ABSTRACT
Zika cases have been reported in 29 out of the 32 states of Mexico. Information regarding which mosquito species might be driving Zika virus transmission/maintenance in nature must be regularly updated. From January 2017 to November 2018, mosquitoes were collected indoors and outdoors using the CDC backpack aspirator in urban and semi-urban areas with evidence of mosquito-borne disease transmission. 3873 mosquito pools were tested for Zika infection using the CDC Trioplex real-time RT-PCR. For each collected specie, maximum likelihood estimator of infection rate (MLE) was estimated. Results showed 492 mosquito pools positive for Zika virus RNA. The majority of the positive pools were Aedes (Stegomyia) aegypti (Linnaeus) (54.6%, MLE = 19) (males and females) and Culex (Culex) quinquefasciatus (Say) (19.5%, MLE = 16.8). For the first time, ZIKV infection was detected in Ae. (Georgecraigius) epactius (Dyar and Knab) (MLE = 17.1), Cx. (Melanoconion) erraticus (Dyar and Knab) (MLE = non-estimable), Culiseta (Culiseta) inornata (Williston) (MLE = non estimable), and Cs (Cs.) particeps (Adams) (MLE = 369.5). Other detected species were: Ae. (Stg.) albopictus (Skuse) (MLE = 90.5), Cx. (Cx.) coronator s.l. (Dyar and Knab) (MLE = 102.8) and Cx. (Cx.) tarsalis (Coquillett) (MLE = 117.2). However, our results do not allow for the incrimination of these species as vectors of ZIKV. Routine surveillance should start to consider other mosquito species across the taxonomic spectrum of the Culicidae.
KEYWORDS: Arbovirus, Culicidae, mosquito-borne diseases, vector control
Introduction
Since 2013, Zika virus (ZIKV) outbreaks have caused great concern due to its association with congenital Zika syndrome [1] and Guillain-Barré Syndrome [2]. After its emergence in Brazil in 2015, ZIKV has spread across the Americas. In Mexico, ZIKV was first documented in 2015 in Chiapas (southern Mexico) [3,4]. Since then, cases have been recorded in 29 out of 32 states in Mexico. By September 2018, more than 12,000 ZIKV infections have been confirmed [5].
ZIKV transmission principally occurs via the bite of infective mosquitoes to a human host. However, venereal transmission has been documented in Aedes aegypti mosquitoes and in humans [6–8]. Worldwide, Ae. aegypti (L.) is the main ZIKV vector in the urban cycle. In Mexico, Aedes spp. and Culex spp. mosquitoes have been found naturally infected with ZIKV [3,9–11]. However, it remains unknown the extent to which species other than Ae. aegypti contribute to Zika transmission in the country.
For appropriate vector management, it is necessary to evaluate the possible roles of various mosquito species in arbovirus circulation. In the present study, we provide an update on mosquito species naturally infected with ZIKV based on 2 years of mosquito surveillance in México.
Methods
Mosquitoes were collected in urban (with high population density, public transportation and minimal green areas) and semi-urban areas where people partially maintain agricultural activities (both areas as described by [12]), with significant numbers of cases of dengue, chikungunya and/or Zika. Collections were performed in 16 states (Baja California Norte, Campeche, Chiapas, Chihuahua, Durango, Estado de México, Guerrero, Hidalgo, Jalisco, México City, Oaxaca, Puebla, Tabasco, Tamaulipas, Veracruz and Yucatán). Adult mosquitoes were collected indoors and outdoors of randomly selected houses using a CDC Backpack Aspirator (Model 1412, John W. Hock Company, Gainesville, USA). Collections were performed by the staff of the Health Ministry from each state during January 2017 to November 2018, according to the Mexican Vector Control Program protocols [13]. Mosquitoes were transported (alive at 4–8°C) to each State Laboratory. Specimens were then separated by species and sex and pooled (25 mosquitoes maximum, one minimum; Table 1) in 1.5 ml tubes containing BD Universal Viral Transport medium (designed to transport viruses at room temperature; Becton, Dickinson Company East Rutherford, NJ, USA). Tubes were sent to the National Reference Laboratory (Instituto de Diagnóstico y Referencia Epidemiológicos, InDRE) for arbovirus detection. Taxonomic identification was carried out using morphological identification keys of Carpenter & LaCasse [14], and Darsie & Ward [15]. Because of the lack of important taxonomic features for some Culex mosquitoes, identification could not be made beyond the genus level.
