Abstract
Context/Objective: Cognitive deficits can impact as many as 60% of individuals with spinal cord injury (SCI). In an effort to identify the nature of cognitive deficits in SCI, we examined neuropsychological test performance in individuals with SCI, age matched healthy controls and older healthy controls.
Design: Participants completed a motor-free neuropsychological test battery assessing attention, working memory, information processing speed, new learning /memory and executive control.
Setting: Outpatient rehabilitation research facility.
Participants: Participants included 60 individuals with chronic spinal cord injury [SCI; 32 with paraplegia (T2-T12) and 28 with tetraplegia (C3-T1)], 30 age-matched healthy controls (AMHC; 30–40 years old) and 20 older healthy controls (OHC; 50–60 years old).
Outcome Measures: Wechsler Intelligence Scale – 3rd edition (WAIS-III) Digit Span and Letter-Number Sequencing; Symbol Digit Modalities Test (SDMT) – oral version; California Verbal Learning Test-II; Paced Auditory Serial Addition Test (PASAT); Wechsler Abbreviated Scale of Intelligence (WASI); Delis-Kaplan Executive Function System; Verbal Fluency subtest.
Results: Significant differences were noted between the SCI and AMHC groups on measures of information processing speed, new learning and memory, and verbal fluency. No significant differences were noted between the groups on tests of attention or working memory.
Conclusion: The current study documented differences in specific realms of cognitive functioning between a chronic SCI sample and AMHC. Implications for cognitive rehabilitation and overall quality of life are discussed. Additional research is needed utilizing a more comprehensive battery of motor-free neuropsychological tests that avoid the confound of upper limb motor limitations on cognitive performance.
Keywords: Spinal cord injury, Cognitive deficits, Memory, Processing speed, Executive functioning
Introduction
Traumatic spinal cord injury (SCI) is a significant public health concern1 and decades of research have focused on the associated physical limitations, as well as therapies for addressing these physical deficits. However, multiple studies have additionally documented cognitive deficits in persons with SCI, noted in attention,2–7 concentration,3–6 new learning and memory (NLM),3–5,7,8 abstract reasoning,3 verbal learning,3–5 and processing speed (PS),9 even in relatively young SCI cohorts (28–45 years).2,3,10,11 As many as 60% of individuals display some degree of impairment.2,3,10–12 The relative risk of cognitive impairment in persons with SCI is 13 times greater than that which has been documented in uninjured individuals.13 Previous research has identified distinct patterns of cognitive impairment in persons with chronic SCI, characterized by deficits in identifiable cognitive domains, namely in PS, NLM and executive functioning.12 Most recently, Molina and colleagues14 demonstrated cognitive deficits in individuals with SCI during the acute period following injury (2–6 months post-SCI) that appear to worsen over time (one or more years post-injury).
Cognitive deficits exert a substantial negative impact on everyday life and quality of life (QOL) in various neurological populations,15–21 having a critical impact on functional outcomes after SCI in particular. Cognitive deficits after SCI have been associated with diminished functional gains during rehabilitation,22 increased aggressive behaviors,23 higher likelihood of re-hospitalization,24 and limited acquisition of novel, day-to-day skills required for successful community re-integration after SCI.9,25 Cognitive deficits thus detract from comprehensive rehabilitation efforts and social integration, and are also associated with poor self-perception and QOL.9,25,26 Indeed, persons living with SCI report significant decreases in cognitive functioning from before to after injury, which relate to decreased overall QOL.27
Although the etiology of the cognitive deficits in SCI remains elusive, several factors have been suggested as contributory including concomitant traumatic brain injury (TBI),2,3,10,11,28–34 secondary trauma as a result of cerebral edema, hypoxia and anoxia27 and cardiovascular and cerebrovascular dysfunction.7,35,36 Recent studies also suggest that factors such as sleep disordered breathing/sleep apnea,31 core body temperature dysregulation,37,38 as well as medications prescribed for symptom management such as pain,39,40 and neurogenic lower urinary tract dysfunction41 may contribute to post-SCI cognitive dysfunction. Recent work by Bombardier and colleagues42 highlight the likelihood that factors other than TBI likely contribute to cognitive deficits post-SCI, because the number of patients reporting cognitive deficits exceeded physician-rated presence of TBI by 80% in their sample of 105 persons with SCI. Clearly, factors other than concomitant TBI are involved.
One candidate explanation for this pattern of increasing cognitive deficits post-SCI is accelerated brain aging. Across a variety of neurological (e.g. TBI; for review43), psychiatric (e.g. schizophrenia,44 alcohol dependence45), and medical (e.g. hypertension,46 cardiac health47) conditions, increasing evidence suggests that neural damage akin to the aging process is exacerbated, with concomitant medical conditions, often resulting in cognitive impairment48 and disability.49 As a number of these conditions are often associated with SCI, these individuals may be at increased risk, as compared to their able-bodied counterparts, for accelerated brain aging and subsequent cognitive aging.
In an effort to identify the nature of the cognitive deficits in persons with SCI, we examined neuropsychological test performance in persons with traumatic SCI compared to both age matched non-SCI healthy controls (AMHC) and a group of older non-SCI healthy controls (OHC). We hypothesized that the neuropsychological performance of persons with SCI would significantly differ from AMHC, with no difference noted between persons with SCI and OHC.
