Abstract
The immune system affects senescence (declines in probabilities of survival or reproduction with age), by shaping late age vulnerability to chronic inflammatory diseases and infections. It is also a dynamic interactive system that must balance competing demands across the life course. Thus, immune system function remains an important frontier in understanding the evolution of senescence.
Here, we review our expanding mechanistic understanding of immune function over the life course, in the context of theoretical predictions from life‐history evolution. We are especially interested in stage‐ and sex‐dependent costs and benefits of investment in the immune system, given differential life‐history priorities of the life stages and sexes.
We introduce the costs likely to govern immune allocation across the life course. We then discuss theoretical expectations for differences between the sexes and their likely consequences in terms of how the immune system is both modulated by and may modulate senescence, building on information from life‐history theory, experimental immunology and demography.
We argue that sex differences in immune function provide a potentially powerful probe of selection pressures on the immune system across the life course. In particular, differences in ‘competing’ and ‘caring’ between the sexes have evolved across the tree of life, providing repeated instances of divergent selection pressures on immune function occurring within the same overall bauplan.
We conclude by detailing an agenda for future research, including development of theoretical predictions of the differences between the sexes under an array of existing models for sex differences in immunity, and empirical tests of such predictions across the tree of life.
A free http://onlinelibrary.wiley.com/doi/10.1111/1365-2435.13458/suppinfo can be found within the Supporting Information of this article.
Keywords: immunity, life history theory, senescence, sex differences
A free http://onlinelibrary.wiley.com/doi/10.1111/1365-2435.13458/suppinfo can be found within the Supporting Information of this article.

1. INTRODUCTION
The twin mysteries of how and why sex differences and senescence (declines in probabilities of survival or reproduction with age) evolved have long fascinated evolutionary biologists. Here, we propose that sex‐ and age‐dependent variation in immune function provide complementary insights into these mysteries. First, we outline the theory underpinning the evolution of senescence, followed by an overview of late age immune responses. We then discuss immune trade‐offs and outline how they may connect early and late life survival or fertility, a key question in the evolution of senescence. Finally, we place this in the context of theoretical expectations for sex differences in immunity, concluding with future directions that could leverage sex differences to illuminate links between senescence and immunity.
2. THE THEORETICAL CONTEXT
Why do we ‘age’? Fisher argued that senescence emerges as a result of the accumulation of deleterious age‐specific traits that cannot be efficiently removed by natural selection (Fisher, 1930). Such ‘mutation accumulation’ will lead to senescence, as further formalized by Haldane (Haldane, 1942) and Medawar (Medawar, 1952). By 1957, Williams introduced the concept of ‘antagonistic pleiotropy’ where a mutation that increased survival or fertility early in life at the expense of survival or fertility later in life would be likely to spread in a population. Williams also laid out theoretical expectations for differences in senescence between the sexes (Williams, 1957), predicting that the sex with ‘the higher [background] mortality rate, and the lesser rate of increase in fecundity’ with age should undergo the most rapid senescence, for example the more rapid increase in mortality with age. While higher rates of actuarial senescence are observed in males in some mammal species for which males have higher mortality (Gaillard & Lemaître, 2017), exceptions can also be found (e.g. large herbivores (Lemaître & Gaillard, 2013)). Theory indicates that higher background mortality alone (within a sex, or a species) should not modulate evolution of changes in mortality (or fertility) over age (Caswell, 2007; Moorad, Promislow, & Silvertown, 2019; Wensink, Caswell, & Baudisch, 2017), so that other features of the life history are likely to drive this empirical pattern (Abrams, 1993). For sex differences, trade‐offs underlying differences in mortality and fertility between the sexes will be key.
Male–female comparisons have long been recognized as a useful axis for considering how selection shapes longevity and senescence (Williams, 1957). Williams noted that two interacting proximate features shape sex differences: the chromosome differences between the sexes set at conception and hormonal differences that develop over ontogeny, which will intersect to define differences between male and female phenotypes. He further posited that the ultimate drivers of sex differences in longevity and senescence will be differences in schedules of mortality and fertility (Williams, 1957), and associated trade‐offs, in turn rooted in differential investment in parental care (Keller, Bayer, Salzburger, & Roth, 2018; Roth, Scharsack, Keller, & Reusch, 2011) or towards sexual competition (Clutton‐Brock & Isvaran, 2007).
What does this theory mean for selection on immune function (i.e. the various roles of immune systems in organismal physiology, including defence against infection) across the sexes? Early experimental work (Bateman, 1948) yielded one simple prediction: the sex that obtained the greatest fitness returns from securing matings (sexual selection) should favour investment away from survival and towards competition. Due to survival benefits of defence against infection, despite predicted resource costs of immune responses, it was subsequently suggested that this might be via reduced investment in immune function (e.g. Rolff, 2002; Sheldon & Verhulst, 1996). In this framework, the sex under stronger sexual selection and thus with higher variance in reproductive success (often males) was predicted to have weaker immune function. This may be an excellent first approximation (Zuk, 2009), with predictive power in a range of settings, but theoretical probing shows that it also hinges on strong assumptions (Stoehr & Kokko, 2006). In particular, the links from immune responses to survival, and indeed survival to female fitness, need not be straightforward (Forbes, 2007). Furthermore, contrasting ‘strong’ versus ‘weak’ immune responses of the two sexes obscures the fact that selection might differentially affect various aspects of immune function (Stoehr & Kokko, 2006), from pathogen detection to the magnitude of a pathogen‐killing response (Metcalf & Graham, 2018; Metcalf, Tate, & Graham, 2017).
Understanding the role of immune function in senescence is also challenging, because the immune system is a master regulator of physiology and homeostasis, and plays varied roles across age (Figure 1). Efforts to identify reliable biomarkers of ageing increasingly encompass measures of immune function (Nussey, Watt, Pilkington, Zamoyska, & McNeilly, 2011). Yet, the diversity of immune cells, and how they affect each other's activity (Figure 2), makes interpreting such measures challenging. One path forward is to leverage clear contrasts: striking sex differences in immune function (Klein & Flanagan, 2016), for which we have a growing mechanistic understanding (Box 1), provide a foundation for probing how (proximately) and why (ultimately) the immune system affects senescence.
Figure 1.
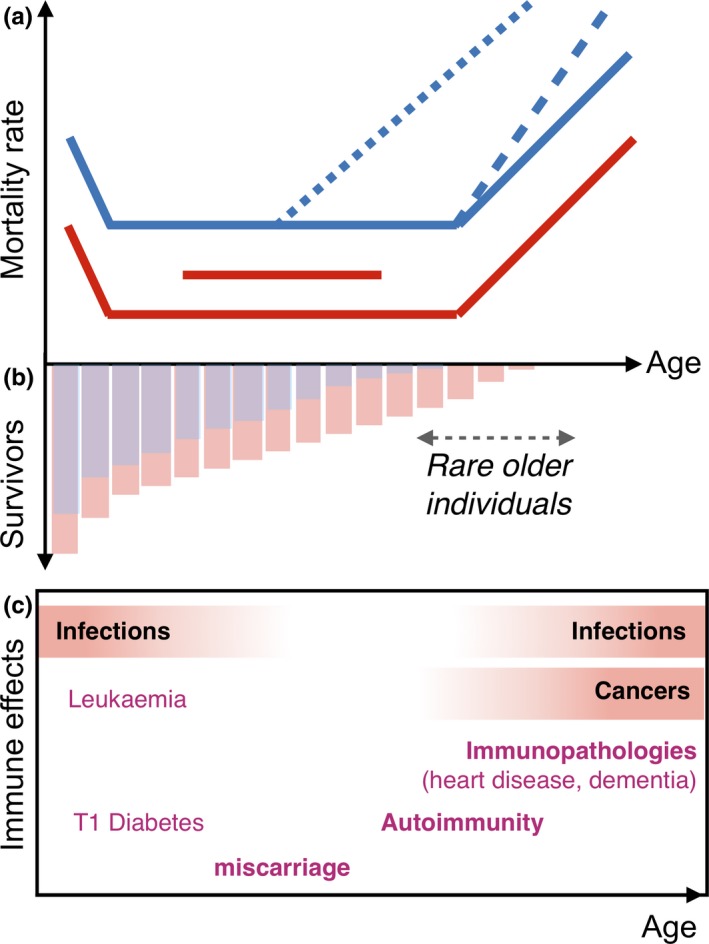
Mortality and immunity across the life course. (a) Mortality rates (y‐axis) tend to decline during the first years of life (x‐axis is age) as individuals grow out of small vulnerable life stages and then increase later in life, a manifestation of senescence or ageing (noting that a wide range of mortality trajectories are possible). Mortality rates are often higher in one sex across all ages (e.g. red vs. blue). On top of this, sex differences in senescence and thus longevity might manifest via an increase in the rate of ageing (blue dashed line) or an earlier age of onset of ageing (blue dotted line) in one or other sex. One sex might also have higher mortality associated with reproductive years (shown as the horizontal red line above the baseline). (b) Differences in age trajectories of mortality will translate into different age profiles (red vs. blue bars, here assuming equal sex ratios at birth), but older individuals are consistently rare, an important driver of the evolution of senescence (noting, however, that late age individuals might have high reproductive value that could counterweight this effect). (c) The immune system is involved in both protection (infectious diseases and cancers) and harmful outcomes (immunopathologies, such as cardiovascular disease, strokes or autoimmunity) across this same time course (x‐axis indicates age, with for example cancers predominantly arising at late ages)
Figure 2.