Table 1.
Mosquitoes naturally infected with ZIKV reported in this study. Culex sp was no considered as a real record, it is only shown for informative reasons. NR = new record.
| Specie | State | Year |
|---|---|---|
| Aedes (Georgecraigius) epactius (NR) | Puebla | 2018 |
| Aedes (Stegomyia) aegyptia | Baja California Norte | 2017 |
| Campeche | 2018 | |
| Chiapas | 2017 | |
| Females | Chihuahua | 2017, 2018 |
| Males | Chihuahua | 2017 |
| Estado de Mexico | 2018 | |
| Guerrero | 2017 | |
| Females | Jalisco | 2017 |
| Oaxaca | 2017, 2018 | |
| Tabasco | 2017 | |
| Females | Tamaulipas | 2017, 2018 |
| Males | Tamaulipas | 2017 |
| Veracruz | 2017 | |
| Yucatán | 2017 | |
| Aedes (Stegomyia) albopictusb | Chiapas | 2017 |
| Culex (Culex) coronator s.l.c | Guerrero | 2017 |
| Culex (Culex) quinquefasciatusd | Baja California Norte | 2017 |
| Chiapas | 2017 | |
| Chihuahua | 2017, 2018 | |
| Guerrero | 2017 | |
| Mexico City | 2017, 2018 | |
| Oaxaca | 2018 | |
| Tamaulipas | 2018 | |
| Veracruz | 2017 | |
| Yucatan | 2017 | |
| Culex (Culex) tarsalise | Chihuahua | 2018 |
| Mexico City | 2018 | |
| Culex (Melanoconion) erraticus (NR) | Chihuahua | 2017 |
| Culex sp. | Chiapas | 2017 |
| Guerrero | 2017 | |
| Jalisco | 2017 | |
| Tabasco | 2017 | |
| Veracruz | 2017 | |
| Culiseta (Culiseta) inornata (NR) | Mexico City | 2018 |
| Culiseta (Culiseta) particeps (NR) | Mexico City | 2018 |
aAedes (St.) aegypti was previously recorded infected in Chiapas by Guerbois et al. [3], in Guerrero by Diaz-Quiñonez et al. [9], in Jalisco by Elizondo-Quiroga et al. [10], in San Luis Potosi by Huerta et al. [11].
bAedes (St.) albopictus was previously recorded infected in San Luis Potosi by Huerta et al. [11].
cCulex (Cx.) coronator s.l. was previously recorded infected in Jalisco by Elizondo-Quiroga et al. [10].
dCulex (Cx.) quinquefasciatus was previously recorded infected in Jalisco by Elizondo-Quiroga et al. [10].
eCulex (Cx.) tarsalis was previously recorded infected in Jalisco by Elizondo-Quiroga et al. [10].
Aedes (Aedimorphus) vexans is other infected species previously recorded by Elizondo-Quiroga et al. [10] in 2016, however, in our study, we were unable to collect this species.
