Abstract
Visceral leishmaniasis (VL) is a life-threatening parasitic disease affecting impoverished people of the developing world; and much effort has been spent on the early case detection and treatment. However, current diagnostics and treatment options are not sufficient for appropriate surveillance in VL elimination setting. Hence, there is a dire need to develop highly sensitive diagnostics and less toxic effective treatments for proper management of cases and to achieve the sustained disease elimination. Although, promising results have been observed with nanomedicines in leishmaniasis; there are great challenges ahead especially in translating this to clinical setting. This review provides updated progress of nanomedicines in VL, and discussed how these innovations and future directions play vital role in achieving VL elimination.
Keywords: : diagnostics, drug resistance, leishmaniasis, nanodiagnostics, nano drug delivery, nanomedicine, nanoparticles, nanotheranostics, treatment, visceral leishmaniasis
Background
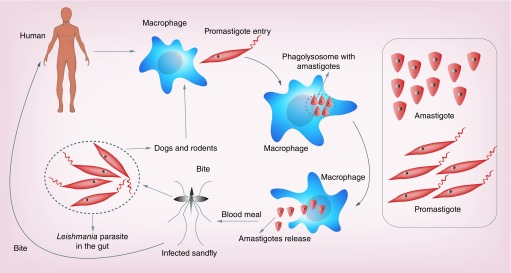
Leishmaniasis currently affects around 12 million people in 98 countries, most of which are in the developing world. The disease is transmitted by sand flies (Phlebotomus spp.), small biting mosquito, which breed in moist soil, forest areas, caves or the burrows of rodents, and feed from infected animal reservoir hosts or humans and Leishmania parasite completes its life cycle [1,2] as shown in Figure 1. It is estimated that about 350 million people are exposed to the risk of infection by the different species of human Leishmania parasites [3]. It comprises of a complex group of diseases ranging from self-healing cutaneous lesions (CL) to severe and systemic visceral leishmaniasis (VL), which is fatal if left untreated. VL (also known as kala-azar) is a parasitic disease affecting an estimated 500,000 new cases per year mostly in the Indian subcontinent and east Africa, though this figure is subjected to substantial uncertainty [4]. It usually occurs in small clusters in remote areas with poor access to health services and exact case numbers are poorly documented. Currently, VL is considered as one of the ‘most neglected tropical diseases’, a term coined to highlight the lack of innovation in both its clinical management and control. There has been an increase in all forms of leishmaniasis worldwide in recent years [5]. New foci from several countries have been reported including Sri Lanka (CL due to Leishmania donovani) [6], Bhutan (VL) [7], several countries of the former USSR (VL and CL) [8,9] and Mali. In Brazil, where VL was a rural disease, it is now reported from urban areas [10]. Africa is the second largest focus (after the Indian subcontinent) of VL accounting for almost 30% of the world’s cases [5]. The endemic regions span southern and eastern Sudan, north-western and southern Ethiopia, Somalia, northern and north-eastern Kenya, north-eastern Uganda and Tunisia.
Figure 1. . Life cycle of the Leishmania parasite inside the host (human), reservoir (dogs & rodents) and vector (sand fly).
Leishmaniasis has recently earned more public attention. The London declaration on Neglected Tropical Diseases has been made to eliminate VL as the public health problem by 2020 [11]. In the Indian subcontinent (India, Nepal and Bangladesh), a joint elimination campaigns are running since 2005 with the aim to reduce the annual incidence of VL less than one case in 10,000 people at public health centre levels in the endemic areas [12]. Early diagnosis and treatment, along with vector management, is one of the vital strategy proposed to achieve this goal of elimination. Significant progress has been made in past years and elimination initiatives have saved many lives in endemic areas. However, this ambitious but laudable goal is threatened by resistance (both to drug and insecticides), transmission by asymptomatic individuals and increasing co-infection. In the following sections, we have described the challenges in diagnosis and treatment of leishmaniasis and further discussed the progress in nanomedical research in terms of their capacity to improve the clinical outcomes of the disease.
Current challenges in diagnostic & treatment options in leishmaniasis
To date there are several commercialized rapid diagnostic kits in different formats available for confirming VL cases. These kits have varying sensitivities and are still far from being ideal. The most widely used test is the rK39 rapid diagnostic tests (rK39 RDT) that has proved to be a better diagnostic guide in the suspected VL cases in the Indian subcontinent and has led to its adoption as a diagnostic test in the VL elimination initiative. However, it showed decreased sensitivity in East African population when compared with the Indian subcontinent [13]. Another limitation of this test is the positivity of the test up to several years after successful treatment, making this test difficult to discriminate between those who still need medical intervention and those who are healthy and do not need treatment anymore [2]. In addition, poor correlation was observed between conventional and molecular methods in identifying asymptomatic and post kala-azar dermal leishmaniasis (PKDL) individuals. Thus innovations in diagnostic development are required to guide the control program.