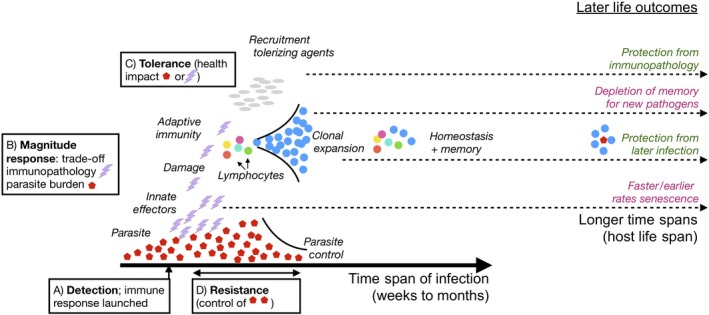
Dynamics of immunity. Many immune defences are inducible, triggered once growing parasite populations (red hexagons) are detected by the pattern recognition receptors of innate immunity identifying either pathogen‐ or damage‐associated molecular patterns (x‐axis is time following parasite arrival). Innate immune effectors are then launched (purple lightning bars). For species that have adaptive immunity, lymphocytes can subsequently be recruited (coloured circles), potentially leading to amplification of specific B‐ or T‐cell clones that recognize the pathogen (blue circles). These early processes generally correspond to a phase of positive feedback. Immune defences are also associated with active downregulation, by production of repressive cytokines, such as IL10, or (for species with adaptive immunity) engagement of T‐regs promoting a tolerizing environment, that is a phase of negative feedback. Infection and the broad return to homeostasis may nevertheless harbour changes that can result in longer term effects (far right) that may negatively (purple) or positively (green) affect survival rates. Background damage shaped by immune effectors could potentially driving earlier or faster senescence; ‘learning’ by immunity will both enhance protection to previously observed pathogens, but deplete memory, reducing ability to ‘remember’ new pathogens. Finally, early infection may enable immunity to develop a broadly tolerizing environment, protecting the organism from late life immunopathology. Each of these phases of induction and return to homeostasis map onto different trade‐offs relevant for balancing costs associated with immunity (alphabetically labelled boxes correspond to labels on Figure 3). The whole process can potentially occur multiple times over the course of an individual's life span, with potential consequences for rates of senescence (see text)
BOX 1. Proximate determinants of sex differences and immunity.
There are two proximate determinants of sex differences: chromosomes and hormones. Although not universal, chromosomal sex determination is widespread. Diverse mechanisms have evolved to prevent double dosage of proteins in the homozygous sex relative to the heterozygous sex (‘dosage compensation’). In mammalian females, one X chromosome in each cell is inactivated: about half the cells express genes derived from the maternal X chromosome, and half express genes from the paternal X chromosome. Female mammals thus have striking physiological immune diversity relative to males, potentially amplifying their ability to survive the onslaught of diverse pathogens (Marais et al., 2018). Additionally, immune genes are highly enriched on the X chromosome (Libert, Dejager, & Pinheiro, 2010), which may further amplify this effect. For example, in humans, two key pattern recognition receptors are encoded on the X, toll‐like receptors 7 and 8 (Jaillon, Berthenet, & Garlanda, 2019), and in Drosophila, the X chromosome encodes an array of immune genes, including peptidoglycan‐recognition protein (Hill‐Burns & Clark, 2009).
Across tetrapods, individuals of the hemizygous sex (e.g. XY in mammals, or ZW in birds, and a variety of patterns among reptiles and amphibians) have lower survival to adulthood (Pipoly et al., 2015). This has been attributed to mechanisms including the ‘unguarded chromosome’ effect, where expression of recessive mutations in the hemizygous sex reduces survival (Maklakov & Lummaa, 2013); the ‘toxic chromosome’ effect, where control of transposable elements on the hemizygous chromosome is lost at late ages; and finally, hemizygous ‘chromosome loss’, a phenomenon that can occur across multiple cell cycles (Marais et al., 2018). The role played by immunity is hard to titrate, as how immune genes cluster across sex chromosomes remains poorly specified across vertebrates, but patterns similar to those observed in well‐described organisms (humans, Drosophila) might contribute to vulnerability of the hemizygous sex. The dangerous side of immunity also means that the sex associated with a concentration of immune genes may be at risk in other ways: for example, biased dosage compensation has been implicated in autoimmunity, as a result of escape from inactivation of specific innate immune genes found on the X (Souyris et al., 2018).
Many species do not have chromosomal sex differences. However, all species with two sexes feature hormonal differences expressed at maturity. Almost every immune cell has hormonal receptors, and there is widespread evidence for hormonal effects on immunity across the tree of life (Foo, Nakagawa, Rhodes, & Simmons, 2017). In vertebrates, testosterone, which is known to stimulate expression of secondary sexual signals while potentially suppressing immune defence, has long been the main proximate candidate to explain decreased immune defence in males (Folstad & Karter, 1992; Foo et al., 2017). While animal models indicate that androgens can reduce aspects such as toll‐like receptor 4 expression on macrophages or natural killer cell activity (Klein & Flanagan, 2016), support for the immunosuppressive effect of testosterone is inconsistent (Owen‐Ashley, Hasselquist, & Wingfield, 2004; Roberts, Buchanan, & Evans, 2004). Furthermore, invertebrates can display sexual immune dimorphism yet lack testosterone, indicating that testosterone cannot be the whole story (Kurtz & Sauer, 2001; McKean & Nunney, 2001; Peters, 2000; Sheridan, Poulin, Ward, & Zuk, 2000).
Female hormonal levels (estradiol, progesterone) are often dynamic and generally change radically during reproductive periods (e.g. pregnancy). These changes affect immune functioning, including both innate and adaptive immunity (Klein & Flanagan, 2016). In vertebrates, estradiol and progesterone receptors are expressed in lymphocytes, macrophages, dendritic cells, etc. Progesterone is generally anti‐inflammatory, while estradiol can have different effects depending on its concentration. Low concentrations of estradiol (e.g. during the follicular stage of the reproductive cycle) can be pro‐inflammatory, whereas high concentrations (e.g. during the luteal phase of the reproductive cycle or during pregnancy) can be anti‐inflammatory (Klein & Flanagan, 2016). Indeed, inflammation is drastically reduced during pregnancy, largely via the effects of hormones (Robinson & Klein, 2012), although immunity during pregnancy is increasingly recognized to be an intricately coordinated process rather than a simple case of immune suppression (Mor, Aldo, & Alvero, 2017). Since hormone levels change with age, these also contribute to changes in immune function over age and its sex differences. For example, in humans, the innate immune system of aged females may be more inflammation‐prone; yet, ageing of the adaptive immune system may occur at a faster rate in men (Bupp, Melanie, Potluri, Fink, & Klein, 2018), presumably as a result of how chromosomal and hormonal differences affect these different branches of immunity.
Systems that have evolved along gradients of parental investment beyond predominantly female care provide an intriguing avenue for probing the effects of hormones on immune function, particularly during pregnancy. For example, syngnathids (pipefish) encompass a spectrum including, uniquely among vertebrates, male pregnancy. In this system, the sex investing more into parental care has a more efficient immune response (Lin, Zhang, Liu, & Xiao, 2016; Roth et al., 2011), and during male pregnancy, male androgens are downregulated, whereas glucocorticoids and prolactin, typically limited to vertebrate females, are upregulated (Scobell & Mackenzie, 2011). Birds with varying extents of paternal investment (Eens & Pinxten, 2000) show similar patterns. Disentangling the mechanistic basis of sex differences in immunity and linking this to ultimate drivers will benefit from such broader perspectives.
