Prior U.S. and most current other hypertension guidelines define treatment-resistant hypertension (TRH) as requiring ≥4 antihypertensive drugs to achieve blood pressure (BP) <140/90 mmHg, and this phenotype has been consistently linked with increased risk of major adverse cardiovascular outcomes, relative to non-resistant hypertension.1,2 In 2017, U.S. hypertension guidelines updated the TRH definition to requiring ≥4 antihypertensive drugs to achieve BP <130/80 mmHg, consistent with the BP target promulgated for most hypertensive individuals.1,3 This expanded definition and recommendations for more intensive treatment, generally, are expected to significantly increase TRH incidence and prevalence by now including individuals with presumably lower-risk profiles, i.e., persons taking 3 antihypertensives with a systolic BP between 130–139 or diastolic BP between 80–89 mmHg.4 Yet, whether these revised TRH criteria identify a population with poor prognosis, similar to that observed with the prior U.S. definition, is unknown. Therefore, we sought to compare updated and prior TRH definitions on risk of adverse cardiovascular events using patient-level data from the Systolic Blood Pressure Intervention Trial (SPRINT) and Action to Control Cardiovascular Risk in Diabetes (ACCORD) trials.
METHODS AND RESULTS
All data supporting the findings of this study are available from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center, accessible at https://biolincc.nhlbi.nih.gov/home/. The design and results of SPRINT and ACCORD have been published previously.5,6 Briefly, both were prospective, randomized, open-label, blinded-endpoint trials comparing an intensive (<120 mmHg) versus standard (<140 mmHg) systolic BP target among high-risk individuals with hypertension and diabetes (ACCORD) or hypertension without diabetes (SPRINT) at baseline (see Supplement for additional detail). Patient-level data from both trials were pooled and harmonized (Supplemental Figure S1); specific data elements included baseline demographics and clinical characteristics (detailed below), clinic BP measurements at each visit, medication use at each visit, and outcomes. Daily BP values between study visits were estimated using linear interpolation. Medication use and adherence were determined via detailed study medication logs, which included information on specific medications, but not doses. Using these data, we determined apparent TRH (aTRH) status for each patient throughout the trial, taking advantage of treatment arm-specific BP targets that allowed definition of aTRH according to previous and updated guidelines. The term “aTRH” is used to signify the fact that data constraints (e.g., lack of antihypertensive dosing information and out-of-office BP) did not allow for exclusion of pseudoresistance. For patients assigned the intensive target, aTRH (hereafter, aTRHupdated) was defined per the 2018 TRH scientific statement1 as a systolic BP ≥130 or diastolic BP ≥80 mmHg with adherent use of ≥3 antihypertensive drugs from different classes, including a diuretic; or, adherent use of ≥4 antihypertensive drugs from different classes, including a diuretic, regardless of BP. For patients assigned the standard target, aTRH (hereafter, aTRHprior) was defined per the 2008 TRH scientific statement7 as having a systolic BP ≥140 or diastolic BP ≥90 mmHg with adherent use of ≥3 antihypertensive drugs, including a diuretic, or adherent use of ≥4 antihypertensive drugs, including a diuretic, regardless of BP. To minimize misclassification following therapy adjustments, we required BP to remain uncontrolled for >7 days following the addition of a 3rd antihypertensive agent before a patient could meet aTRH criteria (aTRHprior in standard arm; aTRHupdated in intensive arm). For patients beginning a 4th antihypertensive drug, aTRH was considered to occur beginning on the date the 4th drug was added, with no lag period. Using daily aTRH status, we then calculated cumulative aTRH exposure, updated throughout follow-up. Patients meeting aTRH criteria at baseline were excluded from the analysis because prior duration of aTRH was unknown.
The primary outcome was first occurrence of myocardial infarction (MI), stroke, heart failure (HF) or cardiovascular death, a hybrid of the SPRINT and ACCORD primary outcomes (see Supplement for additional details). These same events were considered individually as secondary outcomes. Cox proportional hazards models were fit, regressing the outcome on time-updated cumulative aTRH exposure as a 4-level categorical variable (none [referent]; <4 months; 4 months to <1.5 years; ≥1.5 year); these category thresholds were determined using approximate tertile boundaries for cumulative exposure-time among exposed. Separate models were fit for each exposure-outcome comparison, within each treatment cohort stratum (intensive, standard). Adjusted models were fit for each exposure-outcome comparison as above, but with a propensity score summarizing information on original study cohort (SPRINT vs. ACCORD), and potential confounders including baseline age, sex, history of clinical cardiovascular disease, smoking status, estimated glomerular filtration rate, total cholesterol, high-density lipoprotein cholesterol, glucose, and assignment to the intensive glycemia vs. standard glycemia arm in ACCORD; all SPRINT patients were considered as receiving standard glycemia treatment. Finally, in pooled analysis of all patients, we regressed each outcome on time-varying aTRH status, type of aTRH definition (prior vs. updated) and their interaction to determine whether risk differences between aTRHupdated and aTRHprior were significant. Analyses were performed with SAS 9.4 (SAS Institute, Cary, NC). This research was approved by the University of Florida Institutional Review Board.
