Abstract
Aim:
To develop a practical microfluidic 3D hepatocyte chip for hepatotoxicity testing of nanoparticles using proof of concept studies providing first results of the potential hepatotoxicity of superparamagnetic iron oxide nanoparticles (SPION) under microfluidic conditions.
Methods:
A microfluidic 3D hepatocyte chip with three material layers, which contains primary rat hepatocytes, has been fabricated and tested using different concentrations (50, 100 and 200 μg/ml) of SPION in 3-day (short-term) and 1-week (long-term) cultures.
Results:
Compared with standard well plates, the hepatocyte chip with flow provided comparable viability and significantly higher liver-specific functions, up to 1 week. In addition, the chip recapitulates the key physiological responses in the hepatotoxicity of SPION.
Conclusion:
Thus, the developed 3D hepatocyte chip is a robust and highly sensitive platform for investigating hepatotoxicity profiles of nanoparticles.
Keywords: : 3D hepatocyte chip, nanotoxicology, superparamagnetic iron oxide nanoparticles (SPION), primary rat hepatocytes, albumin, urea
Despite the widespread use of nanomaterials in cell and tissue engineering, medical device development, and the encapsulation and delivery of drugs, diagnostics and genes, knowledge on the toxicity and potential health risks associated with nanomaterial use is extremely limited [1,2]. However, safety concerns and the number of studies on health risks associated with nanomaterial exposure continue to grow [1,3].
Hepatotoxicity is a major factor for the biosafety of any formulation before its clinical translation. More than 900 drugs have been implicated in causing liver injury and it is the most common reason for a drug to be withdrawn from the market. Moreover, the liver is the first major organ to be exposed to ingested chemicals and is the most common site prone to toxicity [4,5]. Thus, the investigation of hepatotoxicity profile of nanomaterials is critical to their successful translation into the clinic.
Mice and rats are the most commonly used experimental animal models in preclinical studies to predict metabolic behavior of new compounds in humans, despite the differences in drug metabolism between humans and rodents; the different responses to xenobiotics are explained in part by species differences in Cytochrome P450 subfamilies [6,7]. Due to species differences, extrapolation needs to be done, for example, the human equivalent dose is obtained from the no observed adverse effect level of multi-dose toxicity studies in animal species, and then the human equivalent dose is divided by a factor value of 10, to increase safety of first human dose [8,9]. On the other hand, in vitro screening is the primary alternative to animal testing that is a costly and sometimes inefficient method of generating toxicity data [3,10]. The use of in vitro models provides a significant reduction in the use of animals; allows much smaller quantities of chemicals for testing; shortens the time for assessing toxicity or adverse effects; provides a much higher throughput allowing the rapid evaluation of multiple chemicals at various concentrations and their metabolites; allows to study chemical metabolism, evaluate the mechanisms of toxicity, measure enzyme kinetics and examine dose-response relationships [3,11–13]. Thus, in vitro toxicity tests for screening prior to animal studies are urgently required; numerous in vitro models have been proposed for this purpose, such as liver slices, primary cells, and liver cell lines [3,10]. Compared with liver slices (they exhibit a limited lifespan in culture) and liver cell lines (they do not possess important phenotypic characteristics of the liver tissue with no or very low levels of important drug-metabolizing enzymes and transporters; show much lower expression of liver-specific functions on average; and represent a phenotype from a single donor, which reduces their predictive value for the human population), primary hepatocytes are currently the best option for hepatotoxicity testing [3,10,13]. However, drug-metabolizing enzymes are rapidly altered after their isolation; therefore, to improve the predictive abilities of primary hepatocytes, novel strategies need to be developed to maintain their morphology and function in vitro [10,14].
Primary hepatocytes under conventional 2D culture rapidly lose differentiated functions; on the other hand, 3D microfluidic platforms have been shown to allow the creation of an artificially engineered, physiologically relevant cell culture microenvironment mimicking the in vivo environment of the liver, which is not possible using standard plate cultures [10,15,16]. Beyond providing extensive cell-cell interaction, which is important to maintain the function of biotransformation enzymes and transporter activities [10], 3D microfluidic platforms allow hepatocyte cultures subject to flow illustrate sustained and constant higher albumin and urea secretions for a long period of time compared with static cultures [16–18]. Thus, the aim of the current study is the development of a microfluidic 3D hepatocyte chip for hepatotoxicity testing of nanoparticles that is a critical step to their successful translation into the clinic.
A critical point in organ-on-a-chip development is the balance between complexity and practicality. This balance can be achieved by identifying the minimal subset of cells and micro-environmental factors necessary to create the simplest model possible that recapitulates physiological responses of interest [19]. In this context, a microfluidic 3D hepatocyte chip containing three layers (the bottom layer is a glass slide, the cell culture chamber containing primary rat hepatocytes on the middle layer and the top layer is a piece of plain polydimethylsiloxane [PDMS]) has been fabricated. The fabrication contains a desktop digital craft cutter, which is a low-cost and rapid fabrication method [20].
Superparamagnetic iron oxide nanoparticles (SPION) with unique physicochemical features (e.g., ultra-fine sizes and a large surface area to mass ratio) are currently the only clinically approved metal oxide nanoparticles and the most commonly used superparamagnetic nanoparticles [21–23]. SPION have a vast variety of biomedical applications including MRI, targeted delivery of drugs or genes, tissue engineering, hyperthermia of cancer, magnetic transfections, among others [3,23–30]). With the increasing use in the vast variety of clinical and biomedical applications, safety concerns about SPION have increased, and, to date, there is neither adequate information regarding their safety nor standardized method to establish such parameters [3,31–33].
Thus, due to high popularity of SPION as well as to contribute to establishment of standardized methods by providing first results of the potential hepatotoxicity of SPION under microfluidic conditions, proof of concept studies of the fabricated microfluidic 3D hepatocyte chip were carried out using SPION in different concentrations (50, 100 and 200 μg/ml). By using standard well plates with or without collagen gel cover as competing comparison system for the 1-week on-chip model and 3-day on-chip model, respectively, we investigated effects of the nanoparticles on liver-specific functions of primary rat hepatocytes by analyzing albumin and urea synthesis and effects of the nanoparticles on the viability of the primary rat hepatocytes using live/dead assay.
Materials & methods
Chemicals
Superparamagnetic magnetite (Fe3O4) nanoparticles (10 nm, transmission electron microscopy [TEM]; magnetization: >45 emu/g, at 4500 Oe; 5 mg/ml in H2O) were purchased from Sigma-Aldrich (MO, USA) and used as received. There is no reported toxicity in terms of all toxicity categories (acute toxicity, skin corrosion/irritation, serious eye damage/eye irritation, respiratory or skin sensitization, germ cell mutagenicity, carcinogenicity [except from expression from IARC], reproductive toxicity, specific target organ toxicity – single exposure, specific target organ toxicity – repeated exposure and aspiration hazard) on the ‘safety data sheet’ of the product [34]. We note that while these are not medical grade SPION that are used in the clinic, we used them here to develop an effective in vitro system and identify markers to detect toxicity induced by SPION. Further studies with medical grade SPION need to be conducted in the future to confirm the findings of the current study. Williams’ Medium E, DMSO, and acetaminophen (APAP) were purchased from Sigma-Aldrich; L-glutamine was purchased from Thermo Fisher Scientific (MA, USA). Penicillin/streptomycin was purchased from Invitrogen (CA, USA); albumin ELISA kit and urea colorimetric assay were purchased from Abcam (MA, USA) and Stanbio Laboratory (TX, USA), respectively.