Methods
Participants: Participants consisted of 60 individuals with chronic SCI, including 32 individuals with paraplegia (T2-T12) and 28 individuals with tetraplegia (C3-T1). Thirty (30) age-matched healthy controls (AMHC; 30–40 years old) and 20 older healthy controls (OHC; 50–60 years old) were additionally included; group characteristics may be found in Table 1. The upper age limit in the OHC sample was limited to age 60 to minimize the potential confound of an ongoing dementing processes. All participants were proficient in English and demonstrated visual acuity of at least 20/60 in the worst eye (with prescription eyewear). All participants with SCI were non-ambulatory (used a wheelchair ≥40 hours/week) with American Spinal Injury Association Impairment Scale (AIS) grade A, B or C and were at least 1 year post-injury. Potential participants were excluded in the presence of acute illness or infection or a documented history of chronic hypertension, diabetes mellitus, TBI, stroke, epilepsy or seizure disorders, multiple sclerosis, Parkinson’s disease, psychiatric disorders (post-traumatic stress disorder, schizophrenia, bipolar disorder), illicit drug abuse within the past 6-months, Alzheimer’s disease and dementia. Individuals scoring ≤ 22 the Mini Mental Status Examination (MMSE),50 adapted to eliminate any motor requirements (i.e. changed 3-step command to non-motor tasks, oral sentence generation instead of written, and visuoconstruction task changed to visual discrimination), were also excluded.
Table 1. Demographic and disease characteristics by group.
| SCI N = 60 | AMHC N = 30 | OHC N = 20 | Statistic | P | |
|---|---|---|---|---|---|
| Age (years) | 35.38 (7.01) range: 25–48 | 35.73 (7.35) range: 26–49 | 60.3 (2.68) range: 55–64 | F(2,107) = 117.07 | <0.001a |
| Education (years) | 13.65 (2.24) | 15.50 (1.70) | 16.15 (2.46) | F(2,107) = 13.71 | <0.001b |
| Ethnicity (% Caucasian) | 48.3% | 50% | 90% | χ2(14, N = 110) = 20.17 | 0.125 |
| Age at injury | 25.55 (6.61) range: 16–45 | n/a | n/a | n/a | |
| Years since injury | 9.83 (7.32) range: 1–31 | ||||
| Level of Injury | C3-T1: n = 32 T2-T2: n = 28 | n/a | n/a | n/a | |
| WASI Vocabulary T Score | 48.46 (9.80) | 55.97 (8.67) | 60.30 (11.02) | F(2,107) = 13.37 | <0.001b |
| WASI Matrix Reasoning T Score | 46.95 (10.24) | 52.47 (8.45) | 57.15 (9.20) | F(2,107) = 9.51 | <0.001b |
SCI, spinal cord injury; AMHC, age matched healthy controls; OHC, older healthy controls.
aSCI, AMHC < OHC; P < 0.05 based on ANOVA with LSD post-hoc.
bSCI < AMHC, OHC; P < 0.05 based on ANOVA with LSD post-hoc.
Participants with SCI were recruited first. Individuals with SCI were recruited from the Northern New Jersey SCI Model (NNJSCIS) System database, posted flyers at support groups and inpatient and outpatient treatment facilities. The AMHC and OHC participants were recruited from the local community, hospital personnel and flyers. AMHC and OHC were then matched to the SCI subjects for: sex, race, smoking status, socioeconomic status, IQ, and education (+/− 1 year of educational attainment), to the best of our ability. Participants with SCI were screened for level and severity of injury based on prior evaluation using the International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI).51
There was no significant difference between the SCI group and the AMHC group in age. By definition, there was a significant difference between the OHC group and both the SCI group and the AMHC group (F(2,107) = 117.14, P < 0.001). There was additionally a significant difference between the groups in level of education, with the SCI group having less education than both HC groups (F(2,107) = 13.71, P < 0.001).
Pre-morbid IQ was assessed with the 2-subtest version of Wechsler Abbreviated Scale of Intelligence (WASI)52 which consists of the Vocabulary (verbal IQ; vocabulary and verbal knowledge) and Matrix Reasoning subtests (nonverbal reasoning). The WASI has shown excellent internal consistency reliability, and construct validity has been consistently supported by high intercorrelations between the WASI subtests and WAIS-III IQ scales (range: 0.66–0.92) and factor analyses.53 Significant differences were noted between the groups on the IQ estimates, with the SCI group performing at significantly lower levels than both HC groups [WASI Vocabulary: (F(2,106) = 13.67, P < 0.001); WASI Matrix Reasoning: (F(2,107) = 9.50, P < 0.001), which remained statistically significant after entering education as a covariate (Vocabulary: F(2,106) = 3.53, P < 0.05; Matrix Reasoning: F(2,105) = 4.70, P < 0.05). Education was a significant covariate in both statistical models (both Ps < 0.05). There were no significant differences between the groups on measures of depression (Chicago Multidimensional Depression Inventory) or anxiety (State Trait Anxiety Inventory).
Procedures: This was a prospective, cross-sectional investigation conducted at the Kessler Foundation (KF) in West Orange, NJ and supported by investigators at the James J. Peters Veterans Affair Medical Center (JJPVAMC) in Bronx, NY. Institutional Review Board approvals were obtained at both institutions and informed consent was obtained prior to initiating study procedures. Complete assessment consisted of cardiovascular/cerebrovascular evaluation (heart rate [HR], finger blood pressure [BP], cerebral blood flow velocity [CBFv], and brachial BP at rest), which were collected while the subject was resting in the seated position and during neuropsychological testing. The neuropsychological test battery took approximately 2–2.5 hours to administer and included tasks of memory, attention, working memory, information processing speed and executive functioning. The current paper focuses on neuropsychological test performance; the cardiovascular and cerebrovascular data has been submitted for publication elsewhere.54
Neuropsychological Assessment: A broad-based neuropsychological assessment was administered by a trained research assistant, who was approved for testing via a three-step process including two postdoctorally-trained neuropsychologists. Test choice was limited by the upper limb motor paralysis in participants with tetraplegia. Thus, all measures were motor-free.
