Abstract
Aim
Understanding the complex interaction and relative contributions of factors involved in species and trait diversification is crucial to gain insights into the evolution of Neotropical biodiversity. Here, we investigated the drivers of morphological variation in bromeliads along a latitudinal gradient in a biodiversity hotspot.
Location
Atlantic Forest, Brazil.
Taxon
A species complex in the genus Vriesea (Bromeliaceae).
Methods
We measured shape and size variation for 208 floral bracts and 176 leaves in individuals from 14 localities using geometric morphometrics. We compiled data for two chloroplast regions (matK and trnL‐F) from 89 individuals to assess genetic diversity, population structure and phylogenetic relationships. We tested the influence of climate, altitude and genetic distance on morphological traits using linear statistical models.
Results
Temperature seasonality is a main driver of floral bract shape. Together with precipitation, it also explains changes in leaf size across the latitudinal gradient. Shifts in morphological traits are correlated with genetic structure and partly support the recent taxonomic delimitation proposed for the species complex. The species started to diversify in the Pliocene ca. 5 Mya. We detected a phylogeographical break in species distribution into northern and southern clades between the Bocaina region and the southern portion of the Atlantic Forest.
Main Conclusions
We identify how geography and environmental changes through time shape floral bracts and leaves in similar ways. At highly seasonal sites with lower annual precipitation (in the southern subtropical portion of the Atlantic Forest), leaves are larger and floral bracts are wide‐elliptic, making them better suited for increased water accumulation. In contrast, at less seasonal sites (in the tropical north, where rainfall is more abundant and temperatures are higher), leaves are narrower and floral bracts are lanceolate‐shaped, facilitating water drainage. The biogeographical break we identified suggests a role of tectonic activity and climatic oscillations in promoting species divergence and diversification.
Keywords: biogeography, Bromeliaceae, cpDNA, floral bract shape, geometric morphometrics, leaf size, Vriesea
1. INTRODUCTION
The high biodiversity found in the Neotropical region is driven by multiple abiotic and biotic factors occurring simultaneously over different spatial and temporal scales (Antonelli et al., 2018; Antonelli & Sanmartín, 2011). Variation through space and environment can promote adaptive responses in species (Morales, De‐la‐Mora, & Piñero, 2018; Nosil, 2012). When phenotypes are correlated with environmental gradients, these responses often arise by selection (local adaptation), phenotypic plasticity, or some combination, and can influence the patterns of gene flow and distribution (Wanderley et al., 2017). Understanding the complex interaction and relative contributions of factors involved in the diversification of species and traits is crucial to gain insights into the evolution of the Neotropical biota.
In plants, the large variation of leaf shape and size reflects physiological demands imposed by environment (Givnish, 1979; Nicotra et al., 2011), and is observed both among and within species (Morello, Sassone, & López, 2018). Shape and size variability may have distinct patterns in distinct organisms, with size being considered more labile with variation mostly influenced by environment (phenotypic plasticity), while shape can be more conserved and variation usually genetically structured (Cardini & Elton, 2009; Chitwood et al., 2014; Maestri et al., 2016).
Vegetative and reproductive traits can undergo different selective pressures. The lability of vegetative traits often prevails over that found for reproductive ones (Chalcoff, Ezcurra, & Aizen, 2008). Reproductive accessory organs such as showy floral bracts have an important role in pollination, and hence reproductive isolation (Bergamo et al., 2019). Floral bracts can have a variety of colours, shapes and scents decisive to reproductive success, besides their function to protect the flowers and therefore are often more conserved than leaves (Pélabon, Armbruster, & Hansen, 2011). Unlike the poorly studied floral bracts, leaves present a well‐known pattern, where small‐sized leaves are often found in dry and hot places, as well as at high latitudes and elevations to avoid transpirational water loss, while large sized leaves are typical of high humidity and warm conditions (Wright et al., 2017).
Within the Neotropical region, the Atlantic Forest is exceptionally diverse (Myers, Mittermeier, Mittermeier, da Fonseca, & Kent, 2000). The past and present environmental dynamics have a role in biome diversification and current species distribution. Both the Pliocene geological processes and subsequent Pleistocene climatic oscillations and the strong gradient across its latitudinal range may lead to genetic and morphological divergence not only within species, but also within complexes of closely related taxa (Alvares, Stape, Sentelhas, de Moraes, & Sparovek, 2013; Turchetto‐Zolet, Pinheiro, Salgueiro, & Palma‐Silva, 2013). Vriesea (Bromeliaceae) is one of the most representative genera of Atlantic Forest epiphytes (Ramos et al., 2019), where more than 90% of its 225 species occur (BFG, 2018). Recent diversification of the genus has caused low infrageneric resolution and unclear species boundaries (Costa, Gomes‐da‐Silva, & Wanderley, 2014; Gomes‐da‐Silva & Souza‐Chies, 2017).
One of the many species complexes within Vriesea includes V. incurvata Gaudich., V. taritubensis var. taritubensis E. Pereira & I. A. Penna, V. taritubensis var. brevisepala E. Pereira & I. A. Penna, V. taritubensis var. patens B. Neves & A. F. Costa and V. sucrei L. B. Sm & Read. For decades these taxa have been misidentified with individuals generally assigned to the first described V. incurvata (hereafter, ‘V. incurvata complex’). The Vriesea incurvata complex is widely and continuously distributed across the Atlantic Forest. A recent taxonomic revision using morphological multivariate analysis showed a north–south gradient in the width and length of both leaves and floral bracts and identified five taxa, including a new variety V. taritubensis var. patens (Neves, Uribbe, Jacques, Zanella, & Costa, 2018). Here, we aim to assess the drivers of morphological trait variation in the V. incurvata complex along a latitudinal gradient in the Atlantic Forest. We build upon previous work (Neves et al., 2018) using geometric morphometrics and the estimation of genetic structure and diversity. We evaluate shape and size of floral bracts and leaves of taxa in the complex. We use climatic, altitudinal and plastid genetic data to address the roles of both historical gene flow and environment in shaping morphological traits and species limits.
We test for trait variation in accordance with two hypotheses: (a) if shape is less labile than size (Cardini & Elton, 2009; Maestri et al., 2016) and is diagnostic for taxa (Neves et al., 2018), then we expect genetic structure to be a main predictor; (b) if vegetative characters are more labile than reproductive ones (Pélabon et al., 2011), then we expect variation in leaf traits to be better predicted by environmental shifts, while variation in floral bracts should be better predicted by genetic divergence.
We interpret our results in the light of the traditional taxonomic delimitation of the complex and the historical biogeographical patterns that can predict current distribution of taxa. By identifying the factors driving trait variation along the Atlantic Forest, we identify the importance of seasonality and rainfall regime as predictors of plant morphology and the influence of abiotic processes on the Atlantic Forest, including the effect of Pleistocene climatic oscillations on current species distribution and genetic diversity.
2. MATERIALS AND METHODS
2.1. Species and locality description
Taxa within the V. incurvata complex are abundant and widely distributed throughout the south‐eastern and southern part of the Atlantic Forest. Vriesea incurvata is the most widespread species and V. taritubensis, with its three varieties, is restricted to the northern portion of the distribution range. The large populations of V. taritubensis var. brevisepala are mostly concentrated in the Serra dos Órgãos region, Rio de Janeiro state and V. taritubensis var. taritubensis and V. taritubensis var. patens are restricted to the Serra da Bocaina region, Rio de Janeiro and São Paulo states, both mountains are part of the Serra do Mar mountain chain (see Appendix S2: Table S1, Figure 1a). All individuals are epiphytes generally found near rivers and waterfalls, in altitudes varying from 0 to 1,000 m a.s.l., from Rio de Janeiro (22°31′ S) to Rio Grande do Sul (29°44′ S) states (Neves et al., 2018), Brazil. The most notable attribute of these species is their showy red floral bracts covering the inflorescences, filled with a transparent and odourless mucilage. Leaves are highly polymorphic within and among taxa (Neves et al., 2018). Their spiral disposition forms a rosette, and together with leaf sheath size and shape these factors are important for determining the capacity of leaves for water and nutrient storage (Benzing, 2000). Besides the species V. incurvata and V. taritubensis (with three varieties), Neves et al. (2018) distinguished another species, V. sucrei L.B.Sm. & Read, the most divergent in the complex. Its inclusion was needed to determine the position of another binomial involved in the taxonomic revision but we did not include V. sucrei in the present work as it is clearly morphologically distinct.
Figure 1.
