ABSTRACT
Development of resistance among the vectors to different insecticides poses a potential threat to vector control programme. Regular monitoring of susceptibility status of vector species to commonly used insecticides is recommended for planning appropriate vector control measures. In this communication, we have determined the phenotypic resistance of Anopheles culicifacies s.l., the major malaria vector against commonly used various insecticides in ten highly malaria endemic districts of Odisha State in east-central India. Bioassays were conducted before and after mass distribution of long-lasting insecticidal nets (LLINs) on field caught female mosquitoes with dichlorodiphenyl-trichloroethane (DDT), malathion and deltamethrin following the standard World Health Organization (WHO) guidelines. From the bioassays using 1x diagnostic concentrations (DC) recommended by WHO, we confirmed a higher frequency of resistant phenotype in An. culicifacies s.l. against DDT (range: 72% to 90%; average: 82%) compared to that against malathion(range: 17% to 34%; average: 26.7%) and deltamethrin (range: 14% to 24%; average: 19.1%) during 2018. Since, resistance to pyrethroid is detected; it is recommended to carry out bioassays of An. culicifacies s.l. exposing to higher concentrations of deltamethrin which would yield relevant information on the intensity of resistance and be useful to select suitable insecticide for resistance management vector control interventions.
KEYWORDS: Anopheles culicifacies, deltamethrin, India, Odisha, susceptibility
Introduction
Malaria continues to be a public health burden in India with a reported incidence of 840,838 and 103 deaths during 2017 [1]. Odisha State, a part of east-central India, having only 3.6% of the country’s total population (n = 1,362,015,566), contributed to 41.9% of total malaria cases, 55.8% of Plasmodium falciparum cases and 24.3% of total malaria deaths recorded in India [1]. Among the 30 districts of the State, 10 southern districts, having a population of 12,857,902 (as per 2011 census), are worse affected with high incidence of malaria for many years [1,2]. Of the State’s total, the 10 districts alone reported 66.5% of the malaria cases and 52.0% of the malaria deaths in 2017 [1]. Majority of the malaria infections in human are due to P. falciparum (> 90%) [1,2]. Though transmission is perennial, there are seasonal variations of malaria incidence, peaking during rainy (July–September) and post-rainy (November–January) months. The annual parasite incidence per 1000 population (API) of malaria varied between 17.57 and 29.34 in Koraput, 18.54 and 60.31 in Malkangiri, 31.15 and 65.31 in Rayagada, 10.85 and 19.02 in Nabarangpur, 23.11 and 47.47 in Kandhamal, 9.4 and 24.74 in Gajapati, 12.53 and 28.67 in Kalahandi, 2.18 and 6.47 in Balangir, 4.25 and 10.10 in Nuapada and 1.5 and 4.73 in Ganjam district for the last 5 years (2013–2017) [1]. Anopheles culicifacies s.l. Giles (Diptera: Culicidae) is the predominant vector species found mostly in rural and peri-urban parts of these districts and actively transmits malaria [2,3]. Streams, ponds and paddy fields are the preferential breeding habitats of An. culicifacies s.l [2]. Paddy is the major crop cultivated in these districts. It is grown in two seasons, viz, Rabi (dry) and Kharif (wet). Pesticides of pyrethroid (70%) classes, such as lambda-cyhalothrin, cypermethrin, and organophosphorus classes (30%), such as chlorpyrifos (20% EC), dichlorvos are commonly used by farmers to control the pests of rice crop (Data source: Office of the Deputy Director, Agriculture, Koraput, India, 2017). An. culicifacies s.l. is highly endophilic and zoophagic and comprised of 52.9%, 43.9% and 0.9% of sibling species B, E and C, respectively [2].
Vector control has been the key constituent of all malaria control strategies in these districts [3]. An. culicifacies s.l. and An. fluviatilis s.l. are the major malaria vectors in 10 southern districts of Odisha State [2]. Under the National Vector Borne Disease Control Programme (NVBDCP), yearly two rounds of indoor residual spraying (IRS) with dichlorodiphenyl-trichloroethane (DDT) have been carried out in these districts since 1953. In view of persistent transmission of malaria, the southern districts of Odisha State were included under the enhanced malaria control program in 1998 emphasizing on early diagnosis and prompt treatment (EDPT); selective vector control; insecticide-treated bed nets (ITNs); epidemic response and inter-sectoral collaboration; and institutional strengthening [4,5]. Accordingly, from 2001 onwards, synthetic pyrethroids (SPs) (deltamethrin, alpha-cypermethrin, lambda-cyhalothrin or cyfluthrin) have been used for IRS in the place of DDT in many Community Health Centers (CHCs) (where annual parasite incidence (API) per 1000 population was >10) of the districts [4]. From 2009, long-lasting insecticidal nets (LLINs) have been distributed in many CHCs of the districts in a phased manner [6] and during 2017; mass distribution of LLINs (PermaNet 2.0 LNs treated with deltamethrin and DuraNet LNs treated with alphacypermethrin) was carried out in the districts to provide universal coverage.