Mosquito pools were homogenized manually with a pestle and centrifuged at 595 g for 10 min at 4°C. Viral RNA was extracted using the QIAamp viral RNA Mini Kit (Qiagen, Inc. Hilden, Germany). The RNA samples were tested for the presence of viruses using the CDC Trioplex real-time RT-PCR [16,17]. The kit includes a set of oligonucleotide primers and dual-labeled hydrolysis Taqman® probes for in vitro qualitative detection of DENV, ZIKV and CHIKV. Target-specific control RNA (DENV-1-4 mix, CHIKV, and ZIKV) provided by the kit serves as PCR positive controls for each virus. A second positive control (synthetic RNA from reference virus strains available at InDRE) was also included. Negative controls did not include any template RNA (i.e. Not Template Control, provided by the kit). An internal control was also provided by the kit. This assay was used for the detection and differentiation of RNA from ZIKV, DENV, and CHIKV simultaneously. The reaction was performed following the standard protocol provided with the kit. Reactions were performed in a BioRad CFX96 Real-Time PCR detection system (Bio-Rad Laboratories, Inc., USA) with the following cycling conditions: 50°C for 30 min, 95°C for 2 min, followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min. Samples with CT < 39 were considered positive. All positive samples were confirmed with the Viasure Multipex Zika, Dengue and Chikungunya kit (CERTEST Biotec, Spain). For the purposes of this paper, we only report results related to ZIKV infection.
Species-specific maximum likelihood estimates (MLE) for infection rates were calculated using the the PoolInfRate v. 4.0 add-in for Microsoft Excel (https://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html; [18]).
Results and discussion
A total of 4661 mosquito pools were analyzed of which 492 (10.6%) were positive for ZIKV. Most of the positive pools were Ae. aegypti (n = 260) and Cx. quinquefasciatus (n = 96). Pools of other species were positive <5% of the time. Sixty-two pools of males were collected, 55 of which belonged to Ae. aegypti, 6 belonged to Cx. quinquefasciatus and one pool belonged to Cx. tarsalis. However, only nine positive pools of males (Ae. aegypti) were detected. Given these results, there are now 10 mosquito species that have been reported as naturally infected with ZIKV in Mexico (Table 1). ZIKV-infected mosquitoes were detected in 15 states (Figure 1), with Durango as the only state without any positive samples. No double or triple infections (with additional viruses) were detected in the ZIKV positive samples. Not all species were collected in both years. Moreover, MLE infection rates of species recorded in both years were different (Suppl. Table 1). This fact could indicate that the infection rate is variable and, for enhanced vector control strategies at different localities, it needs to be calculated frequently.
Figure 1.
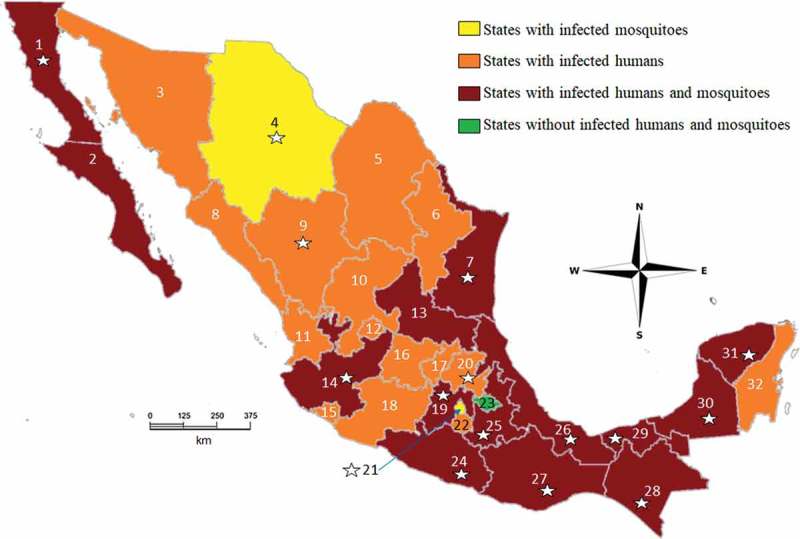
Distribution map summarizing where human and mosquito ZIKV infections have been detected in Mexico. Stars indicate the 16 surveyed states in this study. Infections in both humans and mosquitoes have been detected in 14 states (Baja California Norte (1), Baja California Sur (2), Tamaulipas (7), San Luis Potosí (13), Jalisco (14), Estado de México (19), Guerrero (24), Puebla (25), Veracruz (26), Oaxaca (27), Chiapas (28), Tabasco (29), Campeche (30) and Yucatán (31)), shown in dark red. Human cases alone have been detected in 13 states (Sonora (3), Coahuila (5), Nuevo León (6), Durango (9), Zacatecas (10), Nayarit (11), Aguascalientes (12), Michoacán (18), Guanajuato (16), Querétaro (17), Hidalgo (20), Morelos (22) and Quintana Roo (32)), shown in orange. Two states showed infected mosquitoes but no infected humans (Chihuahua (4) and Mexico City (21)), shown in yellow. Tlaxcala (23, in green) was the only state without infected humans or mosquitoes.