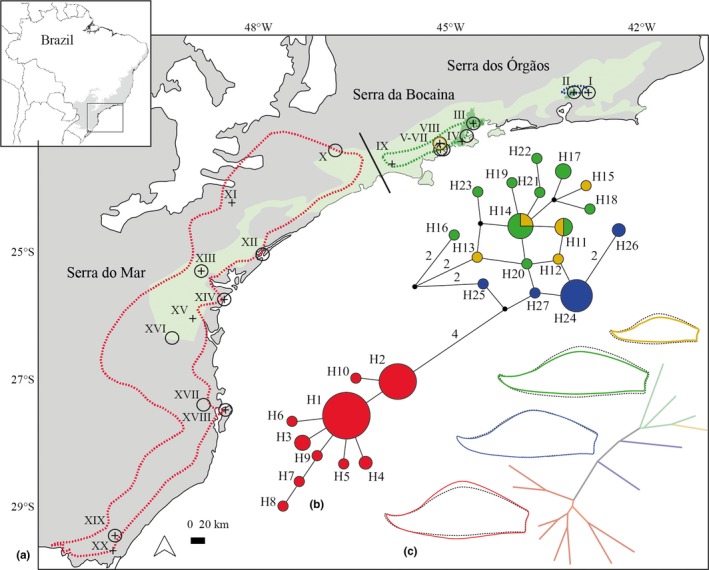
(a) Distribution of taxa based on herbarium records: Vriesea taritubensis var. brevisepala in blue, V. taritubensis var. taritubensis in green, V. taritubensis var. patens in yellow and V. incurvata in red. Sampling localities marked with a circle for morphological data and a cross for genetic data (see codes in Table S1, Appendix S2). The Atlantic Forest (grey), Serra do Mar (light green) and Serra dos Órgãos and Serra da Bocaina (dark green) are shown. The black line shows a major break in species distribution between the Serra da Bocaina region and southern Atlantic Forest. Map of Brazil with the Atlantic Forest area in upper left. (b) A median‐joining network shows two main haplogroups, only V. taritubensis var. taritubensis and V. taritubensis var. patens shared haplotypes; each circle represents one haplotype, with its diameter proportional to its total frequency; more than one mutational step are indicated by numbers. (c) The Neighbour‐joining tree shows the distance in bract shape among localities. The consensus shapes for each taxon (coloured line) and for the whole sampling (black dotted line) display changes from lanceolate to wide‐elliptic [Colour figure can be viewed at http://wileyonlinelibrary.com]
The Atlantic Forest presents high latitudinal (ca. 27°S) and altitudinal (0–2,892 m a.s.l.) variation. Along the study area, the climate ranges from tropical with dry winter at Serra dos Órgãos to humid subtropical with hot summer or temperate summer towards the south, with no distinct dry season. Warmer temperatures are registered in the southeast ranges of both varieties of V. taritubensis, and a cooler and marked seasonal climate is found further south where V. incurvata occurs (Alvares et al., 2013; Ribeiro, Metzger, Martensen, Ponzoni, & Hirota, 2009). Precipitation ranges from 4,000 mm/year in the coast to 1,000 mm/year in inland areas. Higher precipitation levels are registered especially for Serra da Bocaina region (23°S), explained by the orographic effect caused by the mountains, within the range of V. taritubensis var. taritubensis and V. taritubensis var. patens (Table S1, Appendix S2).
2.2. Geometric morphometrics
To explore shape and size variation we sampled and georeferenced individuals from 14 localities, 208 individuals for floral bract and 176 individuals for leaf datasets (Figure 1; Table S1, Appendix S2). We selected localities based on a previous study of herbarium collections (Neves et al., 2018), considering accessibility and availability of individuals. We sampled localities spread out along the whole geographical distribution of species, including the type localities of V. incurvata and V. taritubensis. We randomly sampled 8–19 individuals per locality.
We scanned one floral bract from the middle of the inflorescence and one leaf from the middle of the rosette of each specimen. We then characterized shape by a set of seven landmarks for bracts profile (bracts in side view) and nine for leaves. We discriminate landmarks into anatomical, when located at homologous points with biological meaning; mathematical, when located at extreme points with some geometrical property; and pseudo‐landmarks, when located between other landmarks (Dryden & Mardia, 2016). For bract profile, we placed three anatomical landmarks (1, 2, 5) at the base and apex; two mathematical landmarks (6, 7) and two pseudo‐landmarks (3, 4), at the widest point of the margin and its dorsal opposite and at the midpoint of margin contraction and its dorsal opposite. Such landmarks at the greatest and least width of bract margins reflect greater or lesser cell activity, and their dorsal opposites are needed to minimize the distance among base and tip describing the protuberance they can present (Figure S1a, Appendix S2). The same for leaf, we placed three anatomical landmarks (1, 2, 6) at the sheath base and apex; four mathematical landmarks (3, 4, 8, 9) at sheath widest points and at the transition sheath/blade; and two pseudo‐landmarks (5, 7) at the level 3/4 of blade (Figure S1b, Appendix S2). We used these set of landmarks to better describe the shape of structures without making use of numerous equidistant pseudo‐landmarks along the contour itself (Bensmihen et al., 2008) or the Elliptical Fourier Descriptors (Chitwood & Otoni, 2017), alternatives when homologous points are lacking. To be consistent, a single person (B. Neves) manually digitized the landmarks for each image using TpsDig 2 (Rohlf, 2010). We imported the file containing raw data to MorphoJ 1.06b (Klingenberg, 2011). We then extracted shape information performing a Generalized Procrustes Analysis which removes size, rotation and translation effects (Rohlf & Slice, 1990).
We examined potential bias due to variation within a single individual by testing multiple leaves spirally arranged at the middle of the rosette through a Multivariate Analysis of Variance and Principal Component Analysis (PCA) plot. We also tested for digitization error using Procrustes ANOVA in two sets of data (original and replicated measures of the same leaf) following Klingenberg and McIntrye (1998). We analysed leaves and floral bracts separately, as well as shape and size. We used the centroid size (the square root of the sum of all squared distances from each landmark to the centroid) as a measure of size. We tested allometry by performing a regression analysis between shape and size of each bract and leaf. Because size predicted 47% of leaf shape variation (and 2% for bract), we ran further analyses with the size‐corrected shape matrix resulting from the residuals of the regression. We examined shape and size data performing Procrustes ANOVA, PCA and Canonical Variate Analysis (CVA) (Campbell & Atchley, 1981; Neff & Marcus, 1980), testing for differences among localities and taxa. Due to the marked differentiation in bract shape reflecting the studied taxa, we made a Neighbour‐joining (Saitou & Nei, 1987) tree using Mahalanobis distances, appropriate for morphometric data (Mahalanobis, 1936) and reconstructed the consensus shape for each taxon. All analyses were performed in MorphoJ except for ANOVA and Neighbour‐joining using package ‘vegan’ (Oksanen et al., 2017) in r 3.5.0 (R Core Team, 2018).
2.3. Genetic analyses
To assess genetic diversity and population structure, and to infer the relationships among individuals, we concatenated sequences from two chloroplast regions: trnL‐trnF intron and intergenic spacer and matK gene for 89 individuals in 15 localities. Among the 15 localities, we have morphological data for nine of them. We collected genetic data independently from the morphological data, individuals were randomly sampled according to availability in the field. We used plastidial data (trnL‐trnF and matK) from V. incurvata sequenced by Zanella, Palma‐Silva, Goetze, and Bered (2016) and from 40 new samples of V. taritubensis (Table S1, Appendix S2). Data were deposited in GenBank with accession numbers MN593031 ‐ MN593118. For details about DNA processing and PCR conditions see Zanella et al. (2016). We performed multiple sequence alignment in MUSCLE (Edgar, 2004), implemented in MEGA 7.0 (Kumar, Stecher, & Tamura, 2016). Mononucleotide repeat length variations were excluded and indels of more than 1 bp were coded as single mutational event. Using concatenated sequences, we estimated haplotype (h) and nucleotide (π) diversity (Nei, 1987) for each taxon using ARLEQUIN 3.5.2.2 (Excoffier & Lischer, 2010). The software DnaSP 5.10.01 (Librado & Rozas, 2009) was used to identify haplotypes and calculate distance measures (pairwise F ST; Wright, 1951) for each pair of locality. The pairwise F ST estimatives among localities were displayed in a heat‐map plot using the r package ‘ggfortify’ (Tang, Horikoshi, & Li, 2016). We inferred the genealogical relationships among haplotypes using the median‐joining method (Bandelt, Forster, & Röhl, 1999) with Network 5 (available at http://www.fluxus-engineering.com). We then performed an analysis of molecular variance to assess the partition of genetic diversity among taxa and localities, using ARLEQUIN with 10,000 permutations.
We ran a Bayesian analysis in BEAST 2.5.2 (Bouckaert et al., 2014) to estimate the temporal divergence of species and varieties using the CIPRES Science Gateway 3.3 (Miller, Pfeiffer, & Schwartz, 2010). Alcantarea imperialis (Carrière) Harms, Goudaea chrysostachys (É. Morren) W. Till & Barfuss and Goudaea ospinae (H. Luther) W. Till & Barfuss were included as out‐group. The HKY nucleotide substitution model was used for each marker separately in MEGA. The uncorrelated lognormal relaxed clock and the birth‐death model (Gernhard, 2008) was used to date the tree. Markov chains were run for 150,000,000 steps, sampling every 15,000 steps, totalling 10,000 trees. We used secondary calibration to date the tree based on Givnish et al. (2014) and Kessous et al. (2019): (a) Goudaea + Alcantarea + Vriesea s.s. node (10 ± 2 Mya), (b) Alcantarea + Vriesea s.s. clade (8 ± 2 Mya) as enforced monophyletic group and (c) Vriesea s.s. (5 ± 1.5 Mya). We evaluated that effective sample sizes for all main parameters were above 200 in Tracer 1.6 (Rambaut, Suchard, Xie, & Drummond, 2014). After discarding 10% of the trees as burn‐in we used TreeAnnotator 1.8.4 (Bouckaert et al., 2014) to generate the maximum clade credibility tree. The software FigTree 1.4.2 (Rambaut, 2014) was used to draw the tree.