Rapid development and geographical spread of insecticide resistance among malaria vectors have been reported from various parts of India [6]. For better control of malaria through vector control interventions, updated information on insecticide resistance status of malaria vectors are important. The study conducted during 2010–11 in the 10 southern districts of Odisha State showed that in eight districts, An. culicifacies s.l. was under ‘verification required’ (possible resistance) category when exposed to deltamethrin indicating its tendency toward developing resistance [3]. In areas where resistance or possible resistance has been identified, regular surveillance is necessary to evaluate temporal changes which will guide the efficient use of vector control tools. In view of this, a study was undertaken in the 10 southern districts of Odisha State to verify the resistance status of An. culicifacies s.l. to insecticides used for IRS and LLINs (DDT, malathion and deltamethrin) in 2017 and 2018.
Material and methods
Study area
The study was conducted in the 10 southern districts (latitudes 17° 45ʹ and 21° 5ʹN; longitudes 81° 10ʹ and 85° 05ʹE) of Odisha State, i.e. Koraput, Malkangiri, Rayagada, Nabarangpur, Kandhamal, Gajapati, Kalahandi, Balangir, Nuapada and Ganjam. The area of the 10 districts is 63,483 km [2], which is 40.8% of the total area of the State. Three prevailing seasons in these districts are summer (March to June), rainy (July to October) and winter (November to February). There are altogether 115 CHCs in the districts. Most of the villages are plain or foothills. These districts have been hyper-endemic for malaria for many decades, reporting deaths due to malaria every year (479 reported deaths during 2010–2017) [1].
Mosquito collections
Mosquitoes were collected twice in all the 10 districts and susceptibility tests were carried out prior to mass distribution of LLINs (during March 2017 – April 2017) and one year after the distribution. The frequency and time of testing in a year was according to the abundance of the target vector species so as to obtain adequate number for the tests. Since the rearing facility was not available at the field site to obtain F1 progeny, we used field-collected mosquitoes with known gonotrophic conditions for conducting susceptibility bioassays for both test and control as suggested in the WHO guidelines [7]. Wild caught, preferably blood-fed female mosquitoes were collected from different resting sites (indoors-human dwellings/cattle sheds) in the selected villages using oral aspirator and torch light. The mosquitoes, after collection, were kept in paper cups with 10% glucose solution soaked in cotton pads, wrapped with a wet cloth and transported to the test site. In the laboratory, individual live mosquitoes were transferred to glass tubes and identified morphologically up to species before conducting susceptibility tests. The susceptibility tests were conducted within one to 2 hours of collection of mosquitoes in the field. Attempts were made to collect An. fluviatilis s.l., another major malaria vector in the study area in winter months (the favorable season of this species) for conducting the susceptibility tests. However, the bioassays could not be carried out due to the non-availability of adequate numbers of An. fluviatilis s.l.
Susceptibility tests
Resistance status of An. culicifacies s.l. to DDT, malathion and deltamethrin was studied in all the 10 districts using WHO test kits [7]. Temperature and relative humidity (RH) were maintained at 27 ± 2°C and 75% ±10% RH, respectively, in the airtight field laboratory while conducting the tests. Papers impregnated with 1x diagnostic concentration (DC) of DDT (4%), malathion (5%), deltamethrin (0.05%), and the recommended control papers impregnated with risella oil, olive oil and silicone oil, respectively, for these insecticides were obtained from Vector Control Research Unit, University Sains Malaysia, Penang, Malaysia and used for the susceptibility bioassays conducted by the investigators in the field sites of the 10 districts, following the WHO procedure [7]. For each test, 150 mosquitoes, 100 in test and 50 in control, were exposed. To determine the knock down time 50% (KDT50) and 95% (KDT95), the number knocked down at every 10 min was recorded up to 1 h exposure. After the exposure for 1 h, the mosquitoes were transferred to holding tubes and provided with 10% glucose solution for the next 24-h. The number dead was counted after a 24-h holding period and recorded to determine the resistance status. All the exposed mosquitoes dead/alive were reexamined for further confirmation of the species after conducting each test.
Data analysis
Treated and control mortality were calculated as per the WHO guidelines [7]. Abbott’s formula was not used, as the control mortality remained below 5% in all the tests [8]. The frequency of resistant phenotype was measured in discriminating concentration bioassays as 100 – mortality [9]. From the test mortality, the response of the vector species to different insecticides was categorized according to the WHO criteria: a corrected mortality (CM) of ≥98% was ‘susceptible’, <90% was ‘resistant’ and 90–97% was ‘possible resistance’. Random effect logistic regression model was applied to compare the mortality of mosquitoes between the districts over the years (2017 and 2018). Mortality of An. culicifacies s.l. was modeled as a function of year and district as random effect. This analysis was done separately for each of the three insecticides (DDT, malathion and deltamethrin). The cumulative knockdown time (KDT50 and KDT95) was determined using log-probit method in SPSS 16.0 version. A p-value <0.05 was considered as significant.
