ABSTRACT
Objectives: The long-term goal of our study is to improve the understanding of the biological mechanisms associated with spinal manipulative therapy (SMT) in low back pain.
Methods: This project involved a pilot randomized, blinded clinical trial (ClinicalTrials.gov registration number NCT03078114) of 3-week SMT in chronic nonspecific low back pain (CNSLBP) patients. We recruited 29 participants and randomly assigned them into either a SMT (n = 14) or sham SMT (n = 15) group. Pre- and postintervention, we quantified the effect of SMT on clinical outcomes (Numeric Pain Rating Scale and Oswestry Disability Index) and pressure pain threshold (PPT) at local (lumbar spine), regional (lower extremity), and remote (upper extremity) anatomical sites.
Results: We observed a significant main effect for time signifying reduced hypersensitivity (increased PPT) at local (p = .015) and regional (p = .014) locations at 3 weeks. Furthermore, we found significant main effects of time indicating improvements in pain (p < .001) and disability (p = .02) from baseline among all participants regardless of intervention. However, no between-group differences were observed in PPT, clinical pain, or disability between the SMT and sham SMT groups over 3 weeks.
Conclusions: After 3 weeks of SMT or sham SMT in CNSLBP patients, we found hypoalgesia at local and remote sites along with improved pain and low back-related disability.
Level of Evidence: 1b
KEYWORDS: Spinal manipulation, lumbar spine, manual therapy, low back pain, pressure pain threshold, chronic, treatment outcome
Introduction
Low back pain affects up to 85% of the adult population imposing an economic burden of $86 billion annually or 1% of the United States gross domestic product [1–3]. Chronic low back pain (pain duration > 3 months), although only accounting for 5% of those with low back pain, represents 75% of the total treatment costs [1,2]. Present clinical practice guidelines recommend spinal manipulative therapy (SMT) as a primary intervention for low back pain [4–6]. SMT may reduce pain and disability in chronic low back pain patients [7,8]. A systematic review concluded that improvement in pain and function following SMT, in comparison with other interventions, might not be considered clinically relevant due to limited level of improvement and small effect size [9]. However, in comparison to other therapies, the practical benefits of SMT for managing chronic low back pain may include cost effectiveness, relative safety, and/or clinician or patient preferences [9].
Researchers have investigated changes in pressure pain threshold (PPT) in an attempt to understand how and why SMT impacts peripheral and/or central biological pathways in low back pain; however, the findings have not been conclusive [10–12]. PPT testing may be used as an indirect measure of peripheral and/or central sensitization for musculoskeletal pain [13]. Peripheral and central sensitization may be differentiated by comparing experimental pain responses at sites local and remote to the primary area of injury [14,15]. Peripheral mechanisms such as sensitization of tissue nociceptors may elucidate local tissue hyperalgesia, while central sensitization reflects widespread hyperalgesia at remote anatomical locations [15]. SMT may influence peripheral tissue hyperalgesia through decreased sensitivity within muscles spindles [11,16] and/or central sensitization of dorsal horn neurons through the descending inhibitory pain mechanism (DIPM) via the periaqueductal gray (PAG) region [10,17–21]. Depending on the measurement site, the examined effect of SMT on PPT in chronic LBP patients may reflect local, regional or remote neurophysiological mechanisms [11,12]. Past studies have reported mixed results on changes in PPT after SMT related to the anatomical site of the applied mechanical stimuli in healthy and low back pain subjects [22–26]. Studies in healthy, asymptomatic subjects examining the effects of SMT on PPT reported significant changes in PPT at local, regional, and remote sites [23] along with conflicting results reporting no significant change in PPT at a local site [24]. Investigations in low back pain patients evaluating the effects of SMT on PPT described no significant changes in PPT at regional locations [22,26], while other studies reported significant changes in PPT at a local site [22,25]. Presently, it is unclear whether SMT can reduce PPT in chronic low back pain, and if it does, which pain pathway, local or central, is responsible for changes in PPT. Until these questions are answered, it is neither possible to establish objective neurophysiological evidence of the mechanisms of SMT, nor to gain ubiquitous acceptance of SMT among the scientific and healthcare communities [27,28].
The goal of our study is to further the understanding of the biological effects associated with SMT. As our primary objective, we examined the effect of SMT on PPT at different anatomical sites and specific clinical outcomes. Our central hypothesis was that SMT would reduce hypersensitivity to mechanical stimuli applied at local, regional and remote sites and improve clinical outcomes in chronic nonspecific low back pain (CNSLBP) patients.
Methods
Research design
This project involved a pilot randomized, blinded clinical trial of 3-week spinal manipulative therapy in individuals with CNSLBP (Figure 1). Subjects were randomly assigned to spinal manipulation (SMT) or sham spinal manipulation (sham SMT) groups. We enrolled 29 (n = 29) subjects out of 51 patients who were assessed for inclusion/exclusion criteria. Clinical evaluations and analyses were performed at the University of Saint Mary research lab. Prior to starting treatment, each subject underwent physical and neurological examinations. Physical examination procedures included vital signs, orthopedic testing, palpation, and range of motion testing. Neurological examination comprised testing of muscle strength, deep tendon reflexes, pathological reflexes, and sensation.
Figure 1.
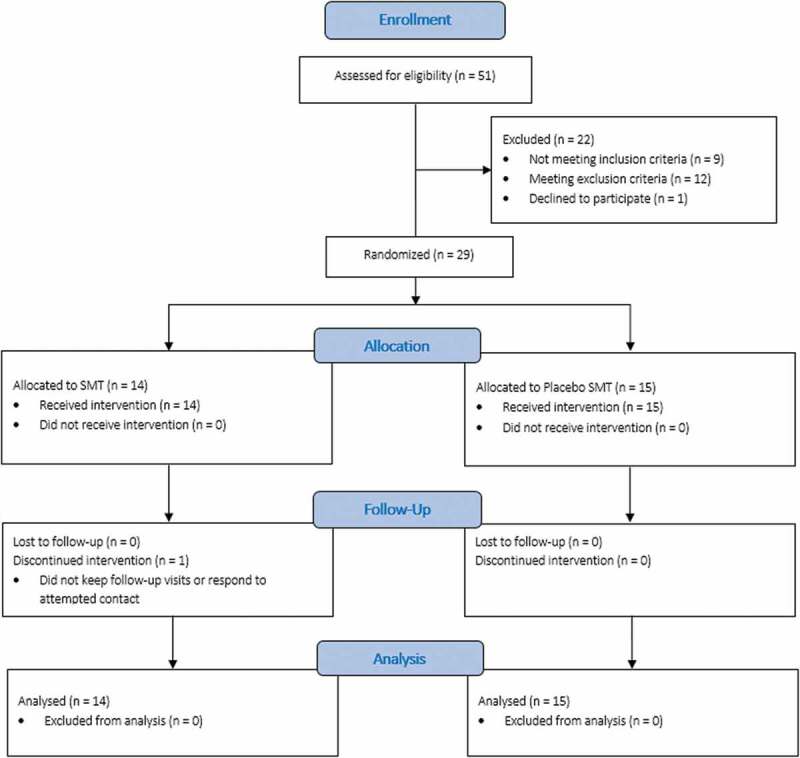
CONSORT diagram of enrollment, allocation, follow-up, and analysis for the study. SMT = spinal manipulative therapy. CONSORT = Consolidated standards of reporting trials.
Participants
We recruited persons with CNSLBP between January 2016 and April 2016 from campuses of two universities. Subjects were screened for fulfilling the inclusion and exclusion criteria. If a subject met these criteria, they were asked to sign an informed consent form approved by the human protection committees of the University of Saint Mary and University of Kansas Medical Center. In addition, we registered this study through ClinicalTrials.gov (registration number NCT03078114). Patients with low back pain were included in this study if they met the following criteria: (1) chronic nonspecific (> 12 weeks duration) low back pain rated ≥ 3/10 at its worst over the past 24 h on a numeric rating scale (NRS) (0 = no pain at all, 10 = worst pain imaginable); (2) male or female subjects between the ages of 18 and 60 years; (3) ability to read and understand English; and (4) currently not involved in litigation. Chronic low back pain patients were excluded if they reported any contraindications for SMT (Appendix 1).