Fabrication of microfluidic device
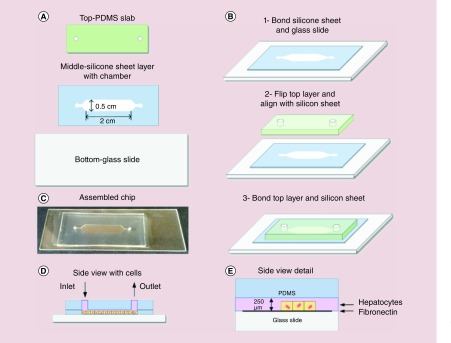
The microfluidic device was fabricated as depicted in Figure 1A–C and described in the following. We used a desktop digital craft cutter to make our microfluidic chip, which is a low-cost and rapid fabrication method [20]. The chip was assembled using three layers where the bottom layer was a glass slide. The cell culture chamber, the middle layer, was carved using the electronic craft cutter (Silhouette CAMEO® 3, Silhouette America, Inc., UT, USA) on a 0.01 inch (250 μm) thickness silicone sheet substrate (BISCO® HT-6240 transparent solid silicone, Rogers Corporation, AZ, USA). Finally, the top layer was a piece of plain PDMS (Sylgard 184, Dow Corning, MI, USA) used to cover the chamber. These three layers were bonded together by using a plasma cleaner (Cute, Femto Science, Inc., Gyeonggi-Do, South Korea) to form a complete microfluidic device.
Figure 1. . Schematic of the various steps during fabrication of the microfluidic device and the 3D hepatocyte culture assembly within the device.
(A) Schematic of the three layers that were used to assemble the device. (B) The bonding process of the three layers by using O2 plasma treatment. (C) Photo of the assembled microfluidic chip. (D) Side view of the chip with seeding of hepatocytes. (E) Detailed side view of the chip with hepatocytes.
Rat hepatocyte isolation & culture
Hepatocytes were isolated from adult female Lewis rats (Charles River Laboratories, MA, USA) weighing 180–200 g, as described previously [18,35]. Routinely, 200–300 million cells with 90–95% viability (determined by trypan blue exclusion) were isolated, and cultured in DMEM (Life Technologies, CA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich), 0.5 U/ml insulin, 7 ng/ml glucagon, 20 ng/ml EGF, 7.5 μg/ml hydrocortisone, 200 U/ml penicillin, 200 μg/ml streptomycin and 50 μg/ml gentamycin.
Rat hepatocytes were seeded in the 6-well tissue culture plates covered with collagen gel (bottom coating: 1 ml/well, nine parts phosphate-buffered saline [PBS]/one part collagen; top gel: 200 μl/well, nine parts collagen/one part 10 × DMEM), as described previously [36]. In the ‘non top gel group’ only bottom coating was carried out, we changed the culture medium after 4–6 h and set this time as T = 0. In the ‘top gel group’, we covered collagen gel on the cells 24 h after cell seeding and set this time as Day = 0. Culture media were changed and collected every 24 h for the albumin and urea assays.
Experimental & control groups
We used two kinds of on-chip experiments to test nanoparticle toxicity. One was a 3-day short-term test and the other lasted for 7 days (long-term). Hepatocytes cultured in collagen-coated 6-well plates with or without collagen gel cover were performed as competing comparison system for the 7-day on-chip model and 3-day on-chip model, respectively.
Hepatocyte culture on chip
Prior to cell seeding, the chips were autoclaved for 20 min at 121°C and the glass slide was coated with 50 μg/ml fibronectin (fibronectin from bovine plasma, Sigma–Aldrich, MA, USA) in PBS and maintained at 37°C for 1 h. Then hepatocytes were perfused into the chamber and allowed to attach as a monolayer of hepatocytes onto the glass slide as shown in (Figure 1D & E).
Three-day on-chip model
We first studied the effect of different flow rates on the hepatocytes cultured in the chip. For the well group, media were changed every 24 h. For the flow group, we started the perfusion at T = 24 h. The syringe pumps were used to continuously perfuse cell culture medium through the chambers with different flow rates 25, 50 and 100 μl/h. The perfusion lasted for 24 h and then the chips were put into the incubator for another 24 h. The detailed schematics of the experimental protocol were shown in Supplementary Figure 1A.
Then, SPION treatment was performed using this model. At T = 24 h, the culture medium with nanoparticles (50 and 100 μg/ml) was applied to the cells cultured both in well and chip. For the flow group, the perfusion lasted for 24 h. Media were collected every 24 h for albumin and urea assays. The detailed schematics of the experimental protocol were shown in Supplementary Figure 1B.
One-week on-chip model
We first studied the effect of the 7-day flow with different flow rates on the hepatocytes cultured in the chip. The perfusion was started at T = 24 h and lasted for 6 days under different flow rates, 25, 50 and 100 μl/h. Media were collected every 24 h for albumin and urea assays, and the live/dead assay was performed at the end of the experiment. For the well group, due to the top collagen coating, the medium collection for the albumin and urea assays was started at forty-eighth hour; the experiment lasted for 192 h, from the beginning. The detailed schematics of the experimental protocol were shown in Supplementary Figure 1C.
Then, SPION treatment was performed using this model. At T = 120 h (day 5), the culture medium with nanoparticles (200 μg/ml) was applied to the cells cultured in the chip with the flow rate of 50 μl/h and the perfusion was lasted for 24 h. The 40 mM of APAP was used as positive control. Media were collected every 24 h for albumin and urea assays, and the live/dead assay was performed at the end of the experiment. The detailed schematics of the experimental protocol were shown in Supplementary Figure 1D.
Albumin ELISA & urea assay
Albumin synthesis and urea production are two widely accepted markers for the evaluation of hepatocyte function. Media were collected from the wells and chips at determined times and stored at -80°C until analyses for albumin and urea secretion, as described previously [3,37].
Albumin analysis
To measure the amount of albumin production, an in-house developed inverse competitive ELISA method was used. In each well of 96-well high-binding plates, 100 μl of 50 μg/ml albumin (MP Biomedicals LLC, CA, USA) was plated and the plates were sealed and kept at 4°C overnight for the albumin to bind. The plates were washed four-times using PBS-Tween and 50 μl of both samples and standards (100–0 μg/ml) were introduced as duplicates in separate wells. Albumin antibody (∼2 μg/ml) was introduced immediately to provide competition for the antibodies between the bound albumin on the plate and albumin in samples, and then the plates were sealed again and incubated at 37°C for 90–120 min. The plates were washed using PBS-Tween, and, by introducing to wells at regular intervals, the plates were developed with an o-phenylenediamine dihydrochloride (OPD) solution containing H2O2; then, by introducing to wells at the same intervals, the reaction was stopped with 8N H2SO4. Using dual readings at 490 and 650 nm with a Benchmark Plus microplate reader (Biorad, Inc., CA, USA), the absorbance at each well was analyzed; and finally, standard curves were constructed for each plate and sample concentrations were calculated accordingly.
Urea analysis
To measure the amount of urea production, a colorimetric urea detection assay (Stanbio, Inc., TX, USA) was used. Following the manufacturer’s instructions, in clear 96-well assay plates, 10 μl of both samples and standards (200–0 μg/ml) were introduced along with 150 μl of blood/urea/nitrogen (BUN) reagent mixture. Then the plates were sealed tightly and incubated at 60°C for 90 min. After the plates were cooled on ice for 20 min, absorbances of the samples were read at 520 and 650 nm with the Benchmark Plus microplate reader (Biorad, Inc.); and finally, standard curves were constructed for each plate and sample concentrations were calculated accordingly.