Digit Span, Wechsler Intelligence Scale–III (WAIS-III) assessed attention and working memory.53 Each segment (forward and backward) consists of seven pairs of random number sequences that the examiner reads aloud at the rate of one per second. In the digit span forward segment, the subject repeats the sequence in the same order presented. Conversely, in the backward segment, the subject repeats the sequence in the reverse order. WAIS III Digit Span has also shown high internal consistency reliability (r = .90) and high construct validity.53
Symbol Digit Modalities Test (SDMT) – oral version55 assessed information processing speed. The examinee substitutes a number for a randomized presentation of a geometric figure. The appropriate number is shown in a key containing the Arabic numbers 1–9, each with a different geometric figure. The SDMT has shown good test-retest (r = .76) and alternate forms (r = .82, r = .84) reliability. The sensitivity of the SDMT to cognitive effects has been demonstrated repeatedly in populations such as learning disabled children, commissurotomy patients (full and frontal), adults with cerebrovascular disease, chronic brain lesions (e.g. TBI), Huntington’s Disease, and Multiple Sclerosis (MS).56 The SDMT has also been shown to be able to distinguish between individuals with depression and those with an organic dementia.55
Letter-Number Sequencing, (WAIS-III LNS)57 assessed auditory working memory. Individuals sequentially order a series of random numbers and letters orally presented in a specified random order. The subjects must first remember the numbers and letters and then reorganize the numbers in ascending order and the letters in alphabetical order. WAIS-III LNS has shown high internal consistency reliability (r = .90), construct validity and differential sensitivity to a variety of neurocognitive disorders.53
The California Verbal Learning Test-II58 assessed verbal new learning and memory. This test consists of a list of 16 words from 4 semantic categories presented orally over 5 trials and includes a 20-minute delayed recall trial as well as a recognition trial. The CVLT-II has demonstrated good reliability and validity, high internal consistency reliability (r = .94), split half reliability (r = .83), test-retest reliability (r = .82) and construct validity.58
The Paced Auditory Serial Addition Test (PASAT)59 assessed information processing, including speed and working memory. The participant adds verbally presented randomized single digits so that each digit is added to the one immediately preceding it. Fifty digits are presented in each of 4 trials via audio recording, with the 4 trials varying in speed of presentation, differing by 0.4 seconds each (1 digit every 1.2 seconds to 1 digit every 2.4 seconds). Performance is evaluated by calculating the number correct or percent correct on each trial. The PASAT has shown good spilt half reliability (r = .96)60 and high internal consistency.61 Factor analytic studies have shown the PASAT to load with other measures of information processing.62 It has also been shown to be sensitive to mild concussions,63,64 diffuse cerebral damage65 and very sensitive to deficits in information processing ability.62
The Verbal Fluency subtest of the Delis-Kaplan Executive Function System (D-KEFS: Delis et al.66) was administered to assess the fluency aspect of executive functioning. This test requires verbal generation of words in 60 seconds to the letter prompts “F”, “A”, and “S”, as well as to the semantic categories of “animals” and switching between “fruits” and “furniture”.
Data Analysis: Statistical analyses were performed using SPSS software (Version 21.0; IBM Corporation, Armonk, NJ, USA) and continuous data are reported as mean ± standard deviation. Analysis of Covariance (ANCOVA) with group as the between subjects variable (SCI, AMHC, OHC) was conducted for scores on each neuropsychological test to examine differences between the groups within the specific cognitive domains of attention, working memory, information processing speed, verbal new learning and memory and verbal fluency. Raw scores (instead of normatively-corrected scores) were utilized for all analyses to facilitate the identification of patterns of performance between AMHC and OHC based on actual performance rather than a decline from previous functioning. Years of education was utilized as a covariate in all analyses due to significant group differences. LSD were calculated for pair-wise comparisons where appropriate. Significance was set at an alpha level of P ≤ 0.05, and analyses were well-powered to detect medium effect sizes (1−β = 0.83 for effect size f = 0.31).
Results
Information processing speed
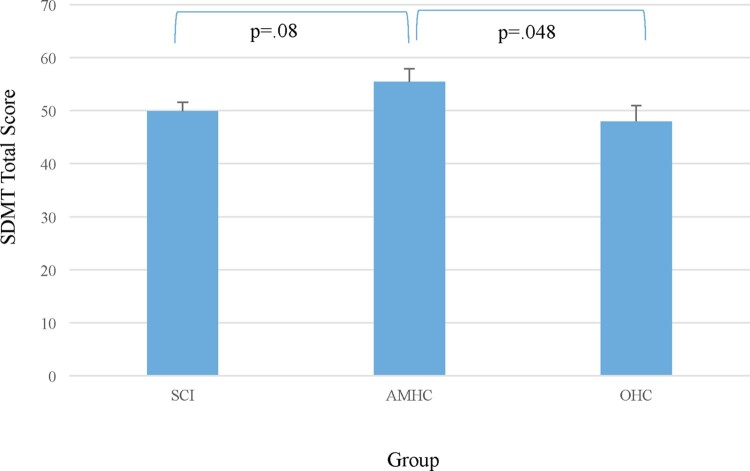
A trend for significance was noted between the groups on the SDMT (F(2,106) = 2.53, P = 0.08). Post-hoc LSD indicated a significant difference between the AMHC and the OHC groups (P < 0.05; Hedge’s g = 0.52; medium effect) and a trend was noted between the SCI group and the AMHC group (P = 0.07; Hedge’s g = 0.55; medium effect; see Fig. 1).
Figure 1.
Information processing speed by group, as determined by the Symbol Digit Modalities Test – Oral Version.
Attention and working memory
No significant group differences were noted between the groups on the PASAT (P = 0.22), letter number sequencing (P = 0.80) or Digit Span (total, forward or backward; all Ps > 0.10).