Randomization and blinding
A computerized random number generator created a random allocation sequence list. Using this list, subjects were randomly allocated to either SMT or sham SMT group. This list was stored in a locked file cabinet with access limited to research personnel. After subject enrollment, a designated research assistant opened the correct numbered, sealed, opaque envelope. Each subject was assigned a unique identification number and the research assistant registered the subject’s name and identification number in a log. This was the only information connecting the patient’s identifying information with study records. Clinicians delivering the intervention were aware of group assignment, but the assessor was blinded to group allocation. A single assessor, with 22 years of experience as a licensed chiropractor, evaluated all outcome measures. Also, subjects were blinded to group allocation and advised to avoid discussing study details with the outcome assessor.
Procedures for clinical assessment
After signing an informed consent, investigators collected information regarding medications, past medical history, education, and demographic data from each subject. We gathered information related to attendance, medications, adverse events, and treatment sessions during the trial. The study coordinator monitored data quality on a weekly basis. In the event of improper data collection, there was immediate resolution of the recognized irregularity. A clinician performed a standard physical examination including vital signs and mobility testing. In addition, subjects underwent a neurological examination.
During the baseline evaluation, subjects completed clinical outcome measures capturing pain and self-reported disability. Information related to pain and disability was ascertained through the Numerical Pain Rating Scale (NPRS) and Oswestry Disability Index (ODI). Clinical changes over 3 weeks (assessed at prefirst intervention and 3 weeks on visit 7) on measures of pain (NPRS) and disability (ODI) served as clinical outcomes. We used the NPRS for screening (inclusion criteria) and as a clinical outcome measure. However, as described by Bialosky et al. (2014), we used the 11-point scale (0–10) during the screening procedure, whereas we used the 101-point (0–100) for measuring clinical outcomes [29]. While using the NPRS, subjects rated their pain intensity using an 101-point scale, with ‘0’ indicating no pain and ‘100’ indicating the worst pain imaginable [30]. The reliability and validity of NPRSs has been established in the scientific literature [31,32]. Scientific literature has established that a change of 1.25 points (on an 11-point NPRS scale) meets the minimal clinically important difference (MCID) for low back pain patients [33]. Alternatively, a 27.9% (raw change/baseline × 100) change in the NPRS realizes the MCID for CLBP patients [34]. The ODI is an efficient (~ 10 min) and generalizable outcome measure [35]. The reliability and validity of the ODI has been reported in the scientific literature [36–39]. The ODI has been found the most sensitive index to detect an improvement in disability associated with manual therapy, yielding large-sized improvements across many studies [30,36,37,40]. Also, MCID change score thresholds for the ODI range from 5 to 10 points [33,41,42].
Assessment of pain sensitivity
During the first visit, CNSLBP subjects underwent pre and immediately posttreatment pressure pain threshold (PPT) assessment. In addition, subjects underwent a third PPT assessment at the 1-week follow-up visit (visit 7) that occurred 3 weeks after the initial session. We determined PPT by applying pressure with a digital algometer (Wagner Instruments, Greenwich, Connecticut) to three anatomical regions allocated as local, regional, or remote. The digital algometer had a 1 cm2 rubber-tipped probe that was applied perpendicular to skin at a rate of 1 kg per second (kg/s) [24]. Marks were placed on the belly (middle third) of the dominant tibialis anterior muscle (regional) [22] and dominant lateral epicondyle of the elbow (remote) [43]. Also, we marked a point 5 cm lateral to the spinous process of L5 (local) on the dominant side [22]. These three anatomical landmarks for pressure application were chosen based on high reliability values reported from previous studies [22,43]. Scientific literature has reported using dominant regions [29] for PPT testing, while a systematic review by Millan et al. [11] reported that SMT consistently demonstrates a bilateral hypoalgesic effect. Thus, we selected the dominant-side for PPT testing.
Subjects were asked to say ‘stop’ the moment the sensation changed from feeling pressure to feeling pain. The pain threshold was defined as the least pressure intensity at which subject’s perceived pain. The pressure threshold in kilograms (kg) causing the perception of pain was recorded for data analysis. Three measurements were collected for each anatomical region with 30 s of rest in between pressure applications. The mean value of the three threshold measurements was used for data analysis [22,24]. Before testing, each subject received three practice measurements with pressure applied to the dorsal aspect of their dominant hand [24]. Previous scientific literature has demonstrated the interrater (ICC = 0.94–0.97), intrarater (ICC = 0.79–0.90), and test–retest (ICC = 0.76–0.79) reliability of PPT measurements [22,44,45]. Prior to data collection, an assessor blinded to group allocation undertook training with the digital algometer to ensure adherence to the specified rate of pressure application and cessation of pressure [24,45]. PPT has been used in previous clinical trials as an outcome measure for response to spinal manipulation [11,12,25,26,29,43,46,47]. Previous scientific literature has established that a 15% reduction in PPT may be considered a clinically relevant change [48,49].
Treatment protocols
After completion of the screening and baseline assessments, both the SMT and sham SMT groups commenced the assigned treatment protocols. The SMT and sham SMT procedures were administered and supervised by licensed clinicians. Subjects received three treatments per week for 2 consecutive weeks (six treatments) with one additional follow-up visit less than 1-week postintervention (visit 7). A written log of attendance, medications, health changes, and injuries/adverse events was maintained for each subject.
Manual interventions
SMT involved the patient lying supine with the spine in a position of lateral bending and rotation followed by a high-velocity low-amplitude force applied to the lumbopelvic region (Appendix 2). This SMT procedure has demonstrated clinical efficacy in previous clinical trials involving low back pain patients [50–53]. This treatment protocol adheres to current United States clinical practice guidelines for managing low back pain with SMT [54,55]. Thus, a 2-week (six treatments) intervention appears sufficient to determine the potential effects of SMT in chronic nonspecific low back pain patients. As reported in previous studies [29,56,57], each subject received two high-velocity low-amplitude thrusts to both sides of the pelvis, alternating between the left and right sides.
Previous clinical trials have used placebo SMT or sham SMT as a comparison group [29,58,59]. Sham SMT placed the patient in the supine position, but without accompanying lateral bending and rotation of the spine (neutral spine position) followed by a high-velocity low amplitude force applied to the table [29]. As reported in previous studies [29,56,57], each subject received two high-velocity low-amplitude thrusts to both sides of the pelvis, alternating between the left and right sides. Thus, each participant (SMT and sham SMT) received four high-velocity low-amplitude thrusts (two left-sided and two right-sided) regardless of whether or not cavitation (‘popping’) occurred during the procedure [29,57]. Both the lumbopelvic SMT and sham SMT procedures were administered by two licensed clinicians (physical therapist and/or chiropractor) with greater than 8 years of manual therapy experience.
Data analyses
We conducted our statistical analyses according to the intention-to-treat analysis principle [60–63]. If data points were missing, we imputed the subject’s missing data by baseline observation carried forward (BOCF) or last observation carried forward (LOCF) methods, dependent upon whether or not we captured postintervention data prior to the dropout [60–62]. We used individual t tests and chi-square tests to assess for postrandomization group differences in demographic measures, clinical measures, and pain sensitivity measures. We set our significance at .05 and performed analyses using the Statistical Package for the Social Sciences (SPSS), version 22.0 (SPSS Inc., Chicago, IL).
Our primary aim consisted of investigating the effect of SMT on PPT in CNSLBP patients. We checked for normality (Kolmogorov-Smirnov Test), homogeneity of variance (Levene’s Test), and sphericity (Mauchly’s Test), making the appropriate statistical corrections (i.e. Greenhouse-Geisser) as indicated by the data. Based on meeting the assumption of normality, we used a mixed analysis of variance to test for a group (SMT, sham SMT) × time (prefirst intervention, immediately postfirst intervention to 3 weeks) interaction for pressure pain threshold. Interaction terms may be considered comparable to the between-group differences or the effect of the intervention. If testing revealed a significant group × time interaction, we performed contrasts to determine within-group changes. We tested within-group pressure pain threshold differences using a paired-samples t test. We repeated these same measures for each pressure pain testing location (lumbar paraspinal musculature, elbow lateral epicondyle, and tibialis anterior muscle).