Live/dead assay
To visualize the viability of hepatocytes exposed to different concentration of nanoparticles both in well and chip, the live/dead assay was performed on the last day by using the Live/Dead Viability/Cytotoxicity Kit (L-3224 Invitrogen, NY, USA) with assay reagents (4 μM EthD-1 and 2 μM Calcein in PBS). The cells were imaged using a Zeiss Axiovert Z200m CFL (Zeiss, Germany) microscope.
Statistical analysis
To determine whether there were any statistically significant differences between the means of the groups, one-way analysis of variance with Tukey's honest significant difference (Tukey's HSD) test was used. Data were expressed as the mean ± SD from three independent experiments with three replicates. For all the statistical analyses, as the threshold for significance, p < 0.05 was used.
Results
Three-day short-term liver-specific functions
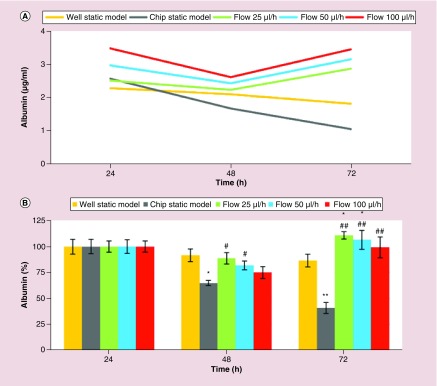
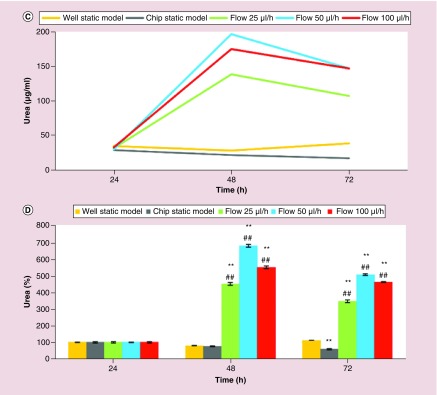
First, the effects of three different flow rates, 25, 50 and 100 μl/h, on the liver-specific functions of the primary rat hepatocytes were assessed by analyzing albumin (Figure 2A & B) and urea (Figure 2C & D) synthesis. Figure 2A & C illustrate the albumin and urea synthesis in μg/ml, respectively. Figure 2B & D illustrate the normalized values of the albumin and urea synthesis, respectively.
Figure 2. . Three-day short-term liver-specific function of primary rat hepatocytes.
Time-course graph (A & C), and bar graph with statistical analyses (B & D) illustrating liver-specific functions (albumin (A & B) and urea (C & D) synthesis) of primary rat hepatocyte cultures in well static model, chip static model (0 μl/h) and chip with flow (25, 50 and 100 μl/h). Significant difference between well static model and chip is denoted as *p-value < 0.05 and **p-value < 0.01; between chip static model and chip with flow is denoted as #p-value < 0.05 and ##p-value < 0.01. Statistical analyses among the flow groups: Albumin: p > 0.05 between any two flow rates at the forty-eighth and seventy-second hour; urea: p < 0.01 between any two flow rates at the forty-eighth and seventy-second hour.
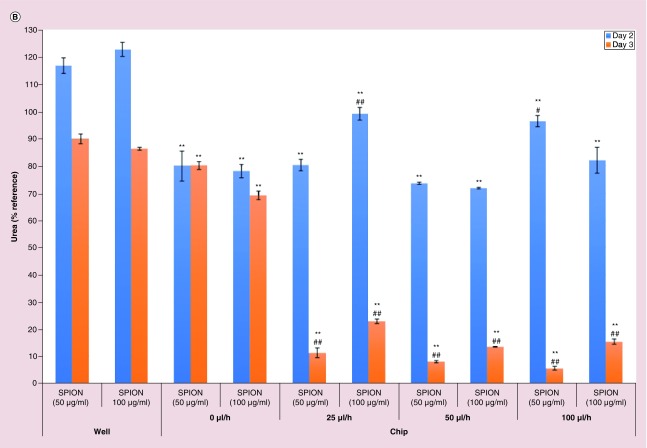
Then, the response capacity of the primary rat hepatocytes in the 3D hepatocyte chip with three different flow rates, 25, 50 and 100 μl/h, was assessed by analyzing albumin (Figure 3A) and urea (Figure 3B) synthesis. The effects of 24 h perfusion of SPION in 50 and 100 μg/ml on the liver-specific functions (albumin and urea synthesis) were analyzed to evaluate the capacity.
Figure 3. . Superparamagnetic iron oxide nanoparticles effect on liver-specific functions of 3-day short-term primary rat hepatocyte cultures.
Albumin (A) and urea (B) assays to quantify the specific function of the primary rat hepatocytes for forty-eighth and seventy-second hour after the seeding (twenty-fourth and forty-eighth hour after the start of treatment with superparamagnetic iron oxide nanoparticles [SPION] in 50 and 100 μg/ml). Significant difference between well static model and chip is denoted as **p-value < 0.01; between chip static model and chip with flow is denoted as #p-value < 0.05 and ##p-value < 0.01. Statistical analyses among the flow groups: (A) forty-eighth hour: SPION (50 μg/ml) treatment: p > 0.05 for 25 versus 50 μl/h; p < 0.01 for 25 versus 100 μl/h; p < 0.05 for 50 versus 100 μl/h; SPION (100 μg/ml) treatment: p < 0.01 for 25 versus 50 μl/h; p < 0.05 for 25 versus 100 μl/h; p > 0.05 for 50 versus 100 μl/h; seventy-second hour: SPION (50 μg/ml) treatment: p > 0.05 between any two flow rates; SPION (100 μg/ml) treatment: p < 0.05 for 25 versus 50 μl/h and for 50 versus 100 μl/h; p < 0.01 for 25 versus 100 μl/h; (B) forty-eighth hour: SPION (50 μg/ml) treatment: p > 0.05 for 25 versus 50 μl/h; p < 0.05 for 25 versus 100 μl/h; p < 0.01 for 50 versus 100 μl/h; SPION (100 μg/ml) treatment: p < 0.01 for 25 versus 50 μl/h; p < 0.05 for 25 versus 100 μl/h; p > 0.05 for 50 versus 100 μl/h; seventy-second hour: SPION (50 μg/ml) treatment: p > 0.05 between any two flow rates; SPION (100 μg/ml) treatment: p < 0.01 for 25 versus 50 μl/h and for 25 versus 100 μl/h; p > 0.05 for 50 versus 100 μl/h.
SPION: Superparamagnetic iron oxide nanoparticles.
One-week long-term liver-specific functions & viability
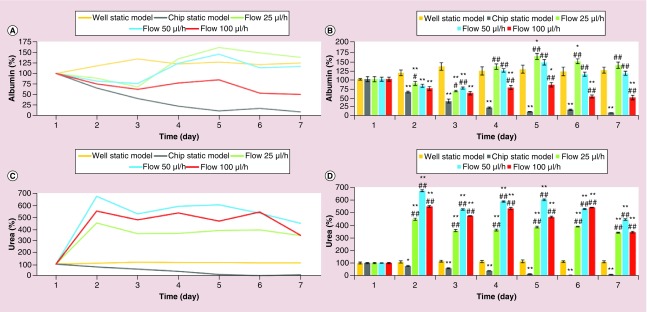
First, the effects of three different flow rates, 25, 50 and 100 μl/h, on the liver-specific functions of the primary rat hepatocytes were assessed by analyzing albumin (Figure 4A & B) and urea (Figure 4C & D) synthesis. At the end of the 1-week experiment, the live/dead assay (Figure 5) was performed to visualize the conditions of the primary rat hepatocyte cultures in the 3D hepatocyte chip with flow rate of 50 μl/h, which was determined as the optimal flow rate from the results of 3-day short-term and 1-week long-term analyses of the liver-specific functions (albumin and urea secretion).