2.4. Climate and topography
To identify the most important environmental variables in explaining trait variation, we extracted a total of 19 bioclimatic variables from 20 localities (Table S1, Appendix S2). Data were obtained from the Worldclim database (Hijmans, Cameron, Parra, Jones, & Jarvis, 2005) with a spatial resolution of 30 arc‐seconds. In addition, data on altitude were extracted from GPS coordinates in the field. We first performed a PCA with the correlation matrix of all environmental variables to avoid colinearity and overfitting the full model. We used the packages ‘raster’ (Hijmans et al., 2015) to extract climate variables and ‘stats’ to perform a PCA in r. The results of the PCA are described in Appendix S1. Because the first three principal components accounted for 96% of variance and were highly correlated with temperature seasonality (BIO4), annual precipitation (BIO12) and altitude (Table S2, Appendix S2), we proceeded the further analyses using these three variables.
To investigate the correlation of the genetic structure, bioclimatic variables and altitude with morphometric traits variation, we ran multiple linear models and to identify the best model in explaining traits variation, we performed model selection based on AIC (Burnham and Anderson 2002). We ran analyses using the R package ‘stats’ and ‘MuMIn’. We included data only for localities where both genetic and morphological data were collected (9 of the 20 localities Table S1, Appendix S2). We used floral bract shape, floral bract size, leaf shape and leaf size, separately, as response variables. The models were fit with genetic distance across localities, temperature seasonality, annual precipitation and altitude as explanatory factors (response variable ~ pco1gen + pco2gen + temp season + annual prec + alt). To describe shape, we used the regression scores from the size versus shape interaction exported from MorphoJ that captured size‐free information, then we performed a PCA with those regression scores and summarized shape in one value using the first PC (which explained 61% of bract shape and 71% of leaf shape variance) as the descriptor. For size, we used the log‐transformed centroid to control for large variation of size in the sampling (Klingenberg, 2016). To describe genetic distance, we first calculated the pairwise F ST between localities using DnaSP. F ST is frequently used as a measure of distance for depicting genetic variation (Wright, 1951). Then we performed a Principal Coordinate Analysis (PCoA) on the pairwise F ST matrix using the r package ‘ape’ (Paradis, Claude, & Strimmer, 2004) and selected the first two PCo following the broken stick criterion (Jackson, 1993; Figure S2a, Appendix S2). We used the scores of PCo 1 and PCo 2 as the descriptors for genetics. The PCo1 separates V. taritubensis from V. incurvata. The PCo2 separates all taxa, both the two species and V.taritubensis varieties (Figure S2a, Appendix S2). For climate we used the raw values of the least correlated variables showing the highest loadings in the first three axes of PCA (Table S2, Appendix S2). All explanatory variables were standardized to zero for the mean and unit variance. Additionally, we tested for spatial autocorrelation in size and shape of bract and leaf with Moran's correlograms using the r package ‘ncf’. Because significant values were found for bract shape, we corrected for spatial autocorrelation effects by performing Principal Coordinates of Neighbourhood Matrices (PCNM; Borcard & Legendre, 2002) with the r package ‘vegan’. The Euclidean distance matrix of the geographical coordinates from the localities was converted into a truncated distance matrix, focusing on neighbouring sites which is then subject to a PCoA. The PCNM axes were used as explanatory variables for bract shape and the one that showed significant explanatory power (PCNM 1) was then incorporated to the model.
3. RESULTS
3.1. Trait variation
3.1.1. Floral bract
The shape of floral bract significantly discriminated three of the four taxa as currently defined morphologically (df = 3, F = 140.8, p < 2e‐16). We identified a shift from lanceolate to wide‐elliptic along the Atlantic Forest latitudinal gradient. In the north, individuals of V. taritubensis var. brevisepala present elliptic bracts (localities I and II); further south individuals of V. taritubensis var. taritubensis and V. taritubensis var. patens have lanceolate bracts (central localities III‐VIII); and individuals of V. incurvata, spread throughout the southern portion of Atlantic Forest, with wide‐elliptic bracts (localities IX‐XX; Figure 1). Based on the Neighbour‐joining tree of shape, V. incurvata and V. taritubensis var. taritubensis plus V. taritubensis var. patens were the most morphologically distant groups (Figure 1c). The PCA and CVA showed clear divergence in floral bract shape, mainly between V. incurvata and V. taritubensis var. taritubensis plus V. taritubensis var. patens, the last two greatly overlapped in morphological space (Figure 2). The first and second PCs accounted for 61 and 17% of total variation and first and second CVs accounted for 88 and 12% of total variation, respectively. The floral bract shape changes in the first axes of both PCA and CVA analyses are congruent with the consensus taxa shapes of Figures 1c and 2c. Shape changes in CV2 relate to a marked contraction of bract apex and an enlargement of the base, found mostly in individuals of V. taritubensis var. brevisepala (Figure 2b).
Figure 2.
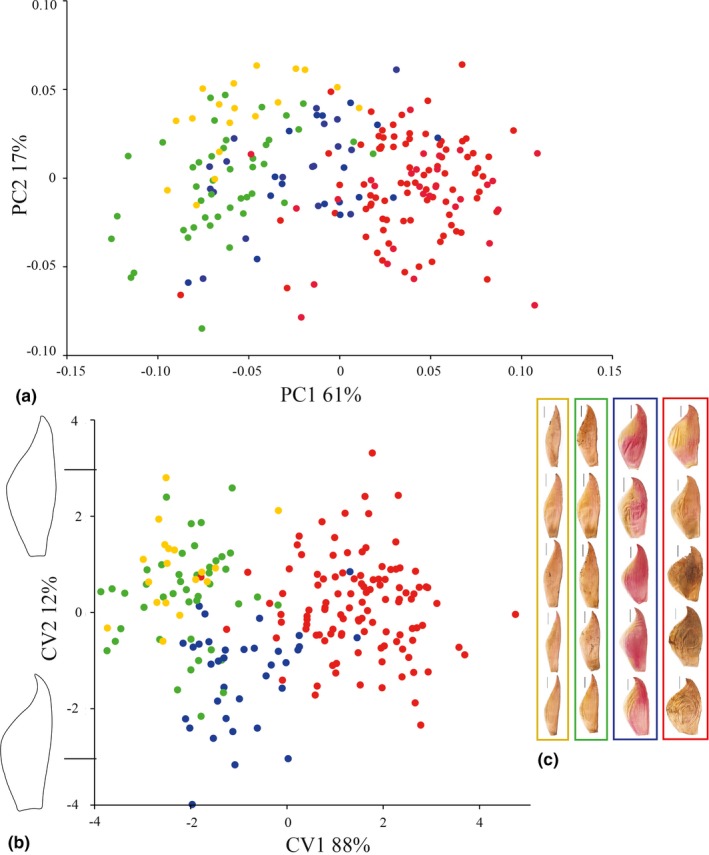
(a) Principal Component Analysis and (b) Canonical Variate Analysis of floral bract landmarks showing taxa divergence. Shape changes in first axes of both analyses are congruent with the consensus taxa shapes in Figure 1. Shape changes in CVA axis 2 relate to a marked contraction in bract apex and an enlargement of the base, found mostly in the blue group. Percentage of total variance for each axis is indicated in Vriesea taritubensis var. patens in yellow, Vriesea taritubensis var. taritubensis in green, V. taritubensis var. brevisepala in blue and V. incurvata in red. (c) A subset of bract profiles for each taxon showing lanceolate, elliptic and wide‐elliptic shape, from the left to the right. [Colour figure can be viewed at http://wileyonlinelibrary.com]
There was significant difference in bract size among taxa (df = 3, F = 3.48, p = .0168) and among localities (df = 13, F = 2.03, p = .0199). Differences in size separated V. taritubensis var. patens, with the smallest bracts (locality VIII), of V. incurvata, with the largest ones. The locality III presented the longest bracts and localities X‐XX had the largest medium centroid size due to the wider bracts (Figure 3a).
Figure 3.
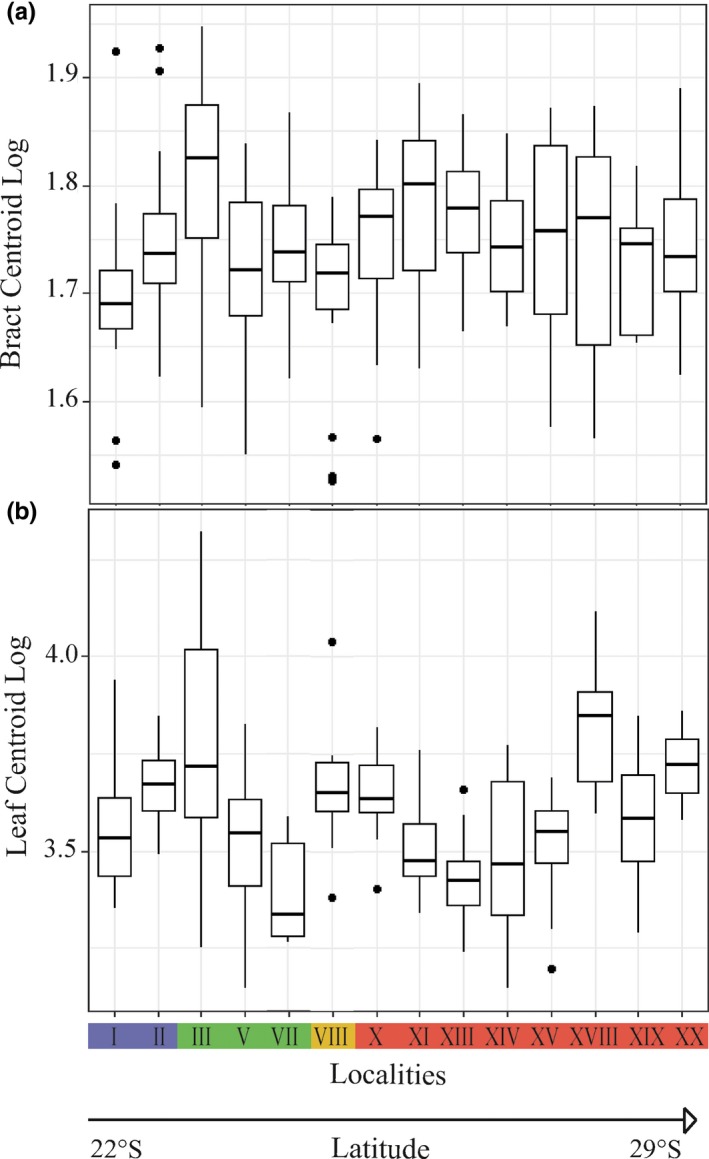
Boxplots of centroid size variation of (a) bracts and (b) leaves in a latitudinal gradient across 14 localities. Vriesea taritubensis var. brevisepala in blue, V. taritubensis var. taritubensis in green, Vriesea taritubensis var. patens in yellow and V. incurvata in red. For localities number see Table S1 in Appendix S2 [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.1.2. Leaf
A significant difference in leaf shape between taxa (df = 3, F = 4.919, p = .00265) and localities (df = 13, F = 2.87, p = .000945) was indicated. The PCA showed shape changes from narrow‐obovate to linear along the first PC, which accounted for 75% of the total variance (Figure 4). Despite the overlap due to the high variation within and between localities, we detected a gradual change pointing to the separation of V. taritubensis var. brevisepala that represents an enlargement of leaf sheaths, from large‐obovate to elliptic (see first CV of CVA in Figure 4b).