Our secondary aim consisted of investigating the effect of SMT on clinical outcomes in CNSLBP patients. We checked for normality (Kolmogorov-Smirnov Test), homogeneity of variance (Levene’s Test), and sphericity (Mauchly’s Test), making the appropriate statistical corrections (i.e. Greenhouse-Geisser) as indicated by the data. Based on meeting the assumption of normality, we used a mixed analysis of variance to test for a group (SMT, sham SMT) × time (prefirst intervention to 3 weeks) interaction for clinical outcomes (NPRS and ODI). If testing revealed a significant group × time interaction, we performed contrasts to determine within-group changes. We tested within-group (pre- and postintervention) clinical differences using a paired-samples t test.
Sample size estimation
Our primary aim was to examine the changes after SMT in pressure pain threshold examined at three different body sites. Bialosky et al. [57] reported an effect size (Cohen’s d) of 1.20 on thermal pain threshold measured on the upper limb after spinal manipulation in comparison to a control group. We assumed that the pressure pain threshold measured at the upper limb may show similar changes after our SMT intervention compared to the control group. Assuming 80% statistical power and .05 alpha level, a sample size of 12 was required for each group in our study. Presuming a drop-out rate of 20%, we needed to recruit a total of 30 subjects.
Results
Baseline demographics and characteristics
We screened 51 individuals for the study and 29 (n = 29) signed the informed consent form (Figure 1). Within our sample 38% of the subjects were females with a mean age of 23.86 (SD = 5.74) years (Table 1). Individual groups did not differ by baseline demographic measures, clinical measures, or pain sensitivity measures.
Table 1.
Baseline comparison of intervention groups.
| SMT | Sham | Total Sample | p Value for Difference | |
|---|---|---|---|---|
| Gender (% female) | 6/14 (43) | 5/15 (33) | 11/29 (38) | .60 |
| Age (years) | 24.29 (7.33) | 23.47 (3.94) | 23.86 (5.74) | .71 |
| Education (years) | 17.00 (1.92) | 17.20 (1.47) | 17.10 (1.68) | .76 |
| Duration of LBP (months) | 45.07 (29.77) | 43.00 (27.40) | 44.00 (28.07) | .85 |
| ODI | 15.93 (6.23) | 15.07 (7.68) | 15.48 (6.91) | .74 |
| NPRS | 41.64 (12.70) | 36.87 (17.25) | 39.17 (15.15) | .41 |
| PPT local | 3.39 (2.02) | 3.36 (1.36) | 3.37 (1.68) | .96 |
| PPT regional | 4.36 (1.78) | 4.88 (1.71) | 4.63 (1.74) | .44 |
| PPT remote | 2.95 (1.33) | 3.19 (1.55) | 3.08 (1.35) | .64 |
All data reported as mean (standard deviation) values. LBP = low back pain. ODI = Oswestry Disability Index (0–100% with smaller numbers indicating less disability). NPRS = numeric pain rating scale (0 = no pain to 100 = worst pain imaginable). PPT = pressure pain threshold expressed in kg/cm2. Local = lumbar paraspinal musculature. Regional = tibialis anterior muscle. Remote = elbow lateral epicondyle.
Pain sensitivity
We did not observe group (SMT, sham SMT) by time (prefirst intervention, immediately postfirst intervention to 3 weeks) differences in PPT assessed at the dominant-side lumbar paraspinal musculature (p = .913) (Table 2). However, we observed a significant main effect for time with PPT at the lumbar paraspinal musculature (p = .015) Post-hoc pairwise comparisons revealed significant (p = .044) within-group differences from prefirst intervention to 3 weeks (Figure 2). We did not observe group (SMT, sham SMT) by time (prefirst intervention, immediately postfirst intervention to 3 weeks) differences in PPT assessed at the dominant-side lateral epicondyle (p = .571) nor did we observe a main effect for time (p = .109). We did not observe group (SMT, sham SMT) by time (prefirst intervention, immediately postfirst intervention to 3 weeks) differences in PPT assessed at the dominant-side tibialis anterior muscle (p = .675). However, we observed a significant main effect for time with PPT at the tibialis anterior muscle (p = .014). Post-hoc pairwise comparisons revealed significant (p = .014) within-group differences from immediately postfirst intervention to 3 weeks.
Table 2.
Changes in PPT.
| Time | PPT Lumbar Paraspinal Musculature (Local) | PPT Tibialis Anterior Muscle (Regional) | PPT Lateral Epicondyle (Remote) | |
|---|---|---|---|---|
| SMT | Prefirst intervention | 3.39 (2.02) | 4.36 (1.78) | 2.95 (1.33) |
| Immediately postfirst intervention | 3.54 (1.95) | 4.28 (2.05) | 2.66 (1.02) | |
| 3 weeks postfirst intervention | 3.88 (1.74)* | 5.12 (2.11)δ | 3.13 (1.32) | |
| Sham | Prefirst intervention | 3.36 (1.36) | 4.88 (1.71) | 3.19 (1.39) |
| Immediately postfirst intervention | 3.42 (1.49) | 4.78 (1.99) | 3.02 (1.64) | |
| 3 weeks postfirst intervention | 3.78 (1.59)* | 5.34 (2.35)δ | 3.17 (1.23) | |
| Total sample | Prefirst intervention | 3.37 (1.68) | 4.63 (1.74) | 3.08 (1.35) |
| Immediately postfirst intervention | 3.48 (1.70) | 4.54 (2.00) | 2.85 (1.36) | |
| 3 weeks postfirst intervention | 3.83 (1.64) | 5.23 (2.20) | 3.15 (1.25) |
All data reported as mean (standard deviation) values. We observed a significant main effect of time for PPT at the lumbar paraspinal and tibialis anterior testing locations, but neither outcome was dependent upon group assignment. PPT = pressure pain threshold expressed in kg/cm2. *significant within-group differences (p < .05) between prefirst intervention and 3 weeks. δsignificant within-group differences (p < .05) between postfirst intervention and 3 weeks.
Figure 2.
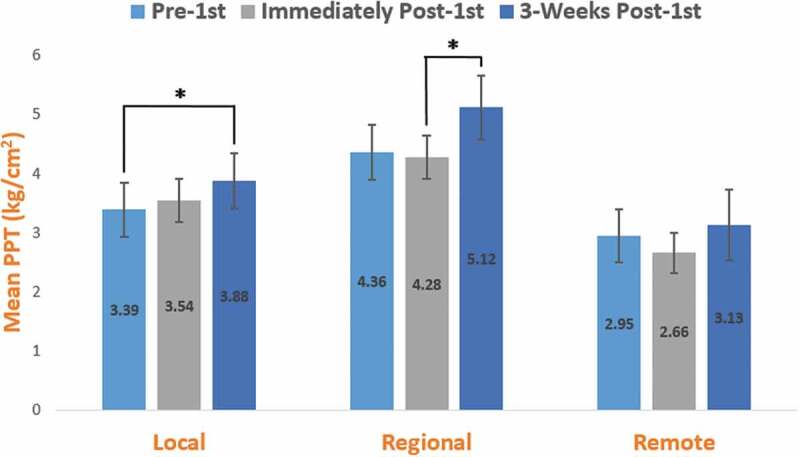
Change in PPT from prefirst intervention to immediately postfirst intervention and 3 weeks postfirst intervention for the SMT group at local, regional, and remote testing locations. We observed a significant main effect of time at the local and regional testing sites, but neither outcome was dependent upon group assignment. SMT = spinal manipulative therapy. PPT = pressure pain threshold expressed in kg/cm2. Local = lumbar paraspinal musculature. Regional = tibialis anterior muscle. Remote = elbow lateral epicondyle. Error bars = standard error. *significant within-group differences (p < .05).
Clinical outcomes
We did not observe a significant group (SMT, sham SMT) by time (baseline to 3 weeks) interaction for low back pain over the 3 weeks of the study (p = .458). However, we observed a significant main effect for time with low back pain (p < .001). Regardless of group assignment, we observed a mean decrease in low back pain of 11.31 (SE = 2.63) across subjects in the study. We did not observe a group (SMT, sham SMT) by time (baseline to 3 weeks) interaction for low back pain-related disability (p = .829). However, we observed a significant main effect for time with disability (p = .02). Regardless of group assignment, we observed a mean decrease in low back pain-related disability of 2.56 (SE = 1.04) across participants in the study.