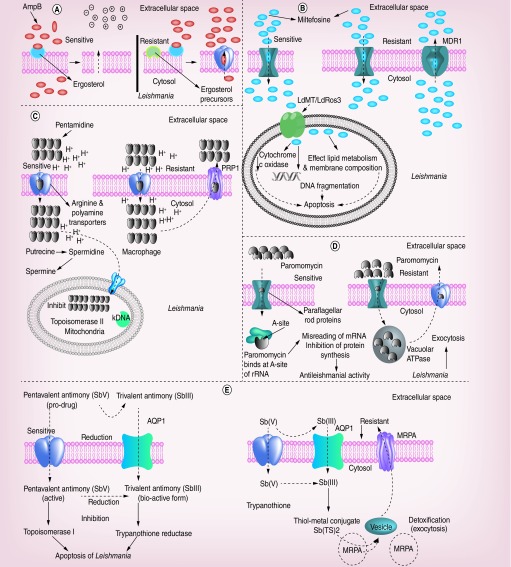
Importantly, the therapeutic options for VL are diverse and depend on different factors, like geographical area of the infection, development of resistance to accustomed treatments, HIV co-infection, undernourishment and other accompanying infections. Further, the intracellular location of the infective amastigote, in the acidic phagolysosomal compartment of different macrophage populations and the varying sensitivities of strains and species renders drugs ineffective [1]. For the past several decades, antimony (sodium stibogluconate) has been the first-line treatment for VL cases despite considerable toxicity and the requirement for 4–5 weeks hospitalization as it is administered by daily intramuscular or intravenous injection [14]. In India, amphotericin B (AmpB) has replaced antimonial in many districts. AmpB is administered as intravenous infusions at 1 mg/kg on alternate days for 15 infusions, necessitating hospitalization for prolonged periods and thus again limiting the number of patients who can be treated [14]. In addition, infusion-related toxicities are very common and an occasional serious adverse events like nephrotoxicity, myocarditis, and even death. Miltefosine, the only oral drug has been chosen for the elimination program but it is contraindicated in pregnancy and breast feeding and due its long half-life there is high potential for development of resistance. Experiences in India have shown a decrease of efficacy from 97% in the Phase III trial to 82% in the Phase IV trial [15]. In the last 5 years, some very important advances occurred in the therapy of Indian VL. In a large study of >300 patients, Sundar et al. showed that a single dose of 10 mg/kg of liposomal AmpB (AmBisome®) cured 95.7% of patients, similar to cure rates with conventional 1-month AmpB deoxycholate treatment [16]. In another important study, Sundar et al. demonstrated combinational therapy given over 8–11 days has led to a cure rate of >97% [17]. These tools, if employed in the control program, could dramatically change the outcome of treatment efforts. An important problem with all antileishmanial drugs is disease relapse following drug treatment either as a recurrence of VL or PKDL, where patients present macular, papular or nodular skin lesions often containing heavily parasitized macrophages [18]. Till date there are no good prognostic markers to identify individuals that might fail drug treatment. The entire major conventional therapeutic, their mode of action and mechanism of drug resistance have been elucidated in Figure 2.
Figure 2. . The mode of action of conventional drugs against leishmaniasis and the mechanisms of drug resistance in Leishmania species.