3. AGEING AND IMMUNE FUNCTION
Late age (dys)function is shaped by mutation accumulation (where rarity of late age individuals allows fixation of deleterious mutations) or trade‐offs playing out across age (whether genetic, as in antagonistic pleiotropy, or physiological, phenotypically plastic, as in allocation of limited resources between physiological functions). While pinpointing specific mechanisms is challenging (e.g. mutation accumulation requires demonstrating that alleles only have deleterious effects, which only manifest at later ages), function is always predicted to decline with age. Immune function displays patterns that align with this prediction, but also ones that (initially) seem at odds with it.
Many innate effectors do decline with age, for example in phagocytic ability, intracellular killing or chemotactic response (Boraschi et al., 2013; Simon, Hollander, & McMichael, 2015; Uciechowski & Rink, 2018). Adaptive immune cells also show declines: for example, individual B lymphocytes accumulate somatic mutations over age that impair repair (Zhang et al., 2019), just as the ability of the whole B‐cell population to generate novel response declines (de Bourcy et al., 2017). However, phenotypes suggestive of higher immune function at late ages are also reported, for example with higher expression of innate immune genes (Landis et al., 2004), or antimicrobial peptides (Zerofsky, Harel, Silverman, & Tatar, 2005) in fruit flies. Nonetheless, high baseline activation coincides with a reduced ability to induce responses upon infection (Zerofsky et al., 2005) and has also been associated with reduced longevity (Fabian et al., 2018). For adaptive immunity, antibody titres (e.g. for influenza (Lessler et al., 2012)) can also be higher with age in humans but it is not clear that this translates into greater protection from infection. These examples illustrate the important point that greater abundance does not necessarily translate into greater functionality for immune effectors. Beyond abundance, the trade‐offs that shape immune function and the dynamic interactions of relevant cell populations must be considered.
4. TRADE‐OFFS ASSOCIATED WITH IMMUNE FUNCTION OVER THE LIFE COURSE
Hosts are prevented from achieving perfect immune defence against all threats by trade‐offs that emerge from allocation of limited resources between different necessary life‐history functions (Sheldon & Verhulst, 1996)—for example, investment towards immune responses might reduce resources available for other life‐history priorities such as growth or fertility. Compounding the problem, protection against pathogens often comes at the cost of ‘self‐harm’ due to collateral damage associated with powerful immune responses (Graham, Allen, & Read, 2005; Sorci, Lippens, Léchenault, & Faivre, 2017). Evolution will select hosts to compromise between competing needs across the life cycle (McKean & Lazzaro, 2011). To this end, the optimal immune response is rarely maximal (Cressler, Graham, & Day, 2015).
At the most basic scale, selection may determine whether hosts do, or do not, invest in immune defence (Rolff & Siva‐Jothy, 2003). For example, Drosophila constitutively able to defend against pathogens show lower larval ability to acquire food (Kraaijeveld, Limentani, & Godfray, 2001). Maintaining constitutive or fixed defences may be costly. For example, resistance to bacterial infection negatively correlates with fecundity of uninfected fruit flies (McKean, Yourth, Lazzaro, & Clark, 2008). Such maintenance costs might select for varied persistence of immune function across age. Early atrophy of the thymus, the organ where T cells are produced in vertebrates, is a striking example of altered functioning with age thought to be associated with the spectacular metabolic costs of random generation of T‐cell receptors (Palmer, 2013; Yates, 2014). Early thymus atrophy could free up resources for other functions (perhaps particularly reproduction, since involution precedes the age at maturity) while the longevity of naive T cells and their capacity for homeostatic proliferation preserve function temporarily. Sex differences in rates of thymus atrophy provide one interesting line of investigation into immune function effects on senescence (Pido‐Lopez, Imami, & Aspinall, 2001).
Beyond the relative benefits of ‘having’ and ‘maintaining’ an immune system, costs of ‘using’ an immune system have presumably been central in shaping the ubiquity of inducibility and active downregulation across the life cycle (McKean & Lazzaro, 2011). Infection or other immune ‘insults’ occur repeatedly through life (Figure 1, Figure 2). If an immune response is induced, costs can escalate rapidly as a result of positive feedback in the immune signalling system (‘cytokine storms’) but equally are subject to negative feedback loops that can swiftly shut the process down (Frank, 2002). These positive and negative feedback dynamics are also associated with legacy effects of potentially great significance in considering how selection plays out across age. For example, because T cells play a key role in immune regulation (Figure 2), when the thymus atrophies and T cells reach their Hayflick limit (Ndifon & Dushoff, 2016) induced immune responses may spiral out of control. In particular, the moderating influence of regulatory T cells upon other cells (Moore, Waal Malefyt, Coffman, & O'Garra, 2001) will wane as they decline. The density and activity of killer and innate cells may then increase, alongside increases in pro‐inflammatory molecules (Okin & Medzhitov, 2012). The tipping of the balance away from regulation towards inflammation is potentially exacerbated by declines in the efficacy of autophagy, or clearance of cellular detritus (Rea et al., 2018), and drives chronic inflammation in older individuals (Okin & Medzhitov, 2012). This syndrome is also referred to as ‘inflammaging’ (Franceschi et al., 2000) and confers greater risk of mortality associated with immunopathology. Indeed, adaptive immune components senesce faster on average than innate components in wild animals (Peters, Delhey, Nakagawa, Aulsebrook, & Verhulst, 2019), though disregulation of innate immunity is likely what kills hosts (Okin & Medzhitov, 2012). Latent or chronic pathogens may play a role—cytomegalovirus has been identified as uniquely important for immunosenescence (Pawelec, 2014) as it monopolizes and exhausts T cells (Schober, Buchholz, & Busch, 2018). Such late age dysregulation of immunity is evidenced in invertebrate systems too (Fabian et al., 2018; Khan, Agashe, & Rolff, 2017). Furthermore, immune pathways have been shown to differ between the sexes (Fabian et al., 2018) RNAi silencing of transcription factors increases male longevity and reduces female longevity. In general, however, sex differences in onset or rate of inflammaging remain understudied.
We can translate the strategic decisions involved around induction of an immune response (Figure 2) into life‐history terms, focusing first on survival. First, induction is associated with a trade‐off based around discriminating whether or not to respond (Metcalf et al., 2017). The cost of false negatives (failing to detect a pathogen that is present) must be balanced with the cost of false positives (launching an immune response in the absence of a threat could result solely in costly immunopathology, Figure 3a). Since the costs of immunopathology (false positives, Figure 3a) will manifest in the absence of any of the hazards immunity is designed to counteract (infection, cancer), optimizing around this discrimination trade‐off will depend on the pattern of hazard over age. Where infection is a key hazard, selection could result in lower probability of responding to infection in long‐lived organisms if most infection occurs early in life (Metcalf & Graham, 2018; Metcalf et al., 2017), since they would otherwise pay the cost of false positives for longer; or declining probability of responding over age if induction probability is tunable (Metcalf et al., 2017). This logic suggests that reduced expression or signalling of many innate immune receptors at late ages (Shaw, Goldstein, & Montgomery, 2013) could actually be adaptive rather than a manifestation of senescence. Sex differences in receptor expression (Jaillon et al., 2019) again provide a useful direction for investigation.
Figure 3.