Additional outcomes
We recorded additional clinical information including adverse events, change in medication, spinal joint cavitation, onset of new injuries/exacerbations, and believability of group assignment. A single adverse event of transient (< 48 h) local, mild joint discomfort was reported in the SMT group, while participants in the sham SMT group related no adverse events during the clinical trial. In addition, no changes in medication were conveyed for participants in either group throughout the study. We calculated the mean number of interventions for each group (SMT =5.7 and sham SMT =6.0), with an independent samples t test indicating no significant difference (p = .336) between the groups. As reported by clinician perception, spinal joint cavitation occurred at 61.25% (49/80 occasions) and 2.22% (2/90 occasions) frequencies in the SMT and sham groups, respectively. We arrived as these values based upon the product of the group size and the number of treatment sessions that each subject attended throughout trial. For example, the sham SMT group consisted of 15 subjects (n = 15) with each participant attending six treatment sessions during trial (i.e. 15 subjects × 6 visits each = 90 occasions). For the sham SMT group, 8/15 (53.3%) of subjects reported an exacerbation of low back pain related to activity at 3-week follow-up session, while only 3/13 (23.1%) of subjects in the SMT group reported an exacerbation during the trial. Based upon a two-sample test for proportions, there was no significant difference (p = .778) between the groups for subjects who felt they received an active form of treatment. Within the SMT group, 38.5% of participants believed that they received an active form of therapy, while 33.3% of subjects in the sham group thought that they received active treatment. Thus, our results indicate that we achieved adequate blinding for both groups and knowledge of treatment did not likely affect outcomes since both groups were similar in perception that they received an active form of intervention.
Discussion
Pain sensitivity
As outlined, our primary aim was to investigate the effect of SMT on PPT in chronic nonspecific low back pain patients, thereby exploring the neurophysiological mechanisms associated with SMT. We tested PPT at three anatomical locations including the lumbar paraspinal musculature [22] (local), tibialis anterior muscle [22] (regional), and lateral epicondyle of the elbow [43] (remote). The application of mechanical stimuli across multiple anatomical regions following SMT may help to differentiate the biological pathways, peripheral and/or central, associated with pain modulation following SMT. The current investigation embodied a novel design by examining the effects of SMT on PPT across multiple anatomical testing locations (local, regional, and remote) in chronic nonspecific low back pain patients.
Based upon our findings, both SMT and sham SMT reduced hypersensitivity (increased PPT) at a local and regional locations from preintervention to 3 weeks. Our results are similar to previous studies [23,25,43,64] that reported reduced hypersensitivity to mechanical stimuli following SMT. Yu et al. [23] reported that lumbopelvic SMT performed on asymptomatic volunteers produced an immediate, significant reduction in hypersensitivity at local, regional, and remote anatomical locations, thus signifying local and widespread hypoalgesia.
Depending on the measurement site, the examined effect of SMT on pressure pain threshold in CLBP patients may reflect local tissue, spinal cord and/or supraspinal biological pathways [12]. Previous studies testing the consequences of lumbopelvic manipulation on pain sensitivity have reported applying stimuli to local, regional, and/or remote anatomical locations [22,24–26,29,56,57]. Coronado et al. [12] published a systematic review and meta-analysis that concluded future research designs should include multi-regional application of stimulus following SMT to differentiate local, specific effects versus general hypoalgesia. Hypoalgesia at a local testing site following SMT might modulate pain via stimulation of peripheral muscle spindles and/or central segmental reflex pathways [11,65]. A regional testing site might be considered an anatomical region within the same or overlapping dermatomes as those influenced by SMT [56]. For example, testing for hypoalgesia following lumbopelvic manipulation only in anatomical locations innervated by lumbosacral nerve roots [22,29]. George et al. [56] reported that pain sensitivity testing only at remote anatomical locations cannot distinguish whether or not the hypoalgesia following SMT is a large, general effect or a specific effect localized to the spinal levels associated with the manipulation. Also, paraspinal muscle reflexes along with motoneuron excitability may be influenced by SMT, perhaps affecting reflex neural output to spinal musculature [11,16]. Thus, modulation of PPT at regional sites following SMT seems likely modulated through central neural mechanisms; however, peripheral mechanisms may also influence the regional pain effects of SMT [11,16]. A systematic review and meta-analysis concluded that increased PPT at remote anatomical sites suggests a general or widespread effect of SMT on central sensitization [12]. In addition, evidence from fMRI imaging suggests that reduced PPT (i.e. hyperalgesia) at a remote site indicates a central, rather than peripheral, cause for CLBP [66].
To the best of our knowledge, this paper represents the first investigation reporting the effects of SMT on PPT across local, regional, and remote locales in CNSLBP patients. In addition to quantifying the immediate (< 30 min) effects of SMT on PPT, our novel design measured the effects of repeated (6 interventions) SMT on PPT at 3 weeks. In our study, we did not find immediate or 3-week hypolagesia at a remote testing site, implying that SMT may not have a significant widespread hypoalgesic effect on CNSLBP patients [56]. In addition, our findings of 3-week hypoalgesia at local and regional sites advocates that SMT may diminish sensitivity within local muscles spindles and/or influence the dorsal horn by means of the removal of subthreshold mechanical stimuli via pain gate mechanisms [10,11,15,16,29]. Based upon previous scientific literature [11,15,29], our findings of local and regional hypoalgesia infer a primarily central-mediated analgesic effect of SMT at the spinal cord, but peripheral mechanisms cannot be excluded from modulating spinal pain. Similar to our results, a previous study reported a local hypoalgesic effect following lumbopelvic SMT in healthy subjects, but no significant widespread hypoalgesic effect on a remote testing site (cervical spine) [56].
Hypolagesia at 3 weeks post-SMT suggests a prolonged analgesic effect beyond the brief, immediate period postintervention reported by previous investigations [23,25,56]. Boal and Gillette [67] suggested that SMT may produce hypoalgesia through stimulation of mechanosensitive afferents that modulate pain via central-mediated pathways. Long-term depression (LTD), initiated by the activation of mechanosensitive afferents, may reverse long-term potentiation (LTP) in dorsal horn neurons through neuronal plasticity [67]. LTD may influence dorsal horn neurons for protracted time intervals, thereby mitigating spinal pain for minutes or hours, and perhaps even for days or weeks [67]. Accordingly, our findings of SMT-induced hypolagesia at 3 weeks implies that manual therapy may modulate pain for an extended period of time through central-mediated neuronal plasticity.
However, interpretation of our results requires a caution. It appears that our SMT and sham SMT had a similar effect on PPT at 3 weeks postintervention. The sham SMT has been previously reported effective in blinding participants [29]. Bialosky et al. [29] compared the effects of SMT in low back pain patients to placebo SMT, ‘enhanced’ placebo SMT, and control groups. Although not significant, Bialosky et al. [29] reported limited, immediate hypoalgesia to mechanical stimuli applied to the posterior superior iliac spine after low back pain subjects received SMT, sham SMT and enhanced sham SMT. The sham SMT used for our experimental design aimed to apply a thrust into the table with the spine positioned in neutral (without trunk lateral bending), unlike the SMT procedure that applied a thrust into rotation with accompanying trunk lateral bending. Bialosky et al. [29] conceded that the sham SMT applied a mechanical load to the spine. Scientific models associated with SMT postulate that a mechanical stimulus may elicit a cascade of potential neurophysiological effects, thereby accounting for the therapeutic benefits associated with manual therapy [13,16,68]. Our findings that both SMT and sham SMT produced hypoalgesia at sites local and distant to the region of pain indicated that the application of a mechanical load to the spine elicited a neurophysiological response, but suggests less importance on how the force is applied.