(A) Amphotericin B affects both stages of the parasite, that is, promastigote and amastigote, mainly by binding parasite cell membrane ergosterol, which enhances cell permeability and ions influx leading to compromised cellular integrity, which eventually results in death of the parasite. In some species due to the different class of ergosterol precursor Amphotericin B fails to bind with parasites membrane leading to drug resistance. (B) Miltefosine works by inhibiting cytochrome-c oxidase beside affecting the membrane potential of mitochondria leading to the death of the parasite through apoptosis. The presence of MDR1 efflux out miltefosine drug from parasites leading to drug resistance beside poor uptake and inactivation of the drug. (C) The inhibition of active transport system through pentamidine is believed to be the mode of action of this drug, which enters the Leishmania parasite through the arginine and polyamine transporters, and gets accumulated in the mitochondria that eventually inhibits topoisomerase II. The poor accumulation of pentamidine in the mitochondria alongside drug effluxed out of the parasite through PRP1; an ATP Binding Cassette (ABC) transporter, which eventually leads to drug resistance.(D) Paromomycin inhibits protein synthesis by binding to the A-site of ribosomal RNA altering the membrane potential of mitochondria, which eventually leads to misreading of mRNA causing the parasite death. An increased vacuolar ATPase activity, which eventually efflux paromomycin out (exocytosis) of the cell in case of resistant strains. (E) Trivalent (SbIII) and pentavalent (Sbv) form of antimony inhibits trypanothione reductase and topoisomerase I enzymes that eventually leads to apoptosis of the Leishmania parasite. The enhanced levels of trypanothione (TSH) conjugates with the tri (SbIII) and pentavalent (Sbv) antimony compounds forming thiol metal conjugates that may eventually form vesicles and these vesicles are effluxed out via multidrug resistance-associated protein ABC transporter (MRPA) during antimony resistance.
How nanomedicine can improve the diagnostics, treatment & aid VL elimination program
Nanomedicine in VL
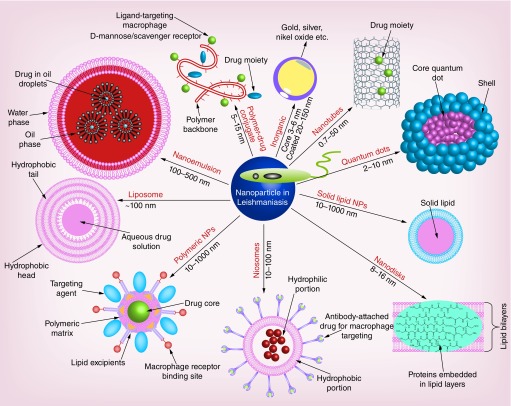
Innovations in interdisciplinary sciences are required to reduce the limitation of drugs alongside enhanced sensitivity of diagnostics that can prove crucial for better health standards. Recent progress in the field of nanotechnology has proven its efficacy in overcoming several shortcomings associated with the medical sciences including diagnostics and therapeutics [19]. The physical and chemical properties of nanoparticles (NPs) may help for the further advancements in diagnosis of VL by cutting the limitations associated with the classical methods of parasite detection. Additionally, usage of lower therapeutic dose of the purified drug within the nanocarrier will be boon to public health in reducing toxicity and cost, which has been a major hindrance in the existing conventional treatment for VL. NP-based diagnostics and drug delivery system (Figure 3) include liposomes, nanoemulsions, niosomes, lipid cochleates, nanodiscs, solid lipid NPs, polymer NPs, polysaccharide polymers, polymeric micelles, quantum dots and inorganic compounds, which have shown encouraging results in detection and delivery against the Leishmania parasite both at the in vitro and in vivo levels [20,21].
Figure 3. . Nanotheranostics/nanomedicines for leishmaniasis.
Nanoparticle-based diagnostics and drug delivery system that include liposomes, nanoemulsions, niosomes, lipid cochleates, nanodiscs, solid lipid NPs, polymer NPs, polysaccharide polymers, polymeric micelles, quantum dots and inorganic compounds, which have shown encouraging results in detection and delivery against the Leishmania parasite both at the in vitro and in vivo levels.
Nanoparticle-based diagnostics for VL
NPs used for detection through biosensors have currently made a vast impact in the field of diagnostics, which can offer cost effective detection of pathogenic infectious agents both in natural and clinical specimens. The first use of NPs in diagnostics occurred way back in 1996 that helped in the detection of anthrax DNA using gold NPs [22]. With the ongoing changes in the field of nanotechnology has made some interesting changes for better detection of infectious diseases caused by Salmonella, Escherichia coli, Staphylococcus aureus, Influenza, Vibrio cholerae, human papillomavirus, Acinetobacter baumanni, etc. [23].