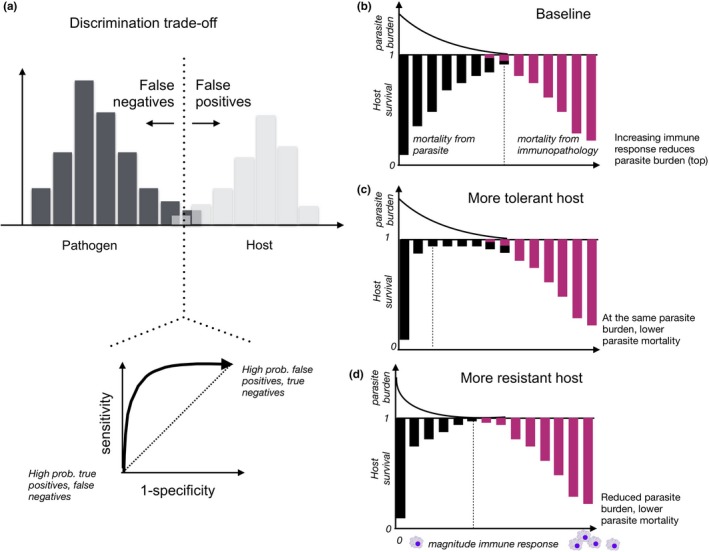
Immune trade‐offs. (a) A discrimination trade‐off: distinguishing between overlapping molecular signatures of the host (grey histogram) and pathogens (black histogram), or deciding where to draw the dashed vertical line, results in a trade‐off between false positive and false negatives (this is framed as a sensitivity/specificity trade‐off by epidemiologists, lower panel). (b) A trade‐off around the magnitude of the immune response: large responses (x‐axis, response magnitude increases left to right) reduce parasite burden (top panel, black line) and thus reduce the impact of parasites on mortality (lower panel, the black bars reflecting how parasites reduce host survival diminish in size) but increase negative effects associated with immunopathology (lower panel, purple bars reflecting how immunopathology reduces host survival increase in size). The optimal response is where the combined burden of parasite‐associated mortality and immunopathology‐related mortality is the smallest (vertical dashed black line). Relative to this baseline, hosts might be (c) more tolerant, where the trajectory of parasite burden (top panel) is unchanged but the impact of parasites on survival is reduced (black bars are smaller, noting that impacts might manifest via fertility instead), or (d) more resistant, where the parasite burden is lower for equivalent magnitude immune responses. The base case (b) represents the trade‐off directly emerging from danger associated with immunity, but both tolerance and resistance will require additional resource allocation, and are thus often found to trade‐off. Tracing these four trade‐offs across the life span is an important direction for future work
Second, once an immune response has been triggered, the optimal magnitude of the response will again be contingent: the benefits of pathogen control must be balanced against the costs of self‐harm (Figure 3b). The magnitude of the response (e.g. number of immune effectors launched) must be large enough to diminish pathogen‐associated mortality yet not sufficiently large as to result in excessive immunopathology. Optimizing around this trade‐off will depend on where organisms lie along the discrimination trade‐off—higher sensitivity (more false positives) will entail more frequent responses, and can thus select for lower magnitude responses (Metcalf & Graham, 2018), which might also vary between the sexes. Figure 3b focusses on survival, but the cost could also be to fertility—for example fallopian tube scarring caused by immune responses to Chlamydia trachomatis infection reduces female fertility (Johnson, Kerr, & Slaven, 2014) suggesting differences in trade‐off structure between the sexes.
In some contexts, the magnitude of induced responses also intersects with another set of trade‐offs (e.g. Raberg, Sim, & Read, 2007). For example, selection may favour the evolution of ‘tolerance’ strategies, via which tolerant hosts suffer considerably lower fitness impacts than intolerant hosts at equivalent pathogen burdens (Figure 3c), and/or ‘resistance’ strategies (Figure 3d) via which hosts minimize fitness impacts of infection by reducing pathogen burden. In systems such as rodent malaria, there is a trade‐off between resistance and tolerance (Raberg et al., 2007); however, the trade‐off between the strategies is not universal. As for optimal discrimination (above), the optimal strategy might depend on timing during the life course: for example a transition from ‘resistance’ to ‘tolerance’ as animal age has been suggested in rodents (Jackson et al., 2014) and sheep (Froy et al., 2019; Garnier et al., 2017).
5. INTERACTIONS BETWEEN IMMUNE FUNCTION EARLY AND LATE IN LIFE
Natural selection should optimize across immune trade‐offs (Figure 3) in the context of life history—with strategies likely to be highly plastic along the life course (Love, Salvante, Dale, & Williams, 2008). Optimization should also increase early life survival and fertility at the expense of later life survival and fertility (Williams, 1957). This prediction aligns with evidence that damage associated with immunopathology resulting from early infection may have long‐lasting negative effects (Figure 2), with reduced longevity of bacteria‐resistant flour beetles relative to RNAi knockouts of a key immune effector (Khan et al., 2017). However, such patterns are not ubiquitous, with no effect of early inflammation on longevity or reproductive output in murine malaria (Lippens et al., 2019) despite evidence that aged mice are more likely to die of inflammation (Belloni et al., 2010).
Indeed, the dynamic nature of immune function means that the opposite can also occur, that is increased early life hazards associated with protection later in life (Figure 2). For example, data from human populations suggest that early infection may be protective as it allows the immune system to learn to ‘curb’ itself (McDade, 2012). Another phenomenon that can result in this pattern is immune memory. Exposure to a pathogen can result in subsequent protection from that same pathogen, via lymphocyte‐mediated memory in vertebrates, or analogues (thus far largely described phenomenologically rather than mechanistically) in invertebrates (Pinaud et al., 2016; Watson et al., 2005). If ‘remembered’ responses provide an important line of immune defence, then, in contrast to theoretical predictions, early life cannot be protected over and above late life (setting effects due to transgenerational immune priming to the side). For example, pathogens like measles contribute little to late age mortality, because most individuals are infected early in life, thus acquiring complete immune protection for the rest of their lives. How much this contributes to emergent late age mortality will depend on the relative risk of encountering novel versus previously experienced pathogens. Since immune memory relies on selection for clonal amplification (in vertebrates), particular T cells can dominate memory (Qi et al., 2014) potentially beyond what would be useful in defence, and in the worst case, strongly targeting self‐antigens (Deshpande, Parrish, and Kuhns, 2015). Both memory imbalances and autoimmune disease could reduce survival at later ages.
6. ULTIMATE DETERMINANTS OF SEX DIFFERENCES IN IMMUNITY: IMPLICATIONS FOR SENESCENCE
To probe how the dynamic (Figure 2) interacting trade‐offs (Figure 3) associated with immune function translate into senescence, we next focus on how strikingly diverse sex differences in immunity (Klein & Flanagan, 2016) might evolve, and infer implications for the evolution of senescence. While the proximate determinants of sex differences can include both chromosomal and hormonal differences (Box 1), ultimate determinants will be rooted in differences in investments in competing and caring between males and females given core trade‐offs (Figure 3) across the life span. Ultimate explanations have been framed around four aspects: quantitative sex differences in immune responses (a); qualitative sex differences in immune responses (b); modified by transfer of immunity between generations for species where this occurs (c); or modified by pregnancy, for relevant species (d). Each of these four framings has different implications for the evolution of sex differences in senescence (Table 1). Empirical studies are sorely needed to test each of these ideas.
Table 1.
Four explanations for sex differences in immunity (columns, see text) framed in terms of expectations in the ‘caring’ sex (generally females), and expected alignment with immune trade‐offs (rows) with implications for senescence (final row)
| Trade‐off | Quantitative differences | Qualitative differences | Effect of transfer of antibodies | Effect of pregnancy |
|---|---|---|---|---|
| Having an immune systema | More expenditure | Either | Either | Either |
| Maintaining an immune systema | More expenditure | Either | Either | Either |
| Discriminating to trigger a response or not (favouring false positives vs. false negatives) | Possibly more triggering (more ‘sensitive’, Figure 3a) | Undefined (for Th1/Th2 contrast), or less triggering, that is less sensitive (for discrimination contrast) | More trigger (more sensitive) | Either |
| Magnitude of the triggered responsea | Larger response | Smaller inflammatory response (for Th1/Th2 contrast) and immune effector response (for discrimination contrast) | Either (but likely smaller) | Larger response to offset females spending time immunosuppressed to tolerate a foetus |
| Tolerating the infection (without reducing burden) versus nota | Possibly greater tolerance to offset greater responses? | Greater tolerance (under Th1 vs. Th2 contrast) or either (discrimination contrast) | Either | Either |
| Resistance, that is excluding infectiona | Larger investment | Either | Either | Either |
| Implications for immune effects on survival in the caring sex at late ages | Greater immunopathology and immune memory depletion; greater survival in the face of late life infections, unless memory depletion has reached problematic levels | Less (for Th1/Th2 contrast) or more (for discrimination contrast) immunopathology; and less memory so less defence against infection for the discrimination contrast | More autoimmunity, immunopathology | More autoimmunity, more immunopathology; assuming that change is in magnitude rather than regulation |
vs. expending resources on another aspect of life history (survival, fertility).
