10 Because PMMA has a higher modulus than trabecular bone, it can lead to stress shielding with consequential bone resorption and disc degeneration adjacent to the reconstruction. Previous biomechanical cadaver studies have experimentally demonstrated that fracture loads of augmented vertebrae could be better conserved using materials with moduli less than that of PMMA cement.26 Fourth, we did not analyze the effect of vertebroplasty on the intervertebral disc. Baraud et al. described the change in loading and stiffness in the intervertebral disc adjacent to the augmented level due to augmentation.9 Using an FE model of a lumbar motion segment they predicted an increase in stiffness of the disc by approximately 11.1%. Fifth, only an axial compressive load of 1,200 N was studied. However, this represents a compressive force on the lumbar spine for an individual standing upright holding an 8.3 kg mass with outstretched arms.25 Further research is required to elucidate the effects of prophylactic vertebroplasty in a dynamic setting. Sixth, it could be argued that metastatic lesions are more common in the thoracic spine rather than in the lumbar spine. However, we aimed to evaluate the effect of axial loading without the influences of the ribs, which can contribute to reduce the effective axial loading applied on the vertebra. Therefore, we decided to study the lumbar spine segment, as recently evaluated in a predictive mechanical model for evaluating vertebral fracture probability in lumbar spine under different osteoporotic drug therapies.32 In future studies, we are planning to study localization of metastasis to the thoracic spine. Finally, our study did not consider metastases on multiple levels. In this condition, the presence of cement could increase the risk of adjacent vertebral fracture since a tumor is present in that level.
Although a preliminary biomechanical study, results suggest that prophylactic vertebroplasty would improve vertebral strength of a metastatically compromised spine without excessively increasing stress on adjacent vertebrae in non-osteoporotic spine. It must be emphasized that further biomechanical testing and randomized controlled clinical trials need to be performed before prophylactic vertebroplasty can be recommended as a treatment modality since possible risks and benefits need to be carefully defined.
Disclaimer statements
Contributors None.
Funding No funding source to declare.
Conflict of interest The authors declare that they have no conflict of interest to declare.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
Funding Statement
No funding source to declare.
References
- 1.Pascal-Moussellard H, Broc G, Pointillart V, Simeon F, Vital JM, Senegas J.. Complications of vertebral metastasis surgery. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 1998;7(6):438–44. doi: 10.1007/s005860050105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denaro V, Longo UG, Denaro L.. Vertebroplasty versus conservative treatment for vertebral fractures. Lancet 2010;376(9758):2071; author reply -2. doi: 10.1016/S0140-6736(10)62289-1 [DOI] [PubMed] [Google Scholar]
- 3.Longo UG, Loppini M, Denaro L, Maffulli N, Denaro V.. Osteoporotic vertebral fractures: current concepts of conservative care. British medical bulletin 2012;102:171–89. doi: 10.1093/bmb/ldr048 [DOI] [PubMed] [Google Scholar]
- 4.Longo UG, Loppini M, Denaro L, Maffulli N, Denaro V.. Conservative management of patients with an osteoporotic vertebral fracture: a review of the literature. The Journal of bone and joint surgery British volume 2012;94(2):152–7. doi: 10.1302/0301-620X.94B2.26894 [DOI] [PubMed] [Google Scholar]
- 5.Fang Z, Giambini H, Zeng H, Camp JJ, Dadsetan M, Robb RA, et al. Biomechanical evaluation of an injectable and biodegradable copolymer P(PF-co-CL) in a cadaveric vertebral body defect model. Tissue engineering Part A 2014;20(5–6):1096–102. doi: 10.1089/ten.tea.2013.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn H, Mousavi P, Roth S, Reidy D, Finkelstein J, Whyne C.. Stability of the metastatic spine pre and post vertebroplasty. Journal of spinal disorders & techniques 2006;19(3):178–82. doi: 10.1097/01.bsd.0000172362.15103.e8 [DOI] [PubMed] [Google Scholar]
- 7.Belkoff SM, Mathis JM, Jasper LE, Deramond H.. An ex vivo biomechanical evaluation of a hydroxyapatite cement for use with vertebroplasty. Spine (Phila Pa 1976). 2001;26(14):1542–6. doi: 10.1097/00007632-200107150-00008 [DOI] [PubMed] [Google Scholar]
- 8.Liebschner MA, Rosenberg WS, Keaveny TM.. Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine 2001;26(14):1547–54. doi: 10.1097/00007632-200107150-00009 [DOI] [PubMed] [Google Scholar]
- 9.Baroud G, Nemes J, Heini P, Steffen T.. Load shift of the intervertebral disc after a vertebroplasty: a finite-element study. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society 2003;12(4):421–6. doi: 10.1007/s00586-002-0512-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furtado N, Oakland RJ, Wilcox RK, Hall RM.. A biomechanical investigation of vertebroplasty in osteoporotic compression fractures and in prophylactic vertebral reinforcement. Spine 2007;32(17):E480-7. [DOI] [PubMed] [Google Scholar]