Our outcomes indicate a small, but potentially clinically relevant change in PPT following SMT in CNSLBP patients. At 3 weeks postintervention, the SMT group demonstrated a 14.5% (± 1.74 SE) increase (hypoalgesia) in PPT at the local site, and a 17.4% (± 2.35 SE) increase in PPT at the regional location (Figure 3). Consequently, the regional PPT testing location reached the 15% change in threshold established as clinically relevant for patient populations, while the local PPT testing location approached the defined threshold [49]. Dorron et al. hypothesized that the immediate analgesia following SMT may further influence clinical outcomes by providing an opportunity to facilitate rehabilitative exercise in patient populations with spinal pain [69]. However, at 3 weeks postintervention, both the SMT and sham SMT groups failed to achieve at least a 15% increase in PPT at the remote location, while the sham SMT group did not meet the established clinical threshold at the local (12.5%) and regional (9.4%) locations. Also, immediately postfirst intervention, both intervention groups did not realize the 15% clinically relevant threshold at any of the three PPT testing locations (local, regional, and remote).
Figure 3.
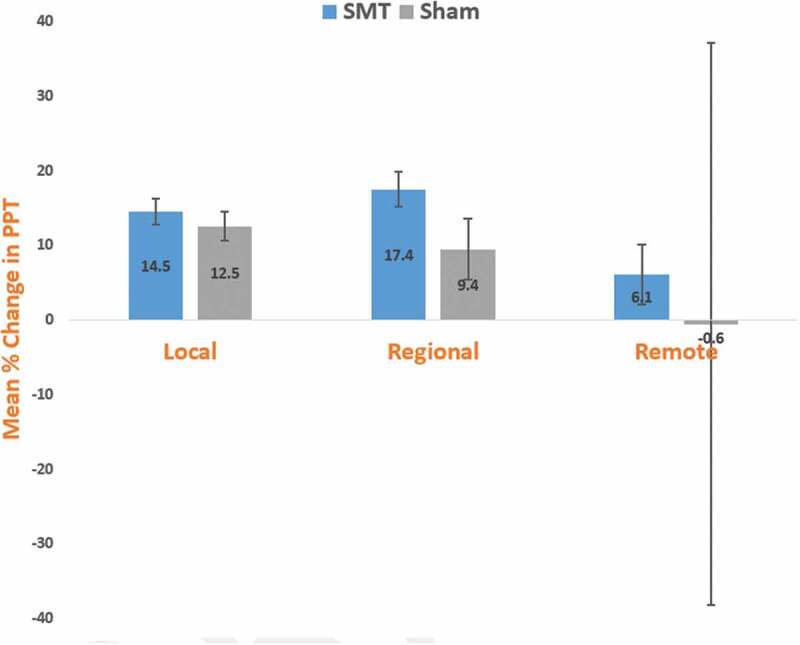
Mean percentage change in PPT from prefirst intervention to 3 weeks postfirst intervention for chronic non-specific low back pain subjects at local, regional, and remote testing locations. For within-group comparisons, negative values indicate reduced PPT (hyperalgesia), while positive values indicate increased PPT (hypoalgesia). A 15% change in PPT may be considered clinically relevant [49]. SMT = spinal manipulative therapy. PPT = pressure pain threshold expressed in kg/cm2. Local = lumbar paraspinal musculature. Regional = tibialis anterior muscle. Remote = elbow lateral epicondyle. Error bars = standard error.
Clinical outcomes
Similarly, our results did not show group-related differences, but a main effect for time in measures of clinical pain and disability over the three weeks of the study. Despite some past clinical trials [7,8] reporting that SMT appears efficacious for managing low back disorders, our results were similar to a previous clinical trial [29]. Bialosky et al. [29] did not observe group-related differences over a 2-week study examining the effects of SMT on clinical pain and disability. However, a significant main effect for time was observed for reduced pain and disability [29]. They cautioned that the design of their trial may have been underpowered to detect clinical treatment effects since their number of subjects were limited across four arms (total n = 110 or ~ 27 per group). Based upon our post-hoc analyses, we obtained an observed power value < 80% for clinical outcomes (NPRS and ODI) between-subjects effects, thus we acknowledge the possibility of a type II error. With alpha set at .05, we estimated post-hoc power using G*Power [70] along with effect sizes using Cohen d [71] (Table 3). Our results signified small, but potentially meaningful changes in patient-rated outcomes. A reduction of 13.29 points (or 1.329 points on an 11-point NPRS scale) in the NPRS score within the SMT group met the MCID of 1.25 [33] points for low back pain patients (Figure 4). However, a reduction of 9.33 points (or 0.933 points on an 11-point NPRS scale) in the NPRS score within the sham SMT group indicated a score below the stated MCID threshold. Alternatively, the SMT group demonstrated a 31.9% reduction in the NPRS score, thereby meeting the MCID of 27.9% reduction [34] (raw change/baseline × 100) for CLBP patients. Again, the sham SMT group failed to achieve the defined MCID with a reduction of 25.3% in the NPRS score. There were 6 (46.2%) subjects in the SMT group and 7 (46.7%) in the sham SMT group that had a pain reduction greater than the MCID.
Table 3.
Effect size (Cohen d) of within-group and between-group changes in pain, disability, and PPT.
| Numeric Pain Rating Scale | Oswestry Disability Index | PPT (Local) | PPT (Regional) | PPT (Remote) | |
|---|---|---|---|---|---|
| Time (within-group comparison) | 0.83 | 0.48 | 0.44 | 0.47 | 0.29 |
| SMT vs. Sham (between-group comparison) | 0.11 | 0.04 | 0.03 | 0.11 | 0.09 |
PPT = pressure pain threshold. Local = lumbar paraspinal musculature. Regional = tibialis anterior muscle. Remote = elbow lateral epicondyle.
Figure 4.
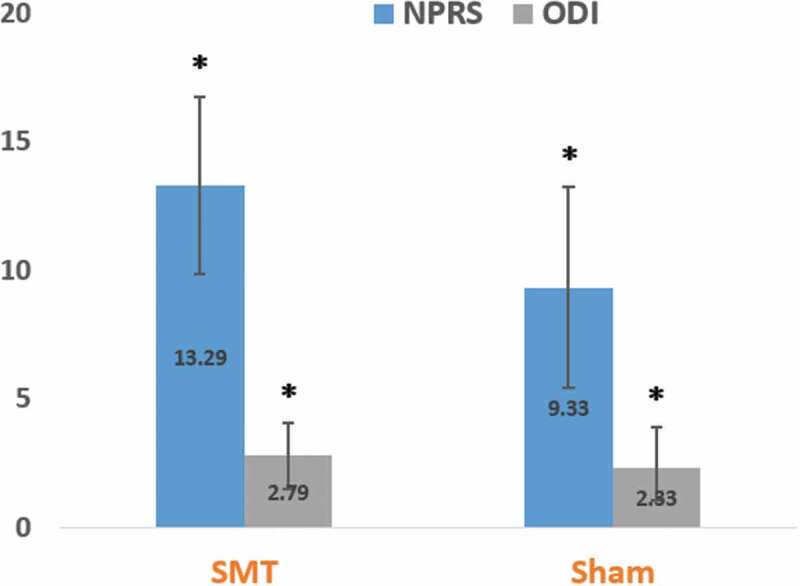
3-week mean change in low back-related pain intensity and disability. Bars signify change in scores (prefirst intervention to 3 weeks postintervention) with positive numbers on the y-axis signifying declining pain and disability following intervention. We observed a significant main effect of time for pain and disability, but neither clinical outcome was dependent upon group assignment. NPRS = numeric pain rating scale (0 = no pain to 100 = worst pain imaginable). ODI = Oswestry disability index (0–100% with smaller numbers representing less disability). SMT = spinal manipulative therapy. Error bars = standard error. *significant within-group differences (p < .05).
Limitations
Pressure may be considered a non-specific stimuli that elicits a response from mechanoreceptors and nociceptors in surrounding tissues [72]. Pressure pain threshold (PPT) may be used as an indirect measure of peripheral and central sensitization for musculoskeletal disorders [13]. In addition, a slow, gradual application of pressure until a pain threshold is reached might reflect a different neural pathway than rapidly applied stimuli [72]. This investigation only examined the effect of SMT in response to mechanical stimuli, but other painful stimuli including thermal and chemical may elicit distinct neural responses or produce unique results after the application of SMT.