Verbal learning & memory
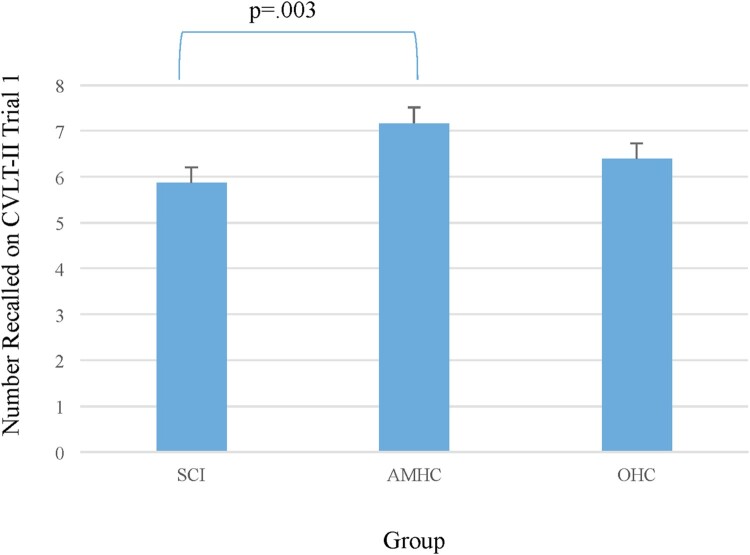
In examining performance on a verbal list learning task, the CVLT-II, several significant differences were noted between the groups. After controlling for education (ns), there was a significant difference between the groups noted on the Trial 1 score (F(2,106) = 4.59, P < 0.01). Post-hoc LSD indicated significant differences between the SCI group and the AMHC (P < 0.01; Hedge’s g = 0.79; large effect). The difference between the SCI group and the OHC was not significant (Fig. 2).
Figure 2.
One-trial verbal learning by group, as determined by the California Verbal Learning Test – Second Edition, Trial 1.
After controlling for education (ns), there was also a significant difference between the groups noted on short delay free recall (F(2,106) = 3.30, P < 0.05). Post-hoc LSD tests indicated a significant difference between the SCI group and the AMHC (P < 0.05; Hedge’s g = 0.69; medium-large). There was a trend toward a significance difference between groups on the CVLT-II Total Learning Score (Trials 1–5; F(2,106) = 2.453; P = 0.09), with a significant difference evident between the SCI group and the AMHC group (P = 0.03; Hedge’s g = 0.66; medium-large effect). There was no significant difference between the SCI and OHC groups There was no significant difference noted on the raw long delay free recall (P = 0.28).
Verbal fluency
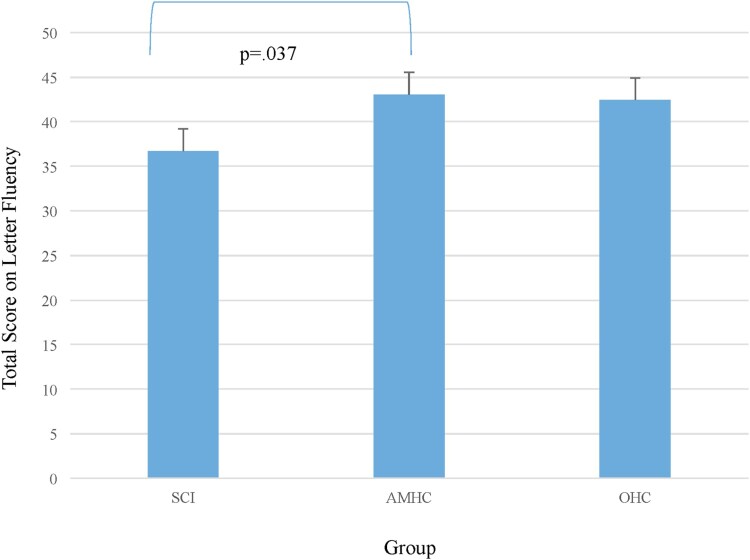
A trend was noted between the groups for the letter fluency total score (F(2,106) = 2.61, P = 0.08) after controlling for variance associated with education (F(1,106) = 7.42, P = 0.008). Significant group differences were noted between the SCI group and AMHC (P < 0.05; Hedge’s g = 0.80; large effect; Fig. 3). No significant difference was noted on category fluency (P = 0.23).
Figure 3.
Verbal fluency (letter) by group, as determined by the Delis-Kaplan Executive Function System – Verbal Fluency Subtest (Condition 1: Letter Fluency).
Discussion
Results indicated several significant differences in specific domains of neuropsychological functioning in persons with SCI as compared with AMHC and OHC. Significant differences from AMHC were noted in information processing speed, verbal learning and memory and verbal fluency, in the absence of significant differences from OHC. In contrast, simple attention and working memory appeared intact in our participants with SCI.
Deficits in information processing speed were noted on the SDMT such that persons with SCI performed at significantly lower levels than AMHC, but did not differ from OHC. The SDMT is a widely-accepted measure of information processing speed in neurological populations and often yields high sensitivity to cognitive impairment in such samples.55,67 Indeed, cognitive slowing is one of the primary deficits observed in normal cognitive aging and is frequently observed in the context of poor white matter integrity. In contrast, all measures of working memory (PASAT, Digit Span backwards, and Letter Number Sequencing) were intact in the SCI group indicating little to no difficulties with working memory. Thus, in the current dataset, the SCI groups performed at expected levels on working memory tests, with a clear deficit in information processing speed noted.