Results
DDT
In 2017, a total of 1500 An. culicifacies s.l. were exposed in the bioassay and the mortality varied from 11.0% (95% CI: 4.9–17.1 in Malkangiri) to 37.0% (95% CI: 27.5–46.4 in Balangir) with an average of 20.2% (95% CI: 17.7–22.7) confirming the resistance of An. culicifacies s.l. to DDT in all the 10 districts (Figure 1). One year later (2018), the mortality of An. culicifacies s.l. (n = 1500) ranged from 10.0% (95% CI: 4.1–15.9 in Kandhamal) to 28.0% (95% CI: 19.2–36.8 in Balangir) with an average of 18.0% (95% CI: 15.6–20.4) (Figure 1). The frequency of resistant phenotype ranged from 63% to 89% with an average of 79.8% during 2017 and from 72% to 90% with an average of 82.0% during 2018. Logistic regression analysis of the data with district as random effect showed that the mortality of An. culicifacies s.l. differed significantly between the districts (χ2 = 12.8, df = 1, p = 0.0002) but not between the years (χ2 = 1.8, df = 1, p = 0.17). The KDT50 and KDT95 of DDT during 2017 ranged from 119.1 min to 209.7 and 330.3 min to 983.8 and in 2018, the respected values ranged from 107.4 min to 194.1 min and 250.2 min to 1099.9 min. The percent knock-down obtained during 1 h exposure in the 10 districts is shown in Figure 2.
Figure 1.
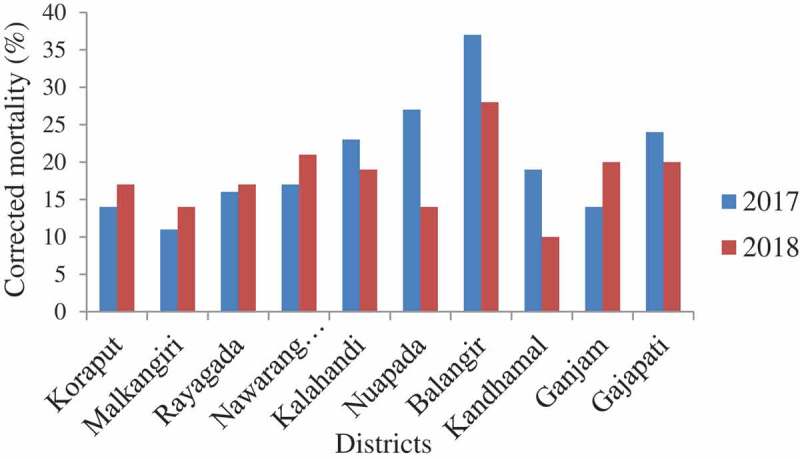
Corrected mortality of An. culicifacies s.l. to DDT 4%.
Figure 2.
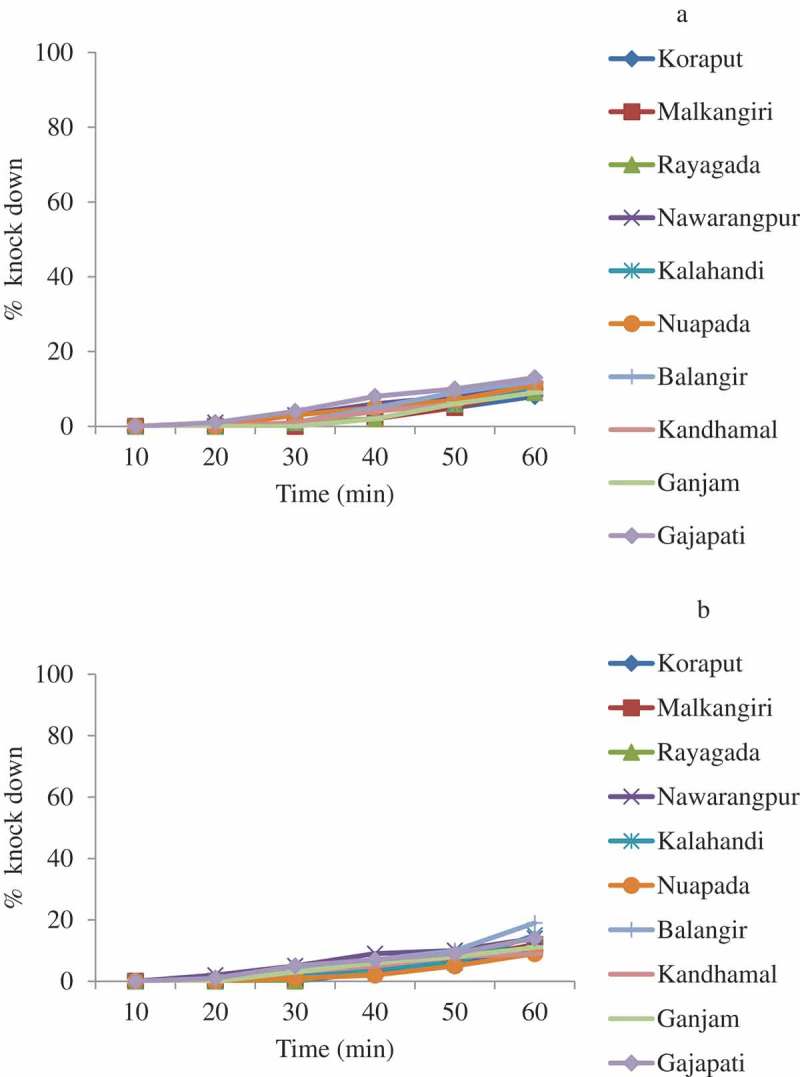
Percent knock-down of An. culicifacies s.l. during 1 h exposure to DDT 4% 2017 (a) and 2018 (b).
