Abstract
T regulatory cells (Tregs) are an important T cell population for immune tolerance, prevention of autoimmune diseases and inhibition of antitumor immunity. The tumor-promoting role played by Tregs in cancer has prompted numerous approaches to develop immunotherapeutics targeting Tregs. One approach to depletion of Treg cells is retargeting the highly potent cytotoxic activity of bacterial toxins. These agents capitalize on the well-characterized bacterial toxins, diphtheria toxin and Pseudomonas aeruginosa exotoxin A–both of which harbor membrane translocation domains and enzymatic domains that catalytically halt protein synthesis within intoxicated eukaryotic cells and act at picomolar or subpicomolar concentrations. In this review, we summarize the preclinical and clinical development of several Treg-depleting cancer immunotherapies based on these two bacterial toxins.
Keywords: : anticancer therapy, denileukin diftitox, fusion protein toxins, immunotherapy, LMB-2, melanoma, targeted toxins, Tregs
Regulatory T cells (Tregs) are a highly immunosuppressive subset of CD4+CD25+ T cells that have been shown to play an essential role in establishment and maintenance of self-tolerance. Their role is critical in establishing tolerance to organ grafts, prevention of graft-versus-host disease after bone marrow transplantation, feto-maternal tolerance, immunity to our own microbial flora, tissue repair and autoimmunity [1–3]. Despite the vital roles that Tregs play in maintaining immune homeostasis, they also have been shown to have detrimental effects on tumor immunosurveillance and antitumor immunity [4]. Tregs are known to suppress effector T-cell mediated antitumor immune responses, and activate and interact with other immunosuppressive cells like myeloid derived suppressor cells and tumor-associated macrophages that also interfere with antitumor immunity [5–7]. Therefore, depleting or downregulating the suppressive functions or destabilization of Tregs has remained an area of intense focus as an anticancer therapeutic strategy for different cancers [4,8]. Besides cancer, Tregs have also attracted attention as potential targets in other fields, including transplantation and autoimmune diseases [9–12].
Potential surface targets of Tregs for immunotherapy
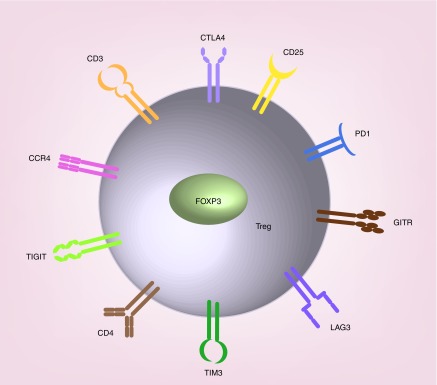
Tregs show increased expression of a transcription factor, Forkhead box protein P3 (FoxP3) that has been shown to repress the expression of several cytokines such as IL-2, IL-4 and IFNγ with concomitant activation of IL-2 receptor (CD25), cytotoxic T lymphocyte–associated protein-4 (CTLA4) and glucocorticoid-induced TNF receptor (GITR) [13–15]. A number of these surface molecules along with inhibitory checkpoint receptors which are expressed on the surface of Tregs such as programmed cell death 1 (PD-1), CC chemokine receptor 4 (CCR4) [16], lymphocyte activation gene-3 (LAG-3) [17], T-cell immunoglobulin and mucin domain-3 protein (TIM-3) [18], T-cell immunoreceptor with Ig and ITIM domains (TIGIT) [19] have been identified as potential targets for the development of various anticancer therapeutics and biomarker development (Figure 1) [7,14,20,21].
Figure 1. . Cartoon depicting antigens and receptors abundant on Treg cells.
CD3: T cell co-receptor which associates with the T cell receptor (TCR) and ζ-chain (zeta-chain) to generate the TCR complex that leads to T cell activation; CD4: Glycoprotein originally known as leu-3 or T4; CD25: IL-2 receptor alpha chain; CTLA4: Cytotoxic T-lymphocyte-associated protein 4 (also known as CD152); FoxP3, forkhead box protein P3; GITR, glucocorticoid-induced TNFR-related protein; LAG3: Lymphocyte-activation gene 3; PD1: Programmed cell death protein 1 (also known as CD279); TIM3: T-cell immunoglobulin and mucin-domain containing-3; TIGIT: T cell immunoreceptor with Ig and ITIM domains.
In general, the immunosuppressive functions of Tregs can be arranged into four groups. First, CTLA-4, the immune checkpoint receptor, interacts with antigen presenting cells (APCs) and inhibits CD28-co-stimulation necessary for T cell activation [22–24]. Second, high level expression of Foxp3 in Tregs leads to the expression and display of high levels of CD25, which forms the heterotrimeric high-affinity IL-2 receptor on the surface of Tregs [13–15]. It has been proposed that the abundance of CD25 on Tregs acts as a cytokine sink that depletes IL-2 in the local environment leading to cytokine deprivation which results in apoptosis of activated effector T cells. Third, Tregs also drive the production of inhibitory cytokines including IL-10, TGF-ß and IL-35 that suppress effector T-cell functions. And the fourth involves the direct cytotoxic killing of APCs and T-effector cells in a granzyme B/perforin-mediated pathway [25].
Additional important immune checkpoint molecules, which are heavily expressed on the surface of Tregs are PD-1 and PDL-1 [15,26]. PD-1 promotes expression of FoxP3 and hence the immunosuppressive activity of Tregs [15,26] and was found to be strongly associated with activated Tregs [27]. PD-1 is known to bind with its ligand PD-L1 expressed on tumor cells as well as on nonmalignant myeloid cells, APCs particularly dendritic cells (DCs), macrophages and lymphocytes [7,15,28,29]. As expected, the blocking of PD-1/PD-L1 interaction has been shown to either deplete or reduce the suppressive functions of Tregs; leading to increased infiltration of effector T-cells along with enhancement of their cytotoxic abilities, reduced expression of IL-10 and elevated production of pro-inflammatory cytokines in the tumor microenvironment [15,30–32].