Among 14,094 individuals enrolled, 12,392 (n=8,353 from SPRINT; n=4,039 from ACCORD) had no evidence of aTRH at baseline and are included herein. Of these, 5,707 (46.1%) met aTRH criteria at some point during follow-up, including 61% of those assigned to the intensive target meeting aTRHupdated criteria, and 32% assigned the standard target meeting aTRHprior criteria. Baseline characteristics are summarized in Supplemental Table S1.
During 49,873 person-years of follow-up, 1007 patients (8.1%) experienced the primary outcome (MI, stroke, HF, or cardiovascular death). Crude incidences appear in Supplemental Tables S2 and S3. In unadjusted analyses, aTRHprior was associated with increased risk for all outcomes. Likewise, aTRHupdated was associated with excess risk of the primary outcome and most individual outcomes, but only with longer exposure periods (Supplemental Figure S2). In propensity score-adjusted analyses, aTRHprior exposure was associated with greater risk of the primary outcome, ranging from 51% increased risk for <4 months exposure versus no exposure (HR 1.51; 95% CI, 1.20–1.92) to more than doubling the risk for ≥1.5 years exposure versus no exposure (HR 2.33; 95% CI, 1.70–3.19). Similar findings were observed for individual outcomes, where the longest exposure-times were associated with the highest point estimates, although the relationship between exposure-time and outcomes was not uniform and confidence intervals overlapped to some degree in all cases (Figure). Conversely, aTRHupdated exposure was associated with more modestly elevated risk of the primary outcome, but only at longer exposure times (HR 1.28; 95% CI, 0.98–1.67 for 0.4 months to <1.5 years vs. no exposure; and, HR 1.41; 95% CI, 1.02–1.95 for ≥1.5 years vs no exposure). Likewise, aTRHupdated exposure ≥1.5 years (versus none) was associated with greater risk of HF (HR, 1.93; 95% CI, 1.15–3.22) and cardiovascular death (HR, 1.98; 95% CI, 1.00–3.95). In pooled models, including an interaction term between aTRH definition and time-varying aTRH status, we found no evidence of significant risk differences among aTRH definitions (all interaction p-values >0.1).
Figure. Adjusted hazard ratios and 95% confidence intervals stratified by apparent treatment-resistant hypertension definition and cumulative exposure category.
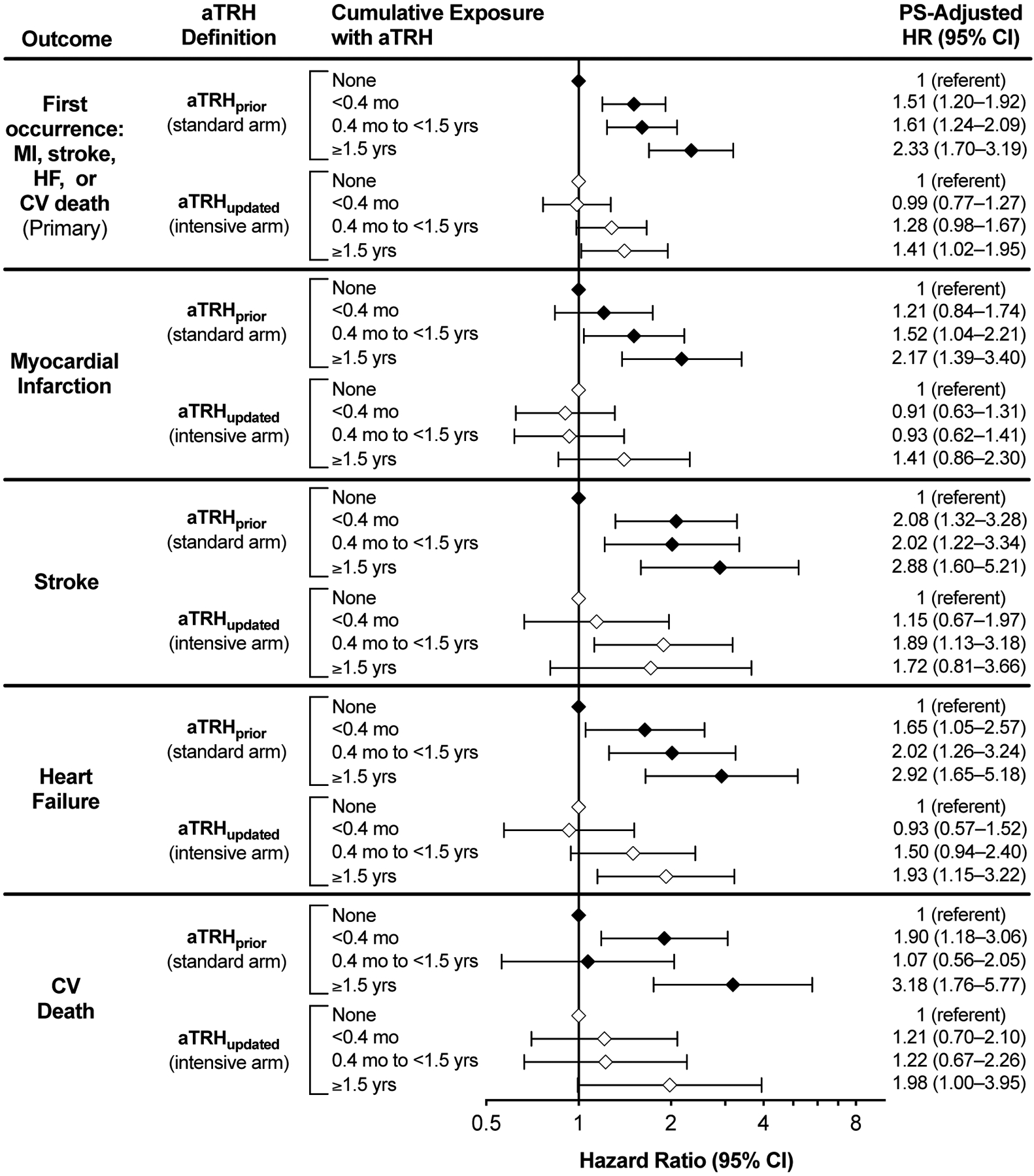
Cumulative exposure is modeled as a time-dependent categorical variable. The model is further adjusted for a propensity score incorporating study cohort (SPRINT vs. ACCORD), age, sex, history of clinical cardiovascular disease, smoking status, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, glucose, estimated glomerular filtration rate and assignment to intensive vs. standard glycemia treatment. aTRH, apparent treatment-resistant hypertension; CV, cardiovascular; HF, heart failure; MI, myocardial infarction; PS, propensity score
COMMENT