Malathion
In 2017, the mortality of An. culicifacies s.l. (n = 1500) against malathion varied from 46.0% (95% CI: 36.2–55.8 in Gajapati) to 68.0% (95% CI: 58.9–77.1 in Ganjam); the average was 59.1% (95% CI: 56.1–62.1) (Figure 3). The resistance frequency ranged from 32% to 54% with an average of 40.9%. After one year, i.e. in 2018, the mortality (n = 1500) ranged from 66.0% (95% CI: 56.7–75.3 in Kalahandi and Rayagada) to 83.0% (95% CI: 75.6–90.4 in Nuapada) with an average of 73.3% (95% CI: 70.6–76.0) (Figure 3). The resistance frequency ranged from 17% to 34% with an average of 26.7%. An. culicifacies s.l. was found to be resistant to malathion in all the districts in 2017 and also in 2018. Logistic regression analysis of the data with district as random effect showed that the mortality of An. culicifacies s.l. did not differ significantly between the districts (χ2 = 1.0, df = 1, p = 0.16) but it differed significantly between the years (χ2 = 44.8, df = 1, p < 0.001). In 2017, the KDT50 and KDT95 ranged from 69.9 min to 99.5 min and 199.8 min to 426.4 min, respectively. In 2018, the KDT50 ranged from 55.1 min to 76.9 min and KDT95 ranged from 123.3 min to 354.2 min. The percent knock-down recorded during 1 h exposure is shown in Figure 4.
Figure 3.
Corrected mortality of An. culicifacies s.l. to malathion 5%.
Figure 4.
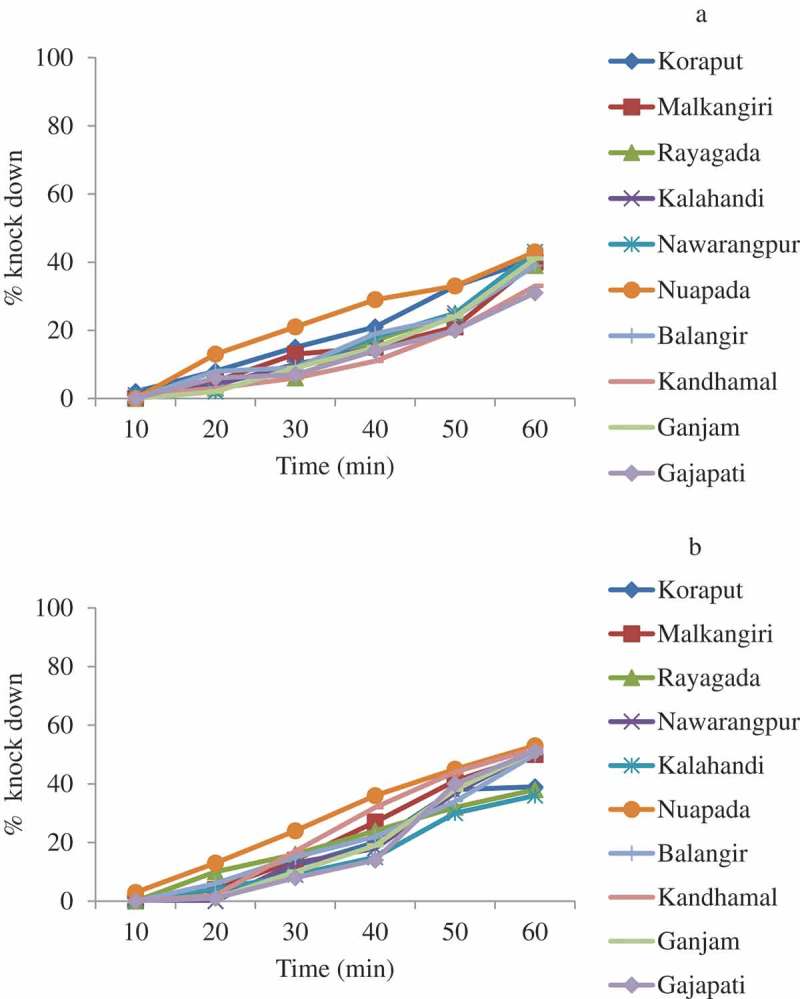
Percent knock-down of An. culicifacies s.l. during 1 h exposure to malathion 5% 2017 (a) and 2018 (b).