CCR4 is expressed on the surface of a more than 75% of Tregs and may be a marker for a subset of Tregs also known as memory-type Tregs. CCR4+ Tregs appear to be innately primed to suppress the proliferation of effector T cells while CCR4 (-) Tregs needs TCR-mediated activation to become fully active or functional [33]. Lymphocyte activation gene-3, LAG-3, another negative co-receptor immune checkpoint, shows 20% sequence similarity to CD4 protein and binds to MHC-II class molecules on APCs with higher affinity than CD4 resulting in downregulation of antigen-specific CD4+ T cell responses that leads to an inhibition of effector T cells proliferation. Activated Tregs express LAG-3, expression of which is further enhanced by the presence of effector T cells [34].
T-cell immunoglobulin and mucin domain-containing protein (TIM-3) is another negative immune regulator that is constitutively expressed on Tregs cells in mice unlike human Tregs where its expression needs T-cell receptor mediated activation. Both mice and human Tregs overexpress TIM-3 upon T-cell receptor activation [35]. While first described in lung cancer, TIM3+ Tregs have been also detected in cervical, ovarian, hepatocellular and colon cancers [36]. TIM-3 expression is found on a variety of T-cells making it an attractive target for cancer immunotherapy. However, TIM-3 is also found on myeloid origin cells such as macrophages, DCs and monocytes leaving the field open to further investigation [18]. TIGIT is a newly identified co-inhibitory molecule expressed on activated Treg cells and it selectively suppresses Th1 and Th17 responses, but not Th2 cell responses [37]. Although multiple immune checkpoint molecules expressed on Tregs play an important role in regulation of immunity, contributing to self-tolerance and preventing autoimmunity, tumors co-opt immune checkpoints as a major immune resistance mechanism to prevent antitumor responses, thereby making them strong candidate targets for anticancer therapeutic discovery (Figure 2).
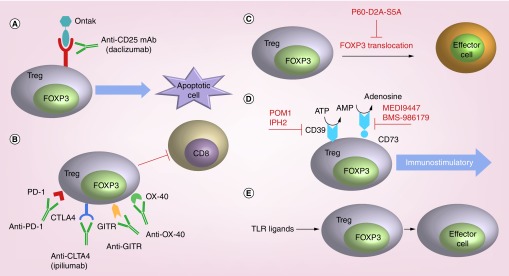
Figure 2. . Current strategies to block or inhibit tumor-associated Treg cells.
(A) In humans, an anti-CD25 mAb, daclizumab, has been used to deplete Treg cells. More recently, the recombinant diphtheria toxin-IL-2 fusion protein denileukin diftitox (Ontak®) has been used to target T cells with high CD25 expression. Upon internalization, diphtheria toxin irreversibly inhibits protein synthesis, ultimately triggering cell death. (B) Inhibition of Treg via targeting of CTLA-4, PD-1, OX40 or GITR alleviates the suppressor activity of these cells on effector CD8 cells. (C) A peptide inhibitor of Foxp3 known as P60-D2A-S5A impairs immunosuppressive activity of murine and human-derived Treg cells leading to enhanced effector T cell function. (D) Treg cells produce adenosine via catabolism of ATP by extracellular ectonucleotidases CD39 and CD73. Adenosine is an immunosuppressive metabolite that may participate in the immunosuppressive activity of Foxp3+ T cells. Inhibitors of CD39 (POM1 and IPH2) and of CD73 (MEDI9447 and BMS-986179) are depicted in red. (E) TLR ligands can directly reverse Treg cell suppressive function.
Different strategies for targeting Treg surface molecules
Numerous approaches have been to selectively deplete tumor-associated Treg cells. These include the use of anti-CD25 monoclonal antibodies [38], anticheckpoint receptor monoclonal antibodies targeting PD-1, CTLA-4, GITR, OX-40 and others [13–15], inhibition of FoxP3 translocation [39], inhibitors of CD39 and/or CD73 to reduce ambient concentrations of adenosine [40] and the use of TLR ligands (Figure 2) [41]. The additional strategy of using targeted bacterial toxins to selectively deplete CD25- or CCR4-bearing cells has led to the development of a successful human cancer immunotherapy known as denileukin diftitox (Ontak® or DAB389IL2).
In this review, we will discuss the use of recombinant bacterial toxin fusion protein to deplete Treg cells with an emphasis on agents that have advanced to the clinic. In general, the targeted toxin approach involves substitution of native receptor binding domains of bacterial toxins such as diphtheria toxin or Pseudomonas aeruginosa exotoxin A with a ligand for a known Treg receptor. This approach exploits the dramatic killing potential of these bacterial toxins – many of which are lethal for cells at subpicomolar concentrations – by redirecting the killing machinery of the toxins specifically to receptors abundant on the Treg cell surface leading to their specific elimination resulting in alleviation of immunosuppression [11,42–46]. While several of the recombinant bacterial toxin fusion proteins discussed in the review are well known in the literature, this review introduces recent advances in combining Treg-depleting fusion toxins with other anticancer therapies as well as exciting developments in toxicity reduction of these agents.