With regard to verbal learning and memory, the SCI group differed significantly from the AMHC but not OHC in performance on several indices of the CVLT-II. Deficits were noted on Trial 1 of the CVLT, which best reflects learning in everyday life, given the fact that daily life learning does not typically provide an opportunity for repetition of the to-be learned information.68 This finding may indicate that persons with SCI demonstrate difficulty learning new information in daily life situations. Deficits were similarly noted (albeit at an omnibus trend level) on total learning across the 5 trials of the CVLT, indicating a deficit in the initial learning of information, even in the presence of repeated presentation of that information. Short delay free recall was similarly impaired. However, it is interesting to note that there was no significant difference between the groups on any measure of memory for the newly learned information after a 20-minute delay. This indicates that although the SCI group demonstrated difficulty with the initial learning of information, the degradation of information over time was equivalent to that observed in both the AMHC and the OHC groups. This pattern of results has significant implications for cognitive rehabilitation. That is, in the presence of an immediate learning impairment, with intact delayed recall, cognitive rehabilitation must be targeted at improving new learning abilities and attempt to improve new learning abilities specifically in individuals with SCI. This would be expected to result in an overall improvement in memory performance, as has been seen in other neurologic populations.69,70
The only indices of executive functioning included in the neuropsychological battery administered assessed verbal fluency. Interestingly, significant differences were noted between the groups on the letter fluency subtest, but not the category fluency subtest. Although it is tempting to attribute deficits in verbal fluency to speed of processing, as fluency measures required rapid speed of processing, the fact that deficits were noted on the letter but not the category fluency argues against this interpretation. Historically, the rapid retrieval of words by initial letter sound has been associated with frontal systems functioning, while such retrieval organized by word category has been associated with temporal lobe functioning. This indicates that persons with SCI are demonstrating a disruption in the frontal executive retrieval system necessary to carry out the task. Indeed, this pattern is consistent with other neurological disorders that have injury patterns secondary to both reduced perfusion (e.g. vascular dementia71) and traumatic injury (e.g. TBI72).
Significant differences were noted between the groups in education level. This was an interesting and unexpected finding, and suggests that perhaps the educational accomplishments of the SCI sample may have been limited by the occurrence of the injury itself. Age at time of injury ranged from 16 to 45 years old (M(SD) = 25.55 (6.61); mode = 24). When examining the individual data, it is notable that of the 60 individuals with SCI included in the study sample only 1 participant reported an education level that would have been completed after the SCI was sustained. Although, the cognitive deficits noted in the SCI group compared to the AMHC are reported after controlling for years of education there is the potential that educational attainment in the SCI group was hindered by the presence of cognitive deficits in the years following the injury. Therefore, early intervention to diagnose and treat cognitive deficits in the SCI population should be considered to facilitate return to school and work and ultimately alleviate long-term cognitive dysfunction.
Beyond the documentation of the pattern of neuropsychological deficits in the current study, the inclusion of two control groups was unique and allowed a comparison of the SCI group to both an AMHC and OHC group. Of the domains assessed in this study, the pattern of deficits in the SCI and OHC samples relative to our younger healthy control sample (AMHC) are consistent with what is consistently reported in the aging literature: dysfunction in information processing speed, new learning and memory, and executive functioning (e.g. Ref. 73; see Ref. 74 for a review). Importantly, we did not use normative scores that pre-corrected for the influence of age on the neuropsychological tests. As cognitive aging is a neurologically normal process, use of normative correction for age would have washed out the raw score differences observed between OHC and AMHC. Additionally, examination of raw scores estimates actual ability on a neuropsychological test, whereas normative corrections are generally utilized to help determine whether a decline from an individual’s previous level of function is likely to have occurred. Given the focus on ability, uncorrected scores can be considered to be a better indicator of real life abilities on daily tasks (i.e. although a decline of 1 standard deviation may be significant for a high-functioning individual, the level of functioning may still be sufficient for performing simple instrumental activities of daily living). As such, acknowledgement of cognitive impairment in this way is important, because treatment focused on these cognitive deficits could have a substantial impact on, not only the deficit itself, but on overall functioning in daily life, including the ability to return to work and school and overall QOL.
There are several study limitations that deserve mention. First, the neuropsychological test battery employed was limited to tests with no motor component. As a result, there was limited availability of measures to reliably assess cognition in this sample. Several cognitive domains were thus not assessed including visuospatial processing, non-verbal memory, and aspects of executive control such as planning and problem solving. Additionally, brain imaging at the time of injury was not available for our participant pool. While there was no documented presence of TBI in any included participant, without neuroimaging we cannot be fully certain that mild TBI was not sustained at the time of injury. Additionally, neuroimaging techniques may more directly help to answer questions related to brain-based pathology, which may or may not confirm the hypothesis of accelerated aging. Furthermore, in order to more rigorously test the hypothesis of accelerated cognitive aging in SCI, a longitudinal study examining the relative cognitive trajectory in the SCI population (compared to their healthy counterparts) is warranted. With regard to generalization, a significant difference was noted between the groups in education. While this was appropriately managed through statistical analysis using years of education as a covariate, the ideal study would have education–matched participant groups. Finally, the relatively small sample size and strict inclusion criteria may preclude generalization of these results to the larger population of individuals with SCI.
Conclusion
Despite these limitations, the current study documented differences in specific realms of cognitive functioning between a chronically injured SCI sample and a sample of AMHC; of note there were no significant differences in cognitive performance comparing the SCI and OHC groups. Cognitive deficits were noted in information processing speed, new learning, and verbal fluency, which is consistent with normal aging. Attention, working memory and memory retrieval processed appear intact. Additional research is needed utilizing a more comprehensive battery of neuropsychological tests that are motor free in nature to avoid the confound of upper extremity motor limitations. Moreover, clinicians in the rehabilitation realm should be aware of the potential presence of cognitive deficits in individuals with SCI and how clinical interactions might be affected. As gleaned from recommendations in other neurological populations with comparable deficits, several ways to minimize the impact of cognitive impairment in treatment settings include: (1) providing information at a slower pace and in multiple modalities (e.g. verbal and written instructions), (2) confirm understanding of medical instructions via independent recitation by the patient, and (3) encouraging use of compensatory strategies to enhance encoding of information to be learned (e.g. spaced self-testing) (see Ref. 75).