Four species were reported as infected for the first time: Ae. epactius (Puebla), Cx. erraticus (Chihuahua), Cs. inornata (Mexico City) and Cs. particeps (Mexico City) (Table 1). To our knowledge, this is the first time that these wild-collected species have been found anywhere in the world infected with ZIKV. Despite the low number of tested pools for Cs. particeps, this species showed the highest estimated infection rate (369.49) (Table 2, Supp. Table 1). Culiseta particeps is a common species in Western USA, Northern and central Mexico [15,19,20]. It can feed on both humans and other organisms [21]. This species has tested positive for West Nile Virus (WNV) RNA in the United States [22]. Culiseta inornata is a common species in the Nearctic region, including Canada, USA, and Northern and central Mexico [14,15]. Female Cs. inornata seem to prefer to feed on large mammals [14]. Individuals of this species have been found to be infected with Western Equine Encephalitis (WEE), La Crosse virus, Melao virus and WNV [22–24]. In urban and semi-urban areas, larvae of Cs. inornata and Cs. particeps can be found in cemeteries, rain collectors, natural lakes, temporary and permanent ponds, wetlands, streams and water traffic corridors [25].
Table 2.
Maximum likelihood estimator of infection rates (MLE) of ZIKV-infected mosquitoes in Mexico. Low Lim = lower limit, Upp Lim = upper limit. NE = non-estimable by the MLE method. Culexspp. were excluded from the analysis.
| Specie | MLE | MLE Low Lim |
MLE Upp Lim |
Pools | Positive pools | % Positive pools | Individuals |
|---|---|---|---|---|---|---|---|
| Aedes aegypti | 19.0 | 16.8 | 21.3 | 3120 | 260 | 69.5 | 14,145 |
| Aedes albopictus | 90.5 | 42.2 | 166.7 | 52 | 7 | 1.87 | 78 |
| Aedes epactius | 17.1 | 0.9 | 89.3 | 13 | 1 | 0.27 | 64 |
| Culex coronator | 102.8 | 25.3 | 306.0 | 4 | 2 | 0.53 | 17 |
| Culex erraticus | NE | NE | NE | 1 | 1 | 0.27 | 1 |
| Culex quinquefasciatus | 16.8 | 13.8 | 20.4 | 664 | 96 | 25.7 | 6054 |
| Culex tarsalis | 117.2 | 46.4 | 241.6 | 15 | 4 | 1.07 | 33 |
| Culiseta inornata | NE | NE | NE | 1 | 1 | 0.27 | 1 |
| Culiseta particeps | 369.5 | 81.4 | 840.2 | 3 | 2 | 0.53 | 6 |
Female Ae. epactius have an aggressive feeding behavior, preferring to feed on mammals including humans [26]. Aedes epactius has been reported as a vector of Jamestown Canyon virus [27] and can transmit St. Louis encephalitis virus transovarially to their progeny [28]. However, Ae. epactius has never been incriminated as a vector of ZIKV, e.g., [10]. This species is a very common species in the Midwestern United States, and northern and central Mexico [15,19], including urban and suburban areas at low, mid and high elevations [29]. In urban and semi-urban areas, larvae of this species can be found in cemeteries, permanent and temporary ponds and water channels with a high content of organic matter [25]. Culex erraticus is a tropical species that occurs from South and Meso America, including southeastern Mexico, to the eastern USA, where this species reaches its northernmost distributional point [15,30]. It feeds primarily on avian hosts, however, it can also bite mammals, reptiles and amphibians [31,32]. It has been proposed that this species may have a role in Eastern Equine Encephalitis (EEE) transmission, St. Louis encephalitis (SLEV) and West Nile virus (WNV) [32]. In urban and semi-urban areas, larvae can be found in rain collectors, ponds and shipping canals [25].