LMB2: a single-chain Fv fragment of an anti-CD25 monoclonal antibody fused to a truncated form of Pseudomonas exotoxin A
LMB-2 is composed of a single-chain Fv fragment of an anti-CD25 mAb fused to a Pseudomonas exotoxin A fragment containing the membrane translocation and enzymatic domains (Figure 3) [47]. LMB-2 administration showed clinical responses in CD25+ hematologic malignancies including adult-T cell leukemia, Hodgkin’s disease, hairy cell leukemia and cutaneous T-cell lymphoma [48,49]. In a recent in vitro study, LMB-2 was evaluated for specific elimination of CD4+ CD25+ Foxp3 expressing human Treg cells from peripheral blood mononuclear cells (PMBCs) [50]. An eightfold reduction was observed in CD25+ expressing CD4+ T cell lymphocytes paralleled by threefold reduction in Foxp3 expression in a population of resting PMBCs treated with LMB-2 for 48 h. Due to short half-life of LMB-2 at its maximum tolerated dose, Attia et al. [50] proposed administration of LMB-2 before the administration of cancer vaccines [48].
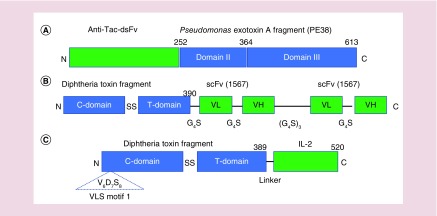
Figure 3. . Cartoon depiction of domains present in Treg-targeting bacterial fusion toxins.
(A) LMB2 is a Treg-targeting fusion protein comprised of a single-chain Fv fragment of an anti-CD25 monoclonal antibody fused to a truncated form of Pseudomonas exotoxin A. The 252 amino acid anti-CD25-targeting single chain Fv fragment, anti-Tac-dsFv, is shown in green. The 361 amino acid Pseudomonas exotoxin A fragment, PE38, comprising domain II (membrane translocation) and domain III (ADP-ribosyltransferase catalytic domain) of exotoxin A is shown in blue. (B) The Treg-cell targeting diphtheria toxin-CCR4 single-chain fold-back diabody fusion protein is shown. The fusion protein is comprised of the N-terminal 390 amino acids of diphtheria toxin which contain the ADP-ribosyltransferase catalytic domain and the transmembrane domain (shown in blue) fused to a dimeric, codon optimized anti-human CCR4 scFv shown in green. The bivalent scFv is comprised of paired VL (variable light chain) VH (variable heavy chains) separated by single or repeated pentapeptide linkers (G4S or Gly4Ser) as shown. (C) The structures of DAB389IL-2 (denileukin diftitox or Ontak®) and DAB389IL-2(V6A) are shown. These Treg-targeting fusion toxins contain the N-terminal 390 amino acids of diphtheria toxin with its ADP-ribosyltransferase catalytic domain and transmembrane domain (shown in blue) fused to IL-2 (green) which is the cognate ligand of CD25. DAB389IL-2(V6A) is a second-generation version of DAB389IL-2 which harbors a valine-to-alanine substitution at residue 6 (V6A) within vascular leak syndrome motif 1 and elicits significantly reduced levels of vascular leak in vitro and in animal models.
In a study by Powell and associates [44], eight patients with metastatic melanoma were treated with LMB-2 followed by vaccination with MART-1 and gp100. A significant reduction in the mean lymphocyte count was observed 1 day after LMB-2 treatment which persisted for 3 days. The lymphocyte count returned to pretreatment level 2 weeks after the first dose of LMB-2. An analysis of T-cell subset indicated nonspecific effects of LMB-2 on both CD4+ and CD8+ types. Although mean frequency of FoxP3+CD4+ T cells was reduced by 50%, LMB-2 mediated cell depletion was transient. About 3 weeks after the first dose of LMB-2, the frequency of FoxP3+CD4+ T cells was mildly elevated as compared with pretreatment level. However, there was significant reduction in the number of circulating CD25+ FOXP3+ CD4+ T cells at the initiation of peptide vaccination on day 5.
Although LMB-2 is a candidate for use in cancer treatment to mediate a transient and partial reduction in circulating and tumor infiltrating Treg cells [44], liver toxicity was found to be a major side effect that must be overcome before clinical introduction [49]. Onda et al. [51] analyzed the mechanism of toxicity of LMB-2 using a mouse model and found that intravenous administration of 0.9 mg/kg of LMB-2 into the NIH Swiss mice induced severe liver damage [51]. Total 18 h postinjection, there was an increase in the level of hepatic transaminases and clear evidence of hepatocyte necrosis. LMB-2 was found to accumulate in Kupffer cells in the liver and was also detected in small resorptive droplets in the apex of proximal tubular epithelial cells of the kidneys. LMB-2-induced induction of TNF-α appeared to mediate hepatotoxicity, since inhibition of TNF-α could prevent LMB-2 induced hepatotoxicity in the mouse model [51]. Most recently, a new derivative of LMB-2, LMB-142, has been constructed which shows cytotoxic activity and lower toxicity in mice [52].
Diphtheria toxin-based antihuman CCR4 fusion protein toxins for targeting Tregs
CCR4 is principally expressed by effector Tregs, but not by naive Tregs or TH1 cells [16]. In contrast, CD8+ T cells, natural killer (NK) cells, CD14+ monocytes, dendritic cells and B cells express barely detectable levels of CCR4 [16]. Since CCR4 is also highly expressed on T-cell lymphomas, this receptor is also a promising therapeutic drug target [53].
Wang et al. [45,54] recently developed three versions of a diphtheria-toxin based antihuman CCR4 fusion protein toxin (monovalent, bivalent and a single-chain fold-back diabody) seeking the optimal isoform for depleting human CCR4+ cells. In their study, a codonoptimized antihuman CCR4 scFv (1567) encoding gene was fused to a gene encoding the N-terminal 390 amino acids of diphtheria toxin (DT390), and the fusion protein toxin was expressed in yeast Pichia pastoris. In this construct, a pentapeptide linker of four glycine and one serine residues (G4S) was used to genetically link DT390 to scFv (1567). In monovalent and bivalent versions, three tandem G4S linkers (G4S)3 linked variable light (VL) chain and variable heavy (VH) chain to generate the scFv. In single-chain fold-back diabody version, VL and VH were linked by only one G4S linker. In the bivalent and single chain fold-back diabody, both scFv were linked by three G4S tandem linkers (see Figure 3). Among the three isoforms, the single-chain fold-back version showed the strongest depletion of CCR4+ Foxp3+ human PBMCs.