Figure 4. . One-week long-term liver-specific function of primary rat hepatocytes.
Time-course graph (A & C), and bar graph with statistical analyses (B & D) illustrating liver-specific functions (albumin (A & B) and urea (C & D) synthesis) of primary rat hepatocyte cultures in well static model, chip static model (0 μl/h) and chip with flow (25, 50 and 100 μl/h). Significant difference between well static model and chip is denoted as *p-value < 0.05 and **p-value < 0.01; between chip static model and chip with flow is denoted as #p-value < 0.05 and ##p-value < 0.01. Statistical analyses among the flow groups: albumin: day 2 and 3: p > 0.05 between any two flow rates; day 4, 5 and 7: p > 0.05 for 25 versus 50 μl/h; p < 0.01 for 25 versus 100 μl/h and for 50 versus 100 μl/h; day 6: p < 0.01 between any two flow rates; urea: day 2, 3, 4 and 5: p < 0.01 between any two flow rates; day 6: p < 0.01 for 25 versus 50 μl/h and for 25 versus 100 μl/h; p > 0.05 for 50 versus 100 μl/h; day 7: p < 0.01 for 25 versus 50 μl/h and for 50 versus 100 μl/h; p > 0.05 for 25 versus 100 μl/h.
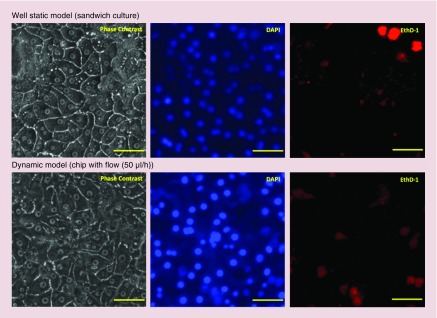
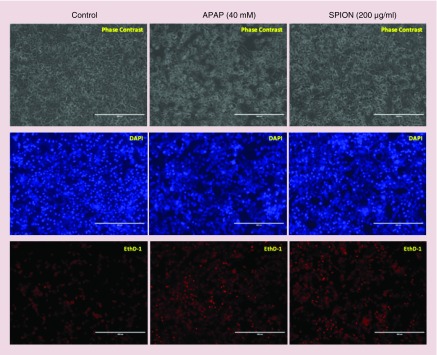
Figure 5. . Live/dead assay at the seventh day after the seeding of primary rat hepatocytes.
Upper panel: primary rat hepatocytes in sandwich culture; lower panel: primary rat hepatocytes in the chip with the flow (50 μl/h). Blue: DNA/nuclei of the cells; red: dead cells; image scale bar: 50 μm.
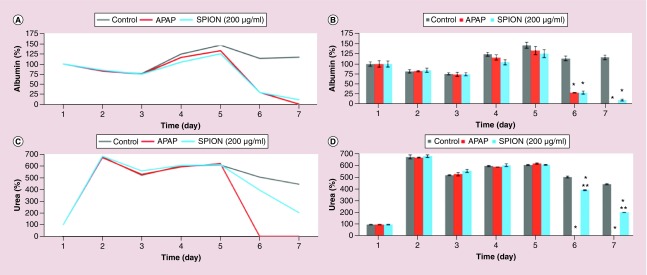
Then, the toxicity detection capacity of the 3D hepatocyte chip with the flow rate of 50 μl/h was assessed by analyzing albumin (Figure 6A & B) and urea (Figure 6C & D) synthesis. Using APAP (40 mM) as the positive control, the effects of 24 h perfusion of SPION (200 μg/ml) from 120th h (5th day) on the liver-specific functions (albumin and urea synthesis) were analyzed to evaluate the capacity. At the end of the 1-week experiment, using APAP (40 mM) as the positive control, the live/dead assay was performed to visualize the capacity of the 3D hepatocyte chip to detect the toxicity of the SPION (200 μg/ml) (Figure 7).
Figure 6. . The effect of superparamagnetic iron oxide nanoparticles on liver-specific functions in 1-week long-term primary rat hepatocyte cultures in the chip with the flow (50 μl/h).
Time-course graph (A & C), and bar graph with statistical analyses (B & D) illustrating liver-specific functions (albumin (A & B) and urea (C & D) synthesis) of primary rat hepatocyte cultures in the chip with the flow (50 μl/h). Positive control acetaminophen (40 mM) and SPION (200 μg/ml) were added at the fifth day; the untreated control cells received medium only. Significant difference between treated and untreated cells is denoted as *p-value < 0.01; between APAP-treated and SPION-treated cells is denoted as **p-value < 0.01.
APAP: Acetaminophen.
Figure 7. . Live/dead assay at the seventh day after the seeding of primary rat hepatocytes that illustrates the effect of superparamagnetic iron oxide nanoparticles on the viability of the hepatocyte cultures in the chip with the flow (50 μl/h).
Positive control acetaminophen (APAP; 40 mM) and SPION (200 μg/ml) were added at the fifth day; the untreated control cells received medium only. Left panel: viability of the untreated control cells; middle panel: viability of the APAP treated cells; right panel: viability of the SPION treated cells. Blue: DNA/nuclei of the cells; red: dead cells; image scale bar: 400 μm.
APAP: Acetaminophen; SPION: Superparamagnetic iron oxide nanoparticle.
Discussion
In the current study, we designed a practical microfluidic 3D hepatocyte chip for hepatotoxicity testing of nanoparticles by considering the balance between complexity and practicality that recapitulates physiological responses of interest. In terms of the creation of the simplicity that provides physiological responses of hepatocytes, the design contains primary rat hepatocyte cultures without extracellular matrix (ECM) protein cover above the fibronectin layer. Previous studies illustrated that two sandwich dimensions with thinner layers promoted up-regulation of some specific characters due to the improvement of gas, nutrient, and toxin exchange [38]. Thus, we hypothesized that instead of culturing the primary rat hepatocytes under a thick second layer of collagen or other ECM barrier, the flow of the growth medium just above the hepatocytes can provide faster toxicological analyses by reducing oxygen, nutrient, and toxic substance diffusion barriers, and facilitating better exchange with the micro-environment. In addition to simplicity and allowing rapid toxicological data, fabrication of the design contains a desktop digital craft cutter, which is a low-cost and rapid fabrication method [20]. Compared with liver slices and liver cell lines, primary hepatocytes are currently the best option for hepatotoxicity testing among in vitro models [3,10,13]. Thus, the hypothetical superior potential of the microfluidic 3D hepatocyte chip for hepatotoxicity testing of nanoparticles was analyzed by focusing on liver-specific functions (albumin and urea secretion) and viability of the primary rat hepatocytes. Hepatocytes, in vivo, do not experience any shear stress since they are isolated from the blood flow by the Space of Disse and the endothelium; on the other hand, in vitro experiments indicated that low shear stress can contribute to the stabilization of hepatocyte functions without affecting cell viability [39,40]. Therefore, here we experimented with flow rates of 25, 50 and 100 μl/h with corresponding low shear stresses of 0.0005, 0.0011, and 0.0021 dynes/cm2 calculated by the following formula:
τ = 6 μQ / h2w
where μ is the flow viscosity (kg/m*s), Q is the volumetric flow rate (m3/s), h is the channel height (m) and w is the channel width (m) [39]. The shear stresses in our microfluidic chip are well below the highest suggested values of 0.3 dynes/cm2 [39,41].