Recently, Anfossi et al. developed a rapid and portable tool for canine VL diagnosis based on the lateral flow immunoassay [24]. This lateral flow immunoassay has two components having a highly specific chimeric recombinant antigen and gold NPs with protein A labeled as reporter. This specific chimeric recombinant antigen binds with antileishmanial antibodies showing 98.4% sensitivity and 98.9% specificity against canine leishmaniasis. A surface plasmon resonance immunosensor has been developed by Souto et al. for the detection of Leishmania infantum hypothetical C1 protein through antibodies immobilized on gold dendrimers [25]. Nanostructured films with Leishmania amazonensis and Trypanosoma cruzi-specific antigen through impedance spectroscopy have been utilized as a detection method for efficient diagnosis of leishmaniasis [26]. Andreadou et al. utilized a combination of cadmium selenite quantum dot and magnetic beads as probe for detection of Leishmania DNA and its specific surface antigens [27]. Magnetic beads capture the analytes from the solution, and cadmium selenite quantum dots help in detection of specific molecule of interest, that is, Leishmania DNA and proteins with 100% sensitivity and specificity in 55 cultured isolates of various microbial pathogens having a lower limit of 3125 ng/μl and 103 cells/ml for Leishmania protein and DNA. A synthesized and characterized quantum dots of magnetic cobalt-zinc ferrite with specific single-stranded DNA probe act like an electrochemical genosensor for Leishmania major detection [28]. This electrochemical genosensor has the ability to detect ssDNA target (10-11-10-18 mol/l with a limit in detection up to 2 × 10-19) and genomic DNA (7.31 × 10-14- 7.31 × 10-6 ng/μl with a limit in detection up to 1.8 × 10-14) with a high selectivity and sensitivity. Additionally, a study by Bose et al. has used plasmonic phenomena of gold NP for naked eye-based cheap, easy and fast detection (DNA) and profiling of sodium antimony gluconate (SAG) resistance during leishmaniasis [29].
Gold NP conjugated with four oligonucleotide probes targeting Leishmania kinetoplastid minicircle DNA is a non-amplification assay detection where the absence of complementary DNA, a red colored turn to purple in acidic environment [30]. While in the presence of Leishmania kinetoplastid DNA, the color of the sample remains red. Tweaking of gold NP-based lateral flow biosensor for visual detection of Leishmania-specific kinetoplast DNA amplification product in infected dog blood samples has showed high specificity [31]. In another study by Bose et al., visual assessment of parasite burden was assessed in amastigotes using gold NPs by plasmonic detection of parasite-specific small subunit rRNA [32]. All these nanodiagnostic studies summarized in Table 1 have been moving in the proper channel in order to enhance the chances toward ultimate challenge toward elimination. Although, a lot has to be explored in case of nanodiagnostics for circumventing VL cases through early detection.
Table 1. . Summary of the nanoparticles-based diagnostics used for the detection of leishmaniasis.
| NPs | Detection | Probe attached | Sensitivity & specificity (%) | Leishmaniasis | Remarks | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Gold | Lateral flow immunoassay | Chimeric recombinant antigen and protein A | CVL endemic region (95.8 & 100) CVL nonendemic region (100 98.8); both regions (98.4 & 98.9) |
Leishmania infantum | 2018 | [25] | |
| Gold | Lateral flow biosensor | Biotin 22-mer 5′-CTTTTCTGGTCCTCCGGGTAGG-3′ upstream primer for Leishmania kinetoplast DNA amplification, 24-mer 5′-CCACCCGGCCCTATTTTACACCAA- downstream primer. 24-mer 5′-TTTTCGCAGAACGCCCCTACCCGC-3′ Leishmania-specific probe |
Leishmania infantum | 2016 | [32] | ||
| Quantum dots of magnetic cobalt-zinc ferrite | Electrochemical genosensor | Specific single-stranded DNA probe (p-ssDNA) sequence: 5′-TGTTGGGTGACGCTTTAGTGGGTT-3′; t-ssDNA sequence: 5′-AACCCACTAAAGCGTCACCCAACA-3′ |
Leishmania major | ssDNA target (10-11 -10-18 mol/l with a limit in detection up to 2×10-19) and genomic DNA (7.31×10-14 - 7.31×10-6 ng/μl with a limit in detection up to 1.8×10-14) | 2016 | [29] | |
| Gold | Plasmonic detection of Leishmania-specific marker RNA | 5′GGACGCACTAAACCCCTCAA3′ of SSU rRNA gene and a-tubulin gene (forward primer-5′TCTGCCTTGA GCACGGCATC3′, reverse primer 5′ACACCAGCTG CTCGGGGTTG3′) |
Leishmania donovani | 2016 | [33] | ||