In addition, Wang et al. also demonstrated binding and in vivo efficacy of antihuman CCR4 protein fusion toxins with nonhuman primate (NHP) CCR4+ Foxp3+ monkey Tregs [54]. The fold-back diabody isoform bound most strongly to CCR4+ Foxp3+ Tregs on monkey PBMCs in a dose-dependent manner. As a result, the fold-back diabody isoform was selected for Treg depletion experiments in monkey. The antihuman CCR4 fusion protein toxin was administered at a dose of 25 μg/kg twice daily intravenously. At this dosage, depletion of 78–89% of CCR4+ Foxp3+ monkey Tregs was achieved that lasted for 10 days. Importantly, other cells including CD8+ T cells, B-cells and NK cells remained unaffected. Taken together, the fold-back diabody isoform of the antihuman CCR4 fusion protein toxin was able to effectively deplete in vivo NHP CCR4+ Foxp3+ Tregs both from lymph nodes and peripheral blood. The CCR4 fusion protein toxin caused an increase in the frequencies of monocytes even as CCR4+ Foxp3+ Tregs were depleted. This study suggested that CCR4+ Tregs have suppressive effect on monocytes that was similar to the suppressive effect on dendritic cells maturation reported earlier [55]. Clinically, the treated animals remained healthy with no observed adverse effect of administration of CCR4 fusion protein toxin.
CD25-targeted, diphtheria toxin-based fusion protein toxin depletes Tregs
Denileukin diftitox (DAB389IL-2, Ontak®) is a fusion protein toxin in which human IL-2 (hIL-2) is genetically fused to the N-terminal 389 amino acids of diphtheria toxin which contains both the catalytic and membrane translocation domains of the toxin (Figure 3) [56]. In this construct, the native diphtheria toxin receptor-binding domain is replaced with hIL-2 sequences that serve to target the fusion protein to only those cells that display the intermediate and high affinity IL-2 receptor on their surface [57]. Once the toxin is bound to the IL-2 receptor, it is internalized by receptor mediated endocytosis into the target host cell. Upon acidification of the endosomal lumen, the translocation domain of the fusion protein toxin inserts into the vesicle membrane forming an 18–22 Å channel, or pore, through which the catalytic domain is translocated to the cytosol. Once delivered into the cytosol, the catalytic domain catalyzes the NAD+-dependent ADP ribosylation of elongation factor 2, which inhibits protein synthesis (Figure 4) [58,59].
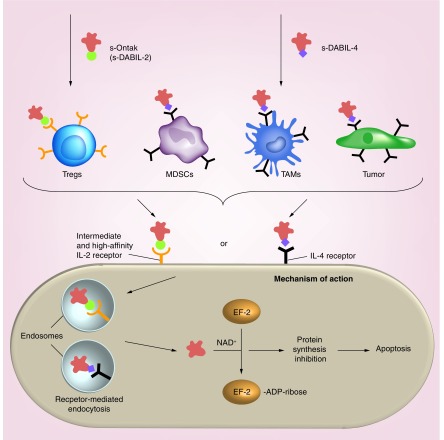
Figure 4. . Cartoon depictions of the mechanism of action of s-DAB389IL-2 and s-DAB389IL-4 which are produced as secreted proteins using Corynebacterium diphtheriae and are soluble, fully monomeric versions of DAB389IL-2 and DAB389IL-4.
Cartoon depiction of Treg-targeting action of s-DABIL-2 (targeting the IL-2 receptor, CD25) and the MDSC-, TAM-, and tumor-targeting action of s-DABIL-4 (targeting the IL-4 receptor, CD124 [Parveen et al., unpublished data]). Both targeted toxins bind to their cognate receptor, undergo receptor-mediated endocytosis with vesicular acidification and subsequently deliver their catalytic domains (red) to the cytosol of the targeted cell. The catalytic domain, a potent ADP-ribosyltransferase of elongation factor-2 (EF-2), enzymatically shuts down protein synthesis leading to apoptotic cell death.
In an early study, Kelley and associates [60] reported immunosuppressive action of denileukin diftitox on CD25+ T cells in vivo in mice model. Mahnke and associates [46] investigated whether denileukin diftitox (Ontak®) could deplete CD25+ Tregs in melanoma patients and how this depletion might augment the immune response against cancer antigens. Ontak® at a concentration of 16 nM was able to kill more than 90% CD4+ CD25+ T cells that were purified from healthy donors. This observation confirmed and extended the observations made by Dannull et al. [61] who reported the killing of approximately 100% of CD4+ CD25+ T cells in vitro with 5 nM Ontak®. Furthermore, Mahnke et al. showed that Ontak® eliminated Tregs by the induction of apoptosis [46]. In that study, a group of seven melanoma patients were selected for assessing the Treg depleting activity of Ontak® in vivo. Before treatment and 3 weeks postinfusion of Ontak®, patients were subjected to leukapheresis. In the study, melanoma patients were also vaccinated with the melanoma protein MelanA (also called MART1) and gp100. PBMC isolated from melanoma patients were analyzed for CD4+ CD25+ T cells and anti-melanoma immune response. An analysis of the CD4+ CD25+ T cells population revealed that the number of CD25+ T cells decreased among the CD4+ T cell population and this drop in CD25+ T cells was not due to an increase in CD4+ CD25- T cells. While there was not a complete depletion of CD4+ CD25+ T cells with successive administration of Ontak® in these melanoma patients, the suppressive capacity of residual Treg population was minimal [46]. Overall, this study indicated not only reduction in the number of Treg population upon Ontak® treatment, but also affected the capacity of Tregs to suppress T cell proliferation. Furthermore, a combination of Ontak®, tumor protein MART1 and gp100 augmented the antitumor activity of killer CD8+ T cells [46].