Disclosure statements
Conflicts of interest The authors report no conflicts of interest.
Funding This work was supported by New Jersey Commission on Spinal Cord Research: [Grant Number CSCR13IRG018]; Rehabilitation Research and Development Service: [Grant Number B2020-C]; Rehabilitation Research and Development Service: [Grant Number B9212-C].
ORCID
Trevor Dyson-Hudson http://orcid.org/0000-0002-0252-2764
References
- 1.Facts and figures. J Spinal Cord Med 2015;38(6):812–13. [Google Scholar]
- 2.Davidoff G, Morris J, Roth E, Bleiberg J.. Cognitive dysfunction and mild closed head injury in traumatic spinal cord injury. Arch Phys Med Rehabil 1985;66(8):489–91. [PubMed] [Google Scholar]
- 3.Wilmot CB, Cope DN, Hall KM, Acker M.. Occult head injury: its incidence in spinal cord injury. Arch Phys Med Rehabil 1985;66(4):227–31. [DOI] [PubMed] [Google Scholar]
- 4.Davidoff G, Roth E, Thomas P, Doljanac R, Dijkers M, Berent S, et al. Depression and neuropsychological test performance in acute spinal cord injury patients: lack of correlation. Arch Clin Neuropsychol 1990;5(1):77–88. [PubMed] [Google Scholar]
- 5.Davidoff GN, Roth EJ, Haughton JS, Ardner MS.. Cognitive dysfunction in spinal cord injury patients: sensitivity of the functional independence measure subscales vs neuropsychologic assessment. Arch Phys Med Rehabil 1990;71(5):326–9. [PubMed] [Google Scholar]
- 6.Roth E, Davidoff G, Thomas P, Doljanac R, Dijkers M, Berent S, et al. A controlled study of neuropsychological deficits in acute spinal cord injury patients. Paraplegia 1989;27(6):480–9. [DOI] [PubMed] [Google Scholar]
- 7.Jegede AB, Rosado-Rivera D, Bauman WA, Cardozo CP, Sano M, Moyer JM, et al. Cognitive performance in hypotensive persons with spinal cord injury. Clin Auton Res 2010;20(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlozzi NE, Goodnight S, Casaletto KB, Goldsmith A, Heaton RK, Wong AWK, et al. Validation of the NIH toolbox in individuals with neurologic disorders. Arch Clin Neuropsychol 2017;32(5):555–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dowler RN, O'Brien SA, Haaland KY, Harrington DL, Feel F, Fiedler K.. Neuropsychological functioning following a spinal cord injury. Appl Neuropsychol 1995;2(3–4):124–9. [DOI] [PubMed] [Google Scholar]
- 10.Davidoff G, Thomas P, Johnson M, Berent S, Dijkers M, Doljanac R.. Closed head injury in acute traumatic spinal cord injury: incidence and risk factors. Arch Phys Med Rehabil 1988;69(10):869–72. [PubMed] [Google Scholar]
- 11.Richards JS, Brown L, Hagglund K, Bua G, Reeder K.. Spinal cord injury and concomitant traumatic brain injury. Results of a longitudinal investigation. Am J Phys Med Rehabil 1988;67(5):211–6. [DOI] [PubMed] [Google Scholar]
- 12.Dowler RN, Harrington DL, Haaland KY, Swanda RM, Fee F, Fiedler K.. Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. J Int Neuropsychol Soc 1997;3(5):464–72. [PubMed] [Google Scholar]
- 13.Craig A, Guest R, Tran Y, Middleton J.. Cognitive impairment and mood states after spinal cord injury. J Neurotrauma 2017;34(6):1156–63. [DOI] [PubMed] [Google Scholar]
- 14.Molina B, Segura A, Serrano JP, Alonso FJ, Molina L, Pérez-Borrego YA, et al. Cognitive performance of people with traumatic spinal cord injury: a cross-sectional study comparing people with subacute and chronic injuries. Spinal Cord 2018;56(8):796–805. [DOI] [PubMed] [Google Scholar]
- 15.Ladavass E, Paolucci S, Umilta C.. Reasons for holding a consensus conference on neuropsychological rehabilitation in adult patients. Eur J Phys Rehabil Med 2011;47(1):91–9. [PubMed] [Google Scholar]
- 16.McKinlay W, Watkiss AJ.. Cognitive and Behavioral Effect of Brain Injury. 3rd ed. Philadelphia (PA): F.A. Davis; 1999. p. 74–86. [Google Scholar]
- 17.Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, et al. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil 2001;16(4):343–55. [DOI] [PubMed] [Google Scholar]
- 18.Rohling ML, Meyers JE, Millis SR.. Neuropsychological impairment following traumatic brain injury: a dose-response analysis. Clin Neuropsychol 2003;17(3):289–302. [DOI] [PubMed] [Google Scholar]
- 19.Schretlen DJ, Shapiro AM.. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry 2003;15(4):341–9. [DOI] [PubMed] [Google Scholar]
- 20.Serino A, Ciaramelli E, Di Santantonio A, Malagu S, Servadei F, Ladavas E.. Central executive system impairment in traumatic brain injury. Brain Inj 2006;20(1):23–32. [DOI] [PubMed] [Google Scholar]
- 21.Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, et al. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil 2000;81(12):1596–615. [DOI] [PubMed] [Google Scholar]
- 22.Macciocchi SN, Bowman B, Coker J, Apple D, Leslie D.. Effect of co-morbid traumatic brain injury on functional outcome of persons with spinal cord injuries. Am J Phys Med Rehabil 2004;83(1):22–6. [DOI] [PubMed] [Google Scholar]
- 23.Bradbury CL, Wodchis WP, Mikulis DJ, Pano EG, Hitzig SL, McGillivray CF, et al. Traumatic brain injury in patients with traumatic spinal cord injury: clinical and economic consequences. Arch Phys Med Rehabil 2008;89(12 Suppl):S77–84. [DOI] [PubMed] [Google Scholar]