The first framing broadly posits that the ‘caring’ sex (females in many species) has been selected to have more ‘robust’ immune responses (Rolff, 2002; Sheldon & Verhulst, 1996; Zuk, 2009). Empirically, this aligns with higher antibody titres following infection or vaccination, greater macrophage activity (Klein & Flanagan, 2016), or higher immunopathology in females from influenza even at equivalent viral titres (Robinson, Lorenzo, Jian, & Klein, 2011). In vertebrates, the ‘immunocompetence—handicap hypothesis’ more specifically postulates that androgens shunt energy away from the immune system towards secondary sexual characteristics, so that males have less robust immune function than females, and thus, only high‐quality males can afford displays (Folstad & Karter, 1992). Various lines of evidence suggest that the effects of testosterone may be more immunomodulatory than immunosuppressive (e.g. (Hodges‐Simeon, Asif, Gurven, Blackwell, & Gaulin, 2019)), and overall, sex differences are more complex than a simple reduction of immune response magnitude in the non‐caring sex (Klein & Flanagan, 2016). However, assuming that this framing provides a reasonable approximation, what is implied for immune‐mediated effects on senescence? The sex with the more ‘robust’ immune response might be expected to suffer more from the negative effects of potent immune responses (e.g. inflammaging) at late ages, but less from infectious disease. Indeed, the concentration of immunopathology‐associated proteins (IL‐6, TNF‐alpha, C‐reactive protein) at late ages is generally higher in females (Bupp et al., 2018). Some evidence also supports lower infectious disease mortality at late ages among female mammals (Simon et al., 2015), although the causes of death in natural populations are often unknown.
The second framing emerges from accounting for qualitative features of immune differences (rather than a quantitative scale of ‘more’ vs. ‘less’ immune). Females may have been selected for a less inflammatory immune response, biased instead towards T‐helper type 2 immune responses (thought to favour pregnancy; e.g. in natural fertility human populations; (Blackwell et al., 2015)), while males are selected for inflammatory responses as being swift although destructive, in line with males being selected for a ‘faster’ lifestyle (Sears, Rohr, Allen, & Martin, 2011); see also (Roved, Westerdahl, & Hasselquist, 2017). This framing broadly suggests opposite sex‐specific pathologies at late ages to the first framing (Table 1). Qualitative immune differences can also be framed in terms of trade‐offs associated with pathogen discrimination and response magnitude (Metcalf & Graham, 2018). Empirically, in birds and mammals, enhanced pathogen detection is suggested for females, where the magnitude of response is enhanced in males. The ‘discriminating females versus responding males’ hypothesis (Metcalf & Graham, 2018) suggests females may deplete pools of naive B and T cells faster, potentially leaving them more vulnerable to infection at late ages (suggested at least for influenza (Kadel & Kovats, 2018)) and potentially also with greater immunopathology. While measures that align with ‘robust’ immunity in females seem to contradict this (e.g. higher immunopathology in infected females), this may in part result from taking static measures from what is inherently a dynamic system—if pathogen incidence is low in females as a result of early detection and exclusion (e.g. influenza in humans (Kadel & Kovats, 2018)), then on the rare occasions that the pathogen escapes the female immune system's vigilance, pathogen growth may be greater, and immunopathology likewise higher.
Third, an important feature of immunity is the potential for transfers between generations via maternal (Boulinier & Staszewski, 2008) or paternal immunity (Olivia Roth et al., 2010; Roth, Klein, Beemelmanns, Scharsack, & Reusch, 2012). The sex responsible for transferring antibodies might be predicted to have a ‘more robust’ (Zuk, 2009), less inflammatory (Sears et al., 2011) or more discriminating (Metcalf & Graham, 2018) immune system. In each scenario, the presence of transfers would be expected to provide another selection pressure to maintain immune function in the face of senescence for as long as offspring were being produced in the transferring sex and thus might lead to modify patterns of senescence between the sexes (Table 1).
Fourth, pregnancy importantly defines female immune system function in mammals. During pregnancy, females must meet the physiological challenge of not responding to the (non‐self) foetus to prevent abortion, driving selection for greater plasticity (as females move in and out of pregnancy) than required by the male immune system (Natri, Garcia, Buetow, Trumble, & Wilson, 2019), a process governed by hormones (Box 1). Inferring how this will affect senescence is not straightforward, in part because some of the most detailed data come from humans—yet human rates of pregnancy in many populations are currently much reduced currently relative to what might have been the case historically (Natri et al., 2019)—and because menopause profoundly alters human hormone levels, and thereby late age immune function, but is extremely rare across the vertebrate tree of life (otherwise only found in a few cetacean species (Whitehead, 2015)). Beyond the fascinating but rare example of menopause, tight dependence of immune function on female hormones could result in mutually exclusive scenarios at late ages: networks that are robust to perturbations, including declines occurring over senescence, or sensitive networks, leading to accelerated inflammaging in females. Emergent sex differences in senescence have the potential to importantly illuminate the links between immunity and senescence. Identifying empirical measures to discriminate between such predictions is a key future direction, urgently requiring associated longitudinal data (Peters et al., 2019).
7. FUTURE DIRECTIONS
Immune function is unique: its dangerous side (from inflammation to autoimmunity) requires careful regulation, while its role in pathogen defence calls for swift reaction and a capacity for memory. The interdependent system that has evolved to meet these needs means that immune changes at one age have intricate implications for the pattern of immune function both concurrently and at later ages. This complexity makes it hard to determine how declining selection pressures with age have altered immune function in ways that modify senescence. Sex differences provide one avenue to probing this question—differing selection pressures on the sexes will shape differences in immune function that are nevertheless occurring within the same bauplan. While we have laid out some broad expectations under existing theories for ultimate drivers of sex differences in immunity (Table 1, last row), they remain necessarily vague, given issues in interpreting what the various models imply. Effectively leveraging the distinction between the sexes will require careful theoretical framing of the various ultimate explanations and extensive empirical study. Measuring outcomes in terms of causes of death has the merit of being tractable, but is also comparable across models (e.g. whatever the nuance of detail in immune system function incorporated within models).
Developing the relevant theory will clearly not be straightforward. One important challenge will be in establishing how resource costs are paid (Schwenke, Lazzaro, & Wolfner, 2016), including the issue of defining the shape of trade‐offs between investment in immune system maintenance/activation/etc versus investment in survival and fertility (likely themselves sex‐specific), as well as resource allocation between immune functions. Another challenge will be reflecting the dynamical aspects of immune function (Figure 2). While generic models contrasting these broad framings may not lend themselves to teasing apart nuances (e.g. resources playing different roles for the two sexes (Rapkin et al., 2018)), predictions about immune differences between males and females at different ages (e.g. at differing resource availability, or for species with very different life spans, or under different frequencies of pathogen return) could launch quantitative tests of the explanatory power of the various models.
The paucity of data at this stage means that we are in little danger of ‘hypothesizing after the results are known’, but empirical measures will be key to advancing understanding. Novel tools (e.g. from CRISPR/CAS9 knockouts to novel immune measures) with unique model systems that encompass an array of life histories (e.g. the Syngnathiformes system ranging from no parental care to paternal pregnancy), and a more comprehensive array of hormones that affect immunity (beyond androgens), will also contribute insight into patterns of immune function across the life course, and between the sexes. Detailing drivers of these patterns between the sexes will allow us to further refine our understanding of core trade‐offs across the life span.
AUTHORS' CONTRIBUTIONS
C.J.E.M., A.L.G. and O.R. conceived the ideas and wrote the manuscript.
Supporting information
ACKNOWLEDGEMENTS
We thank the editors for the invitation to contribute and Brittany Sears for comments on an earlier draft of this manuscript.
Metcalf CJE, Roth O, Graham AL. Why leveraging sex differences in immune trade‐offs may illuminate the evolution of senescence. Funct Ecol. 2020;34:129–140. 10.1111/1365-2435.13458
DATA AVAILABILITY STATEMENT
There are no data associated with this manuscript.