A Phase II clinical trial study of Ontak® to deplete regulatory T cells in the treatment-refractory stage III/IV ovarian cancer was conducted by Curiel and associates [62]. Ontak® at a dose of 12 μg/kg bodyweight was administered once monthly in the treatment-refractory stage III/IV ovarian cancer patients for four cycles. In addition, patients received chemotherapy for next 2 weeks or radiotherapy for next 4 weeks. Data analysis of this study confirmed that Ontak® treatment was associated with reduction in phenotypic CD4+CD25hi Tregs and an increase in CD3+IFN-γ+ T cells [63]. Ontak® has also been shown to be effective as a monotherapy for transient depletion of T regulatory cells in solid tumors. Both Phase I [64] and II [65] studies in patients presenting with unresectable stage IV malignant melanoma have shown that Ontak® as a monotherapy is also effective as an immunotherapeutic. In these trials, Ontak® was shown to transiently deplete T regulatory cells, apparently break tolerance and allow for a more robust host mounted T-effector cell antitumor response against the tumors. In the Phase II study [65], 45% of the immunotherapy-hemotherapy naive patients responded partially. About 1-year survival was also significantly higher in patients who responded (80 ± 11.9%) compared with patients with progressive disease (23.7 ± 6.5%). Moreover, more than 40% of the patients (40 ± 6.2%) treated with Ontak® were alive at 1 year.
In a more recent study, Lutz and associates [66] investigated the effect of Ontak® on activated Tregs and tumor antigen specific conventional T cells, and the study demonstrated that the cytotoxicity of Ontak® correlated with both cell types. In the case of resting T cells, high doses of Ontak® were required to induce apoptosis in both cell types; whereas, low doses were found to be antiapoptotic. In marked contrast, activated Tregs and antigen activated conventional T cells were readily killed by Ontak® treatment.
Despite the clinical efficacy of denileukin diftitox, the drug was placed on clinical hold due to presence of drug aggregates and variations in batch-to-batch formulations of the drug in 2011. In addition, there are reports of dose-limiting toxicity of denileukin diftitox leading to the induction of vascular leak syndrome (VLS) [67], that was unrelated to its CD25 targeted toxicity. The mechanism of VLS induced by denileukin diftitox is not clear, but disruption of tight junctions between endothelial cells leads to extravasation of fluid into the surrounding tissues. VLS can be severe, leading to peripheral edema, hypotension, hypovolemia, and pleural and pericardial effusions sometimes leading to death.
To address the problem of aggregate formation by denileukin diftitox, Cheung et al. [68] recently described the production of a fully folded monomeric form of the protein that was expressed and secreted from Corynebacterium diphtheriae C7(-). In addition, to address the problem of vascular leak, a V6A mutation was introduced into one of the three VLS-inducing motifs found in the diphtheria toxin portion of the fusion toxin protein [68]. This V6A substitution significantly reduced the capacity of the fusion protein toxin to induce vascular leak while retaining almost full biologic activity.
Combining Treg-depleting, second-generation denileukin diftitox therapy with immune checkpoint inhibitors
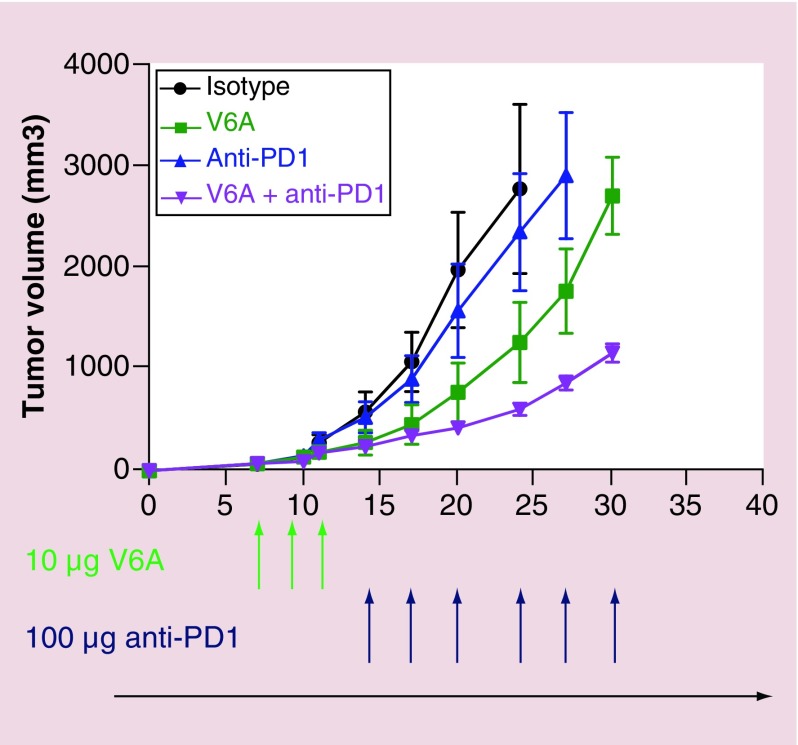
Cheung et al. [68] study further examined the effectiveness of V6A mutant denileukin diftitox, both as monotherapy and in a sequential immunotherapeutic regimen with the immune checkpoint inhibitor, anti-PD-1 antibody, in the B16F10 murine melanoma model. The overlapping combination of both drugs was found to deplete Tregs both from lymph nodes and spleen of tumor bearing mice combined with a remarkable inhibition of tumor growth. More recent studies from our group reveal that fully sequential (nonoverlapping) dual combined therapy with s-DABIL-2(V6A) and anti-PD1 leads to superior tumor control than either monotherapy alone (Figure 5). To examine if the depletion of Tregs could potentiate CD8+ T cells mediated antitumor immunity in tumor microenvironment, the study also analyzed the tumor infiltrating lymphocytes and found a significant increase in IFN-γ production by CD8+ cytotoxic T cells both in spleen and tumor from mice treated with V6A substituted form of s-Ontak. Overall, this study demonstrated that Treg-depleting denileukin diftitox fusion toxin could potentially enhance immune checkpoint inhibitor therapy.