- 24.Davidoff G, Schultz JS, Lieb T, Andrews K, Wardner J, Hayes C, et al. Rehospitalization after initial rehabilitation for acute spinal cord injury: incidence and risk factors. Arch Phys Med Rehabil 1990;71(2):121–4. [PubMed] [Google Scholar]
- 25.Davidoff GN, Roth EJ, Richards JS.. Cognitive deficits in spinal cord injury: epidemiology and outcome. Arch Phys Med Rehabil 1992;73(3):275–84. [PubMed] [Google Scholar]
- 26.Weber E, Wecht JM, Katzelnick C, Dyson-Hudson T, Chiaravalloti N.. Learning and Memory Profile of Individuals with Spinal Cord Injury. Washington (DC): International Neuropsychological Society; 2018. [Google Scholar]
- 27.Murray RF, Asghari A, Egorov DD, Rutkowski SB, Siddall PJ, Soden RJ, et al. Impact of spinal cord injury on self-perceived pre- and postmorbid cognitive, emotional and physical functioning. Spinal Cord 2007;45:429–36. [DOI] [PubMed] [Google Scholar]
- 28.Kessler HR, Cohen RA, Lauer K, Kausch DF.. The relationship between disability and memory dysfunction in multiple sclerosis. Int J Neurosci 1991;62(1–2):17–34. [DOI] [PubMed] [Google Scholar]
- 29.Silver JR, Morris WR, Otfinowski JS.. Associated injuries in patients with spinal injury. Inj 1980;12(3):219–24. [DOI] [PubMed] [Google Scholar]
- 30.Davidoff G, Morris J, Roth E, Bleiberg J.. Closed head injury in spinal cord injured patients: retrospective study of loss of consciousness and post-traumatic amnesia. Arch Phys Med Rehabil 1985;66(1):41–3. [PubMed] [Google Scholar]
- 31.Schembri R, Spong J, Graco M, Berlowitz DJ, team Cs . Neuropsychological function in patients with acute tetraplegia and sleep disordered breathing. Sleep 2017;40(2):1–6. [DOI] [PubMed] [Google Scholar]
- 32.Dubo H, Delaney G.. 101 Spinal cord injuries due to motor vehicle accidents. Proceedings of the American Spinal Injury Association Annual Meeting 1984:35–8.
- 33.Schueneman AL, Morris J.. Neuropsychological deficits associated with spinal cord injury. Spinal Cord Inj Dig 1982;4(35):64. [Google Scholar]
- 34.Wagner H Jr., Parkinson DR, Madoc-Jones H, Sternick ES, Vrusho K, Krasin F.. Combined effect of diethyldithiocarbamate (DDC) and modest hyperthermia on Chinese hamster (V79) cell survival and DNA strand break repair following photon irradiation. Int J Radiat Oncol Biol Phys 1984;10(9):1575–9. [DOI] [PubMed] [Google Scholar]
- 35.Wecht JM, Weir JP, Radulovic M, Bauman WA.. Effects of midodrine and L-NAME on systemic and cerebral hemodynamics during cognitive activation in spinal cord injury and intact controls. Physiol Rep 2016;4(3):e12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wecht JM, Rosado-Rivera D, Jegede A, Cirnigliaro CM, Jensen MA, Kirshblum S, et al. Systemic and cerebral hemodynamics during cognitive testing. Clin Auton Res 2012;22(1):25–33. [DOI] [PubMed] [Google Scholar]
- 37.Handrakis JP, Liu SA, Rosado-Rivera D, Krajewski M, Spungen AM, Bang C, et al. Effect of mild cold exposure on cognition in persons with tetraplegia. J Neurotrauma 2015;32(15):1168–75. [DOI] [PubMed] [Google Scholar]
- 38.Handrakis JP, Guan ZN, Nulty JW, Tascione O, Rosado-Rivera D, White D, et al. Effect of heat exposure on cognition in persons with tetraplegia. J Neurotrauma 2017;34(24):3372–80. [DOI] [PubMed] [Google Scholar]
- 39.Engelberts NH, Klein M, van der Ploeg HM, Heimans JJ, Jolles J, Kasteleijn-Nolst Trenite DG.. Cognition and health-related quality of life in chronic well-controlled patients with partial epilepsy on carbamazepine monotherapy. Epilepsy Behav 2002;3(4):316–21. [DOI] [PubMed] [Google Scholar]
- 40.Krystal JH, Perry EB Jr., Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, et al. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry 2005;62(9):985–94. [DOI] [PubMed] [Google Scholar]
- 41.Krebs J, Scheel-Sailer A, Oertli R, Pannek J.. The effects of antimuscarinic treatment on the cognition of spinal cord injured individuals with neurogenic lower urinary tract dysfunction: a prospective controlled before-and-after study. Spinal Cord 2018;56(1):22–7. [DOI] [PubMed] [Google Scholar]
- 42.Bombardier CH, Lee DC, Tan DL, Barber JK, Hoffman JM.. Comorbid traumatic brain injury and spinal cord injury: screening validity and effect on outcomes. Arch Phys Med Rehabil 2016;97(10):1628–34. [DOI] [PubMed] [Google Scholar]
- 43.Wood RL. Accelerated cognitive aging following severe traumatic brain injury: a review. Brain Inj 2017;31(10):1270–8. [DOI] [PubMed] [Google Scholar]
- 44.Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, et al. Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull 2014;40(5):1140–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guggenmos M, Schmack K, Sekutowicz M, Garbusow M, Sebold M, Sommer C, et al. Quantitative neurobiological evidence for accelerated brain aging in alcohol dependence. Transl Psychiatry 2017;7(12):1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzourio C, Laurent S, Debette S.. Is hypertension associated with an accelerated aging of the brain? Hypertens 2014;63(5):894–903. [DOI] [PubMed] [Google Scholar]