The presence of ZIKV-infected mosquitoes in Mexico City (in Central Mexico) is intriguing since the city is considered to be free of any Ae. aegypti and Ae. albopictus populations and mosquito-borne viral autochthonous diseases. Fortunately, until now, the risk of introduction and local spread of ZIKV by mosquitoes has been practically nil in the city. The average altitude (2,240 m) and climate characteristics (humid/subhumid/dry and cold/template climates) of the city have historically rendered unlikely the establishment of Ae. aegypti and Ae. albopictus colonies, thus the possibility of locally transmitted Aedes-borne diseases was considered unlikely. As such, it is possible that ZIKV-infected travelers (local or foreign) could have infected the mosquitoes reported here.
Chihuahua (North Mexico) has no reported cases of Zika, although dengue virus (DENV) transmission is common. A large proportion of ZIKV infection has been estimated to be asymptomatic [33]. In regions without reported human cases, but with infected mosquitoes, it is possible that asymptomatic cases exist and could be playing a role in transmission to mosquitoes with a manner reflective of other vector-borne diseases (for a similar claim see [34]). For example, it has been recognized that DENV infections with no clinical symptoms can contribute to virus transmission dynamics by infected mosquitoes [35]. In addition, the clinical and serological diagnosis of Zika can be confused with dengue, and this could also explain the absence of reported Zika cases in the state.
ZIKV detected in male Ae. aegypti (9 positive pools, 16%) indicates possible transovarial and/or venereal transmission. Vertical and venereal transmission of ZIKV has been previously reported in Ae. aegypti and Ae. albopictus [36–38]. These results suggest that inter-vectorial ZIKV transmission in mosquitoes could contribute to the maintenance of virus circulation in Mexico. These results are significant because vector surveillance and control programs mainly target the females of vector species. However, the relevance of infected males to the maintenance of virus transmission should be further evaluated (see also [39]).
In several states, ZIKV infections were common in Cx. quinquefasciatus. However, the role of this species in ZIKV transmission remains controversial. Recent studies have shown that Cx. quinquefasciatus could have a role in the transmission [40,41]. Nevertheless, other studies concluded the opposite, where Cx. quinquefasciatus was not a ZIKV vector [42–44]. Despite this controversy, virological surveys should continue to include Culex spp. in Mexico.
Our results do not permit the incrimination of any of these species as competent vectors of ZIKV. Infection could represent residual, viremic blood from prior human feeding, or weak infections that did not disseminate to the salivary glands and therefore do not represent transmission risk. However, Elizondo-Quiroga et al. [45], have suggested that Ae. vexans, Cx. quinquefasciatus, Cx. coronator and Cx. tarsalis, could be probable vectors for ZIKV since all these species were found with the presence of infection in the salivary glands. The necessary next step is to isolate and sequence the viruses found in each mosquito species to confirm the ZIKV infection and perform experimental infections under laboratory conditions to assess the vector potential of these reported species.
Some of the reported species are not thought to feed frequently on humans, however, for some of these species (e.g. Ae. albopictus, Cx. coronator, Cx. tarsalis, and Cs. particeps) the MLE infected rate was high (compared to Ae. aegypti) (Table 2). Agriculture, development and urbanization can decrease forest cover, wetlands and other natural habitats, increasing the possibility of contact between sylvatic mosquito species and humans. This could increase the risk of disease transmission in semi-urban areas because of human exposure to opportunistic vectors, the adaption of vectors to disrupted or newly created niches (see also [46]) and migration of non-human hosts. For a control program, the ability to predict the risk of infection more accurately is essential. Opportunistic, generalist or ‘bridge’ mosquitoes, which feed on a greater variety of hosts, may transmit ZIKV to incidental hosts including humans and domesticated (possible reservoir) animals. The early detection of viruses in mosquitoes through routine virus surveillance could allow for early and targeted vector control actions.