Figure 4.
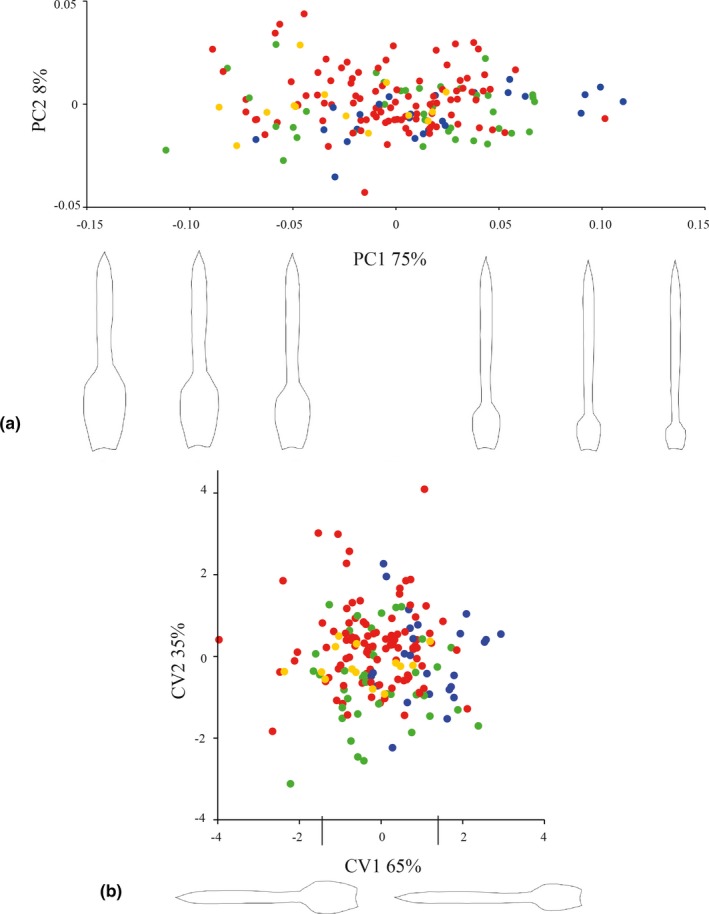
(a) Principal Component Analysis and (b) Canonical Variate Analysis computed on leaf landmarks. Variation of shape from narrow‐obovate to linear is shown along the first PC. The first CV relate to a change of leaf sheaths from large‐obovate to elliptic which separate blue and red groups. Percentage of total variance for each axis is indicated. Vriesea taritubensis var. taritubensis in green, Vriesea taritubensis var. patens in yellow, V. taritubensis var. brevisepala in blue and V. incurvata in red [Colour figure can be viewed at http://wileyonlinelibrary.com]
Leaf size did not discriminate taxa (df = 3, F = 0.603, p = .614), but differed among localities (df = 13, F = 7.24, p < .0001). The strongest differentiation was among the individuals of V. taritubensis var. taritubensis (with the largest leaves found in locality III and the smallest in locality VII) and within V. incurvata (locality XIII presents the smallest, and localities XVIII and XX the largest leaves) (Figure 3b). Changes in size translated into long, narrow leaves in V. taritubensis var. taritubensis and V. taritubensis var. patens and short and wide leaves in V. incurvata.
3.2. Genetic structure and diversity
We identified 24 polymorphic sites (8 transitions, 4 transversions and 12 indels) resulting in 27 haplotypes from the cpDNA dataset (Table S1, Appendix S2). Vriesea incurvata, the most widespread taxon, presented the highest number of haplotypes, followed by Vriesea taritubensis var. taritubensis. The four taxa showed high haplotype and nucleotide diversity (Table 1). The main source of genetic variation was found among taxa (79%), followed by 17% within localities and 2% among localities within taxa (Table S3, Appendix S2). We found high genetic differentiation among taxa through pairwise F ST estimates, excepted between V. taritubensis var. taritubensis and V. taritubensis var. patens that showed low genetic differentiation (Table 2). On the other hand, low genetic distance across localities within each taxon was observed (Figure S2, Appendix S2). Two main haplogroups were found: one corresponding to V. incurvata, another to V. taritubensis. A subgroup can also be identified dividing V. taritubensis var. brevisepala from V. taritubensis var. taritubensis and V. taritubensis var. patens (Figure 1b). Haplotype H2 from V. incurvata is separated from H25 and H27 belonging to V. taritubensis var. brevisepala by five mutational steps and one median vector. A slight divergence was found among the varieties of V. taritubensis (Figure 1b). V. taritubensis var. taritubensis and V. taritubensis var. patens shared two haplotypes (H11 and H14, Figure 1b).
Table 1.
Number of specimens (N), number of haplotypes (NH), haplotype diversity (h) and nucleotide diversity (π) for each taxon and total sampling
| Taxa | N | NH | h (SD) | π (SD) |
|---|---|---|---|---|
| V. incurvata | 49 | 10 | 0.6922 (0.0499) | 0.000404 (0.000299) |
| V. taritubensis var. taritubensis | 18 | 9 | 0.8627 (0.0609) | 0.000672 (0.000453) |
| V. taritubensis var. brevisepala | 16 | 4 | 0.4417 (0.1446) | 0.000350 (0.000282) |
| V. taritubensis var. patens | 6 | 6 | 1.0000 (0.0962) | 0.001299 (0.000886) |
| Total | 89 | 27 | 0.8828 (0.0207) | 0.001802 (0.000982) |
Table 2.
Pairwise genetic divergence (F ST values) for Vriesea incurvata complex species based on plastid sequence data (trnL‐trnF + matK)
| V. incurvata | V. taritubensis var. taritubensis | V. taritubensis var. brevisepala | V. taritubensis var. patens | |
|---|---|---|---|---|
| V. incurvata | ||||
| V. taritubensis var. taritubensis | 0.85024 | |||
| V. taritubensis var. brevisepala | 0.85073 | 0.62717 | ||
| V. taritubensis var. patens | 0.82459 | 0.09474 | 0.48859 |
The p‐values determined by permutation test with 10,000 permutations are all significant.
The Bayesian consensus tree showed that V. taritubensis and V. incurvata likely diverged around 5 million years ago (Mya) during Late Quaternary (Pliocene), and V. taritubensis varieties more recently (Figure 5). Two well‐supported clades comprised both V. taritubensis (PP = 0.99) and V. incurvata (PP = 1) specimens. Vriesea taritubensis individuals tend to cluster in two major subclades splitting V. taritubensis var. taritubensis and V. taritubensis var. brevisepala, with few exceptions. While individuals V. taritubensis var. patens are spread along the two subclades. However, the clade comprising most individuals from V. taritubensis var. taritubensis lacks strong posterior probability support (Figure 5).
Figure 5.