REFERENCES
- Abrams, P. A. (1993). Does increased mortality favor the evolution of more rapid senescence? Evolution; International Journal of Organic Evolution, 47(3), 877–887. 10.1111/j.1558-5646.1993.tb01241.x [DOI] [PubMed] [Google Scholar]
- Bateman, A. J. (1948). Intra‐sexual selection in Drosophila. Heredity, 2(3), 349–368. 10.1038/hdy.1948.21 [DOI] [PubMed] [Google Scholar]
- Belloni, V. , Faivre, B. , Guerreiro, R. , Arnoux, E. , Bellenger, J. , & Sorci, G. (2010). Suppressing an anti‐inflammatory cytokine reveals a strong age‐dependent survival cost in mice. PLoS ONE, 5(9), e12940 10.1371/journal.pone.0012940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell, A. D. , Tamayo, M. A. , Beheim, B. , Trumble, B. C. , Stieglitz, J. , Hooper, P. L. , … Gurven, M. (2015). Helminth infection, fecundity, and age of first pregnancy in women. Science, 350(6263), 970–972. 10.1126/science.aac7902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi, D. , Aguado, M. T. , Dutel, C. , Goronzy, J. , Louis, J. , Grubeck‐Loebenstein, B. , … Del Giudice, G. (2013). The gracefully aging immune system. Science Translational Medicine, 5(185), 185ps8 10.1126/scitranslmed.3005624 [DOI] [PubMed] [Google Scholar]
- Boulinier, T. , & Staszewski, V. (2008). Maternal transfer of antibodies: Raising immuno‐ecology issues. Trends in Ecology & Evolution, 23(5), 282–288. 10.1016/j.tree.2007.12.006 [DOI] [PubMed] [Google Scholar]
- Bupp, G. , Melanie, R. , Potluri, T. , Fink, A. L. , & Klein, S. L. (2018). The confluence of sex hormones and aging on immunity. Frontiers in Immunology, 9(June), 1269 10.3389/fimmu.2018.01269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell, H. (2007). Extrinsic mortality and the evolution of senescence. Trends in Ecology & Evolution, 22(4), 173–174. 10.1016/j.tree.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Clutton‐Brock, T. H. , & Isvaran, K. (2007). Sex differences in ageing in natural populations of vertebrates. Proceedings of the Royal Society B: Biological Sciences, 274(1629), 3097–3104. 10.1098/rspb.2007.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressler, C. E. , Graham, A. L. , & Day, T. (2015). Evolution of hosts paying manifold costs of defence. Proceedings of the Royal Society B: Biological Sciences, 282(1804), 20150065 10.1098/rspb.2015.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bourcy, C. F. A. , Angel, C. J. L. , Vollmers, C. , Dekker, C. L. , Davis, M. M. , & Quake, S. R. (2017). Phylogenetic analysis of the human antibody repertoire reveals quantitative signatures of immune senescence and aging. Proceedings of the National Academy of Sciences of the United States of America, 114(5), 1105–1110. 10.1073/pnas.1617959114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, N. R. , Parrish, H. L. , & Kuhns, M. S. (2015). Self-recognition drives the preferential accumulation of promiscuous CD4+ T-cells in aged mice. Elife, 4, e05949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eens, M. , & Pinxten, R. (2000). Sex‐role reversal in vertebrates: Behavioural and endocrinological accounts. Behavioural Processes, 51(1–3), 135–147. 10.1016/S0376-6357(00)00124-8 [DOI] [PubMed] [Google Scholar]
- Fabian, D. K. , Garschall, K. , Klepsatel, P. , Santos‐Matos, G. , Sucena, É. , Kapun, M. , … Flatt, T. (2018). Evolution of longevity improves immunity in Drosophila. Evolution Letters, 2(6), 567–579. 10.1002/evl3.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, R. A. (1930). The genetical theory of natural selection. Oxford, UK: Oxford University Press. [Google Scholar]
- Folstad, I. , & Karter, A. J. (1992). Parasites, bright males, and the immunocompetence handicap. The American Naturalist, 139(3), 603–622. 10.1086/285346 [DOI] [Google Scholar]
- Foo, Y. Z. , Nakagawa, S. , Rhodes, G. , & Simmons, L. W. (2017). The effects of sex hormones on immune function: A meta‐analysis. Biological Reviews of the Cambridge Philosophical Society, 92(1), 551–571. 10.1111/brv.12243 [DOI] [PubMed] [Google Scholar]
- Forbes, M. R. (2007). On sex differences in optimal immunity. Trends in Ecology & Evolution, 22(3), 111–113. 10.1016/j.tree.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Franceschi, C. , Bonafè, M. , Valensin, S. , Olivieri, F. , De Luca, M. , Ottaviani, E. , & De Benedictis, G. (2000). Inflamm‐aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences, 908(June), 244–254. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- Frank, S. A. (2002). Immune response to parasitic attack: Evolution of a pulsed character. Journal of Theoretical Biology, 219(3), 281–290. 10.1006/jtbi.2002.3122 [DOI] [PubMed] [Google Scholar]
- Froy, H. , Sparks, A. M. , Watt, K. , Sinclair, R. , Bach, F. , Pilkington, J. G. , … Nussey, D. H. (2019). Senescence in immunity against helminth parasites predicts adult mortality in a wild mammal. Science, 365(6459), 1296–1298. [DOI] [PubMed] [Google Scholar]
- Gaillard, J.‐M. , & Lemaître, J.‐F. (2017). The Williams' legacy: A critical reappraisal of his nine predictions about the evolution of senescence. Evolution; International Journal of Organic Evolution, 71(12), 2768–2785. 10.1111/evo.13379 [DOI] [PubMed] [Google Scholar]
- Garnier, R. , Cheung, C. K. , Watt, K. A. , Pilkington, J. G. , Pemberton, J. M. , & Graham, A. L. (2017). Joint associations of blood plasma proteins with overwinter survival of a large mammal. Ecology Letters, 20(2), 175–183. 10.1111/ele.12719 [DOI] [PubMed] [Google Scholar]
- Graham, A. L. , Allen, J. E. , & Read, A. F. (2005). Evolutionary causes and consequences of immunopathology. Annual Review of Ecology, Evolution, and Systematics, 36(1), 373–397. 10.1146/annurev.ecolsys.36.102003.152622 [DOI] [Google Scholar]
- Haldane, J. B. S. (1942). New paths in genetics. George Allen & Unwin. [Google Scholar]
- Hill‐Burns, E. M. , & Clark, A. G. (2009). X‐linked variation in immune response in Drosophila melanogaster . Genetics, 183(4), 1477–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges‐Simeon, C. R. , Asif, S. , Gurven, M. , Blackwell, A. D. , & Gaulin, S. J. C. (2019). Testosterone is positively and estradiol negatively associated with mucosal immunity in Amazonian adolescents. American Journal of Human Biology, e23284. [DOI] [PubMed] [Google Scholar]
- Jackson, J. A. , Hall, A. J. , Friberg, I. M. , Ralli, C. , Lowe, A. , Zawadzka, M. , … Begon, M. (2014). An immunological marker of tolerance to infection in wild rodents. PLoS Biology, 12(7), e1001901 10.1371/journal.pbio.1001901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillon, S. , Berthenet, K. , & Garlanda, C. (2019). Sexual dimorphism in innate immunity. Clinical Reviews in Allergy & Immunology, 56(3), 308–321. 10.1007/s12016-017-8648-x [DOI] [PubMed] [Google Scholar]
- Johnson, R. M. , Kerr, M. S. , & Slaven, J. E. (2014). An atypical CD8 T‐cell response to Chlamydia muridarum genital tract infections includes T cells that produce interleukin‐13. Immunology, 142(2), 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadel, S. , & Kovats, S. (2018). Sex hormones regulate innate immune cells and promote sex differences in respiratory virus infection. Frontiers in Immunology, 9(July), 1653 10.3389/fimmu.2018.01653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, I. S. , Bayer, T. , Salzburger, W. , & Roth, O. (2018). Effects of parental care on resource allocation into immune defense and buccal microbiota in mouthbrooding cichlid fishes. Evolution; International Journal of Organic Evolution, 72(5), 1109–1123. 10.1111/evo.13452 [DOI] [PubMed] [Google Scholar]