- 11.Tschirhart CE, Roth SE, Whyne CM.. Biomechanical assessment of stability in the metastatic spine following percutaneous vertebroplasty: effects of cement distribution patterns and volume. Journal of biomechanics 2005;38(8):1582–90. doi: 10.1016/j.jbiomech.2004.07.023 [DOI] [PubMed] [Google Scholar]
- 12.Tschirhart CE, Finkelstein JA, Whyne CM.. Optimization of tumor volume reduction and cement augmentation in percutaneous vertebroplasty for prophylactic treatment of spinal metastases. Journal of spinal disorders & techniques 2006;19(8):584–90. doi: 10.1097/01.bsd.0000211236.76093.b0 [DOI] [PubMed] [Google Scholar]
- 13.Georgy BA. Metastatic spinal lesions: state-of-the-art treatment options and future trends. AJNR Am J Neuroradiol 2008;29(9):1605–11. doi: 10.3174/ajnr.A1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiras J, Depriester C, Weill A, Sola-Martinez MT, Deramond H.. [Percutaneous vertebral surgery. Technics and indications]. J Neuroradiol 1997;24(1):45–59. [PubMed] [Google Scholar]
- 15.Wenger M. Vertebroplasty for metastasis. Med Oncol 2003;20(3):203–9. doi: 10.1385/MO:20:3:203 [DOI] [PubMed] [Google Scholar]
- 16.Denaro L, Longo UG, Denaro V.. Vertebroplasty and kyphoplasty: reasons for concern? The Orthopedic clinics of North America 2009;40(4):465–71, viii. doi: 10.1016/j.ocl.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 17.Denaro V, Longo UG, Maffulli N, Denaro L.. Vertebroplasty and kyphoplasty. Clin Cases Miner Bone Metab 2009;6(2):125–30. [PMC free article] [PubMed] [Google Scholar]
- 18.Longo UG, Denaro V.. Spinal augmentation: what have we learnt? Lancet 2009;373(9679):1947; author reply -8. doi: 10.1016/S0140-6736(09)61065-5 [DOI] [PubMed] [Google Scholar]
- 19.Polikeit A, Nolte LP, Ferguson SJ.. The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit: finite-element analysis. Spine 2003;28(10):991–6. [DOI] [PubMed] [Google Scholar]
- 20.Elnoamany H. Percutaneous Selective Vertebroplasty: State of the Art Management in Well-Confined Metastatic Vertebral Lesions. Asian Spine J 2016;10(5):869–76. doi: 10.4184/asj.2016.10.5.869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Instability of the lumbar spine . Cambridge, England, April 1983. Spine 1985;10(3):252–91. [PubMed] [Google Scholar]
- 22.Izzo R, Guarnieri G, Guglielmi G, Muto M.. Biomechanics of the spine. Part I: spinal stability. Eur J Radiol 2013;82(1):118–26. doi: 10.1016/j.ejrad.2012.07.024 [DOI] [PubMed] [Google Scholar]
- 23.Ellingson AM, Shaw MN, Giambini H, An KN.. Comparative role of disc degeneration and ligament failure on functional mechanics of the lumbar spine. Computer methods in biomechanics and biomedical engineering 2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corp. DSS, editor . Abaqus Analysis User's Manual. Providence, RI.2008. a.
- 25.Schultz A, Andersson G, Ortengren R, Haderspeck K, Nachemson A.. Loads on the lumbar spine. Validation of a biomechanical analysis by measurements of intradiscal pressures and myoelectric signals. The Journal of bone and joint surgery American volume 1982;64(5):713–20. doi: 10.2106/00004623-198264050-00008 [DOI] [PubMed] [Google Scholar]
- 26.Chae SW, Kang HD, Lee MK, Lee TS, Park JY.. The effect of vertebral material description during vertebroplasty. Proceedings of the Institution of Mechanical Engineers Part H, Journal of engineering in medicine 2010;224(1):87–95. doi: 10.1243/09544119JEIM654 [DOI] [PubMed] [Google Scholar]
- 27.Whyne CM, Hu SS, Workman KL, Lotz JC.. Biphasic material properties of lytic bone metastases. Annals of biomedical engineering 2000;28(9):1154–8. doi: 10.1114/1.1313773 [DOI] [PubMed] [Google Scholar]
- 28.Trout AT, Kallmes DF, Kaufmann TJ.. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol 2006;27(1):217–23. [PMC free article] [PubMed] [Google Scholar]
- 29.Tschirhart CE, Nagpurkar A, Whyne CM.. Effects of tumor location, shape and surface serration on burst fracture risk in the metastatic spine. Journal of biomechanics 2004;37(5):653–60. doi: 10.1016/j.jbiomech.2003.09.027 [DOI] [PubMed] [Google Scholar]
- 30.Whyne CM, Hu SS, Lotz JC.. Burst fracture in the metastatically involved spine: development, validation, and parametric analysis of a three-dimensional poroelastic finite-element model. Spine 2003;28(7):652–60. [DOI] [PubMed] [Google Scholar]
- 31.Mizrahi J, Silva MJ, Hayes WC.. Finite element stress analysis of simulated metastatic lesions in the lumbar vertebral body. Journal of biomedical engineering 1992;14(6):467–75. doi: 10.1016/0141-5425(92)90098-6 [DOI] [PubMed] [Google Scholar]
- 32.Lopez E, Ibarz E, Herrera A, Puertolas S, Gabarre S, Mas Y, et al. A predictive mechanical model for evaluating vertebral fracture probability in lumbar spine under different osteoporotic drug therapies. Comput Methods Programs Biomed 2016;131:37–50. doi: 10.1016/j.cmpb.2016.04.006 [DOI] [PubMed] [Google Scholar]