Three-day short-term liver-specific functions
Since albumin and urea are considered to be the two of the most reliable markers for assessing the metabolic competency of the liver [3,42], the levels of albumin and urea secretion of the primary rat hepatocyte cultures were analyzed to assess viability and functionality of the cultures. Albumin synthesis and secretion of the primary rat hepatocyte cells in the microfluidic chip were comparable to the static model (Figure 2A & B). On the other hand, urea excretion in the microfluidic cultures was much higher compared with the static model (Figure 2C & D). The superior liver-specific functions of the primary rat hepatocytes in the chip were induced with the flow for only 1 day between twenty-fourth and forty-eighth hour. Specifically, compared with standard well plates, there were statistically significant increases in albumin secretion (p < 0.05) in the chip with flow rates of 25 and 50 μl/h at seventy-second hour, and there were statistically significant increases in urea secretion (p < 0.01) in the chip with all flow rates (25, 50 and 100 μl/h) at forty-eighth and seventy-second hour. Compared with chip static model (0 μl/h), except for flow rate of 100 μl/h at forty-eighth hour for albumin secretion, all of the flow rates induced statistically significant increases in liver-specific functions (level of significances were p < 0.01, except for p < 0.05 for flow rates of 25 and 50 μl/h for albumin secretion at forty-eighth hour) (Figure 2B & D). There was no statistically significant difference (p > 0.05) between the three different flow rates for albumin secretion. In contrast, urea excretion indicated that the flow rate of 50 μl/h was superior, and each flow rate was significantly different (p < 0.01) from the others at forty-eighth and seventy-second hour (Figure 2). Therefore, based on the results of 3-day short-term primary rat hepatocyte cultures, 50 μl/h was the optimal flow rate in our model.
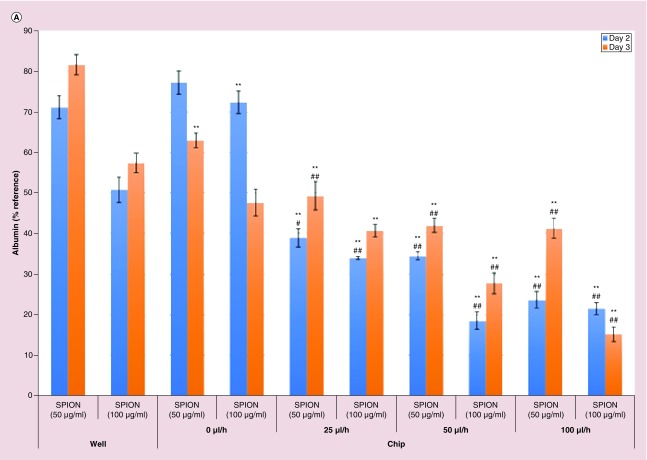
After obtaining superior liver-specific functions of the primary rat hepatocytes in dynamic microfluidic conditions, the response capacity of the hepatocytes to nanoparticles (SPION) was assessed in this environment via changes in liver-specific functions. In our recent study [3], effects of 100, 200 and 400 μg/ml of SPION on viability and liver-specific functions of primary rat hepatocytes in sandwich culture were investigated and 100 μg/ml of SPION was found safe in terms of liver-specific functions except for albumin secretion in the case of treatment in cumulative style. Thus, in terms of liver-specific functions, the response capacity of the primary rat hepatocytes in the chip to nanoparticles was first tested using SPION in concentrations of 50 and 100 μg/ml. SPION treatment was applied for 1 day between twenty-fourth and forty-eighth hour and albumin and urea secretion were measured at forty-eighth and seventy-second hour and presented in Figure 3A & B as percentages of the values of the twenty-fourth hour. More statistically significant effects were observed in a concentration- and time-dependent manner in dynamic conditions (chip with flow rates of 25, 50 and 100 μl/h). Compared with static models (standard well plates and chip static model [0 μl/h]), SPION treatment in all of the flow rates caused significantly more deleterious effects in albumin secretion at forty-eighth and seventy-second hour; significances were p < 0.01, except for in the case of the treatment of SPION (50 and 100 μg/ml) in flow rate of 25 μl/h compared with chip static model (Figure 3A). On the other hand, especially at the seventy-second hour, compared with static models (standard well plates and chip static model [0 μl/h]), highly significant (p < 0.01) damages were observed in urea secretion in the case of SPION treatment in all of the flow rates (Figure 3B). The increase in flow rate causes an increase in shear stress; this factor may explain the lowest albumin and urea secretion observed with the flow rate of 100 μl/h 72 h after the treatment with 100 and 50 μg/ml of SPION, respectively (Figure 3).
One-week long-term liver-specific functions & viability
Quite effective liver-specific functions achieved with only 1-day flow in 3-day short-term cultures in the 3D hepatocyte chip, triggered us to conduct longer-term analyses in the same chip for 7 days. Consistently with the results of 3-day short-term cultures, the albumin secretion for the 1-week microfluidic chip culture was comparable to static model (Figure 4A & B), whereas urea excretion for the 1-week microfluidic chip culture was much higher compared with the static model (Figure 4C & D). Due to providing extensive cell-cell interaction and allowing hepatocyte cultures subject to flow, previous studies have reported that 3D microfluidic platforms illustrated sustained and constant higher albumin and urea secretions for a long period of time compared with static cultures [6,16–18,43]. Thus, the higher liver-specific functions of the primary rat hepatocytes obtained for both 3-day short-term and 1-week long-term cultures in the 3D hepatocyte chip are consistent with the previous findings. Three different flow rates, 25, 50 and 100 μl/h, were also compared for the 1-week long-term cultures in the 3D hepatocyte chip. In terms of albumin secretion, 25 and 50 μl/h were superior (except for the 6th day, there was no statistically significant difference between the two flow rates). On the other hand, in terms of urea excretion, 50 and 100 μl/h were superior (except for the 6th day, 50 μl/h caused significantly (p < 0.01) more urea production compared with 100 μl/h) (Figure 4). Therefore, based on the results of 1-week long-term primary rat hepatocyte cultures, 50 μl/h was the optimal flow rate in our model. Albumin is synthesized primarily in the liver and it is the most abundant protein in plasma, whereas urea is the chief metabolic product of nitrogen metabolism in mammals, and the urea cycle is the metabolic pathway responsible for ammonia detoxification in which ammonia (formed in the breakdown of amino acids) is converted to urea, a less toxic and more water-soluble product which is easily excreted into urine [3]. Thus, the differences in the profiles of albumin and urea synthesis can result from their different sensitivities to cell polarity and cell-cell interactions [10]; the different sensitivities of albumin and urea synthesis to cell polarity and cell-cell interactions caused by different flow rates may explain the differences for albumin and urea secretion at different flow rates in 3-day short-term and 1-week long-term analyses.
From the results of 3-day short-term and 1-week long-term analyses of the liver-specific functions (albumin and urea secretion), we determined the best flow rate as 50 μl/h, and we continued further analyses with this flow rate; the higher albumin and urea production rates observed at this flow rate provides a more sensitive measurement of toxicity. Figure 5 illustrates the representative live/dead images at the seventh day of the primary rat hepatocyte cultures in the sandwich model and in the 3D hepatocyte chip with this optimum flow rate of 50 μl/h. As evidenced by phase contrast images and the signals of DAPI and EthD-1 channels, morphology and viability (>90% for both static and dynamic model) of the primary rat hepatocytes in the 3D hepatocyte chip are comparable to that of the sandwich model (Figure 5).