Deltamethrin
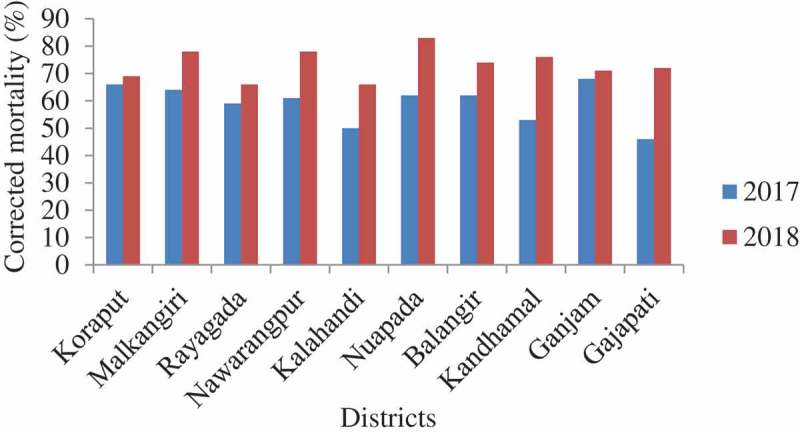
A total of 3000 An. culicifacies s.l. were tested in the bioassays in 2017 and also in 2018. The mortality of An. culicifacies s.l. varied from 77.0% (95% CI: 68.8–85.2 in Kalahandi) to 85.0% (95% CI: 78.0–91.9 in Nuapada and Ganjam) with an average of 82.2% (95% CI: 79.8–84.6) in 2017 (Figure 5). Thus, An. culicifacies s.l. was resistant to deltamethrin in all the 10 districts. One year later, in 2018, the mortality ranged from 76.0% (95% CI: 67.6–84.4 in Malkangiri) to 86.0% (95% CI: 79.2–92.8 in Kalahandi); the average was 80.9% (95% CI: 78.5–83.3), further confirming its resistance to deltamethrin (Figure 5). The frequency of resistance phenotype during 2017 ranged from 15% to 23% with an average of 17.8% and in 2018 ranged from 14% to 24% with an average of 19.1%. Logistic regression analysis of the data with district as random effect showed that the mortality of An. culicifacies s.l. did not differ significantly between the districts (p > 0.5) as well as between the years (p > 0.9). In 2017, KDT50 for deltamethrin ranged from 55.3 min to 71.6 min and KDT95 ranged from 146.1 min to 258.5 min, respectively. In 2018, the KDT50 and KDT95 ranged from 54.5 min to 64.5 min and 142.1 min to 183.6 min. The percent knock-down recorded during the 1 h exposure in the 10 districts is shown in Figure 6.
Figure 5.

Corrected mortality of An. culicifacies s.l. to deltamethrin 0.05%.
Figure 6.
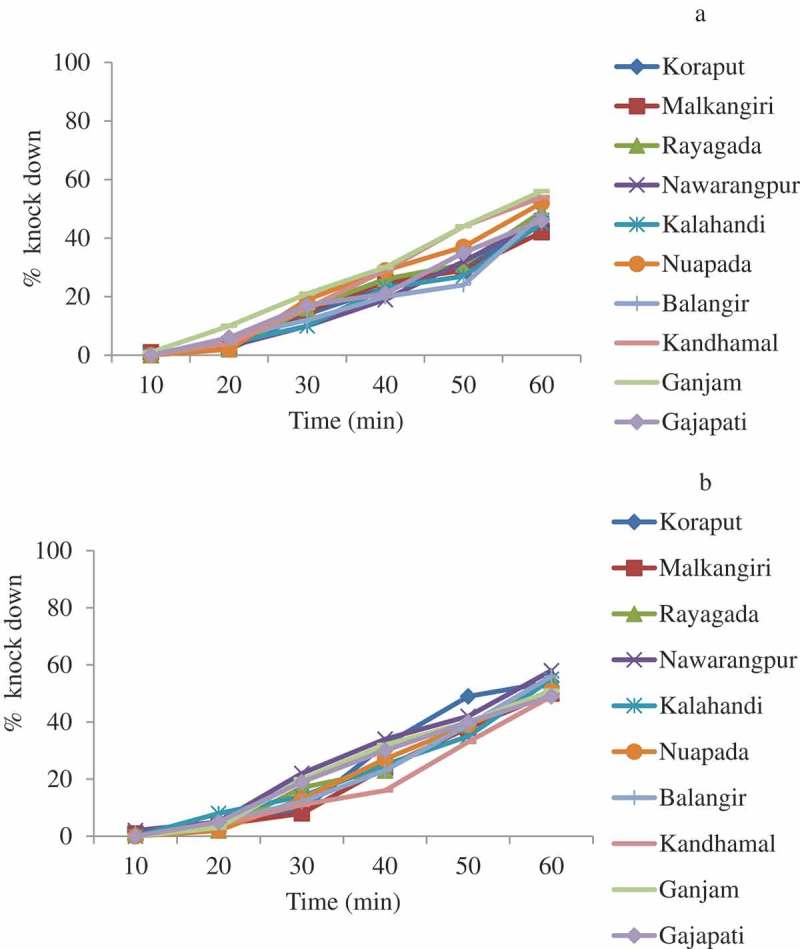
Percent knock-down of An. culicifacies s.l. during 1 h exposure to deltamethrin 0.05% 2017 (a) and 2018 (b).