- Khan, I. , Agashe, D. , & Rolff, J. (2017). Early‐life inflammation, immune response and ageing. Proceedings of the Royal Society B: Biological Sciences, 284(1850), 20170125 10.1098/rspb.2017.0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, S. L. , & Flanagan, K. L. (2016). Sex differences in immune responses. Nature Reviews. Immunology, 16(10), 626–638. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld, A. R. , Limentani, E. C. , & Godfray, H. C. (2001). Basis of the trade‐off between parasitoid resistance and larval competitive ability in Drosophila melanogaster . Proceedings of the Royal Society B: Biological Sciences, 268(1464), 259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz, J. , & Sauer, K. P. (2001). Gender differences in phenoloxidase activity of Panorpa vulgaris hemocytes. Journal of Invertebrate Pathology, 78(1), 53–55. 10.1006/jipa.2001.5040 [DOI] [PubMed] [Google Scholar]
- Landis, G. N. , Abdueva, D. , Skvortsov, D. , Yang, J. , Rabin, B. E. , Carrick, J. , … Tower, J. (2004). Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America, 101(20), 7663–7668. 10.1073/pnas.0307605101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaître, J.‐F. , & Gaillard, J.‐M. (2013). Male survival patterns do not depend on male allocation to sexual competition in large herbivores. Behavioral Ecology, 24(2), 421–428. 10.1093/beheco/ars179 [DOI] [Google Scholar]
- Lessler, J. , Riley, S. , Read, J. M. , Wang, S. , Zhu, H. , Smith, G. J. D. , … Cummings, D. A. T. (2012). Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS Path, 8(7), e1002802 10.1371/journal.ppat.1002802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert, C. , Dejager, L. , & Pinheiro, I. (2010). The X chromosome in immune functions: When a chromosome makes the difference. Nature Reviews. Immunology, 10(8), 594–604. [DOI] [PubMed] [Google Scholar]
- Lin, T. , Zhang, D. , Liu, X. , & Xiao, D. (2016). Parental care improves immunity in the seahorse (Hippocampus erectus). Fish & Shellfish Immunology, 58, 554–562. 10.1016/j.fsi.2016.09.065 [DOI] [PubMed] [Google Scholar]
- Lippens, C. , Guivier, E. , Reece, S. E. , O'Donnell, A. J. , Cornet, S. , Faivre, B. , & Sorci, G. (2019). Early plasmodium‐induced inflammation does not accelerate aging in mice. Evolutionary Applications, 12(2), 314–323. 10.1111/eva.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, O. P. , Salvante, K. G. , Dale, J. , & Williams, T. D. (2008). Sex‐specific variability in the immune system across life‐history stages. The American Naturalist, 172(3), E99–E112. 10.1086/589521 [DOI] [PubMed] [Google Scholar]
- Maklakov, A. A. , & Lummaa, V. (2013). Evolution of sex differences in lifespan and aging: Causes and constraints. BioEssays, 35(8), 717–724. 10.1002/bies.201300021 [DOI] [PubMed] [Google Scholar]
- Marais, G. A. B. , Gaillard, J.‐M. , Vieira, C. , Plotton, I. , Sanlaville, D. , Gueyffier, F. , & Lemaitre, J.‐F. (2018). Sex gap in aging and longevity: Can sex chromosomes play a role? Biology of Sex Differences, 9(1), 33 10.1186/s13293-018-0181-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade, T. W. (2012). Early environments and the ecology of inflammation. Proceedings of the National Academy of Sciences of the United States of America, 109(Suppl. 2), 17281–17288. 10.1073/pnas.1202244109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean, K. A. , & Lazzaro, B.P. (2011). The costs of immunity and the evolution of immunological defense mechanisms In Heyland A. & Flatt T. (Eds.), Mechanisms of life history evolution (pp. 299–310). Oxford, UK: Oxford University Press; 10.1093/acprof:oso/9780199568765.003.0023 [DOI] [Google Scholar]
- McKean, K. A. , & Nunney, L. (2001). Increased sexual activity reduces male immune function in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America, 98(14), 7904–7909. 10.1073/pnas.131216398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean, K. A. , Yourth, C. P. , Lazzaro, B. P. , & Clark, A. G. (2008). The evolutionary costs of immunological maintenance and deployment. BMC Evolutionary Biology, 8(March), 76 10.1186/1471-2148-8-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar, P. B. (1952). An Unsolved Problem of Biology: An Inaugural Lecture Delivered at University College, London, 6 December, 1951. [Google Scholar]
- Metcalf, C. J. E. , & Graham, A. L. (2018). Schedule and magnitude of reproductive investment under immune trade‐offs explains sex differences in immunity. Nature Communications, 9(1), 4391 10.1038/s41467-018-06793-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf, C. J. E. , Tate, A. T. , & Graham, A. L. (2017). Demographically framing trade‐offs between sensitivity and specificity illuminates selection on immunity. Nature Ecology & Evolution, 1(11), 1766–1772. 10.1038/s41559-017-0315-3 [DOI] [PubMed] [Google Scholar]
- Moorad, J. , Promislow, D. , & Silvertown, J. (2019). Evolutionary ecology of senescence and a reassessment of Williams' ‘Extrinsic Mortality’ hypothesis. Trends in Ecology & Evolution, 34(6), 519–530. 10.1016/j.tree.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, K. W. , de Waal Malefyt, R. , Coffman, R. L. , & O'Garra, A. (2001). Interleukin‐10 and the interleukin‐10 receptor. Annual Review of Immunology, 19, 683–765. [DOI] [PubMed] [Google Scholar]
- Mor, G. , Aldo, P. , & Alvero, A. B. (2017). The unique immunological and microbial aspects of pregnancy. Nature Reviews. Immunology, 17(8), 469–482. [DOI] [PubMed] [Google Scholar]
- Natri, H. , Garcia, A. R. , Buetow, K. H. , Trumble, B. C. , & Wilson, M. A. (2019). The pregnancy pickle: Evolved immune compensation due to pregnancy underlies sex differences in human diseases. Trends in Genetics: TIG, 35(7), 478–488. 10.1016/j.tig.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndifon, W. , & Dushoff, J. (2016). The Hayflick limit may determine the effective clonal diversity of naive T cells. Journal of Immunology, 196(12), 4999–5004. 10.4049/jimmunol.1502343 [DOI] [PubMed] [Google Scholar]
- Nussey, D. H. , Watt, K. , Pilkington, J. G. , Zamoyska, R. , & McNeilly, T. N. (2011). Age‐related variation in immunity in a wild mammal population. Aging Cell, 11(1), 178–180. 10.1111/j.1474-9726.2011.00771.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okin, D. , & Medzhitov, R. (2012). Evolution of inflammatory diseases. Current Biology: CB, 22(17), R733–R740. 10.1016/j.cub.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen‐Ashley, N. T. , Hasselquist, D. , & Wingfield, J. C. (2004). Androgens and the immunocompetence handicap hypothesis: Unraveling direct and indirect pathways of immunosuppression in song sparrows. The American Naturalist, 164(4), 490–505. 10.1086/423714 [DOI] [PubMed] [Google Scholar]
- Palmer, D. B. (2013). The effect of age on thymic function. Frontiers in Immunology, 4, 316 10.3389/fimmu.2013.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec, G. (2014). Immunosenenescence: Role of cytomegalovirus. Experimental Gerontology, 54(June), 1–5. 10.1016/j.exger.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Peters, A. (2000). Testosterone treatment is immunosuppressive in superb fairy–wrens, yet free–living males with high testosterone are more immunocompetent. Proceedings of the Royal Society of London. Series B: Biological Sciences, 267(1446), 883–889. 10.1098/rspb.2000.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, A. , Delhey, K. , Nakagawa, S. , Aulsebrook, A. , & Verhulst, S. (2019). Immunosenescence in wild animals: Meta‐analysis and outlook. Ecology Letters, 22(10), 1709–1722. 10.1111/ele.13343 [DOI] [PubMed] [Google Scholar]
- Pido‐Lopez, J. , Imami, N. , & Aspinall, R. (2001). Both age and gender affect thymic output: More recent thymic migrants in females than males as they age. Clinical and Experimental Immunology, 125(3), 409–413. 10.1046/j.1365-2249.2001.01640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud, S. , Portela, J. , Duval, D. , Nowacki, F. C. , Olive, M.‐A. , Allienne, J.‐F. , … Gourbal, B. (2016). A shift from cellular to humoral responses contributes to innate immune memory in the vector snail Biomphalaria glabrata . PLoS Path, 12(1), e1005361 10.1371/journal.ppat.1005361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipoly, I. , Bókony, V. , Kirkpatrick, M. , Donald, P. F. , Székely, T. , & Liker, A. (2015). The genetic sex‐determination system predicts adult sex ratios in tetrapods. Nature, 527(7576), 91–94. 10.1038/nature15380 [DOI] [PubMed] [Google Scholar]