Figure 5. . Antitumor effects of s-DABIL-2(V6A)-His6 when used as monotherapy and as dual sequential therapy with anti-PD1 in the mouse B16-F10 syngeneic melanoma tumor model.
Groups C57BL/six mice (eight mice per group) were inoculated with B16-F10 murine melanoma cells on day 0. They were treated with intraperitoneal doses on the indicated days of: (A) anti-PD1 isotype control antibody, (B) 10 μg doses of purified s-DABIL-2(V6A)-His6, abbreviated V6A (C) 100 μg doses of anti-PD1 antibody or (D) a sequential combination of purified s-DABIL-2(V6A)-His6 followed by anti-PD1 antibody. Tumor volume was measured with electronic callipers.
Conclusion
Treg cells remain a promising target cancer immunotherapy. Denileukin diftitox has been shown to deplete human Treg cells and has been used successfully in several human malignancies. In this review, we have discussed the development and use of denileukin diftitox, the development of related bacterial toxin fusion proteins targeting either CD25 or CCR4, as well as second generation improvements of DAB389IL2 to decrease the toxicity using protein engineering approach. Targeted bacterial toxins to selectively deplete tumor-associated Treg cells remain a promising strategy in cancer immunotherapy.
Future perspective
Targeted bacterial toxin fusion proteins offer significant promise toward new anticancer therapies. They have been shown to successfully target a particular cell population or subset with few off-target toxicities; be readily internalized by target cells; have potent cytotoxicity for the targeted cells (picomolar IC50 values) enabling significant activity at low doses. One of bacterial toxin fusion proteins, DAB389IL-2 (denileukin diftitox or Ontak®), was the first targeted bacterial fusion toxin protein to be approved by US FDA, and it has been shown to transiently deplete Treg cells in several human studies. As a monotherapy or in combination therapy with melanoma antigen vaccines, DAB389IL2 shows antitumor efficacy in human melanoma, and recently it has shown potent additive therapeutic benefit when combined with immune checkpoint inhibitors in mouse melanoma models. The major side effect of DAB389IL2, VLS, which is unrelated to CD-25 targeted toxicity, has been addressed by V6A substitution in the DAB389IL2. With the recent demonstration that combined therapy with s-DABIL-2(V6A) and anti-PD1 is superior than either monotherapy alone in a murine melanoma model, Treg-depleting immunotherapy appears to give additive benefit to immune checkpoint blockade.
Furthermore, as noted in this review, combinations of targeted bacterial toxin-based fusion proteins that deplete Tregs with other cancer drugs offers promise as a generalizable anticancer strategy. Evaluating alternative Treg antigens may further improve the specificity of targeted Treg depleting drugs and lead to better solutions for cancer treatment.
Executive summary.
Depleting or downregulating suppressive functions of Tregs are an area of intense focus as an anticancer therapeutic strategy.
Potential surface targets of Tregs cells for immunotherapy & strategies to target them
There are several surface molecules on Tregs that can be targeted by cell-depleting, targeted strategies.
Targeting CCR4 or CD25 on the surface of Tregs currently remains the most effective approach using bacterial toxin fusion proteins.
LMB2, a single-chain Fv fragment of an anti-CD25 monoclonal antibody fused to a truncated form of Pseudomonas exotoxin A, is a bacterial toxin fusion protein candidate that shows partial reduction in tumor infiltrating Tregs. Liver toxicity was major side effect of the drug. A new engineered version of LMB2, LMB-142, has been developed which shows improved Treg cytotoxicity and reduced toxicity in mice.
A CCR4 fusion protein toxin, comprised of a codon-optimized antihuman CCR4 scFv fused to a gene encoding the N-terminal 390 amino acids of diphtheria toxin (DT390), targets CCR4+ Tregs that exert suppressive effects on monocytes and dendritic cell maturation in the tumor microenvironment.
The CD25-targeted diphtheria toxin-based fusion protein toxin, denileukin diftitox (Ontak®) selectively depletes CD25+ Tregs. Ontak® has also been shown to be effective as a monotherapy for transient depletion of T regulatory cells in solid tumors. A dose-limiting toxicity of Ontak was the induction of vascular leak syndrome that was unrelated to its CD25 targeted toxicity.
The expression of a new monomeric form of denileukin diftitox (s-DABIL-2) and a V6A mutation in vascular leak syndrome-inducing motifs of s-DABIL-2 significantly reduced the capacity of the fusion protein toxin to induce vascular leak while retaining almost full biologic activity.
A sequential (nonoverlapping) dual combined therapy with s-DABIL-2(V6A) and anti-PD1 leads to superior tumor control than either monotherapy alone.
Conclusion
Combination therapies using targeted fusion protein toxins with other immunotherapies, cancer vaccines, and anticancer drugs offer considerable promise.
Acknowledgments
The authors gratefully acknowledge the editorial assistance of Dr Geetha Srikrishna.