Discussion
Control of malaria vectors through the use of insecticides for IRS and bed nets is the keystone of interrupting malaria transmission in India [10]. Currently, the vector control program in the country mainly depends on SPs, which have been used in malaria control program in 10 falciparum hyper endemic districts of Odisha State since 2001 both in IRS and insecticide-treated nets [1–3]. From 2009 to 2012, LLINs were distributed in a phased manner and during 2017, a total of 5,725,225 LLINs (1,922,416 PermaNet 2.0 with 100 denier (40 g/m2 fabric weight) and 3,802,809 DuraNet with 150 denier (45 g/m2 fabric weight)) were distributed achieving universal coverage (approximately one net for two persons) (Source: NVBDCP, Odisha). As these districts are under enormous pressure of SPs in addition to DDT since last 16 years, the quantitative information on resistance status of the field populations of vectors is necessary to understand the operational significance of resistance and to select appropriate insecticide for malaria vector control. The current study investigated the resistance status of one of the malaria vectors, An. culicifacies s.l. to 1x DC of DDT, malathion and deltamethrin in the 10 southern districts of Odisha State which are badly affected by falciparum malaria in east-central India.
The results of the current study conducted on two occasions, 2017 and 2018, showed that An. culicifacies s.l. frequently survived after 1 h exposure to 1x DC of DDT. This could be due to the extensive and prolonged use (since 1953) of this insecticide in public health as well as in agricultural fields leading to high selection pressure [11–13]. The resistance frequency of An. culicifacies s.l. to malathion was relatively lower than that to DDT in all the 10 districts on both the occasions, 2017 and 2018. Malathion was not included in the public health program in Odisha, but the resistance developed in malaria vector might be due to the moderate use of this insecticide in agricultural sector [12]. An. culicifacies s.l. when exposed to deltamethrin was found to be less resistant (based on frequency of resistant phenotypes in the mosquito population after exposure to the 1x DC) in all the districts on both the occasions than DDT and malathion.
In India, resistance in An. culicifacies s.l. to DDT at several places has been reported [14–19]. It was first reported in Panchmahal district in Bombay State, India in 1959 and later in 1962 in a village of Uttar Pradesh [17]. Double or triple resistance to DDT, dieldrin and malathion was found in An. culicifacies s.l. in 30 districts of Maharashtra [18]. So far, resistance to malathion in An. culicifacies s.l. is reported in 13 States (Andhra Pradesh, Chhattisgarh, Gujarat, Haryana, Jharkhand, Madhya Pradesh, Maharashtra, Odisha, Rajasthan, Tamil Nadu, Telangana, Uttar Pradesh and West Bengal) of India [6]. Malathion resistance in An. culicifacies s.l. was first observed in Gujarat in 1973 [19] and subsequently in Andhra Pradesh [20]. The last report of malathion resistance in this species was from Chhattisgarh State in 2016 [6]. The susceptibility studies with An. culicifacies s.l. to SPs conducted so far in India showed that resistance development in this species was not widespread. Among the available reports, the resistance to deltamethrin in this species was reported first in Surat, Gujarat in 2001 and subsequently in Chhattigarh (2002), Tamil Nadu (2006), Andhra Pradesh (2009), Assam (2009), Madhya Pradesh (2009), Telangana (2009) and Odisha (2014) [6,21]. The results of the current study indicated that the species has developed resistance to pyrethroids in all the 10 districts of Odisha State during 2017–2018, which is of great concern to Indian vector control program as this insecticide is being used extensively in public health program at present. Similar to the findings of the current study, resistance to DDT, malathion and deltamethrin in An. culicifacies s.l. was recently (2016–2017) found in Gadchiroli district of Maharashtra [22].
There are a few earlier reports available regarding the resistance status of An. culicifacies s.l. to commonly used insecticides in the current study area. A study conducted during 1995 in Koraput district showed that An. culicifacies s.l. was resistant to DDT but susceptible to malathion and deltamethrin [23]. Subsequent studies carried out during 2004 in eight southern districts of Odisha State reported that An. culicifacies s.l. was resistant to DDT in all the eight districts, to malathion in four districts and was under ‘verification required’ category to deltamethrin in three districts [24]. A study conducted in these 10 districts during 2010–2011 showed a similar resistance status in An. culicifacies s.l. to DDT and malathion [3]. On exposure to deltamethrin, this species was found susceptible in two districts and was under ‘verification required’ category in the other eight districts [3]. However, the deltamethrin resistance recorded in the current study showed that it took nearly 16 years for An. culicifacies s.l. to develop wide-spread resistance at a low frequency after extensive use of this insecticide since 2001 both in public health and in agricultural sectors in east-central India [12]. Though An. culicifacies s.l. was reported to be resistant to all the three insecticides in the current study, the resistance was lesser to SPs than DDT and malathion.