Acknowledgments
We would like to thank all the staff from each of the Mexican Specialized Bioassay Units of in the surveyed states for their assistance with field work. We are grateful to all the staff of in the Department of Virology InDRE and to the Red Nacional de Laboratorios de Salud Publica (RNLSP) for molecular work. Luis M. Hernández-Triana would like to thank the EU Framework Horizon 2020 Innovation Grant, European Virus Archive (EVAg, grant no. 653316) and the UK Department for Environment and Rural Affairs (DEFRA), Scottish Government and Welsh Government (grant nos. SV3045, SE4113) for travel expenses. We thank Selene Garcia-Luna for assisting us with the MLE analysis. We are extremely grateful to Audrey Lenhart for her comments, suggestions and proofreading this paper for the use of English. We thank two anonymous reviewers for invaluable comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Cordeiro MT, Pena LJ, Brito CA, et al. Positive IgM for Zika virus in the cerebrospinal fluid of 30 neonates with microcephaly in Brazil. Lancet. 2016;387:1811–1812. [DOI] [PubMed] [Google Scholar]
- [2].Cao-Lormeau V-M, Blake A, Mons S, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guerbois M, Fernandez-Salas I, Azar SR, et al. Outbreak of Zika Virus infection, Chiapas State, Mexico, 2015, and first confirmed transmission by Aedes aegypti mosquitoes in the Americas. J Infect Dis. 2016;214:1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Secretaría de Salud de México Boletin Epidemiologico Semana 47. Dir. Gen. Epidemiol. Mexico: Direccion General Adjunta de Epidemiologia; 2015. Available from: https://www.gob.mx/salud/acciones-y-programas/historico-boletin-epidemiologico [Google Scholar]
- [5].Secretaría de Salud de México Casos Confirmados de Infección por Virus Zika 2018. Mexico: Direccion General Adjunta de Epidemiologia; 2018. Available from: https://www.gob.mx/cms/uploads/attachment/file/388152/Cuadro_Casos_ZIKA_y_Emb_SE35_2018.pdf [Google Scholar]
- [6].Grischott F, Puhan M, Hatz C, et al Non-vector-borne transmission of Zika virus: A systematic review. Travel Med Infect Dis. 2016;14:313–330. [DOI] [PubMed]
- [7].Campos SS, Fernandes RS, Dos Santos A, et al. Zika virus can be venereally transmitted between Aedes aegypti mosquitoes. Parasit Vectors. 2017;10:605. [DOI] [PMC free article] [PubMed]
- [8].Pereira-Silva JW, Nascimento V, Belchior H, et al. First evidence of Zika virus venereal transmission in Aedes aegypti mosquitoes. Mem. Inst. Oswaldo Cruz. 2018;113:56–61. [DOI] [PMC free article] [PubMed]
- [9].Diaz-Quinonez JA, Lopez-Martinez I, Torres-Longoria B, et al. Evidence of the presence of the Zika virus in Mexico since early 2015. Virus Genes. 2016;52:855–857. [DOI] [PubMed] [Google Scholar]
- [10].Elizondo-Quiroga D, Medina-Sánchez A, Sánchez-González JM, et al. Zika Virus in salivary glands of five different species of wild-caught mosquitoes from Mexico. Sci Rep. 2018;8:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huerta H, González-Roldán JF, Sánchez-Tejeda G, et al. Detection of Zika virus in Aedes mosquitoes from Mexico. Trans R Soc Trop Med Hyg. 2017;111:328–331. [DOI] [PubMed] [Google Scholar]
- [12].Diaz-Badillo A, Bolling BG, Perez-Ramirez G, et al. The distribution of potential West Nile virus vectors, Culex pipiens pipiens and Culex pipiens quinquefasciatus (Diptera: Culicidae), in Mexico City. Parasit Vectors. 2011;4:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].CENAPRECE Guia Metodologica para la Vigilancia Entomovirologica. Subsecretaria Prevencion y Promocion la Salud. Mexico: Centro Nacional de Programas Preventivos y Control Enfermedades. Secretaria de Salud; 2018. Available from: https://www.gob.mx/cms/uploads/attachment/file/354682/Guia_Metodol_gica_para_la_Vigilancia_Entomovirologica.pdf [Google Scholar]