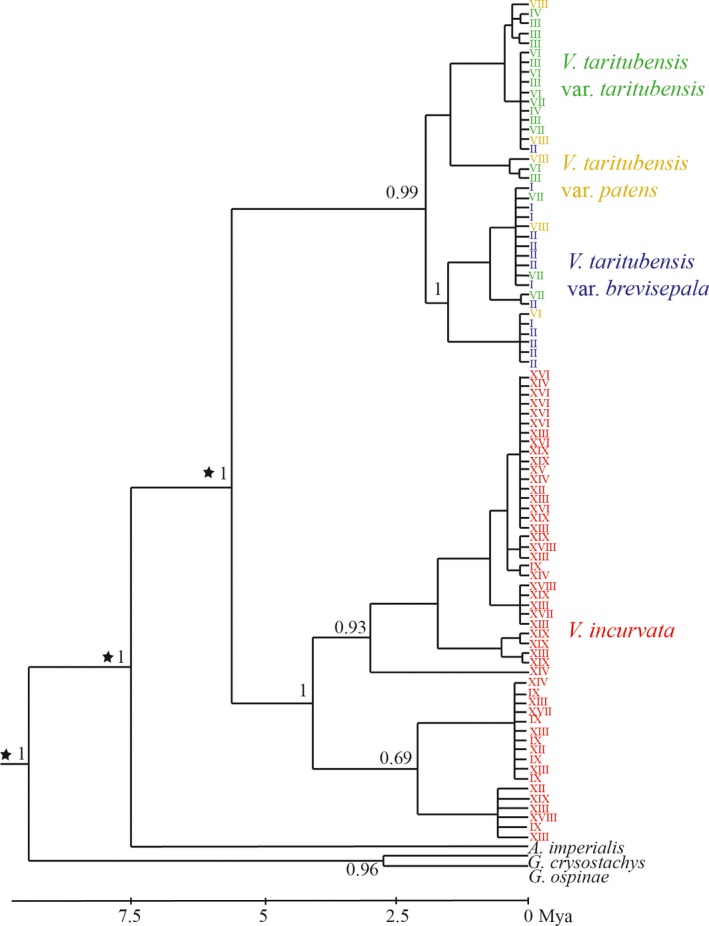
Maximum Clade Credibility tree from BEAST based on trnL‐trnF and matK sequences with posterior probabilities above 0.8 shown for the main clades. Two major clades splitting V. taritubensis (green, yellow and blue) and V. incurvata (red) specimens are recovered. Stars represent calibration points. For localities number see Table S1 in Appendix S2 [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.3. Predictors of morphological variation
Temperature seasonality and genetics were the main drivers of floral bract shape and together explained a large proportion of the trait variance (66%). Annual precipitation, temperature seasonality and genetics were recovered as drivers of leaf size changes and our best fit model for leaf size explained 28% of overall variation. Only a small portion of the variation was predicted by bract size and leaf shape models (6% and 9%, respectively) and both traits correlated with genetics and altitude (Table 3). For complete information on best models selected see Table S4, Appendix S2.
Table 3.
Results of the best models selected based on AIC to explain morphological trait variation in Vriesea incurvata complex along the Atlantic Forest
| Full initial model: response variable ~ tempseason + annualprec + alt + pco1gen + pco2gen | ||||
|---|---|---|---|---|
| Selected model: bractshape ~ tempseason + pco2gen | R 2adj = 0.66 | |||
| Estimate | SE | Pr(>t) | Sample size = 122 | |
| tempseason | 0.5004 | 0.1243 | 0.0001*** | |
| pco2gen | 0.3158 | 0.0534 | <0.0001*** | |
| Selected model: bractsize ~ annualprec + alt + pco2gen | R 2adj = 0.06 | |||
| Estimate | SE | Pr(>t) | Sample size = 122 | |
| annualprec | −0.2290 | 0.1325 | 0.0864 | |
| alt | −0.2409 | 0.0925 | 0.0104 * | |
| pco2gen | −0.3147 | 0.1290 | 0.0162 * | |
| Selected model: leafshape ~ alt + pco2gen | R 2adj = 0.09 | |||
| Estimate | SE | Pr(>t) | Sample size = 109 | |
| alt | −0.2557 | 0.0917 | 0.00625 ** | |
| pco2gen | 0.2431 | 0.0917 | 0.00922 ** | |
| Selected model: leafsize ~ tempseason + annualprec + pco1gen + pco2gen | R 2 adj = 0.28 | |||
| Estimate | SE | Pr(>t) | Sample size = 122 | |
| tempseason | −0.6515 | 0.1990 | 0.00143 ** | |
| annualprec | −0.6104 | 0.1233 | <0.0001*** | |
| pco1gen | −0.3656 | 0.2068 | 0.0799 | |
| pco2gen | −0.4916 | 0.1168 | <0.0001*** | |
The variance explained by the selected model for each response variable is given (R 2). The explaining factors are marked according to significance level (*0.01, **0.001, ***0). In bract shape model we included PCNM1 to control for spatial autocorrelation (not shown, see methods section). The full initial model included all explanatory factors: tempseason, temperature seasonality; annualprec, annual precipitation; alt, altitude; pco1gen, PCo 1 of genetics; pco2gen, PCo 2 of genetics.
4. DISCUSSION
We used an interdisciplinary approach to investigate the factors underlying morphological trait variation in bromeliads across a latitudinal gradient in the Atlantic Forest. Temperature seasonality is the main driver of floral bract shape and together with annual precipitation explains leaf size variation. The genetic structure (Figure 1b and Figure S2a) correlates with morphological trait variation and all models indicate an influence of genetic inheritance (Table 3). This finding rejects our two hypotheses as follows (a) vegetative and reproductive characters are both labile and (b) shape is not more conserved than size, and they both vary with environmental heterogeneity. Our results also reveal a north–south phylogeographical break between the Bocaina region and southern portion of Atlantic Forest (Figures 1 and 5), from where V. taritubensis and V. incurvata likely diverged around 5 Mya. Divergence was likely a consequence of tectonic events that continued shaping the complex topography of the Serra do Mar at that time, resulting in marked habitat heterogeneity (Brunes, Sequeira, Haddad, & Alexandrino, 2010; Ribeiro, 2006).
4.1. Climatic drivers of morphological traits
We found clear trends in shape and size that are congruent with climatic variation along the Atlantic Forest latitudinal gradient (Figure 1). Floral bracts are wide‐elliptic and leaf sizes larger (wider blades and sheaths) in localities at highly seasonal sites with lower precipitation levels (V. incurvata distribution) in the southern, subtropical portion of the Atlantic Forest (Figures 1, 2, 3 and 4). This phenotype relates to bracts and rosettes with greater capacity to intercept and accumulate water, ensuring survival in dryer periods. In contrast, our data show lanceolate bracts and narrower leaf blades and sheaths in localities at the Bocaina region (V. taritubensis var. taritubensis and V. taritubensis var. patens distribution), where climate is hot most of the year and annual precipitation reaches the highest levels in the studied area (up to 2,587 mm). The high rainfall availability throughout the year selects for narrow bracts and leaf blades, behaving like gutters for water drainage. An intermediate morphology of elliptic bracts is found at the northernmost distribution (V. taritubensis var. brevisepala distribution), where annual precipitation is moderate (ranging from 1,336 to 1,740 mm) and there is little seasonal temperature variation (Figures 1 and 2).
The size and shape of leaves have been widely associated with temperature and moisture variables (Nicotra et al., 2011; Royer, Wilf, Janesko, Kowalski, & Dilcher, 2005; Wright et al., 2017). A global assessment of climatic drivers of leaf size demonstrated no effective thermal constraint acting in warm and ever‐wet tropical climates, as sufficient water is commonly available for transpirational cooling and plants are not exposed to frost damage (Wright et al., 2017). At a small scale and focusing on a clade of understorey epiphytes in a humid mountain tropical forest, we still detect climate influencing leaf size changes (Table 3). Besides considering leaf area itself, bromeliad tanks store water that is then available for a longer time period than a single rainfall event. Being large leaved at seasonal sites with lower mean annual precipitation is advantageous if this size translates into wider blades and sheaths to form a robust tank to retain water. Our results contrast to the pattern of a decrease in size at sites with lower rainfall and hotter temperatures with increased irradiance (Peppe et al., 2011; Ribeiro et al., 2016; Wright et al., 2017), but is consistent if interpreted in a more comprehensive way regarding plant architecture. Furthermore, the rainwater drainage capacity of the narrow‐leaved plants generally found in humid regions can prevent leaf surface water retention, which can affect gas exchange and inhibit photosynthesis (Benzing, 2000; Martin & Siedow, 1981). Bromeliads show a large variety of leaf shape and size and tank forms (Benzing, 2000). We hypothesize the pattern we found here to occur in another epiphytic tank‐forming bromeliads which are widely distributed across Atlantic Forest.
We show bract shape changes follow similar trends as leaf size in being strongly correlated with temperature seasonality (Table 3; Figures 1 and 2). Contrary to our findings, Pélabon et al. (2011) found leaf size to be more sensitive to environment than floral bract size. The bracts of V. incurvata complex are leaf‐like, boat‐shaped and distichally arranged along the inflorescence axis. Such display is similar to the water impound tank of most bromeliads and likely controls flower humidity, reflecting the similar pattern of variation found for the leaves.
Floral bract size and leaf shape variation were poorly explained by our models (Table 3). Both traits show high local and intraspecific variation that could be influenced by other abiotic local conditions and/or biotic interaction, or still by stochastic factors not accounted for in this study. Leaf shape is shown to be driven by both evolutionary and environmental forces (Chitwood & Sinha, 2016). We identified some influence of both genetic structure and climate driving leaf shape, despite the diffuse pattern recovered among taxa and along the latitudinal gradient in the forest (Table 3, Figure 4). Finally, most morphometric studies assessing leaf shape variation have been focused on individual taxa and eudicotyledons net‐veined leaves with distinct petioles (Royer, Meyerson, Robertson, & Adams, 2009). Our study is one of the first to present an integrative and ecological assessment of shape of leaves and floral bracts in bromeliads and, as far as we know, in monocotyledons.
4.2. Genetic structure and diversity reflect morphological and taxon divergence
Our models show a genetic component for all the traits tested (Table 3) and genetic structure corresponds with morphological trait variation in the V. incurvata complex. We found strong genetic structure among taxa with the exception of V. taritubensis var. taritubensis and V. taritubensis var. patens, which show low genetic differentiation (Table 2, Figures 1 and 5). Vriesea incurvata and V. taritubensis started to diverge in the Pliocene (5 Mya; Figure 5) and probably experienced a long period of genetic isolation in consequence of geographical and climatic barriers, and/or influenced by low or absent gene flow.