The success of the malaria control program in a country depends mainly on how effectively the vector control interventions are implemented. Currently, pyrethroid class of insecticides are being extensively used in public health and agriculture as they show low mammalian toxicity and excito-repellent effects [12]. As most insecticides used in agricultural fields are of the same chemical classes as those used for vector control, close alliance between malaria control programs and the agricultural sector is required. Since An. culicifacies s.l. has developed resistance to pyrethroids as reported in the current study and screening of insecticide resistance is scarce and geographically limited in India [3]; it is recommended that longitudinal monitoring of insecticide-resistance development in malaria vectors to the commonly used insecticides at regular intervals is reasonable to understand the extent of its geographical spread [7,25].
Conducting bioassays with discriminating concentrations is widely adopted for monitoring insecticide resistance in mosquitoes [7]. In the current study, the resistance phenotype of An. culicifacies s.l. was detected using the discriminating concentrations of DDT, malathion and deltamethrin which did not provide information in terms of efficacy failure of insecticides in the field. If any vector is found resistant to a particular insecticide, it is necessary to expose subsequent samples from the same target vector population to substantially higher concentrations (5x and 10x) of the same insecticide to know the intensity of resistance and its operational significance [7]. A study conducted during 2012–2016 in five countries (Benin, Cameroon, India, Kenya, and Sudan), representing a range of pyrethroid resistance and malaria transmission scenarios showed that LLINs provided protection against malaria in all study countries except Sudan and found no evidence that the amount of protection provided by LLINs differed by the frequency of resistance as measured by WHO bioassays. They also observed no evidence of an association between infection prevalence with higher pyrethroid resistance [26]. Therefore, it is recommended to carry out the bioassays with 5x and 10x concentrations of DDT, malathion and deltamethrin in the current study area for taking appropriate operational decisions.
One of the limitations of the current study was that the resistance status of individual sibling species of An. culicifacies s.l. could not be tested, as wild caught mosquitoes were directly tested and rearing facility was not available at the field site to obtain F1 progeny. The second limitation of the study was that the information on quality control aspects of the insecticide papers were not available as the insecticide impregnated papers used for the susceptibility tests were prepared at VCRU in Malaysia. Another limitation of the study was that the intensity bioassays were not carried out after recording resistance on 1x DC of the insecticides. The study districts have been endemic for malaria and the NVBDCP has distributed LLINs to all the districts. Therefore, it was not possible or ethical to include a control arm without LLINs in the study; this was also a limitation.
Conclusion
The current study confirmed development of multiple resistance in An. culicifacies s.l. in east-central India. The pyrethroid resistance in An. culicifacies s.l. assumes significance in view of the recent universal LLIN coverage in Odisha State that is likely to further hasten the spread of resistance in the vector species as observed in a study conducted in one tribal district of Chhattisgarh State, India during 2014–2015 [27]. The development of pyrethroid resistance in An. culicifacies s.l. may pose a challenge to the ongoing success of pyrethroid-based malaria vector control program in the study region in near future. At present, after SPs, no alternative and effective insecticide molecule has been approved by the WHO for use in LLINs [28]. However, new classes of insecticides like neonicotinoids recently recommended by WHO could be considered for IRS in pyrethroid-resistant areas [29]. It is also recommended to conduct bioassays of An. culicifacies s.l. exposing to higher concentrations of deltamethrin which would yield relevant information on the intensity of resistance and be useful to select suitable insecticide for resistance management vector control interventions.
Funding Statement
The study was funded by National Vector Borne Disease Control Programme, Bhubaneswar, Odisha.
Acknowledgments
The technical assistance rendered by the staff of ICMR-VCRC Field Station, Koraput, Odisha is gratefully acknowledged. We are grateful to NVBDCP, Bhubaneswar, Odisha for funding this project.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Malaria situation in India National Vector Borne Disease Control Programme. 2018. Accessed on 2019 October 1. Available from: https://www.nvbdcp.gov/
- [2].Sahu SS, Gunasekaran K, Krishnamoorthy N, et al. Bionomics of Anopheles fluviatilis and An. culicifacies in relation to transmission of malaria and its control in east-central India. J Med Entomol. 2017;54(4):821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sahu SS, Gunasekaran K, Raju HK, et al. Response of the malaria vectors to the conventional insecticides in the southern districts of Odisha state, India. Indian J Med Res. 2014;139(2):294–300. [PMC free article] [PubMed] [Google Scholar]
- [4].Jambulingam P, Sahu SS, Manonmani A.. Reappearance of Anopheles minimus in Singhabhum hills of east-central India. Acta Trop. 2005;96(1):31–35. [DOI] [PubMed] [Google Scholar]