After achieving high and stable liver-specific functions and viability rates in dynamic conditions in the 1-week period, the 3D hepatocyte chip (with 50 μl/h) was tested with a SPION concentration of 200 μg/ml using a positive control (APAP) and an untreated control group. APAP, also known as paracetamol, is a frequently used pain killer. Both acute and chronic overdosing of APAP can cause liver toxicity and liver failure, mediated by the formation of the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) [44–49]. Consistently with previous findings [45] that reported concentration-dependent decreases in albumin and urea production of primary rat hepatocytes upon treatment with APAP, the treatment of APAP (40 mM) caused a complete loss of liver-specific functions (albumin and urea secretion) of the primary rat hepatocytes in the 3D hepatocyte chip at 48 h after the start of treatment (complete loss of the secretion of urea and albumin took place 24 and 48 h after the start of treatment, respectively) (Figure 6). The cell viabilities were evaluated by counting the cell number after live/dead staining (Figure 7), and after the APAP (40 mM) treatment, the viability percentage was determined as 28.57 ± 2.91% of the untreated control. 40 mM is commonly regarded as a lethal dose for APAP, and is at the upper limit of APAP concentration derived from in vivo rodent pharmacokinetic properties, and is consistent with those commonly used for in vivo and in vitro studies [50–54]. Previous studies reported that 1 day after culturing of primary rat hepatocytes in dynamic [44] and static [46] conditions, exposure of 40 mM of APAP for 24 h induced complete loss of viability of primary rat hepatocytes. On the other hand, a detailed study [46] investigating effects of culture time on sensitivity of primary rat hepatocytes toward APAP induced toxicity reported that EC50 (half maximal effective concentration) values were 6, 12.5 and 18.8 mM in the case of treatment of APAP at 24, 48 and 72 h after seeding, respectively. Thus, the longer time of culture as we conduct here can increase the resistance of hepatocytes; longer time of culture in the 3D hepatocyte chip (5 days) before APAP (40 mM) treatment explains the higher viability percentage (28.57 ± 2.91%).
On the other hand, SPION (200 μg/ml) treatment on the fifth day of culture for 24 h caused 76.62% decrease and 91.19% decrease in albumin secretion 24 h after the start of treatment (from 125.47 ± 9.75 at 5th day to 29.33 ± 3.84 at 6th day) and 48 h after the start of treatment (from 125.47 ± 9.75 at 5th day to 11.06 ± 2.60 at 7th day), respectively (Figure 6 A & B). The treatment caused 35.44% decrease and 66.59% decrease in urea secretion 24 h after the start of treatment (from 609.83 ± 4.16 at 5th day to 393.71 ± 4.01 at 6th day) and 48 h after the start of treatment (from 609.83 ± 4.16 at 5th day to 203.77 ± 1.14 at 7th day), respectively (Figure 6C & D). The cell viabilities were evaluated by counting the cell number after live/dead staining (Figure 7), and after the SPION (200 μg/ml) treatment, the viability percentage was determined as 58.82 ± 4.71 % of the untreated control.
In our recent study [3], compared with single dosing, cumulative dosing of SPION (100, 200 and 400 μg/ml) caused significantly (p < 0.05) more deleterious effects in liver-specific functions (albumin and urea secretion) (except for urea secretion 48 h after the start of treatment with 200 μg/ml of SPION) of the primary rat hepatocytes in sandwich culture; according to our hypothesis the different results in different treatment groups were caused by different agglomeration of SPION due to the treatment styles (compared with single dosing, cumulative dosing caused less agglomeration of SPION). Compared with the sandwich model in the mentioned study (SPION [200 μg/ml] treatment caused approximately 55 and 70% decrease in albumin secretion 48 h after the start of treatment in single dose and cumulative dose, respectively; SPION (200 μg/ml) treatment caused approximately 15 and 2% decrease in urea secretion 48 h after the start of treatment in single dose and cumulative dose, respectively) [3], in the current study, SPION (200 μg/ml) treatment in dynamic model characterized by the designed 3D hepatocyte chip with flow rate of 50 μl/h caused more dramatic damages in liver-specific functions (91.19% decrease in albumin secretion and 66.59% decrease in urea secretion) of the primary rat hepatocytes 48 h after the start of treatment. In addition, compared with the sandwich model in the mentioned study (SPION [200 μg/ml] treatment caused ∼20% decrease in viability 48 h after the start of treatment in single dose and cumulative dose) [3], in the current study, SPION (200 μg/ml) treatment in the dynamic conditions caused more dramatic damages in viability (∼40% decrease) of the primary rat hepatocytes 48 h after the start of treatment. Since, cumulative treatment style used in the sandwich culture [3] is far from the real situation characterized by low exposure to nanoparticles that accumulate in cells over time, changing the growth medium every 24 h forms an obstacle to mimic the real cumulative treatment style. On the other hand, microfluidic 3D hepatocyte chip in the current study is a much better platform to mimic real cumulative exposure; thus, this platform allowed interaction of the primary rat hepatocytes with smaller SPION by minimizing possible agglomeration of SPION. Nanoparticle size is an important factor affecting cytotoxicity, smaller nanoparticles are more toxic than larger ones; due to a high surface area relative to their total mass, small nanoparticles have higher chances to interact with surrounding biomolecules (e.g., biomolecules involved in pathways of albumin and urea synthesis) and, as a consequence, to trigger adverse responses [3,55]. These findings are consistent with the previous studies reporting more sensitive responses of hepatocytes to xenobiotics in perfusion models compared with static models [44,54]. The higher sensitivity observed in the microfluidic hepatocyte chip might, at first sight, be attributed to the higher cumulative exposure to SPION, under flow. Nevertheless, the clearance rate of nanoparticles is also relatively higher in the flow conditions, where nanoparticles that are uninternalized by the cells are cleared in 15 min (100 μl/h) to 1 h (25 μl/h), compared with static wells where nanoparticles are available for the whole 24 h. This reflects a balance between introduction and clearance and thus comparable average exposure of cells to nanoparticles in either culture system. Moreover, in Figure 3, we observed that there is no linear correlation between toxicity and flow rate, and in some instances only mild effects from the increased flow rates toward toxicity, in other words, depression of the functional markers, albumin and urea. This corroborates the notion that increased sensitivity to nanoparticles in the microfluidic hepatocyte chip cannot be attributed to a hypothetical increased exposure.
Conclusion
In conclusion, low-cost, rapidly fabricated and practical microfluidic 3D hepatocyte chip was designed for hepatotoxicity testing of nanoparticles by considering the balance between complexity and practicality that recapitulates physiological responses of interest; the 3D hepatocyte chip offers two main advantages: continuous perfusion and thus a cumulative nanoparticle exposure, and a stable model that does not require a second ECM layer which can present a transport barrier, thereby it allows faster toxicological analyses by reducing oxygen, nutrient, and toxic substance diffusion barriers; the 3D hepatocyte chip recapitulates the key physiological responses in the hepatotoxicity of SPION; cumulative exposure of the nanoparticles via the 3D hepatocyte chip results in more dramatic damages in hepatocytes; thus, importance of exposure styles during evaluation of nanoparticle-induced hepatotoxicity have been illustrated; the proof of concept studies providing first results of the potential hepatotoxicity of SPION under microfluidic conditions illustrates that the developed 3D hepatocyte chip is a robust platform that can be used also for investigating hepatotoxicity profiles of other nanoparticles.