On the other hand, the V. incurvata and V. taritubensis varieties showed moderate to high intraspecific genetic diversity and intraspecific gene flow (Table 1; Tables S1 and S3, Figure S2, Appendix S2), as has been observed for other bromeliad species (Goetze, Zanella, Palma‐Silva, Büttow, & Bered, 2017; Palma‐Silva et al., 2009; Zanella et al., 2016). The low genetic variation among localities within taxa suggests that intra‐taxon gene flow has been maintained (Figure S2, Appendix S2). The V. incurvata complex is pollinated by hummingbirds such as Phaethornis eurynome, generalist and non‐territorial long‐distance dispersers (Machado & Semir, 2006). Seeds are wind dispersed, which may also facilitate the maintenance of gene flow and localities connectivity.
We show floral bract shape and chloroplast divergence support different taxa, except for V. taritubensis var. taritubensis and V. taritubensis var. patens that share haplotypes and the same floral bract shape (Figure 1). The two species V. incurvata and V. taritubensis are clearly distinct from each other and the varieties of V. taritubensis are not monophyletic (Figure 5). Our results partly corroborate the recent taxonomic circumscription proposed by Neves et al. (2018) using traditional morphometrics.
The new variety V. taritubensis var. patens proposed by these authors was described based on size and orientation of the inflorescence. We found three exclusive haplotypes for V. taritubensis var. patens and a difference in size separating it from V. incurvata (Figures 1b and 3a). Our PCoA results based on pairwise F ST distance across all localities also showed a separation of V. taritubensis var. patens (locality VIII) along PCo 2 (Figure S2a, Appendix 1). Despite this lack of evidence we find here for V. taritubensis var. patens, we suggest taxonomic decisions be made using more robust molecular data.
4.3. Phylogenetic and biogeographical patterns in the Vriesea incurvata complex
We identify a north–south phylogeographical break between the Bocaina region and southern Atlantic Forest (Figures 1 and 5). The divergence between V. incurvata and V. taritubensis started around 5 Mya, followed by the split of the V. taritubensis varieties around 2.5 Mya, during the Pliocene–Early Pleistocene (Figure 5). This temporal framework is in accordance with that estimated by Kessous et al. (2019) for Vriesea species diversification (starting ca. 3 Mya and increasing during Pleistocene) and also coincides with the divergence of other widely distributed Atlantic Forest lineages such as reptiles and amphibians (Brunes et al., 2010; Fitzpatrick, Brasileiro, Haddad, & Zamudio, 2009; Grazziotin, Monzel, Echeverrigaray, & Bonatto, 2006).
The topography of the Brazilian east coast, specifically the Serra do Mar mountains, was formed during the Cretaceous and has been continuously modified by tectonic activity and erosional processes until the Pliocene (ca. 5–3 Mya) (Almeida, 1976). These geological processes together with the subsequent Pleistocene climatic oscillations likely facilitated species divergence (e.g. Turchetto‐Zolet et al., 2013).
The central region of the Atlantic Forest was recognized as a “hotspot within a hotspot” based on palaeoclimatic data (Carnaval, Hickerson, Haddad, Rodrigues, & Moritz, 2009). The phylogeographical break that split V. incurvata and V. taritubensis occurs in this region (between 15° and 23°S) and was reported for other Vriesea species, although at different locations (Palma‐Silva et al., 2009; Zanella, 2013). We suggest the break to be the result of increased topographic discontinuity of the Serra do Mar mountains, which led to geographical isolation. Furthermore, the central Atlantic Forest is a convergence zone of numerous distinct bromeliad lineages and is highly species rich (Fontoura, Scudeller, & Costa, 2012), consistent with the higher biodiversity levels found in the central portion (Carnaval et al., 2009).
Pollen data from Holocene vegetation support the existence of historical refugia in the Serra da Bocaina and Serra dos Órgãos (Behling, Dupont, Safford, & Wefer, 2007; Behling & Safford, 2010), where three varieties of V. taritubensis are distributed. Niche models for V. incurvata showed climatic stability of the southern portion of the Atlantic Forest during Last Glacial Maximum (Aguiar‐Melo et al., 2019). Lineages in the V. incurvata complex have likely survived in stable areas during the more recent climatic oscillations of Quaternary and from there colonized its current range.
5. CONCLUSIONS
Our findings identify the role of climate, genetics and topography in shaping morphological traits in the Atlantic Forest. We provide a broad‐scale quantification of trait variability at both inter‐ and intraspecific levels. Importantly, we show that reproductive (floral bracts) and vegetative (leaves) traits respond in similar ways to abiotic factors, contradicting previous suggestions. Temperature seasonality and rainfall regime are associated with the morphological diversification of these bromeliads as they drive traits variation that are diagnostic for taxa and have an ecological role. Morphological variation and genetic structure patterns coincided with recurrent biogeographical breaks in space (central region) and time (Late Pliocene) with other widely distributed Atlantic Forest organisms. We shed new light on the species delimitation of one of many species complexes of morphologically similar taxa within Vriesea. Because the Atlantic Forest is highly threatened and suffers continued habitat destruction, studies like this help to unveil combined evolutionary and ecological dynamics generating species diversification, before much biodiversity is lost.
BIOSKETCH
Beatriz Neves is interested in the evolution and diversification of bromeliads in the Neotropics. The research team studies systematics of Bromeliaceae, population genetics of Neotropical plants, and the past, present and future of biodiversity.
Author contributions: B.N. collected the morphological data, and C.M.Z. the genetic data. A.F.C., B.N., C.D.B. and A.A. conceived the ideas. B.N. and C.M.Z. performed the analyses. I.M.K. built the phylogeny. All authors contributed to interpreting and discussing results and writing the manuscript.
Supporting information
ACKNOWLEDGEMENTS
We thank Simon Mayo, Renan Maestri, and members of the Antonelli Lab for feedback and discussions, Camila Ritter, Vinícius Nunes Lima, Paulo Paiva and Søren Faurby for statistical assistance, and Museu Nacional of Universidade Federal do Rio de Janeiro for infrastructural support for field collections. The study was supported by the Coordenação de Aperfeiçoamento Pessoal—Brasil (CAPES)—Finance Code 001 to B.N. (data collected during masters, and analysed during PhD, PROTAX II 88887.199918/2018‐00), I.M.K. (PROTAX II 88887.199917/2018‐00) and F.P.U., the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) through a post‐doc fellowship for C.M.Z. (E‐26/201.854/2015), the European Research Council under the European Union's Seventh Framework Programme (FP/2007‐2013, ERC Grant Agreement 331024), the Swedish Foundation for Strategic Research, the Swedish Research Council and a Wallenberg Academy Fellowship to A.A., and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to A.F.C. (478345/2013‐5 and 305704/2018‐4).
Neves B, Zanella CM, Kessous IM, et al. Drivers of bromeliad leaf and floral bract variation across a latitudinal gradient in the Atlantic Forest. J Biogeogr. 2020;47:261–274. 10.1111/jbi.13746
Beatriz Neves and Camila M. Zanella shared first authorship.
Handling Editor: Simon Scheiter
DATA AVAILABILITY STATEMENT
The data that support the findings of this study will be openly available in the DRYAD Digital Repository. Neves, B., Zanella, C. M., Kessous, I. M., Uribbe, F. P., Salgueiro, F., Bered, F., Antonelli, A., Bacon, C. D., & Costa, A. F. (2019). Seasonality drives bromeliad leaf and floral bract variation across a latitudinal gradient in the Atlantic Forest. (https://doi.org/10.5061/dryad.sj3tx9619).