| Cadmium selenide quantum dot and magnetic beads | Leishmania DNA and its specific surface antigens | Oligonucleotide probes (LeishQD1 (5′-3′): biotin-AAGAGGCGGTGTCACAGAGATGGG; LeishQD2 (5′-3′): biotin-ACAGCGACGTCCGTGGAAAG) anti-Leishmania LPG (IgM); anti-Leishmania gp63 (IgG1a); biotin-labeled polyclonal antimouse antibody (IgM); biotinlabeled polyclonal antimouse IgG1 | 100 100 |
Leishmania infantum | Lower limit of detection 3125 ng/μl | 2016 | [28] |
| Gold & dendrimers | Surface plasmon resonance immunosensor | Antibodies against hypothetical C1 protein | Leishmania infantum | Lower limit of detection 7.37 nmol/l; low limits of quantification 7.83 nmol/l |
2015 | [26] | |
| Gold | Plasmonic detection of PCR amplified genetic biomarker | Chr13 Pos442924 (sensitive-5′GCCGACCAAGCCTAATTT3′, resistance-5GCCGACCAAGCCTAATTA3′) Chr35 Pos1192217 (sensitive- 5′ GCGACGACTGCGGCGGTG3′, resistance-5′ GCGACGACTGCGGCGGTA3′) Chr35 Pos1619958 (sensitive- 5′ GGGCGTAGAGGAAGCTCG3’, resistance-5′ GGGCGTAGAGGAAGCTCA3′) Chr35 Pos1656169 (sensitive- 5′ CAGCGAACGTACTGCCTC 3′, resistance-5′ CAGCGAACGTACTGCCTT 3′), kDNA (5′ GGCTTAGTGTTAGCCGTGTGT 3′) |
Leishmania donovani | Lower limit of detection 212.5 –6.64 ng | 2015 | [30] | |
| Gold | Targeting kinetoplastid minicircle DNA |
4 Oligonucleotide sequence (5′–3′) LeishAu1 (GTTAGCCGATGGTGGTCTTG) LeishAu2 (ACGGGTGTCTTTGATGATGC) LeishAu3 (TAGTCTGGTGGGATGCTTCG) LeishAu4 (GTGCCTTTGATGTGGGTGTT) |
92 100 |
Leishmania infantum | Lower limit of detection 11.5 ng/μl | 2014 | [31] |
| Nanostructured films | Specific Leishmania amazonensis and Trypanosoma cruzi antigens and employing impedance spectroscopy | Purified anti-IgG | Leishmania amazonensis | 2010 | [27] |
CVL: Canine visceral leishmaniasis; NP: Nanoparticle; SSU: Small subunit.
Nanoparticle-based drug delivery system for VL
Introduction of nanotechnology in treatment of VL has a great potential to overcome most of the limitations associated with conventional therapy including development of multidrug resistance, toxicity concern of the drug and most importantly the cost factor. Improved biodegradability and nonimmunogenic properties of nanocarrier-conjugated drugs make it a suitable candidate for research and therapeutic application. Nanoparticulate carrier systems may allow preferential delivery of the drugs to the macrophage system, which is the reservoir of infection. It can enhance the therapeutic success with reduction of the dosage of drug, toxicity and cost of medication [33].
Amphotericin B (AmpB), an antifungal drug, has been extensively used for treatment of VL including the endemic regions of Indian subcontinent [14,34]. This leishmanicidal drug affects both stages of the parasite, that is, promastigote and amastigote, mainly by targeting parasite cell membrane ergosterol that enhances cell permeability and ions influx leading to compromised cellular integrity, which eventually results in death of the parasite [1]. Besides its antileishmanial activity, AmpB use has been restricted because of its high toxicity with aftermath such as nephrotoxicity and infusion reactions [14]. These adverse effects have been successfully underrated by its liposomal formulation, that is, AmBisome, in diseased endemic countries. The cost burden of AmBisome has also been a concern for the subjects in poverty. In order to circumvent adverse effects of AmpB and high cost of AmBisome besides enhancing the efficacy and oral delivery, several research groups have been in the process of developing NP-based AmpB payloads along with other classical drugs with specific properties and pharmacokinetics targeted toward parasite-infected macrophages.
In view of above limitations, many research groups have worked on several antileishmanial nonmacrophagic and macrophage-directed nanometric delivery systems of drugs, both at the in-vitro and in-vivo level for the treatment of leishmaniasis [35]. Our research group has also studied the efficacy of amine-functionalized carbon nanotubes and graphene NPs for the delivery of AmpB administered intraperitoneally and orally, which have proved to be more potent antileishmanial activity than conventional AmpB and AmBisome with no toxic side effects [36–39]. Several in vitro and in vivo studies have used AmpB attached with NPs (chitosan, thiolated chitosan, gelatin, poly lactic-co-glycolic acid) tagged with ligands (D-mannose, lactoferrin, 1,2-diacyl-sn-glycero-3-phospho-l-serine, p-aminophenyl-a-D-mannoside) for macrophages-specific receptor (mannose/scavenger)-mediated delivery, which have shown excellent leishmanicidal activity with minimal toxicity in comparison with the AmpB treated [40–43].