- 47.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, et al. Cardiac index is associated with brain aging: the framingham heart study. Circ 2010;122(7):690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu PH, Lee GJ, Raven EP, Tingus K, Khoo T, Thompson PM, et al. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J Clin Exp Neuropsychol 2011;33(10):1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inzitari D, Pracucci G, Poggesi A, Carlucci G, Barkhof F, Chabriat H, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. Br Med J 2009;339:b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 51.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wechsler D. Wechsler Abbreviated Scale of Intelligence Manual (WASI). San Antonio (TX): Harcourt Assessment; 1999. [Google Scholar]
- 53.Wechsler D. Wechsler Memory Scale. 3rd ed. San Antonio (TX): The Psychological Corporation; 1997. [Google Scholar]
- 54.Wecht JM, Weir JP, Katzelnick C, Wylie G, Eraifej M, Nguyen N, et al. Cognitive test performance in persons with spinal cord injury: contribution of blood pressure and cerebral blood flow responses. J Neurotrauma (in submission). [Google Scholar]
- 55.Smith A. Symbol Digit Modalities Test: Manual. Los Angeles (CA). Western Psychological Services; 1982. [Google Scholar]
- 56.Chiaravalloti ND, DeLuca J, Moore NB, Ricker JH.. Treating learning impairments improves memory performance in multiple sclerosis: a randomized clinical trial. Mult Scler 2005;11(1):58–68. [DOI] [PubMed] [Google Scholar]
- 57.Wechsler D, editor Wechsler Adult Intelligences Scale-revised Manual. New York (NY): Hartcourt Brace Jovanovich; 1981. [Google Scholar]
- 58.Delis D, Kramer JH, Kaplan E, Ober BA, editors California Verbal Learning Test: Adult Version. San Antonio (TX): The Psychological Corporation; 1987. [Google Scholar]
- 59.Brittain JL, La Marche JA, Reeder KP, Roth DL, Boll TJ.. Effects of age and IQ on paced auditory serial addition task (PASAT) performance. Clin Neuropsychol 1991;5(2):163–75. [Google Scholar]
- 60.Egan V. PASAT: observed correlations with IQ. Pers Indiv Differ 1988;9(1):179–80. [Google Scholar]
- 61.Spreen O, Strauss E, editors. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York (NY): Oxford University Press; 1991. [Google Scholar]
- 62.Gronwall D, Wrightson P.. Memory and information processing capacity after closed head injury. J Neurol Neurosurg Psychiatry 1981;44(10):889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gronwall D, Sampson H, editors. The Psychological Effects of Concussion. New Zealand: Auckland University Press/Oxford University Press; 1974. [Google Scholar]
- 64.Gronwall D, Wrightson P.. Delayed recovery of intellectual function after minor head injury. Lancet 1974;304(7881):605–9. [DOI] [PubMed] [Google Scholar]
- 65.Roman DD, Edwall GE, Buchanan RJ, Patton JH.. Extended norms for the paced auditory serial addition task. Clin Neuropsychol 1991;5(1):33–40. [Google Scholar]
- 66.Delis DC, Kaplan E, Kramer JH, editors. Delis-Kaplan Executive Function System. San Antonio (TX): The Psychological Corporation; 2000. [Google Scholar]
- 67.Chiaravalloti ND, DeLuca J.. The influence of cognitive dysfunction on benefit from learning and memory rehabilitation in MS: a sub-analysis of the MEMREHAB trial. Mult Scler J 2015;21(12):1575–82. [DOI] [PubMed] [Google Scholar]
- 68.Krch D, Sumowski JF, Deluca J, Chiaravalloti N.. Subjective memory in multiple sclerosis is associated with initial-trial learning performance. J Int Neuropsychol Soc 2011;17(3):557–61. [DOI] [PubMed] [Google Scholar]
- 69.Chiaravalloti ND, Moore NB, Nikelshpur OM, Deluca J.. An RCT to treat learning impairment in multiple sclerosis: the MEMREHAB trial. Neurol 2013;81(24):2066–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiaravalloti ND, Sandry J, Moore NB, DeLuca J.. An RCT to treat learning impairment in traumatic brain injury: the TBI-MEM trial. Neurorehabil Neural Repair 2016;30(6):539–50. [DOI] [PubMed] [Google Scholar]
- 71.Jones S, Laukka EJ, Bäckman L.. Differential verbal fluency deficits in the preclinical stages of Alzheimer's disease and vascular dementia. Cortex 2006;42(3):347–55. [DOI] [PubMed] [Google Scholar]
- 72.Capitani E, Rosci C, Saetti MC, Laiacona M.. Mirror asymmetry of category and letter fluency in traumatic brain injury and Alzheimer's patients. Neuropsychologia 2009;47(2):423–9. [DOI] [PubMed] [Google Scholar]
- 73.Salthouse TA. Decomposing age correlations on neuropsychological and cognitive variables. J Int Neuropsychol Soc 2009;15(5):650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc 2010;16(5):754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Postal K, Armstrong K.. Feedback that Sticks: The Art of Effectively Communicating Neuropsychological Assessment Results. New York (NY): Oxford University Press; 2013. [Google Scholar]