In addition, the mean age of our sample at 23.86 (± 5.74) years may not be representative of the CNSLBP population. Previous scientific literature has reported the mean ages of CNSLBP patients seeking SMT ranging from 31.68 (± 11.85) [29] to ‘middle-aged’ [9]. Thus, our study sample may have been younger than reported in previous studies examining the effects of SMT, perhaps limiting the generalizability of our results. However, epidemiological data indicates that between 1992 and 2006, the prevalence of chronic low back pain increased by 201% in the 21 to 34 year age group, while the peak incidence rate for low back pain in the United States occurs between 25 and 29 years of age [73,74]. In addition, our baseline pain (NPRS) and low back-related disability (ODI) values across both study groups were similar to a previous study [29] investigating the effects of SMT on pain sensitivity.
This study may have been more clinically meaningful if we had monitored our subjects at some further time interval (6 months or 1 year). Our study only examined the immediate effects of SMT on CNSLBP patients, so it is feasible that neurophysiological and biomechanical adaptations manifest over a longer duration. Thus, long-term follow-up may have provided us with a more consequential measure of the effect of SMT on CNSLBP, thereby contributing to the development of more comprehensive evidence-based practice guidelines for managing low back disorders.
Conclusions
Following a 3-week course of SMT or sham SMT in CNSLBP patients, we found hypoalgesia at local and regional sites along with improved pain and low back-related disability. However, there was no difference between the two interventions in terms of PPT or clinical outcomes (NPRS and ODI) indicating that the method of SMT force application may not signify a fundamental influence on biological outcomes.
Biographies
Bryan M. Bond holds the position of professor in the physical therapy program at the University of Saint Mary. His research interest includes exploring the biology of manual therapies.
Chris D. Kinslow, PT, DC, OCS has been an assistant professor at the University of Saint Mary in the physical therapy program since 2014. His clinical expertise include manual therapies and dry needling with over 19 years of experience
Adam W. Yoder is an assistant professor in the physical therapy program at the University of Saint Mary. He is board certified in orthopedics and a fellow of the American Academy of Orthopedic Manual Physical Therapists.
Wen Liu, PhD, is an associate professor in the department of physical therapy at the University of Kansas Medical Center. Liu is responsible for teaching in the DPT and PhD programs. He is currently conducting research in the area of motor learning, postural control, and gait disorders in individuals with age-related diseases such as stroke, and Parkinson’s disease.
Appendix 1. Contraindications for Spinal Manipulative Therapy Used in our Study
Exclusion criteria include the occurrence of any of the following conditions or findings as determined by examination:
previous low back surgery;
severe structural spinal deformity;
neurological compromise/spinal cord compression;
severe spinal instability;
severe osteoporosis/osteopenia;
head trauma (recent);
spinal infection (recent);
known neurological, neuromuscular, systemic or orthopedic problems that might prevent them from participating in manual therapy interventions;
pregnancy;
obesity;
pain or paresthesia below the knees;
systemic illness known to affect sensation, i.e. diabetes;
acute and/or chronic pain condition unrelated to low back pain; and
spinal manipulation within the past 4 weeks.
Appendix 2.
Spinal manipulative therapy and sham spinal manipulative therapy [29].

Funding Statement
This work was supported by the National Chiropractic Mutual Insurance Company Foundation.
Acknowledgments
BB was supported by National Chiropractic Mutual Insurance Company Foundation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Luo X, Pietrobon R, Sun SX, et al. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine (Phila Pa 1976). 2004;29(1):79–86. Epub 2003/ 12/31. PubMed PMID: 14699281. [DOI] [PubMed] [Google Scholar]
- [2].Frymoyer JW, Cats-Baril WL.. An overview of the incidences and costs of low back pain. Orthop Clin North Am. 1991;22(2):263–271. Epub 1991/04/01.PubMed PMID: 1826550. [PubMed] [Google Scholar]
- [3].Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. Jama. 2008;299(6):656–664. Epub 2008/ 02/14. doi: 299/6/656 [pii]. PubMed PMID: 18270354 [DOI] [PubMed] [Google Scholar]
- [4].Dagenais S, Tricco AC, Haldeman S. Synthesis of recommendations for the assessment and management of low back pain from recent clinical practice guidelines. Spine J. 2010;10(6):514–529. Epub 2010/ 05/25. doi: S1529-9430(10)00288-3 [pii]. PubMed PMID: 20494814. [DOI] [PubMed] [Google Scholar]
- [5].Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American college of physicians and the American pain society. Ann Intern Med. 2007;147(7):478–491. Epub 2007/ 10/03. doi: 147/7/478 [pii]. PubMed PMID: 17909209. [DOI] [PubMed] [Google Scholar]
- [6].Negrini S, Giovannoni S, Minozzi S, et al. Diagnostic therapeutic flow-charts for low back pain patients: the Italian clinical guidelines. Eura Medicophys. 2006;42(2):151–170. Epub 2006/ 06/13.PubMed PMID: 16767064. [PubMed] [Google Scholar]
- [7].Ghroubi S, Elleuch H, Baklouti S, et al. Chronic low back pain and vertebral manipulation. Ann Readapt Med Phys. 2007;50(7):570–576. Epub 2007/03/27. PubMed PMID: 17382426. [DOI] [PubMed] [Google Scholar]
- [8].Senna MK, Machaly SA. Does maintained spinal manipulation therapy for chronic nonspecific low back pain result in better long-term outcome? Spine (Phila Pa 1976). 2011;36(18):1427–1437. Epub 2011/ 01/20. PubMed PMID: 21245790. [DOI] [PubMed] [Google Scholar]
- [9].Rubinstein SM, van Middelkoop M, Assendelft WJ, et al. Spinal manipulative therapy for chronic low-back pain: an update of a cochrane review. Spine (Phila Pa 1976). 2011;36(13):E825–46. Epub 2011/ 05/20. PubMed PMID: 21593658. [DOI] [PubMed] [Google Scholar]
- [10].Savva C, Giakas G, Efstathiou M. The role of the descending inhibitory pain mechanism in musculoskeletal pain following high-velocity, low amplitude thrust manipulation. A review of the literature. J Back Musculoskelet Rehabil. 2014;27(4):377–382. Epub 2014/05/29. PubMed PMID: 24867897. [DOI] [PubMed] [Google Scholar]
- [11].Millan M, Leboeuf-Yde C, Budgell B, et al. The effect of spinal manipulative therapy on experimentally induced pain: a systematic literature review. Chiropr Man Therap. 2012;20(1):26. Epub 2012/ 08/14. PubMed PMID: 22883534; PubMed Central PMCID: PMCPMC3527169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Coronado RA, Gay CW, Bialosky JE, et al. Changes in pain sensitivity following spinal manipulation: a systematic review and meta-analysis. J electromyogr kinesiol. 2012;22(5):752–767. Epub 2012/ 02/03. PubMed PMID: 22296867; PubMed Central PMCID: PMCPmc3349049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bialosky JE, Bishop MD, Price DD, et al. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Manual Ther. 2009;14(5):531–538. Epub 2008/ 11/26. doi: S1356-689X(08)00159-8 [pii]. PubMed PMID: 19027342; PubMed Central PMCID: PMC2775050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coronado RA, Simon CB, Valencia C, et al. Experimental pain responses support peripheral and central sensitization in patients with unilateral shoulder pain. Clin J Pain. 2014;30(2):143–151. Epub 2013/ 04/27. PubMed PMID: 23619203; PubMed Central PMCID: PMCPMC3732495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Graven-Nielsen T, Arendt-Nielsen L. Peripheral and central sensitization in musculoskeletal pain disorders: an experimental approach. Curr Rheumatol Rep. 2002;4(4):313–321. Epub 2002/ 07/20.PubMed PMID: 12126583. [DOI] [PubMed] [Google Scholar]