Highly pressurized AmpB having 10–20 nm particle size have shown significantly inhibiting parasite replication and parasite suppression results in comparison with AmpB [44]. Gelatin NPs were modified and then complexed with AmpB, have shown targeted delivery with enhanced uptake in macrophage-rich organs such as spleen and liver alongside the J774A.1 cell lines, which have shown significant antileishmanial activity when compared with the native form of AmpB in experimental VL [41]. AmpB loaded on poly (α-glutamic acid), which is biodegradable, nontoxic and biocompatible polymer have shown excellent leishmanicidal activity against intracellular L. donovani amastigotes same as the AmBisome with reduced toxicity in comparison with fungizone [45]. A nanoreservoir encapsulated with AmpB of lactoferrin appended poly (lactic-co-glycolic acid) (PLGA) has showed significant suppression of the parasite in comparison with conventional antileishmanial drugs used in experimental model of VL [43]. PLGA NPs loaded with Bis-naphthal-imido-propyl-dia-amino-octane (BNIPDaoct) by nanoprecipitation method showed selective and effective leishmanicidal activity against intracellular L. infantum of experimental VL [46]. AmpB-based drug delivery system using nanospheres has shown visceral organs accumulation and immune modulation, which are stable over 60 days at 30°C, and have reduced the parasite load [47]. Nanocochleates-loaded dual drug conjugate of Miltefosine and AmpB have showed good results for noninvasive drug release in the intestine [48]. PLGA nanospheres functionalized with mannan loaded with AmpB have shown substantial reduction in parasite burden both at in vivo and in vitro levels with increased proinflammatory Th1 response and nitric oxide [49]. Polyester NPs for delivery of AmpB delivered by oral route have shown significantly more activity in bone marrow-derived macrophages infected with L. donovani than the AmpB [50].
Moreover, several in vitro and in vivo studies have used rifampicin, doxorubicin, antimony, curcumin and andrographolide-tagged NPs for macrophages-specific receptor delivery, which have shown better antileishmanial activity in comparison with their respective NP nontagged drug-treated groups [51–56] and are summarized in Table 2.
Table 2. . Summary of the nanoparticles-based classical drugs and some other active compound targeted specifically and nonspecifically toward infected macrophages for the treatment of experimental leishmaniasis.
| Nanoparticle | Drug delivered | Ligand attached | Targeted receptor | Leishmania strain | In vitro & in vivo (adminstration) | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Chitosan | Rifampicin | D-Mannose | Mannose receptor | Albino rats ex vivo macrophages |
2014 | [53] | |
| Gelatin | Amphotericin B | 1,2-diacyl-sn-glycero- 3-phospho-l-serine | Scavenger receptors | L. donovani | J774.1 macrophage cell line hamster (intraperitoneally) | 2014 | [43] |
| CNT functionalized amine | Amphotericin B | – | – | L. donovani | J774.1 macrophage cell line hamster (oral & intraperitoneally) | 2011 | [38,40] |
| Thiolated chitosan | Amphotericin B | D-Mannose | Mannose receptor | L. donovani | J774.1 macrophage cell line BALB/c mice (intravenously) |
2017 | [42] |
| Gelatin | Amphotericin B | D-Mannose | Mannose receptor | L. donovani | J774.1 macrophage cell line | 2010 | [56] |
| PLGA | Amphotericin B | Lactoferrin | Mannose receptor | L. donovani | J774.1 macrophage cell line hamster (intraperitoneally) Swiss mice (intravenous) |
2015 | [45] |
| Liposome | Antimony | Phosphatidylserine | Scavenger receptors | L. chagasi | Hamsters (intraperitoneal) BALB/c peritoneal macrophages |
2004 | [54] |
| Nanocapsules | Doxorubicin | Phosphatidylserine | Scavenger receptors | L. donovani | J774.1 macrophage cell line hamsters (intraperitoneally) Wistar rats (intravenously) |
2012 | [55,57] |