The 2017 U.S. hypertension guidelines redefined TRH to be consistent with the threshold for uncontrolled BP in the general population, but without empiric assessment of whether this new definition still identifies high-risk individuals who may benefit from closer follow-up and additional therapeutic intervention. We compared this new U.S. aTRH definition, now employed under more intensive treatment recommendations, with the prior U.S. aTRH definition employed under prior treatment norms, in terms of cardiovascular outcome risk. We found that the new U.S. definition appears to effectively discriminate a higher-risk population, for most cardiovascular outcomes studied, when aTRH persists long-term. Although we observed no significant difference in risk comparing the two definitions, the generally lower hazard ratio point estimates for aTRHupdated versus aTRHprior are consistent with prior findings of a benefit with intensive treatment among persons with aTRH using the updated definition.8
Major strengths of this analysis are that we used pooled clinical trial data with similar standardized treatment algorithms and adjudicated outcomes. And, we compared the aTRH definitions approximating the treatment context within which they would be used, that is, the updated definition under more intensive BP targets, and the prior definition under previously standard targets. However, it must be noted that our estimates of risk with the new aTRH definition were derived under a systolic BP goal <120 mmHg as employed in SPRINT/ACCORD, whereas the new U.S. systolic BP goal is <130 mmHg.
In sum, our data suggest that the current U.S. definition of TRH will significantly broaden the population now classified as having TRH, and that such patients remain at elevated risk of cardiovascular outcomes relative to those with non-resistant hypertension. Future research, using real world data subsequent to implementation of the 2017 U.S. hypertension guidelines, is needed to further assess risk-benefit.
Supplementary Material
Acknowledgements
This manuscript was prepared using ACCORD and SPRINT Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the ACCORD and SPRINT investigators or the NHLBI.
Funding
No funding was provided directly for this study. Dr. Smith is funded by the National Heart, Lung, and Blood Institute (K01 HL138172). Dr. Pepine is also funded by the National Heart, Lung and Blood Institute (R01 HL132448 and R01 HL033610).
Footnotes
Disclosures
The authors report no conflicts of interest related to this work.
References
- 1.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension. 2018;72:e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carris NW, Ghushchyan V, Libby AM, Smith SM. Health-related quality of life in persons with apparent treatment-resistant hypertension on at least four antihypertensives. J Hum Hypertens. 2016;30:191–196. [DOI] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Gurka MJ, Winterstein AG, Pepine CJ, Cooper-DeHoff RM. Incidence, prevalence, and predictors of treatment-resistant hypertension with intensive blood pressure lowering. J Clin Hypertens (Greenwich). 2019;21:825–34. 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 8.Smith SM, Gurka MJ, Calhoun DA, Gong Y, Pepine CJ, Cooper-DeHoff RM. Optimal systolic blood pressure target in resistant and non-resistant hypertension: A pooled analysis of patient-level data from SPRINT and ACCORD. Am J Med 2018;131:1463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