- [5].Patil RR, Kumar RK.. World bank EMCP malaria project in Orissa, India – a field reality. Trop Parasitol. 2011;1(1):26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Raghavendra K, Velamuri PS, Verma V, et al. Tempero-spacial distribution of insecticide-resistance in Indian malaria vectors in the last quarter-century: need for regular resistance monitoring and management. J Vector Borne Dis. 2017;54(2):111–130. [PubMed] [Google Scholar]
- [7].Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Second ed. World Health Organisation; 2016. Available from: https://apps.who.int›irisrest›bitstreams›retrieve [Google Scholar]
- [8].Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. [Google Scholar]
- [9].Global report on insecticide resistance in malaria vectors: 2010–2016. World Health Organisation; 2018. Available from: https://apps.who.int›iris›bitstreams›handle›9789241514057-eng [Google Scholar]
- [10].Gunasekaran K, Sahu SS, Vijayakumar T, et al. Bio-efficacy of LifeNet, a deltamethrin incorporated long-lasting insecticidal net, as assessed in experimental huts against Anopheles fluviatilis, a major malaria vector in east-central India. Acta Trop. 2018;187:151–157. [DOI] [PubMed] [Google Scholar]
- [11].Diabate A, Baldet T, Chandre F, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67(6):617–622. [DOI] [PubMed] [Google Scholar]
- [12].Sahu SS, Dash S, Sonia T, et al. Outbreak of Japanese Encephalitis in Malkangiri district of Odisha State, India, inhabited predominantly by tribes and endemic for falciparum malaria. Mem Inst Oswaldo Cruz. 2018;113(6):e170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dhiman S, Yadav K, Rabha B, et al. Evaluation of insecticides susceptibility and malaria vector potential of Anopheles annularis s.l. and Anopheles vagus in Assam, India. PLoS One. 2016;11(3):e0151786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mittal PK, Adak T, Singh OP, et al. Reduced susceptibility to deltamethrin in Anopheles culicifacies sensu lato in Ramanathapuram district, Tamil Nadu: selection of a pyrethroid resistant strain. Curr Sci. 2002;82:185–188. [Google Scholar]
- [15].Singh OP, Raghavendra K, Nanda N, et al. Pyrethroid resistance in Anopheles culicifacies in Surat district Gujarat, west India. Curr Sci. 2002;82:547–550. [Google Scholar]
- [16].Mishra AK, Chand SK, Barik TK, et al. Insecticide resistance status in Anopheles culicifacies in Madhya Pradesh, central India. J Vector Borne Dis. 2012;49:39–41. [PubMed] [Google Scholar]
- [17].Singh RK, Mittal PK, Gourshettiwar MP, et al. Susceptibility of malaria vectors to insecticides in Gadchiroli district (Maharashtra), India. J Vector Borne Dis. 2012;49:42–44. [PubMed] [Google Scholar]
- [18].Vittal M, Deshpande LB. Development of malathion resistance in a DDT, HCH resistant Anopheles culicifacies population in Thane district (Maharashtra). J Commun Dis. 1983;15:144–145. [PubMed] [Google Scholar]
- [19].Rajagopal R. Malathion resistance in Anopheles culicifacies in Gujarat. Indian J Med Res. 1977;66:27–28. [PubMed] [Google Scholar]
- [20].Raghavendra K, Vasantha K, Subbarao SK, et al. Resistance in Anopheles culicifacies sibling species B and C to malathion in Andhra Pradesh and Gujarat states in India. J Am Mosq Control Assoc. 1991;7:255–259. [PubMed] [Google Scholar]
- [21].Raghavendra K, Barik TK, Sharma SK, et al. A note on the insecticide susceptibility status of principal malaria vector Anopheles culicifacies in four states of India. J Vector Borne Dis. 2014;51:230–234. [PubMed] [Google Scholar]
- [22].Chand G, Behera P, Bang A, et al. Status of insecticide resistance in An. culicifacies in Gadchiroli (Maharashtra) India. Pathog Glob Health. 2017;111(7):362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sahu SS, Patra KP. A study on insecticides resistance in Anopheles fluviatilis and An. culicifacies to HCH and DDT in Malkangiri district of Odisha. Indian J Malariol. 1995;32(3):112–118. [PubMed] [Google Scholar]
- [24].Sharma SK, Upadhyay AK, Haque MA, et al. Insecticide susceptibility status of malaria vectors in some hyperendemic tribal districts of Odisha. Curr Sci. 2004;87:1722–1726. [Google Scholar]
- [25].Orjuela LI, Morales JA, Ahumada ML, et al. Insecticide resistance and its intensity in populations of malaria vectors in Colombia. Biomed Res Int. 2018;2018(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kleinschmidt I, Bradley J, Knox TB, et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: a WHO-coordinated, prospective, international, observational cohort study. Lancet Infect Dis. 2018;18:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kona MP, Kamaraju R, Donnelly MJ, et al. Characterization and monitoring of deltamethrin-resistance in Anopheles culicifacies in the presence of a long-lasting insecticide-treated net intervention. Malar J. 2018;17(1):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nauen R. Insecticide resistance in disease vectors of public health importance. Pest Manag Sci. 2007;63(7):628–633. [DOI] [PubMed] [Google Scholar]
- [29].WHO recommended insecticides for indoor residual spraying against malaria vectors. World Health Organisation; 2018. Available from: https://www.who.int/neglected_diseases/vector_ecology/vectorcontrol/Insecticides_IRS_22_September_2018.pdf?ua=1 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Malaria situation in India National Vector Borne Disease Control Programme. 2018. Accessed on 2019 October 1. Available from: https://www.nvbdcp.gov/