- [16].Pickar JG. Neurophysiological effects of spinal manipulation. Spine J. 2002;2(5):357–371. Epub 2003/ 11/01. S152994300200400X [pii]. PubMed PMID: 14589467. [DOI] [PubMed] [Google Scholar]
- [17].Wright A. Hypoalgesia post-manipulative therapy: a review of a potential neurophysiological mechanism. Manual Ther. 1995;1(1):11–16. Epub 1995/ 11/01. PubMed PMID: 11327789. [DOI] [PubMed] [Google Scholar]
- [18].Salom-Moreno J, Ortega-Santiago R, Cleland JA, et al. Immediate changes in neck pain intensity and widespread pressure pain sensitivity in patients with bilateral chronic mechanical neck pain: a randomized controlled trial of thoracic thrust manipulation vs non-thrust mobilization. J Manipulative Physiol Ther. 2014;37(5):312–319. Epub 2014/ 06/02. PubMed PMID: 24880778. [DOI] [PubMed] [Google Scholar]
- [19].Skyba DA, Radhakrishnan R, Rohlwing JJ, et al. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. Pain. 2003;106(1–2):159–168. Epub 2003/10/29.PubMed PMID: 14581123; PubMed Central PMCID: PMCPMC2732015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sluka KA, Skyba DA, Radhakrishnan R, et al. Joint mobilization reduces hyperalgesia associated with chronic muscle and joint inflammation in rats. J Pain. 2006;7(8):602–607. Epub 2006/08/04. PubMed PMID: 16885017. [DOI] [PubMed] [Google Scholar]
- [21].Sluka KA, Wright A. Knee joint mobilization reduces secondary mechanical hyperalgesia induced by capsaicin injection into the ankle joint. Eur J Pain. 2001;5(1):81–87. Epub 2001/ 06/08. PubMed PMID: 11394925. [DOI] [PubMed] [Google Scholar]
- [22].de Oliveira RF, Liebano RE, Costa Lda C, et al. Immediate effects of region-specific and non-region-specific spinal manipulative therapy in patients with chronic low back pain: a randomized controlled trial. Phys Ther. 2013;93(6):748–756. Epub 2013/ 02/23. PubMed PMID: 23431209. [DOI] [PubMed] [Google Scholar]
- [23].Yu X, Wang X, Zhang J, et al. Changes in pressure pain thresholds and basal electromyographic activity after instrument-assisted spinal manipulative therapy in asymptomatic participants: a randomized, controlled trial. J Manipulative Physiol Ther. 2012;35(6):437–445. Epub 2012/ 08/21. PubMed PMID: 22902139. [DOI] [PubMed] [Google Scholar]
- [24].Thomson O, Haig L, Mansfield H. The effects of high-velocity low-amplitude thrust manipulation and mobilisation techniques on pressure pain threshold in the lumbar spine. Int J Osteopath Med. 2009;12(2):56–62. [Google Scholar]
- [25].Shearar KA, Colloca CJ, White HL. A randomized clinical trial of manual versus mechanical force manipulation in the treatment of sacroiliac joint syndrome. J Manipulative Physiol Ther. 2005;28(7):493–501. Epub 2005/09/27. PubMed PMID: 16182023. [DOI] [PubMed] [Google Scholar]
- [26].Cote P, Mior SA, Vernon H. The short-term effect of a spinal manipulation on pain/pressure threshold in patients with chronic mechanical low back pain. J Manipulative Physiol Ther. 1994;17(6):364–368. Epub 1994/ 07/01.PubMed PMID: 7964196. [PubMed] [Google Scholar]
- [27].Bronfort G, Haas M, Evans R, et al. Evidence-informed management of chronic low back pain with spinal manipulation and mobilization. Spine J. 2008;8(1):213–225. [DOI] [PubMed] [Google Scholar]
- [28].Cramer G, Budgell B, Henderson C, et al. Basic science research related to chiropractic spinal adjusting: the state of the art and recommendations revisited. J Manipulative Physiol Ther. 2006;29(9):726–761. Epub 2006/ 12/05. doi: S0161-4754(06)00277-6 [pii]. PubMed PMID: 17142166. [DOI] [PubMed] [Google Scholar]
- [29].Bialosky JE, George SZ, Horn ME, et al. Spinal manipulative therapy-specific changes in pain sensitivity in individuals with low back pain (NCT01168999). J Pain. 2014;15(2):136–148. Epub 2013/ 12/24. PubMed PMID: 24361109; PubMed Central PMCID: PMCPMC3946602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Maughan EF, Lewis JS. Outcome measures in chronic low back pain. Eur Spine J. 2010. DOI: 10.1007/s00586-010-1353-6. Epub 2010/ 04/17. PubMed PMID: 20397032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gagliese L, Weizblit N, Ellis W, et al. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117(3):412–420. Epub 2005/ 09/13. PubMed PMID: 16153776. [DOI] [PubMed] [Google Scholar]
- [32].Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. Epub 1986/ 10/01.PubMed PMID: 3785962. [DOI] [PubMed] [Google Scholar]
- [33].Cleland JA, Whitman JM, Houser JL, et al. Psychometric properties of selected tests in patients with lumbar spinal stenosis. Spine J. 2012;12(10):921–931. Epub 2012/ 07/04. PubMed PMID: 22749295. [DOI] [PubMed] [Google Scholar]
- [34].Farrar JT, Young JP Jr., LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. Epub 2001/11/03.PubMed PMID: 11690728. [DOI] [PubMed] [Google Scholar]
- [35].Fritz JM, Hebert J, Koppenhaver S, et al. Beyond minimally important change: defining a successful outcome of physical therapy for patients with low back pain. Spine (Phila Pa 1976). 2009;34(25):2803–2809. Epub 2009/11/17. PubMed PMID: 19910868. [DOI] [PubMed] [Google Scholar]
- [36].Changulani M, Shaju A. Evaluation of responsiveness of oswestry low back pain disability index. Arch Orthop Trauma Surg. 2009;129(5):691–694. Epub 2008/ 06/04. PubMed PMID: 18521617. [DOI] [PubMed] [Google Scholar]
- [37].Frost H, Lamb SE, Stewart-Brown S. Responsiveness of a patient specific outcome measure compared with the oswestry disability index v2.1 and Roland and Morris disability questionnaire for patients with subacute and chronic low back pain. Spine (Phila Pa 1976). 2008;33(22):2450–2457. discussion 8. Epub 2008/10/01. PubMed PMID: 18824951. [DOI] [PubMed] [Google Scholar]
- [38].Fairbank JC, Pynsent PB. The oswestry disability index. Spine (Phila Pa 1976). 2000;25(22):2940–2952. discussion 52. Epub 2000/11/14. PubMed PMID: 11074683. [DOI] [PubMed] [Google Scholar]
- [39].Fritz JM, Irrgang JJ. A comparison of a modified oswestry low back pain disability questionnaire and the quebec back pain disability scale. Phys Ther. 2001;81(2):776–788. Epub 2001/02/15.PubMed PMID: 11175676. [DOI] [PubMed] [Google Scholar]
- [40].Filiz M, Cakmak A, Ozcan E. The effectiveness of exercise programmes after lumbar disc surgery: a randomized controlled study. Clin Rehabil. 2005;19(1):4–11. Epub 2005/02/12.PubMed PMID: 15704503. [DOI] [PubMed] [Google Scholar]
- [41].Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33(1):90–94. Epub 2008/ 01/01. 00007632-200801010-00015 [pii]. PubMed PMID: 18165753. [DOI] [PubMed] [Google Scholar]
- [42].Schwind J, Learman K, O’Halloran B, et al. Different minimally important clinical difference (MCID) scores lead to different clinical prediction rules for the Oswestry disability index for the same sample of patients. J Man Manip Ther. 2013;21(2):71–78. Epub 2014/ 01/15. PubMed PMID: 24421616; PubMed Central PMCID: PMCPMC3649353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fernandez-de-Las-Penas C, Perez-de-Heredia M, Brea-Rivero M, et al. Immediate effects on pressure pain threshold following a single cervical spine manipulation in healthy subjects. J Orthop Sports Phys Ther. 2007;37(6):325–329. Epub 2007/07/07.PubMed PMID: 17612359. [DOI] [PubMed] [Google Scholar]
- [44].Walton DM, Macdermid JC, Nielson W, et al. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J Orthop Sports Phys Ther. 2011;41(9):644–650. Epub 2011/09/03. PubMed PMID: 21885906. [DOI] [PubMed] [Google Scholar]