Future perspective
The most promising application of magnetic iron oxide particles is targeted delivery of anticancer drugs for cancer therapy (to improve stability, biodistribution and pharmacokinetics of the anticancer drugs, and to decrease adverse effects and toxicity thanks to the selective accumulation of the drug in tumor sites) [3,23,30], which contains surface modification of SPION characterized in mainly four structures, core-shell structure, matrix-dispersed structure, janus structure, shell-core-shell structure [56,57]. Thus, the results revealed by the current study should be supported by further investigations on hepatotoxicity profiles of surface functionalized SPION.
Compared with recently reported more complicated liver chips such as hepatic lobule equivalent models, cord-like liver equivalent models and co-culture models [16,58], the fabrication process of our 3D hepatocyte chip model is simple, fast and inexpensive. Moreover, our model allows direct exposure of the hepatocytes to the flowing media, thus it can provide faster toxicological analyses by reducing oxygen, nutrient, and toxic substance diffusion barriers, and facilitating better exchange with the micro-environment. The majority of the liver consists of parenchymal cells, in other words, hepatocytes (80% of the liver volume), while the nonparenchymal cells, Kupffer cells, sinusoidal endothelial cells, and stellate cells, represent only 6.5% of the organ volume [3]. However, nonparenchymal cells have also important functions, for example, activation of the major specialized tissue-resident macrophages, Kupffer cells, directly or indirectly by toxic agents results in the release of an array of inflammatory mediators, growth factors and reactive oxygen species [59,60]. Thus, although our hepatocyte chip enables robust detection of nanoparticle-induced hepatotoxicity, a liver-on-a-chip platform containing both parenchymal (hepatocytes) and nonparenchymal cells (endothelial, stellate and Kupffer cells) would be useful in further mechanistic analyses of the response of nonparenchymal cells and their interactions with the hepatocytes when exposed to nanoparticles. In addition, this platform would be useful in further mechanistic analyses of the time-course internalization and metabolization of nanoparticles by the liver.
Summary points.
Background
To develop a practical microfluidic 3D hepatocyte chip for hepatotoxicity testing of nanoparticles using proof of concept studies providing first results of the potential hepatotoxicity of superparamagnetic iron oxide nanoparticles (SPION) under microfluidic conditions.
Materials & methods
A microfluidic 3D hepatocyte chip containing three layers (the bottom layer is a glass slide, the cell culture chamber containing primary rat hepatocytes on the middle layer and the top layer is a piece of plain PDMS) was fabricated.
Proof of concept studies of the fabricated hepatocyte chip were carried out using SPION in different concentrations (50, 100 and 200 μg/ml), by focusing on liver-specific functions and viability.
Standard well plates with or without collagen gel cover were used as controls for the 1-week on-chip model and 3-day on-chip model, respectively.
Results & discussion
Due to providing extensive cell-cell interaction and allowing hepatocyte cultures subject to flow, the hepatocyte chip with flow provided comparable viability and significantly higher liver-specific functions up to 1 week, compared with standard well plates.
Compared with static models (standard well plates and chip static model [0 μl/h]), SPION treatment with flow caused significantly more deleterious effects in liver-specific functions (albumin and urea secretion) and viability, in a dose- and time-dependent manner.
The microfluidic 3D hepatocyte chip, a much better platform to mimic real cumulative exposure compared with static models, allowed interaction of the primary rat hepatocytes with smaller SPION by minimizing possible agglomeration of SPION, which caused more dramatic effects.
The findings are consistent with the previous studies reporting more sensitive responses of hepatocytes to xenobiotics in perfusion models compared with static models.
Conclusion
The developed low-cost, rapidly fabricated, practical 3D hepatocyte chip recapitulates the key physiological responses in the hepatotoxicity of SPION; thus, the developed 3D hepatocyte chip is a robust platform for investigating hepatotoxicity profiles of nanoparticles.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.future-science.com/doi/suppl/10.2217/nnm-2019-0086
Financial & competing interests disclosure
L Li was supported by the China Scholarship Council and by the National Natural Science Foundation of China (grant no. 31770107 and 21874116). K Gokduman was supported by the Scientific and Technological Research Council of Turkey (TUBITAK). The authors acknowledge funding support from the National Institutes of Health through the following grants: NIH# 1R21EB020192, NIH# 5R01EB023812 and NIH# 5P41EB002503 (BioMEMS Resource Center). The authors would also like to acknowledge the MGH Cell Resource Core; Proteomics & Genomics Core Shared Facility, Morphology and Imaging Shared Facility, Nano-Micro Core Special Shared Facility and Translational Regenerative Medicine Shared Facility provided at the Shriners Hospitals for Children. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small 4(1), 26–49 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Linkov I, Satterstrom FK, Corey LM. Nanotoxicology and nanomedicine: making hard decisions. Nanomedicine 4(2), 167–171 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Gokduman K, Bestepe F, Li L, Yarmush ML, Usta OB. Dose-, treatment- and time-dependent toxicity of superparamagnetic iron oxide nanoparticles on primary rat hepatocytes. Nanomedicine (Lond.) 13(11), 1267–1284 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first report describing toxicity of superparamagnetic iron oxide nanoparticles on primary rat hepatocytes in static conditions.
- 4.Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc. Natl Acad. Sci. USA 106(37), 15714–15719 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajan B, Sathish S, Balakumar S, Devaki T. Synthesis and dose interval dependent hepatotoxicity evaluation of intravenously administered polyethylene glycol-8000 coated ultra-small superparamagnetic iron oxide nanoparticle on Wistar rats. Environ. Toxicol. Pharmacol. 39(2), 727–735 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2(6), 875–894 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Yoshizato K, Tateno C. A human hepatocyte-bearing mouse: an animal model to predict drug metabolism and effectiveness in humans. PPAR Res. 2009, 476217(2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contrera JF, Matthews EJ, Kruhlak NL, Benz RD. Estimating the safe starting dose in Phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose. Regul. Toxicol. Pharmacol. 40(3), 185–206 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7(2), 27–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Hu K, Wang Y. Primary hepatocytes cultured on a fiber-embedded PDMS chip to study drug metabolism. Polymers 9(6), 215 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris AJ, Dial SL, Casciano DA. Comparison of basal gene expression profiles and effects of hepatocarcinogens on gene expression in cultured primary human hepatocytes and HepG2 cells. Mutat. Res. 549(1–2), 79–99 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Beigel J, Fella K, Kramer PJ, Kroeger M, Hewitt P. Genomics and proteomics analysis of cultured primary rat hepatocytes. Toxicol. In Vitro 22(1), 171–181 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Soldatow VY, Lecluyse EL, Griffith LG, Rusyn I. In vitro models for liver toxicity testing. Toxicol. Res. (Camb.) 2(1), 23–39 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive review on various traditional and novel in vitro liver models for toxicity testing.
- 14.Langsch A, Bader A. Longterm stability of Phase I and Phase II enzymes of porcine liver cells in flat membrane bioreactors. Biotechnol. Bioeng. 76(2), 115–125 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Usta OB, Mccarty WJ, Bale S. et al. Microengineered cell and tissue systems for drug screening and toxicology applications: evolution of in-vitro liver technologies. Technology (Singap. World Sci.) 3(1), 1–26 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prodanov L, Jindal R, Bale SS. et al. Long-term maintenance of a microfluidic 3D human liver sinusoid. Biotechnol. Bioeng. 113(1), 241–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the recapitulation of a liver sinusoid-on-a-chip, for a period of 28 days.