Footnotes
Financial & competing interests disclosure
JR Murphy and W Bishai are cofounders of Sonoval, LLC which holds rights to the commercialization of s-DABIL-2(V6A). The authors acknowledge financial support of a NIH grant AI 130595 and grants from Maryland Tedco, the Cigarette Restitution Fund, and the Abell Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No funded writing assistance was utilized in the production of this manuscript.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 133(5), 775–787 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10(7), 490–500 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11(1), 7–13 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Shitara K, Nishikawa H. Regulatory T cells: a potential target in cancer immunotherapy. Ann. NY Acad. Sci. 1417(1), 104–115 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Vasievich EA, Huang L. The suppressive tumor microenvironment: a challenge in cancer immunotherapy. Mol. Pharm. 8(3), 635–641 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138(2), 105–115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming V, Hu X, Weber R. et al. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front. Immunol. 9, 398 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat. Revi. Clin. Oncol. 16, 356–371 (2019). [DOI] [PubMed] [Google Scholar]
- 9.LeMaistre CF, Craig FE, Meneghetti C. et al. Phase I trial of a 90-minute infusion of the fusion toxin DAB486IL-2 in hematological cancers. Cancer Res. 53(17), 3930–3934 (1993). [PubMed] [Google Scholar]
- 10.LeMaistre CF, Meneghetti C, Rosenblum M. et al. Phase I trial of an interleukin-2 (IL-2) fusion toxin (DAB486IL-2) in hematologic malignancies expressing the IL-2 receptor. Blood 79(10), 2547–2554 (1992). [PubMed] [Google Scholar]
- 11.Arellano B, Graber DJ, Sentman CL. Regulatory T cell-based therapies for autoimmunity. Disc. Med. 22(119), 73–80 (2016). [PMC free article] [PubMed] [Google Scholar]
- 12.von Boehmer H, Daniel C. Therapeutic opportunities for manipulating T(Reg) cells in autoimmunity and cancer. Nat. Rev. Drug Discov. 12(1), 51–63 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Vent-Schmidt J, Han JM, MacDonald KG, Levings MK. The role of FOXP3 in regulating immune responses. Int. Rev. Immunol. 33(2), 110–128 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 27(1), 109–118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianchecchi E, Fierabracci A. Inhibitory receptors and pathways of lymphocytes: the role of PD-1 in Treg development and their involvement in autoimmunity onset and cancer progression. Front. Immunol. 9, 2374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugiyama D, Nishikawa H, Maeda Y. et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl Acad. Sci. USA 110(44), 17945–17950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long L, Zhang X, Chen F. et al. The promising immune checkpoint LAG-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer 9(5–6), 176–189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Cao J, Zhao C, Li X, Zhou C, Hirsch FR. TIM-3, a promising target for cancer immunotherapy. OncoTargets Ther. 11, 7005–7009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtulus S, Sakuishi K, Ngiow SF. et al. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Invest. 125(11), 4053–4062 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol. Cell Biol. 96(1), 21–33 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp. Mol. Med. 50(12), 165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walunas TL, Lenschow DJ, Bakker CY. et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1(5), 405–413 (1994). [DOI] [PubMed] [Google Scholar]
- 23.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J. Immunol. 162(10), 5813–5820 (1999). [PubMed] [Google Scholar]
- 24.Walker LS, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 36(2), 63–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol. 8(7), 523–532 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francisco LM, Salinas VH, Brown KE. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206(13), 3015–3029 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J. Immunol. 176(5), 2808–2816 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Lin H, Wei S, Hurt EM. et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J. Clin. Invest. 128(2), 805–815 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang F, Zheng P. Tumor cells versus host immune cells: whose PD-L1 contributes to PD-1/PD-L1 blockade mediated cancer immunotherapy? Cell Biosc. 8, 34–34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okiyama N, Furumoto Y, Villarroel VA. et al. Reversal of CD8 T-cell-mediated mucocutaneous graft-versus-host-like disease by the JAK inhibitor tofacitinib. J. Invest. Dermatol. 134(4), 992–1000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J. Immunother. Cancer 2, 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boutros C, Tarhini A, Routier E. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nature Rev. Clin. Oncol. 13(8), 473–486 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Baatar D, Olkhanud P, Sumitomo K, Taub D, Gress R, Biragyn A. Human peripheral blood T regulatory cells (Tregs), functionally primed CCR4+ Tregs and unprimed CCR4- Tregs, regulate effector T cells using FasL. J. Immunol. 178(8), 4891–4900 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang CT, Workman CJ, Flies D. et al. Role of LAG-3 in regulatory T cells. Immunity 21(4), 503–513 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Gautron AS, Dominguez-Villar M, de Marcken M, Hafler DA. Enhanced suppressor function of TIM-3+ FoxP3+ regulatory T cells. Eur. J. Immunol. 44(9), 2703–2711 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan J, Zhang Y, Zhang JP, Liang J, Li L, Zheng L. Tim-3 expression defines regulatory T cells in human tumors. PLoS ONE 8(3), e58006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joller N, Lozano E, Burkett PR. et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 40(4), 569–581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris JC, Waldmann TA. Advances in interleukin 2 receptor targeted treatment. Ann. Rheum. Dis. 59(Suppl 1, i109–114 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casares N, Rudilla F, Arribillaga L. et al. A peptide inhibitor of FOXP3 impairs regulatory T cell activity and improves vaccine efficacy in mice. J. Immunol. 