| Graphene | Amphotericin B | – | – | L. donovani | J774.1 macrophage cell line hamster (oral & intraperitoneally) | 2014 2016 |
[39,41] |
| Liposome | Andrographolide | p-Aminophenyl-α-D-Mannopyranoside | Mannosyl receptors | L. donovani | Hamster BALB/c peritoneal macrophages |
2000 | [58] |
| Chitosan | Curcumin | D-Mannose | Mannose receptor | L. donovani | J774.1 macrophage cell line hamster (oral & intraperitoneally) | 2018 | [59] |
| Nanocapsules | Doxorubicin | Chondroitin sulfate | Mannose receptor | L. donovani | J774.1 macrophage cell line hamster (intravenous) | 2015 | [60] |
| – | Nano-amphotericin B | – | – | L. donovani | J774.1 macrophage cell line hamster (intraperitonially) | 2008 | [46] |
| Poly-l-glutamic acid | Amphotericin B | – | – | L. donovani | THP-1 BALB/c mice (intravenous) |
2013 | [47] |
| PLGA | Bisnaphthalimidop-ropyldiaaminoocta-ne | – | – | L. infantum | THP1 cell line BALB/c mice (intraperitoneal) |
2012 | [48] |
| PLGA | Amphotericin B | – | – | L. infantum | THP1 cell line BALB/c mice (intravenous) |
2014 | [49] |
| PLGA | Artemisinin | – | – | L. donovani | BALB/c mice (intraperitoneally) primary splenocytes | 2015 | [61] |
| Nanoliposomes | Artemisinin | – | – | L. donovani | BALB/c mice (intraperitoneally) BALB/c peritoneal macrophages |
2017 | [62] |
| Polyester | Amphotericin B | – | – | L. donovani | Bone marrow-derived macrophages BALB/c female mice (oral) | 2012 | [52] |
| Polymer | Resiquimod | – | – | L. donovani | RAW 264.7 macrophage cell line BALB/c mice (intravenous) |
2013 | [63] |
| Liposome | Resiquimod | – | – | L. donovani | RAW 264.7 macrophage cell line BALB/c mice (intravenous) |
2013 | [64] |
CNT: Carbon nanotube; PLGA: Poly(lactic-co-glycolic acid).
Artemisinin tagged to PLGA NPs and nanoliposomal artemisinin have advocated superior in vivo antileishmanial activity besides restoring the host immune response in BALB/c mice model of VL in comparison with artemisinin [58,59]. Doxorubicin loaded on nanocapsules showed significant leishmanicidal activity with mitigation in Th2 immune response (IL-10, IL-4 and TGF-β) and improved Th1 immune proinflammatory response (TNF-α, IL-12 and INF-γ) [61]. Doxorubicin-based stable emulsion showed improved antileishmanial efficacy in comparison with the drug in native form against intracellular amastigotes of L. donovani [62]. Chitosan microparticles-loaded doxorubicin directed against intramacrophagic amastigote-directed delivery has shown pH resistance of lysosomal macrophage and activation of macrophages [60]. Chitosan particles conjugated with mannose and rifampicin have shown significant effect for VL treatment [51]. Mannosylated chitosan NPs-loaded curcumin has showed significant uptake by macrophages by endocytosis [56]. Intravenous admiration of resiquimod in polymer microparticles formed by electron spray in mice has shown significant reduction in L. donovani load [57]. Liposomal resiquimod by extrusion method has showed significant clearance in experimental VL without any hepatic and renal toxicity [65]. Furthermore, NPs-based combinational drug delivery, receptor-mediated targeted drug delivery and bi-functionalized nanocarrier system may overcome the difficulties faced by the conventional drugs by precisely targeting macrophage phagosomal Leishmania parasite.
Lessons learnt from preclinical in vivo studies: common pitfalls in nanotechnology
Limitations of nanodiagnostics
Although VL nanodiagnostic platforms seem to be dependable for both developed and developing nation for diagnosis applications, their limitations include detecting accuracy and sensitivity beside the false-negative results observed in a clinical setting [63] make dark clouds loom over nanodiagnostics for infectious diseases including VL. Hence, more and more novel approaches could help in overcoming these challenges in the clinical samples that could develop an ideal strategy for the unmet demands for VL diagnosis.