- Qi, Q. , Liu, Y. , Cheng, Y. , Glanville, J. , Zhang, D. , Lee, J. Y. , … Goronzy, J.J. (2014). Diversity and clonal selection in the human T-cell repertoire. Proceedings of the National Academy of Sciences, 111(36), 13139–13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raberg, L. , Sim, D. , & Read, A. F. (2007). Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science, 318(5851), 812–814. 10.1126/science.1148526 [DOI] [PubMed] [Google Scholar]
- Rapkin, J. , Kim Jensen, C. , Archer, R. , House, C. M. , Sakaluk, S. K. , del Castillo, E. , & Hunt, J. (2018). The geometry of nutrient space‐based life‐history trade‐offs: Sex‐specific effects of macronutrient intake on the trade‐off between encapsulation ability and reproductive effort in decorated crickets. The American Naturalist, 191(4), 452–474. 10.1086/696147 [DOI] [PubMed] [Google Scholar]
- Rea, I. M. , Gibson, D. S. , McGilligan, V. , McNerlan, S. E. , Denis Alexander, H. , & Ross, O. A. (2018). Age and age‐related diseases: Role of inflammation triggers and cytokines. Frontiers in Immunology, 9(April), 586 10.3389/fimmu.2018.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, M. L. , Buchanan, K. L. , & Evans, M. R. (2004). Testing the immunocompetence handicap hypothesis: A review of the evidence. Animal Behaviour, 68(2), 227–239. 10.1016/j.anbehav.2004.05.001 [DOI] [Google Scholar]
- Robinson, D. P. , & Klein, S. L. (2012). Pregnancy and pregnancy‐associated hormones alter immune responses and disease pathogenesis. Hormones and Behavior, 62(3), 263–271. 10.1016/j.yhbeh.2012.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D. P. , Lorenzo, M. E. , Jian, W. , & Klein, S. L. (2011). Elevated 17β‐estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Path, 7(7), e1002149 10.1371/journal.ppat.1002149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolff, J. (2002). Bateman's principle and immunity. Proceedings of the Royal Society of London. Series B: Biological Sciences, 269(1493), 867–872. 10.1098/rspb.2002.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolff, J. , & Siva‐Jothy, M. T. (2003). Invertebrate ecological immunology. Science, 301(5632), 472–475. 10.1126/science.1080623 [DOI] [PubMed] [Google Scholar]
- Roth, O. , Joop, G. , Eggert, H. , Hilbert, J. , Daniel, J. , Schmid‐Hempel, P. , & Kurtz, J. (2010). Paternally derived immune priming for offspring in the red flour beetle, Tribolium castaneum . The Journal of Animal Ecology, 79(2), 403–413. [DOI] [PubMed] [Google Scholar]
- Roth, O. , Klein, V. , Beemelmanns, A. , Scharsack, J. P. , & Reusch, T. B. H. (2012). Male pregnancy and biparental immune priming. The American Naturalist, 180(6), 802–814. 10.1086/668081 [DOI] [PubMed] [Google Scholar]
- Roth, O. , Scharsack, J. P. , Keller, I. , & Reusch, T. B. H. (2011). Bateman's principle and immunity in a sex‐role reversed pipefish. Journal of Evolutionary Biology, 24(7), 1410–1420. 10.1111/j.1420-9101.2011.02273.x [DOI] [PubMed] [Google Scholar]
- Roved, J. , Westerdahl, H. , & Hasselquist, D. (2017). Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Hormones and Behavior, 88(February), 95–105. [DOI] [PubMed] [Google Scholar]
- Schober, K. , Buchholz, V. R. , & Busch, D. H. (2018). TCR repertoire evolution during maintenance of CMV‐specific T‐cell populations. Immunological Reviews, 283(1), 113–128. 10.1111/imr.12654 [DOI] [PubMed] [Google Scholar]
- Schwenke, R. A. , Lazzaro, B. P. , & Wolfner, M. F. (2016). Reproduction–immunity trade‐offs in insects. Annual Review of Entomology, 61(1), 239–256. 10.1146/annurev-ento-010715-023924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobell, S. K. , & Mackenzie, D. S. (2011). Reproductive endocrinology of syngnathidae. Journal of Fish Biology, 78(6), 1662–1680. 10.1111/j.1095-8649.2011.02994.x [DOI] [PubMed] [Google Scholar]
- Sears, B. F. , Rohr, J. R. , Allen, J. E. , & Martin, L. B. (2011). The economy of inflammation: When is less more? Trends in Parasitology, 27(9), 382–387. 10.1016/j.pt.2011.05.004 [DOI] [PubMed] [Google Scholar]
- Shaw, A. C. , Goldstein, D. R. , & Montgomery, R. R. (2013). Age‐dependent dysregulation of innate immunity. Nature Reviews. Immunology, 13(12), 875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon, B. C. , & Verhulst, S. (1996). Ecological immunology: Costly parasite defences and trade‐offs in evolutionary ecology. Trends in Ecology & Evolution, 11(8), 317–321. 10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Sheridan, L. A. D. , Poulin, R. , Ward, D. F. , & Zuk, M. (2000). Sex differences in parasitic infections among arthropod hosts: Is there a male bias? Oikos, 88(2), 327–334. 10.1034/j.1600-0706.2000.880211.x [DOI] [Google Scholar]
- Simon, A. K. , Hollander, G. A. , & McMichael, A. (2015). Evolution of the immune system in humans from infancy to old age. Proceedings of the Royal Society B: Biological Sciences, 282(1821), 20143085 10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorci, G. , Lippens, C. , Léchenault, C. , & Faivre, B. (2017). Benefits of immune protection versus immunopathology costs: A synthesis from cytokine KO models. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases, 54(October), 491–495. 10.1016/j.meegid.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Souyris, M. , Cenac, C. , Azar, P. , Daviaud, D. , Canivet, A. , Grunenwald, S. , … Guéry, J.‐C. (2018). TLR7 escapes X chromosome inactivation in immune cells. Science Immunology, 3(19), eaap8855 10.1126/sciimmunol.aap8855 [DOI] [PubMed] [Google Scholar]
- Stoehr, A. M. , & Kokko, H. (2006). Sexual dimorphism in immunocompetence: What does life‐history theory predict? Behavioral Ecology: Official Journal of the International Society for Behavioral Ecology, 17(5), 751–756. 10.1093/beheco/ark018 [DOI] [Google Scholar]
- Uciechowski, P. , & Rink, L. (2018). Neutrophil, basophil, and eosinophil granulocyte functions in the elderly In Fulop T., Franceschi C., Hirokawa K., & Pawelec G. (Eds.), Handbook of immunosenescence (pp. 1–27). Springer Switzerland AG; 10.1007/978-3-319-64597-1_22-1 [DOI] [Google Scholar]
- Watson, F. L. , Püttmann‐Holgado, R. , Thomas, F. , Lamar, D. L. , Hughes, M. , Kondo, M. , … Schmucker, D. (2005). Extensive diversity of Ig‐superfamily proteins in the immune system of insects. Science, 309(5742), 1874–1878. 10.1126/science.1116887 [DOI] [PubMed] [Google Scholar]
- Wensink, M. J. , Caswell, H. , & Baudisch, A. (2017). The rarity of survival to old age does not drive the evolution of senescence. Evolutionary Biology, 44(1), 5–10. 10.1007/s11692-016-9385-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead, H. (2015). Life history evolution: What does a menopausal killer whale do? Current Biology: CB, 25(6), R225–R227. 10.1016/j.cub.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Williams, G. C. (1957). Pleiotropy, natural selection, and the evolution of senescence. Evolution; International Journal of Organic Evolution, 11(4), 398 10.1111/j.1558-5646.1957.tb02911.x [DOI] [Google Scholar]
- Yates, A. J. (2014). Theories and quantification of thymic selection. Frontiers in Immunology, 5(February), 13 10.3389/fimmu.2014.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerofsky, M. , Harel, E. , Silverman, N. , & Tatar, M. (2005). Aging of the innate immune response in Drosophila melanogaster . Aging Cell, 4(2), 103–108. 10.1111/j.1474-9728.2005.00147.x [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Dong, X. , Lee, M. , Maslov, A. Y. , Wang, T. , Vijg, J. (2019). Single‐cell whole‐genome sequencing reveals the functional landscape of somatic mutations in B lymphocytes across the human lifespan. Proceedings of the National Academy of Sciences of the United States of America, 116, 9014–9019. 10.1101/535906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk, M. (2009). The sicker sex. PLoS Path, 5(1), e1000267 10.1371/journal.ppat.1000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are no data associated with this manuscript.