REFERENCES
- Aguiar‐Melo, C. , Zanella, C. M. , Goetze, M. , Palma‐Silva, C. , Hirsch, L. D. , Neves, B. , … Bered, F. (2019). Ecological niche modeling and a lack of phylogeographic structure in Vriesea incurvata suggest historically stable areas in the southern Atlantic Forest. American Journal of Botany, 106, 971–983. 10.1002/ajb2.1317 [DOI] [PubMed] [Google Scholar]
- Almeida, F. F. M. (1976). The system of continental rifts bordering the Santos basin. Anais Da Academia Brasileira De Ciências, 48, 15–26. [Google Scholar]
- Alvares, C. A. , Stape, J. L. , Sentelhas, P. C. , De Moraes, J. L. , & Sparovek, G. (2013). Köppen's climate classification map for Brazil. Meteorologische Zeitschrift, 22, 711–728. 10.1127/0941-2948/2013/0507 [DOI] [Google Scholar]
- Antonelli, A. , Ariza, M. , Albert, J. , Andermann, T. , Azevedo, J. , Bacon, C. , … Edwards, S. (2018). Conceptual and empirical advances in Neotropical biodiversity research. PeerJ, 6, e5644 10.7717/peerj.5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli, A. , & Sanmartín, I. (2011). Why are there so many plant species in the Neotropics? Taxon, 60, 403–414. 10.1002/tax.602010 [DOI] [Google Scholar]
- Bandelt, H. J. , Forster, P. , & Röhl, A. (1999). Median‐joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution, 16, 37–48. 10.1093/oxfordjournals.molbev.a026036 [DOI] [PubMed] [Google Scholar]
- Behling, H. , Dupont, L. , Safford, H. D. , & Wefer, G. (2007). Late Quaternary vegetation and climate dynamics in the Serra da Bocaina, southeastern Brazil. Quaternary International, 161, 22–31. 10.1016/j.quaint.2006.10.021 [DOI] [Google Scholar]
- Behling, H. , & Safford, H. D. (2010). Late‐glacial and Holocene vegetation, climate and fire dynamics in the Serra dos Órgãos, Rio de Janeiro State, southeastern Brazil. Global Change Biology, 16, 1661–1671. 10.1111/j.1365-2486.2009.02029.x [DOI] [Google Scholar]
- Bensmihen, S. , Hanna, A. I. , Langlade, N. B. , Micol, J. L. , Bangham, A. , & Coen, E. S. (2008). Mutational spaces for leaf shape and size. HFSP Journal, 2, 110–120. 10.2976/1.2836738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing, D. H. (2000). Bromeliaceae: Profile of an adaptive radiation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Bergamo, P. J. , Wolowski, M. , Telles, F. J. , Brito, V. L. G. , Varassini, I. G. , & Sazima, M. (2019). Bracts and long‐tube flowers of hummingbird‐pollinated plants are conspicuous to hummingbirds but not to bees. Biological Journal of the Linnean Society, 126, 533–544. 10.1093/biolinnean/bly217 [DOI] [Google Scholar]
- BFG . (2018). Brazilian Flora 2020: Innovation and collaboration to meet Target 1 of the Global Strategy for Plant Conservation (GSPC). Rodriguésia, 69, 1513–1527. [Google Scholar]
- Borcard, D. , & Legendre, P. (2002). All‐scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecological Modellling, 153, 51–68. 10.1016/S0304-3800(01)00501-4 [DOI] [Google Scholar]
- Bouckaert, R. , Heled, J. , Kuhnert, D. , Vaughan, T. , Wu, C. H. , Xie, D. , … Drummond, A. J. (2014). BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Computational Biology, 10, 1–6. 10.1371/journal.pcbi.1003537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunes, T. O. , Sequeira, F. , Haddad, C. F. B. , & Alexandrino, J. (2010). Gene and speciation trees of a Neotropical group of treefrogs: Genetic diversification in the Brazilian Atlantic forest and the origin of a polyploid species. Molecular Phylogenetics and Evolution, 57, 1120–1133. 10.1016/j.ympev.2010.08.026 [DOI] [PubMed] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information-theoretic approach. New York, NY: Springer-Verlag. [Google Scholar]
- Campbell, N. A. , & Atchley, W. R. (1981). The geometry of canonical variate analysis. Systematic Zoology, 30, 268–280. 10.2307/2413249 [DOI] [Google Scholar]
- Cardini, A. , & Elton, S. (2009). Geographical and taxonomic influences on cranial variation in red colobus monkeys (Primates, Colobinae): Introducing a new approach to ‘morph’ monkeys. Global Ecology and Biogeography, 18, 248–263. 10.1111/j.1466-8238.2008.00432.x [DOI] [Google Scholar]
- Carnaval, A. C. , Hickerson, M. J. , Haddad, C. F. B. , Rodrigues, M. T. , & Moritz, C. (2009). Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science, 323, 785–789. 10.1126/science.1166955 [DOI] [PubMed] [Google Scholar]
- Chalcoff, V. R. , Ezcurra, C. , & Aizen, M. A. (2008). Uncoupled geographical variation between leaves and flowers in a South‐Andean Proteaceae. Annals of Botany, 102, 79–91. 10.1093/aob/mcn057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. , & Otoni, W. C. (2017). Morphometric analysis of Passiflora leaves: The relationship between landmarks of the vasculature and elliptical Fourier descriptors of the blade. GigaScience, 6, giw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. , Ranjan, A. , Martinez, C. C. , Headland, L. R. , Thiem, T. , Kumar, R. , … Downs, N. (2014). A modern ampelography: A genetic basis for leaf shape and venation patterning in grape. Plant Physiology, 164, 259–272. 10.1104/pp.113.229708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D. H. , & Sinha, N. R. (2016). Evolutionary and environmental forces sculpting leaf development. Current Biology, 26, 297–306. 10.1016/j.cub.2016.02.033 [DOI] [PubMed] [Google Scholar]
- Costa, A. F. , Gomes‐da‐Silva, J. , & Wanderley, M. G. L. (2014). Vriesea (Bromeliaceae, Tillandsioideae): Taxonomic history, and morphology of the Brazilian lineage. Journal of the Torrey Botanical Society, 141, 338–352. 10.3159/torrey-d-13-00070.1 [DOI] [Google Scholar]
- Dryden, I. L. , & Mardia, K. V. (2016). Statistical shape analysis: With applications in R. Chichester, UK: John Wiley & Sons. [Google Scholar]
- Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier, L. , & Lischer, H. E. (2010). Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources, 10, 564–567. 10.1111/j.1755-0998.2010.02847.x [DOI] [PubMed] [Google Scholar]
- Fitzpatrick, S. W. , Brasileiro, C. A. , Haddad, C. F. B. , & Zamudio, K. R. (2009). Geographical variation in genetic structure of an Atlantic Coastal Forest frog reveals regional differences in habitat stability. Molecular Ecology, 18, 2877–2896. 10.1111/j.1365-294X.2009.04245.x [DOI] [PubMed] [Google Scholar]
- Fontoura, T. , Scudeller, V. V. , & da Costa, A. F. (2012). Floristics and environmental factors determining the geographic distribution of epiphytic bromeliads in the Brazilian Atlantic Rain Forest. Flora‐Morphology, Distribution, Functional Ecology of Plants, 207, 662–672. 10.1016/j.flora.2012.05.003 [DOI] [Google Scholar]
- Gernhard, T. (2008). The conditioned reconstructed process. Journal of Theoretical Biology, 253, 769–778. 10.1016/j.jtbi.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Givnish, T. J. (1979). On the adaptive significance of leaf form In Solbrig O. T., Jain S., Johnson G. B., & Raven P. H. (Eds.), Topics in plant population biology (pp. 375–407). New York, NY: Columbia University Press. [Google Scholar]
- Givnish, T. J. , Barfuss, M. H. J. , Ee, B. V. , Riina, R. , Schulte, K. , Horres, R. , … Sytsma, K. J. (2014). Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Molecular Phylogenetics and Evolution, 71, 55–78. 10.1016/j.ympev.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Goetze, M. , Zanella, C. M. , Palma‐Silva, C. , Büttow, M. V. , & Bered, F. (2017). Incomplete lineage sorting and hybridization in the evolutionary history of closely related, endemic yellow‐flowered Aechmea species of subgenus Ortgiesia (Bromeliaceae). American Journal of Botany, 104, 1073–1087. 10.3732/ajb.1700103 [DOI] [PubMed] [Google Scholar]
- Gomes‐da‐Silva, J. , & Souza‐Chies, T. T. (2017). What actually is Vriesea? A total evidence approach in a polyphyletic genus of Tillandsioideae (Bromeliaceae, Poales). Cladistics, 2017, 1–19. [DOI] [PubMed] [Google Scholar]
- Grazziotin, F. G. , Monzel, M. , Echeverrigaray, S. , & Bonatto, S. L. (2006). Phylogeography of the Bothrops jararaca complex (Serpentes: Viperidae): Past fragmentation and island colonization in the Brazilian Atlantic Forest. Molecular Ecology, 15, 3969–3982. 10.1111/j.1365-294x.2006.03057.x [DOI] [PubMed] [Google Scholar]
- Hijmans, R. J. , Cameron, S. E. , Parra, J. L. , Jones, P. G. , & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978. 10.1002/joc.1276 [DOI] [Google Scholar]
- Hijmans, R. J. , van Etten, J. , Cheng, J. , Mattiuzzi, M. , Sumner, M. , Greenberg, J. A. , … Hijmans, M. R. J. (2015). Package ‘raster’. R package Retrieved from https://cran.rproject.org/web/packages/raster/index.html
- Jackson, D. A. (1993). Stopping rules in principal component analysis: A comparison of heuristical and statistical approaches. Ecology, 74, 2204–2214. 10.2307/1939574 [DOI] [Google Scholar]
- Kessous, I. M. , Neves, B. , Couto, D. R. , Paixão‐Souza, B. , Pederneiras, L. C. , Moura, R. L. , … Costa, A. F. (2019). Historical biogeography of a Brazilian lineage of Tillandsioideae (subtribe Vrieseinae, Bromeliaceae): The Paranaean Sea hypothesized as the main vicariant event. Botanical Journal of the Linnean Society. 10.1093/botlinnean/boz038 [DOI] [Google Scholar]
- Klingenberg, C. P. (2011). MorphoJ: An integrated software package for geometric morphometrics. Molecular Ecology Resources, 11, 353–357. 10.1111/j.1755-0998.2010.02924.x [DOI] [PubMed] [Google Scholar]