- [14].Carpenter SJ, LaCasse WJ.. Mosquitoes of North America (North of Mexico). Berkeley, CA: University of California Press; 1955. [Google Scholar]
- [15].Darsie RF, Ward RA. Identification and geographical distribution of the mosquitoes of North America, North of Mexico. 2nd ed. Gainesville, FL: University Press of Florida; 2005. [Google Scholar]
- [16].CDC Trioplex Real-time RT-PCR assay. Atlanta, GA: Centers for Disease Control and Prevention; 2018. Available from https://www.cdc.gov/zika/pdfs/trioplex-real-time-rt-pcr-assay-instructions-for-use.pdf [Google Scholar]
- [17].Santiago GA, Vázquez J, Courtney S, et al. Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses. Nat Commun. 2018;9:1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Biggerstaff BJ. PooledInfRate, version 4.0: a Microsoft add-in to compute prevalence estimates from pooled samples. Fort Collins, CO: Centers of Disease Control and Prevention; 2012. [Google Scholar]
- [19].Ibanez-Bernal S, Martinez-Campos C. Clave para la identificación de larvas de mosquitos comunes en las áreas urbanas y suburbanas de la República Mexicana (Diptera: Culicidae). Folia Entomológica Mex. 1994;92:43–73. [Google Scholar]
- [20].Jackson M, Howay T, Belton P. The first record of Culiseta particeps (Diptera: Culicidae) in Canada. Can Entomol. 2013;145:115–116. [Google Scholar]
- [21].Schreiber ET, Chaney JD, Mulla MS, et al. Bionomics of Culiseta particeps in southern California. J Am Mosq Control Assoc. 1989;5:434–435. [PubMed] [Google Scholar]
- [22].CDC Mosquito species in which West Nile virus has been detected, United States, 1999–2012. Atlanta, GA; 2012. Available from: https://www.cdc.gov/westnile/resources/pdfs/Mosquito%20Species%201999-2012.pdf [Google Scholar]
- [23].Foster WA, Walker ED. Mosquitoes (Culicidae) In: Mullen G, Durden L, editors. Medical and Veterinary Entomology. San Diego, CA: Academic Press; 2002. p. 203–262. [Google Scholar]
- [24].Hammon W, Reeves W, Benner S, et al. Human encephalitis in the Yakima Valley, Washington, 1942: with forty-nine virus isolations (Western Equine and St. Louis types) from mosquitoes. J Am Med Assoc. 1945;128:1133–1139. [Google Scholar]
- [25].Davalos-Becerril E, Correa-Morales F, González-Acosta C, et al. Urban and semi-urban mosquitoes of Mexico City: A risk for endemic mosquito-borne disease transmission. PLoS One. 2019;14:e0212987. [DOI] [PMC free article] [PubMed]
- [26].Farajollahi A, Price DC. A rapid identification guide for larvae of the most common North American container-inhabiting Aedes species of medical importance. J Am Mosq Control Assoc. 2013;29:203–221. [DOI] [PubMed] [Google Scholar]
- [27].Heard PB, Zhang M, Grimstad PR. Laboratory transmission of Jamestown Canyon virus and snowshoe hare virus (Bunyaviridae: California serogroup) by several species of mosquitoes. J Am Mosq Contr Assoc. 1991;7:94–102. [PubMed] [Google Scholar]
- [28].Hardy JL, Rosen L, Kramer LD, et al. Effect of rearing temperature on transovarial transmission of St. Louis encephalitis virus in mosquitoes. Am J Trop Med Hyg. 1980;29:963–968. [DOI] [PubMed] [Google Scholar]
- [29].Lozano-Fuentes S, Welsh-Rodriguez C, Hayden MH, et al. Aedes (Ochlerotatus) epactius along an elevation and climate gradient in Veracruz and Puebla States, México. J Med Entomol. 2012;49:1244–1253. [DOI] [PubMed] [Google Scholar]