- 17.Hegde M, Jindal R, Bhushan A. et al. Dynamic interplay of flow and collagen stabilizes primary hepatocytes culture in a microfluidic platform. Lab Chip 14(12), 2033–2039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarty WJ, Usta OB, Luitje M. et al. A novel ultrathin collagen nanolayer assembly for 3-D microtissue engineering: Layer-by-layer collagen deposition for long-term stable microfluidic hepatocyte culture. Technology (Singap. World Sci.) 2(1), 67–74 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 14(4), 248–260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuen PK, Goral VN. Low-cost rapid prototyping of flexible microfluidic devices using a desktop digital craft cutter. Lab Chip 10(3), 384–387 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Singh N, Jenkins GJ, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dey S, Maiti TK. Superparamagnetic nanoparticles and RNAi-mediated gene silencing: evolving class of cancer diagnostics and therapeutics. J. Nanomater. 2012, 15(2012). [Google Scholar]
- 23.Gokduman K. Strategies targeting DNA topoisomerase I in cancer chemotherapy: camptothecins, nanocarriers for camptothecins, organic non-camptothecin compounds and metal complexes. Curr. Drug Targets 17(16), 1928–1939 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Wu C, Li J, Pang P. et al. Polymeric vector-mediated gene transfection of MSCs for dual bioluminescent and MRI tracking in vivo. Biomaterials 35(28), 8249–8260 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Bradshaw M, Clemons TD, Ho D. et al. Manipulating directional cell motility using intracellular superparamagnetic nanoparticles. Nanoscale 7(11), 4884–4889 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Estelrich J, Escribano E, Queralt J, Busquets MA. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 16(4), 8070–8101 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kandasamy G, Maity D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 496(2), 191–218 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Quinto CA, Mohindra P, Tong S, Bao G. Multifunctional superparamagnetic iron oxide nanoparticles for combined chemotherapy and hyperthermia cancer treatment. Nanoscale 7(29), 12728–12736 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K, Kievit FM, Sham JG. et al. Iron-oxide-based nanovector for tumor targeted siRNA delivery in an orthotopic hepatocellular carcinoma xenograft mouse model. Small 12(4), 477–487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gokduman K, Demir AS. Camptothecin anticancer drug loaded iron oxide micro and nanoparticles: tuning targeted and sustained release of the drug in bioactive form#. Curr. Nanosci. 9(6), 704–710 (2013). [Google Scholar]
- 31.Levy M, Luciani N, Alloyeau D. et al. Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials 32(16), 3988–3999 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Kuang H, Zhang W. et al. Size dependent biodistribution and toxicokinetics of iron oxide magnetic nanoparticles in mice. Nanoscale 7(2), 625–636 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Soenen SJ, Rivera-Gil P, Montenegro J-M, Parak WJ, De Smedt SC, Braeckmans K. Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 6(5), 446–465 (2011). [Google Scholar]
- 34.Sigma-Aldrich. Iron oxide(II,III), magnetic nanoparticles solution safety data sheet (2019).
- 35.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol. Prog. 7(3), 237–245 (1991). [DOI] [PubMed] [Google Scholar]
- 36.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 3(2), 174–177 (1989). [DOI] [PubMed] [Google Scholar]
- 37.Usta OB, Kim Y, Ozer S. et al. Supercooling as a viable non-freezing cell preservation method of rat hepatocytes. PLoS ONE 8(7), e69334 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Marí-Buyé N, Muiños TF, Borrós S, Favia P, Semino CE. Nanometric self-assembling peptide layers maintain adult hepatocyte phenotype in sandwich cultures. J. Nanobiotechnology 8(1), 29 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang YBA, Eo J, Mert S, Yarmush ML, Usta OB. Metabolic patterning on a chip: towards in vitro liver zonation of primary rat and human hepatocytes. Sci. Rep. 8(1), 8951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka Y, Yamato M, Okano T, Kitamori T, Sato K. Evaluation of effects of shear stress on hepatocytes by a microchip-based system. Meas. Sci. Technol. 17, 3167–3170 (2006). [Google Scholar]
- 41.Tilles AW, Berthiaume F, Yarmush ML, Tompkins RG, Toner M. Bioengineering of liver assist devices. J. Hepatobiliary Pancreat. Surg. 9(6), 686–696 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Borlak J, Chougule A, Singh PK. How useful are clinical liver function tests in in vitro human hepatotoxicity assays? Toxicol. In Vitro 28(5), 784–795 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Tong WH, Fang Y, Yan J. et al. Constrained spheroids for prolonged hepatocyte culture. Biomaterials 80, 106–120 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Yu F, Deng R, Hao Tong W. et al. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. 7(1), 14528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports more sensitive responses of hepatocytes in perfusion models compared with static models.
- 45.Ukairo O, Mcvay M, Krzyzewski S. et al. Bioactivation and toxicity of acetaminophen in a rat hepatocyte micropatterned coculture system. J. Biochem. Mol. Toxicol. 27(10), 471–478 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Jemnitz K, Veres Z, Monostory K, Kobori L, Vereczkey L. Interspecies differences in acetaminophen sensitivity of human, rat, and mouse primary hepatocytes. Toxicol. In Vitro 22(4), 961–967 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Lane JE, Belson MG, Brown DK, Scheetz A. Chronic acetaminophen toxicity: a case report and review of the literature. J. Emerg. Med. 23(3), 253–256 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Black M. Acetaminophen hepatotoxicity. Annu. Rev. Med. 35, 577–593 (1984). [DOI] [PubMed] [Google Scholar]
- 49.Corcoran GB, Mitchell JR, Vaishnav YN, Horning EC. Evidence that acetaminophen and N-hydroxyacetaminophen form a common arylating intermediate, N-acetyl-p-benzoquinoneimine. Mol. Pharmacol. 18(3), 536–542 (1980). [PubMed] [Google Scholar]
- 50.Orbach SM, Cassin ME, Ehrich MF, Rajagopalan P. Investigating acetaminophen hepatotoxicity in multi-cellular organotypic liver models. Toxicol. In Vitro 42, 10–20 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Bachmann K, Pardoe D, White D. Scaling basic toxicokinetic parameters from rat to man. Environ. Health Perspect. 104(4), 400–407 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belanger PM, Lalande M, Dore F, Labrecque G. Time-dependent variations in the organ extraction ratios of acetaminophen in rat. J. Pharmacokinet. Biopharm. 15(2), 133–143 (1987). [DOI] [PubMed] [Google Scholar]
- 53.Lee HB, Blaufox MD. Blood volume in the rat. J. Nucl. Med. 26(1), 72–76 (1985). [PubMed] [Google Scholar]
- 54.Allen JW, Khetani SR, Bhatia SN. In vitro zonation and toxicity in a hepatocyte bioreactor. Toxicol. Sci. 84(1), 110–119 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Shang L, Nienhaus K, Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J. Nanobiotechnology 12, 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive review on interaction of engineered nanoparticles with cells by focusing on nanoparticle size.
- 56.Zhu N, Ji H, Yu P. et al. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials (Basel) 8(10), 810 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu W, Wu Z, Yu T, Jiang C, Kim WS. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 16(2), 023501 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes main styles of surface functionalization of superparamagnetic iron oxide nanoparticles.
- 58.Materne EM, Tonevitsky AG, Marx U. Chip-based liver equivalents for toxicity testing – organotypicalness versus cost-efficient high throughput. Lab Chip 13(18), 3481–3495 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol. Sci. 96(1), 2–15 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Arami H, Khandhar A, Liggitt D, Krishnan KM. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 44(23), 8576–8607 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.