- [45].Walton DM, Levesque L, Payne M, et al. Clinical pressure pain threshold testing in neck pain: comparing protocols, responsiveness, and association with psychological variables. Phys Ther. 2014;94(6):827–837. Epub 2014/ 02/22. PubMed PMID: 24557645; PubMed Central PMCID: PMCPMC4040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fernandez-Carnero J, Cleland JA, Arbizu RL. Examination of motor and hypoalgesic effects of cervical vs thoracic spine manipulation in patients with lateral epicondylalgia: a clinical trial. J Manipulative Physiol Ther. 2011;34(7):432–440. Epub 2011/ 08/31. PubMed PMID: 21875517. [DOI] [PubMed] [Google Scholar]
- [47].Fernandez-de-Las-Penas C, Alonso-Blanco C, Cleland JA, et al. Changes in pressure pain thresholds over C5-C6 zygapophyseal joint after a cervicothoracic junction manipulation in healthy subjects. J Manipulative Physiol Ther. 2008;31(5):332–337. Epub 2008/06/19. PubMed PMID: 18558274. [DOI] [PubMed] [Google Scholar]
- [48].Chesterton LS, Barlas P, Foster NE, et al. Gender differences in pressure pain threshold in healthy humans. Pain. 2003;101(3):259–266. Epub 2003/02/14.PubMed PMID: 12583868. [DOI] [PubMed] [Google Scholar]
- [49].Moss P, Sluka K, Wright A. The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Manual Ther. 2007;12(2):109–118. Epub 2006/ 06/17. PubMed PMID: 16777467. [DOI] [PubMed] [Google Scholar]
- [50].Flynn T, Fritz J, Whitman J, et al. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine (Phila Pa 1976). 2002;27(24):2835–2843. Epub 2002/12/18. PubMed PMID: 12486357. [DOI] [PubMed] [Google Scholar]
- [51].Flynn TW, Childs JD, Fritz JM. The audible pop from high-velocity thrust manipulation and outcome in individuals with low back pain. J Manipulative Physiol Ther. 2006;29(1):40–45. Epub 2006/ 01/07. doi: S0161-4754(05)00347-7 [pii]. PubMed PMID: 16396728. [DOI] [PubMed] [Google Scholar]
- [52].Flynn TW, Fritz JM, Wainner RS, et al. The audible pop is not necessary for successful spinal high-velocity thrust manipulation in individuals with low back pain. Arch Phys Med Rehabil. 2003;84(7):1057–1060. Epub 2003/07/26.doi: S0003999303000480 [pii]. PubMed PMID: 12881834. [DOI] [PubMed] [Google Scholar]
- [53].Childs JD, Fritz JM, Flynn TW, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med. 2004;141(12):920–928. Epub 2004/ 12/22. doi: 141/12/920 [pii]. PubMed PMID: 15611489. [DOI] [PubMed] [Google Scholar]
- [54].Globe GA, Morris CE, Whalen WM, et al. Chiropractic management of low back disorders: report from a consensus process. J Manipulative Physiol Ther. 2008;31(9):651–658. Epub 2008/ 11/26. PubMed PMID: 19028249. [DOI] [PubMed] [Google Scholar]
- [55].Globe G, Farabaugh RJ, Hawk C, et al. Clinical practice guideline: chiropractic care for low back pain. J Manipulative Physiol Ther. 2016;39(1):1–22. Epub 2016/ 01/26. PubMed PMID: 26804581. [DOI] [PubMed] [Google Scholar]
- [56].George SZ, Bishop MD, Bialosky JE, et al. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord. 2006;7(68). Epub 2006/ 08/17. doi: 1471-2474-7-68 [pii]. DOI: 10.1186/1471-2474-7-68 PubMed PMID: 16911795; PubMed Central PMCID: PMC1578563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bialosky JE, Bishop MD, Robinson ME, et al. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys Ther. 2009;89(12):1292–1303. Epub 2009/ 10/03. doi: ptj.20090058 [pii]. PubMed PMID: 19797305; PubMed Central PMCID: PMC2794479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Han L, Zhao P, Guo W, et al. Short-term study on risk-benefit outcomes of two spinal manipulative therapies in the treatment of acute radiculopathy caused by lumbar disc herniation: study protocol for a randomized controlled trial. Trials. 2015;16:122. Epub 2015/ 04/16. PubMed PMID: 25872929; PubMed Central PMCID: PMCPMC4380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Molins-Cubero S, Rodriguez-Blanco C, Oliva-Pascual-Vaca A, et al. Changes in pain perception after pelvis manipulation in women with primary dysmenorrhea: a randomized controlled trial. Pain Med. 2014;15(9):1455–1463. Epub 2014/ 03/29. PubMed PMID: 24666560. [DOI] [PubMed] [Google Scholar]
- [60].Joseph R, Sim J, Ogollah R, et al. A systematic review finds variable use of the intention-to-treat principle in musculoskeletal randomized controlled trials with missing data. J Clin Epidemiol. 2015;68(1):15–24. [DOI] [PubMed] [Google Scholar]
- [61].Armijo-Olivo S, Warren S, Magee D. Intention to treat analysis, compliance, drop-outs and how to deal with missing data in clinical research: a review. Phys Ther Rev. 2009;14(1):36–49. [Google Scholar]
- [62].Del Re AC, Maisel NC, Blodgett JC, et al. Intention-to-treat analyses and missing data approaches in pharmacotherapy trials for alcohol use disorders. BMJ open. 2013;3(11):e003464. Epub 2013/11/15. PubMed PMID: 24227870; PubMed Central PMCID: PMCPMC3831108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109–112. Epub 2011/ 09/08. PubMed PMID: 21897887; PubMed Central PMCID: PMCPMC3159210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Fernandez-Carnero J, Fernandez-de-Las-Penas C, Cleland JA. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epicondylalgia. J Manipulative Physiol Ther. 2008;31(9):675–681. Epub 2008/ 11/26. doi: S0161-4754(08)00275-3 [pii]. PubMed PMID: 19028251. [DOI] [PubMed] [Google Scholar]
- [65].Clark BC, Goss DA Jr., Walkowski S, et al. Neurophysiologic effects of spinal manipulation in patients with chronic low back pain. BMC Musculoskelet Disord. 2011;12:170. Epub 2011/07/26. PubMed PMID: 21781310; PubMed Central PMCID: PMCPMC3149032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheumatism. 2004;50(2):613–623. Epub 2004/02/12. PubMed PMID: 14872506. [DOI] [PubMed] [Google Scholar]
- [67].Boal RW, Gillette RG. Central neuronal plasticity, low back pain and spinal manipulative therapy. J Manipulative Physiol Ther. 2004;27(5):314–326. Epub 2004/06/15. PubMed PMID: 15195039. [DOI] [PubMed] [Google Scholar]
- [68].Cramer GD, Ross K, Pocius J, et al. Evaluating the relationship among cavitation, zygapophyseal joint gapping, and spinal manipulation: an exploratory case series. J Manipulative Physiol Ther. 2011;34(1):2–14. Epub 2011/01/18. PubMed PMID: 21237402; PubMed Central PMCID: PMCPMC3582390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dorron SL, Losco BE, Drummond PD, et al. Effect of lumbar spinal manipulation on local and remote pressure pain threshold and pinprick sensitivity in asymptomatic individuals: a randomised trial. Chiropr Man Therap. 2016;24:47. Epub 2016/ 12/17. PubMed PMID: 27980726; PubMed Central PMCID: PMCPMC5137207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. Epub 2007/08/19.PubMed PMID: 17695343. [DOI] [PubMed] [Google Scholar]
- [71].Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. Epub 1992/07/01.PubMed PMID: 19565683. [DOI] [PubMed] [Google Scholar]
- [72].Kardouni JR, Shaffer SW, Pidcoe PE, et al. Immediate changes in pressure pain sensitivity after thoracic spinal manipulative therapy in patients with subacromial impingement syndrome: A randomized controlled study. Manual Ther. 2015;20(4):540–546. Epub 2015/ 01/18. PubMed PMID: 25595413. [DOI] [PubMed] [Google Scholar]
- [73].Waterman BR, Belmont PJ Jr., Schoenfeld AJ. Low back pain in the United States: incidence and risk factors for presentation in the emergency setting. Spine J. 2012;12(1):63–70. Epub 2011/ 10/08. PubMed PMID: 21978519. [DOI] [PubMed] [Google Scholar]
- [74].Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Internal Med. 2009;169(3):251–258. Epub 2009/02/11. PubMed PMID: 19204216; PubMed Central PMCID: PMCPMC4339077. [DOI] [PMC free article] [PubMed] [Google Scholar]