- Klingenberg, C. P. (2016). Size, shape, and form: Concepts of allometry in geometric morphometrics. Development Genes and Evolution, 226, 113–137. 10.1007/s00427-016-0539-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg, C. P. , & McIntyre, G. S. (1998). Geometric morphometrics of developmental instability: Analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution, 52, 1363–1375. 10.1111/j.1558-5646.1998.tb02018.x [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado, P. , & Rozas, J. (2009). DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics, 25, 1451–1452. 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- Machado, C. G. , & Semir, J. (2006). Fenologia da floração e biologia floral de bromeliáceas ornitófilas de uma área da Mata Atlântica do Sudeste brasileiro. Revista Brasileira De Botânica, 29, 163–174. 10.1590/S0100-84042006000100014 [DOI] [Google Scholar]
- Maestri, R. , Fornel, R. , Gonçalves, G. L. , Geise, L. , Freitas, T. R. O. , & Carnaval, A. C. (2016). Predictors of intraspecific morphological variability in a tropical hotspot: Comparing the influence of random and non‐random factors. Journal of Biogeography, 43, 2160–2172. 10.1111/jbi.12815 [DOI] [Google Scholar]
- Mahalanobis, P. C. (1936). On the generalized distance in statistics. Proceedings of the National Institute of Science of India, 2, 49–55. [Google Scholar]
- Martin, C. E. , & Siedow, J. N. (1981). Crassulacean acid metabolism in the epiphyte Tillandsia usneoides L. (Spanish moss). Responses of CO2 exchange to controlled environmental conditions. Plant Physiology, 68, 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. A. , Pfeiffer, W. , & Schwartz, T. (2010). Creating the CIPRES science gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments Workshop (GCE) (pp. 1–8). Retrieved from https://ieeexplore.ieee.org/document/5676129
- Morales, A. E. , De‐la‐Mora, M. , & Piñero, D. (2018). Spatial and environmental factors predict skull variation and genetic structure in the cosmopolitan bat Tadarida brasiliensis . Journal of Biogeography, 45, 1529–1540. 10.1111/jbi.13243 [DOI] [Google Scholar]
- Morello, S. , Sassone, A. B. , & López, A. (2018). Leaflet shape in the endemic South American Oxalis sect. Alpinae: An integrative approach using molecular phylogenetics and geometric morphometrics. Perspectives in Plant Ecology, Evolution and Systematics, 35, 22–30. 10.1016/j.ppees.2018.09.003 [DOI] [Google Scholar]
- Myers, N. , Mittermeier, R. A. , Mittermeier, C. G. , da Fonseca, G. A. , & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Neff, N. A. , & Marcus, L. F. (1980). A survey of multivariate methods for systematics. New York, NY: American Museum of Natural History Press. [Google Scholar]
- Nei, M. (1987). Molecular evolutionary genetics. New York, NY: Columbia University Press. [Google Scholar]
- Neves, B. , Uribbe, F. P. , Jacques, S. S. A. , Zanella, C. M. , & Costa, A. F. (2018). Species boundaries in the Vriesea incurvata (Bromeliaceae) complex after a broad morphometric and taxonomic study. Systematic Botany, 43, 870–888. 10.1600/036364418x697670 [DOI] [Google Scholar]
- Nicotra, A. B. , Leigh, A. , Boyce, C. K. , Jones, C. S. , Niklas, K. J. , Royer, D. L. , & Tsukaya, H. (2011). The evolution and functional significance of leaf shape in the angiosperms. Functional Plant Biology, 38, 535–552. 10.1071/FP11057 [DOI] [PubMed] [Google Scholar]
- Nosil, P. (2012). Ecological speciation. New York, NY: Oxford University Press. [Google Scholar]
- Oksanen, J. , Blanchet, F. G. , Kindt, R. , Legendre, P. , Minchin, P. R. , O'Hara, R. B. , … Wagner, H. (2017). Vegan: Community ecology package. R package version. 2.3–3. Retrieved from https://cran.r-project.org/web/packages/vegan/
- Palma‐Silva, C. , Lexer, C. , Paggi, G. M. , Barbará, T. , Bered, F. , & Bodanese‐Zanettini, M. H. (2009). Range‐wide patterns of nuclear and chloroplast DNA diversity in Vriesea gigantea (Bromeliaceae), a neotropical forest species. Heredity, 103, 503–512. 10.1038/hdy.2009.116 [DOI] [PubMed] [Google Scholar]
- Paradis, E. , Claude, J. , & Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289–290. [DOI] [PubMed] [Google Scholar]
- Pélabon, C. , Armbruster, W. S. , & Hansen, T. F. (2011). Experimental evidence for the Berg hypothesis: Vegetative traits are more sensitive than pollination traits to environmental variation. Functional Ecology, 25, 247–257. 10.1111/j.1365-2435.2010.01770.x [DOI] [Google Scholar]
- Peppe, D. J. , Royer, D. L. , Cariglino, B. , Oliver, S. Y. , Newman, S. , Leight, E. , … Adams, J. M. (2011). Sensitivity of leaf size and shape to climate: Global patterns and paleoclimatic applications. New Phytologist, 190, 724–739. 10.1111/j.1469-8137.2010.03615.x [DOI] [PubMed] [Google Scholar]
- R Core Team . (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Retrieved from https://www.R-project.org/ [Google Scholar]
- Rambaut, A. (2014). FigTree vol 1.4.2: Tree figure drawing. Retrieved from http://tree.bio.ed.ac.uk/software/figtree/
- Rambaut, A. , Suchard, M. A. , Xie, W. , & Drummond, A. J. (2014). Tracer v 1.6. Retrieved from https://github.com/beast-dev/tracer/releases
- Ramos, F. N. , Mortara, S. R. , Monalisa‐Francisco, N. , Elias, J. P. C. , Neto, L. M. , Freitas, L. , … Alcantara, S. (2019). Atlantic epiphytes: A data set of vascular and non‐vascular epiphyte plants and lichens from the Atlantic Forest. Ecology, 100, e02541. [DOI] [PubMed] [Google Scholar]
- Ribeiro, A. C. (2006). T ectonic history and the biogeography of the freshwater fishes from the coastal drainages of eastern Brazil: an example of faunal evolution associated with a divergent continental margin. Neotropical Ichthyology, 4, 225–246. [Google Scholar]
- Ribeiro, M. C. , Metzger, J. P. , Martensen, A. C. , Ponzoni, F. J. , & Hirota, M. M. (2009). The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation, 142, 1141–1153. 10.1016/j.biocon.2009.02.021 [DOI] [Google Scholar]
- Ribeiro, P. C. , Souza, M. L. , Muller, L. A. , Ellis, V. A. , Heuertz, M. , Lemos‐Filho, J. P. , & Lovato, M. B. (2016). Climatic drivers of leaf traits and genetic divergence in the tree Annona crassiflora: A broad spatial survey in the Brazilian savannas. Global Change Biology, 22, 3789–3803. 10.1111/gcb.13312 [DOI] [PubMed] [Google Scholar]
- Rohlf, F. J. (2010). Tps series. Stony Brook, NY: Department of Ecology and Evolution, State University of New York. [Google Scholar]
- Rohlf, F. J. , & Slice, D. (1990). Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39, 40–59. 10.2307/2992207 [DOI] [Google Scholar]
- Royer, D. L. , Meyerson, L. A. , Robertson, K. M. , & Adams, J. M. (2009). Phenotypic plasticity of leaf shape along a temperature gradient in Acer rubrum . PLoS ONE, 4, e7653 10.1371/journal.pone.0007653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer, D. L. , Wilf, P. , Janesko, D. A. , Kowalski, E. A. , & Dilcher, D. L. (2005). Correlations of climate and plant ecology to leaf size and shape: Potential proxies for the fossil record. American Journal of Botany, 92, 1141–1151. 10.3732/ajb.92.7.1141 [DOI] [PubMed] [Google Scholar]
- Saitou, N. , & Nei, M. (1987). The neighbor‐joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Tang, Y. , Horikoshi, M. , & Li, W. (2016). ggfortify: Unified interface to visualize statistical results of popular R packages. The R Journal, 8, 474–489. 10.32614/RJ-2016-060 [DOI] [Google Scholar]
- Turchetto‐Zolet, A. C. , Pinheiro, F. , Salgueiro, F. , & Palma‐Silva, C. (2013). Phylogeographical patterns shed light on evolutionary process in South America. Molecular Ecology, 22, 1193–1213. 10.1111/mec.12164 [DOI] [PubMed] [Google Scholar]
- Wanderley, A. M. , Machado, I. C. S. , de Almeida, E. M. , Felix, L. P. , Galetto, L. , Benko‐Iseppon, A. M. , & Sork, V. L. (2017). The roles of geography and environment in divergence within and between two closely related plant species inhabiting an island‐like habitat. Journal of Biogeography, 45, 381–393. 10.1111/jbi.13137 [DOI] [Google Scholar]
- Wright, I. J. , Dong, N. , Maire, V. , Prentice, I. C. , Westoby, M. , Díaz, S. , … Wilf, P. (2017). Global climatic drivers of leaf size. Science, 357, 917–921. 10.1126/science.aal4760 [DOI] [PubMed] [Google Scholar]
- Wright, S. (1951). The genetical structure of populations. Annals of Eugenics, 15, 323–354. 10.1111/j.1469-1809.1949.tb02451.x [DOI] [PubMed] [Google Scholar]
- Zanella, C. (2013). Padrões históricos e processo de hibridação entre duas espécies simpátricas de bromélias da Mata Atlântica. PhD Thesis. Porto Alegre, Brazil: Universidade Federal do Rio Grande do Sul. [Google Scholar]
- Zanella, C. M. , Palma‐Silva, C. , Goetze, M. , & Bered, F. (2016). Hybridization between two sister species of Bromeliaceae: Vriesea carinata and V. incurvata . Botanical Journal of Linnean Society, 181, 491–504. 10.1111/boj.12424 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study will be openly available in the DRYAD Digital Repository. Neves, B., Zanella, C. M., Kessous, I. M., Uribbe, F. P., Salgueiro, F., Bered, F., Antonelli, A., Bacon, C. D., & Costa, A. F. (2019). Seasonality drives bromeliad leaf and floral bract variation across a latitudinal gradient in the Atlantic Forest. (https://doi.org/10.5061/dryad.sj3tx9619).