- [30].Martinez-Palacios A. Notas sobre la distribucion de los mosquitos Culex en Mexico (Dipetra: Culicidae). Rev Soc Mex Hist Nat. 1952;13:75–87. [Google Scholar]
- [31].Burkett-Cadena ND, McClure CJW, Ligon RA, et al. Host Reproductive phenology drives seasonal patterns of host use in mosquitoes. PLoS One. 2011;6:e17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mendenhall IH, Tello SA, Neira LA, et al. Host preference of the arbovirus vector Culex erraticus (Diptera: Culicidae) at Sonso Lake, Cauca Valley Department, Colombia. J Med Entomol. 2012;49:1092–1102. [DOI] [PubMed] [Google Scholar]
- [33].Ladhani SN, O’Connor C, Kirkbride H, et al. Outbreak of Zika virus disease in the Americas and the association with microcephaly, congenital malformations and Guillain–barré syndrome. Arch Dis Child. 2016;101(600):LP–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gutierrez-Bugallo G, Piedra LA, Rodriguez M, et al. Vector-borne transmission and evolution of Zika virus. Nat. Ecol. Evol. 2018;3:561–569. [DOI] [PMC free article] [PubMed]
- [35].Duong V, Lambrechts L, Paul RE, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci. 2015;112:14688–14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ciota AT, Bialosuknia SM, Ehrbar DJ, et al. Vertical Transmission of Zika Virus by Aedes aegypti and Ae. albopictus mosquitoes. Emerg Infect Dis. 2017;23:880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gutiérrez-Bugallo G, Piedra LA, Rodriguez M, et al. Vector-borne transmission and evolution of Zika virus. Nat Ecol Evol. 2019;3:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thangamani S, Huang J, Hart CE, et al. Vertical transmission of Zika Virus in Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2016;95:1169–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Boyer S, Calvez E, Chouin-Carneiro T, et al. An overview of mosquito vectors of Zika virus. Microbes Infect. 2018;20:646–660. [DOI] [PubMed] [Google Scholar]
- [40].Guedes DR, Paiva MH, Donato MM, et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg Microbes Infect. 2017;6:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Smartt CT, Shin D, Kang S, et al. Culex quinquefasciatus (Diptera: Culicidae) from Florida transmitted Zika Virus. Front Microbiol. 2018;9:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kenney JL, Romo H, Duggal NK, et al. Transmission incompetence of Culex quinquefasciatus and Culex pipiens pipiens from North America for Zika Virus. Am J Trop Med Hyg. 2017;96:1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lourenco-de-Oliveira R, Marques JT, Sreenu VB, et al. Culex quinquefasciatus mosquitoes do not support replication of Zika virus. J Gen Virol. 2018;99:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Roundy CM, Azar SR, Brault AC, et al. Lack of evidence for Zika virus transmission by Culex mosquitoes. Emerg Microbes Infect. 2017;6:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Elizondo-Quiroga D, Medina-Sánchez A, Sánchez-González JM, et al. Zika Virus in salivary glands of five different species of wild-caught mosquitoes from Mexico. Sci. Rep. 2016;8:809. [DOI] [PMC free article] [PubMed]
- [46].Kamdem C, Tene Fossog B, Simard F, et al. Anthropogenic habitat disturbance and ecological divergence between incipient species of the malaria mosquito Anopheles gambiae. PLoS One. 2012;7:e39453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