185(9), 5150–5159 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Perrot I, Michaud HA, Giraudon-Paoli M. et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 27(8), 2411–2425 e2419 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Shukla NM, Chan M, Hayashi T, Carson DA, Cottam HB. Recent advances and perspectives in small-molecule TLR ligands and their modulators. ACS Med. Chem. Lett. 9(12), 1156–1159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arce Vargas F, Furness AJS, Solomon I. et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity 46(4), 577–586 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann. NY Acad. Sci. 1174, 99–106 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Powell DJ, Jr., Felipe-Silva A, Merino MJ. et al. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J. Immunol. 179(7), 4919–4928 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Wei M, Zhang H. et al. Diphtheria-toxin based anti-human CCR4 immunotoxin for targeting human CCR4(+) cells in vivo. Mol. Oncol. 9(7), 1458–1470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahnke K, Schonfeld K, Fondel S. et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int. J. Cancer 120(12), 2723–2733 (2007). [DOI] [PubMed] [Google Scholar]
- 47.FitzGerald DJ, Waldmann TA, Willingham MC, Pastan I. Pseudomonas exotoxin-anti-TAC. Cell-specific immunotoxin active against cells expressing the human T cell growth factor receptor. J. Clin. Invest. 74(3), 966–971 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kreitman RJ, Wilson WH, Robbins D. et al. Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood 94(10), 3340–3348 (1999). [PubMed] [Google Scholar]
- 49.Onda M, Kreitman RJ, Vasmatzis G, Lee B, Pastan I. Reduction of the nonspecific animal toxicity of anti-Tac(Fv)-PE38 by mutations in the framework regions of the Fv which lower the isoelectric point. J. Immunol. 163(11), 6072–6077 (1999). [PubMed] [Google Scholar]
- 50.Attia P, Powell DJ, Jr., Maker AV, Kreitman RJ, Pastan I, Rosenberg SA. Selective elimination of human regulatory T lymphocytes in vitro with the recombinant immunotoxin LMB-2. J. Immunother. 29(2), 208–214 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onda M, Willingham M, Wang QC. et al. Inhibition of TNF-alpha produced by Kupffer cells protects against the nonspecific liver toxicity of immunotoxin anti-Tac(Fv)-PE38, LMB-2. J. Immunol. 165(12), 7150–7156 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Mazor R, Kaplan G, Park D. et al. Rational design of low immunogenic anti CD25 recombinant immunotoxin for T cell malignancies by elimination of T cell epitopes in PE38. Cell. Immunol. 313, 59–66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshie O, Matsushima K. CCR4 and its ligands: from bench to bedside. Int. Immunol. 27(1), 11–20 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Pratts SG, Zhang H. et al. Treg depletion in non-human primates using a novel diphtheria toxin-based anti-human CCR4 immunotoxin. Mol. Oncol. 10(4), 553–565 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayry J, Tchilian EZ, Davies MN. et al. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc. Natl Acad. Sci. USA 105(29), 10221–10226 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams DP, Parker K, Bacha P. et al. Diphtheria toxin receptor binding domain substitution with interleukin-2: genetic construction and properties of a diphtheria toxin-related interleukin-2 fusion protein. Protein Eng. 1(6), 493–498 (1987). [DOI] [PubMed] [Google Scholar]
- 57.Re GG, Waters C, Poisson L, Willingham MC, Sugamura K, Frankel AE. Interleukin 2 (IL-2) receptor expression and sensitivity to diphteria fusion toxin DAB389IL-2 in cultured hematopoietic cells. Cancer Res. 56(11), 2590–2595 (1996). [PubMed] [Google Scholar]
- 58.Williams DP, Snider CE, Strom TB, Murphy JR. Structure/function analysis of interleukin-2-toxin (DAB486-IL-2). Fragment B sequences required for the delivery of fragment A to the cytosol of target cells. J. Biol. Chem. 265(20), 11885–11889 (1990). [PubMed] [Google Scholar]
- 59.Bacha P, Williams DP, Waters C, Williams JM, Murphy JR, Strom TB. Interleukin 2 receptor-targeted cytotoxicity. Interleukin 2 receptor-mediated action of a diphtheria toxin-related interleukin 2 fusion protein. J. Exp. Med. 167(2), 612–622 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelley VE, Bacha P, Pankewycz O, Nichols JC, Murphy JR, Strom TB. Interleukin 2-diphtheria toxin fusion protein can abolish cell-mediated immunity in vivo. Proc. Natl Acad. Sci. USA 85(11), 3980–3984 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dannull J, Su Z, Rizzieri D. et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 115(12), 3623–3633 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Curiel TJ, Coukos G, Zou L. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10(9), 942–949 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Brian G, Barnett JR, Cheng PJ. et al. Phase II trial of Ontak to deplete regulatory T cells in treatment-refractory stage III/IV ovarian cancer. Proc. Amer. Assoc. Cancer Res. 66(8), 1148 (2006). [Google Scholar]
- 64.Rasku MA, Clem AL, Telang S. et al. Transient T cell depletion causes regression of melanoma metastases. J. Transl. Med. 6, 12 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Telang S, Rasku MA, Clem AL. et al. Phase II trial of the regulatory T cell-depleting agent, denileukin diftitox, in patients with unresectable stage IV melanoma. BMC Cancer 11, 515 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lutz MB, Baur AS, Schuler-Thurner B, Schuler G. Immunogenic and tolerogenic effects of the chimeric IL-2-diphtheria toxin cytocidal agent Ontak((R)) on CD25(+) cells. Oncoimmunology 3, e28223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olsen E, Duvic M, Frankel A. et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J. Clin. Oncol. 19(2), 376–388 (2001). [DOI] [PubMed] [Google Scholar]
- 68.Cheung LS, Fu J, Kumar P. et al. Second-generation IL-2 receptor-targeted diphtheria fusion toxin exhibits antitumor activity and synergy with anti-PD-1 in melanoma. Proc. Natl Acad. Sci. USA 116(8), 3100–3105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]