Limitations of nano drug carrier systems
Most of the in vivo studies of the NPs delivery system have been mainly focused through intraperitoneal and intravenous administration for Leishmania treatment. Much of the focus now has to be shifted for the oral delivery of NPs with utmost efficacy in order to overcome the limitation of parenteral administration allied infusion reactions of AmpB. This may help us to step ahead for the new noninvasive therapeutics. Previously cited studies have extensively gone through the leishmanicidal activity but a very limited pharmacokinetics, nanotoxicity and NP biodistribution at both in vivo and in vitro has been explored. None of these studies have shown the biodegradability and post exposure biopersistence of these NPs in liver, spleen, kidney and other tissues, and their associated changes using histopathology, inflammation and alterations in overall gene expression. The fate of nanoparticulate system post treatment still remains unclear. Alongside these extensive nanotoxicity studies to be done, much of the focus has to shift toward these inorganic NPs (metals) and their role as epimutagenes that can affect gene regulations [64]. A much extensive toxicity and post exposure biopersistence of these NPs studies using techniques such as hyper spectral mapping, confocal laser scanning microscopy, scanning electron microscope, transmission electron microscopy, methylation sequencing, etc., must be done to know the repercussions associated with nanotoxicity at both tissue and cellular level. Although, several cancer studies associated with NPs have done these extensive observations for nanotoxicity, biodistribution and post exposure biopersistence [66], which could be translated in case of NPs-based drug delivery for Leishmania infection for future translational medicine in case of VL.
Additionally, some of the most common pitfalls observed during nanoformulations include compromised sterility and endotoxin, without proper and adequate physicochemical characterization, residual manufacturing components, nonactive pharmaceutical ingredients, compromised batch-to-batch validations, in vivo NP instability and improper drug release rate [67] making them inefficient or toxic for oral/intravenous/intraperitoneal administration. So the future objective of biomedical research has to be focused on NPs-based drugs candidates, which are safe, efficient and affordable with a shorter duration of antileishmanial therapy.
Technical issues & possible way forward: from science to policy
Challenges ahead for nanotechnology for diagnosis, treatment, prophylaxis and their economic sustainability in case of leishmaniasis for the betterment of nanomedicine in translational to human drug trials, have been discussed in detail in the latter sections.
Nanodiagnostic development
For the detection of Leishmania, improvement in current methods and the discovery of suitable biomarkers are very much crucial for the early identification and better therapy. For this, several key features such as sensitivity, specificity, speed, accuracy, accessibility, ease of usage and field applicability beside their cost–effectiveness, versatility and discriminatory capacity should be considered for detection and quantification of 21 Leishmania species that are pathogenic to humans [2]. Although, the progress of nanodiagnostics in case of VL remains at a snail pace with prime focus on DNA/RNA-based detections using inorganic NPs using gold, cobalt-zinc ferrite and cadmium selenide quantum dots have sparked a ray of hope for betterment in near feature for the detection of parasite [27–29,31] and much of the focus has to be shifted toward proteomic and glycomic approaches. In near future, NPs having the ability for simultaneous detection of leishmaniasis and its drug resistance would be a great addition for the present line of diagnosis. Although progress has been done in case of SAG resistance [29], much of the progress has to be shifted in case of other drug resistant VL. These advancements in the field of nanomedicine may decrease the cost burden for kala-azar diagnosis making it easy, fast, sensitive and a cheaper way for visual diagnosis of the parasite in the affected subjects.
Nanomedicine for oxidative burst
As reported by several studies, Leishmania parasite is highly sensitive to the reactive oxygen and reactive nitrogen species, which has to be balanced by the parasite trypanothione/trypanothione reductase system with linked spermidine (polyamine), as it lacks alternate antioxidative machinery [1]. Subsequently, targeting this system may help in enhancing the efficacy of antimony drug resulting in clearance of the parasite through oxidative/nitrosative burst.
In order to expand the potential use of nitric oxide (NO) donor materials and to increase the NO-loading capacity, Fan J et al. developed a self-assembled sandwich nanomedicine to manipulate NO release on demand by near-infrared light [68]. Such strategies could be a very good option in near future for treatment of VL in the clinical settings as Leishmania parasite is quite sensitive to nitric oxide. Importantly, toxicity of carbon-based NPs remains a concern for its translation in clinical settings, hence a strategy using biocompatible NPs attached to some novel NO donors can serve the purpose for the proper parasite killing.
Recently, mannose-tagged meglumine antimoniate-loaded thiolated polymer-based NPs have shown leishmanicidal activity through the inhibition of trypanothione reductase system and P-glycoproteins (P-gp) efflux pump (ABC transporter) [69]. In near future, this type of nanoformulation may help in enhanced efficacy of antimonial drug-resistant leishmaniasis. This strategy could also be translated to other antileishmanial drugs for their better activity against VL. Although resistance in leishmaniasis is multifactorial contributed by several factors, hence the drug delivery (with one or more than one drug) should be aimed at obstructing at multiple levels rather than focusing on single check point for VL treatment without any reoccurrence.
Nanomedicine for molecular targets & metabolic pathways
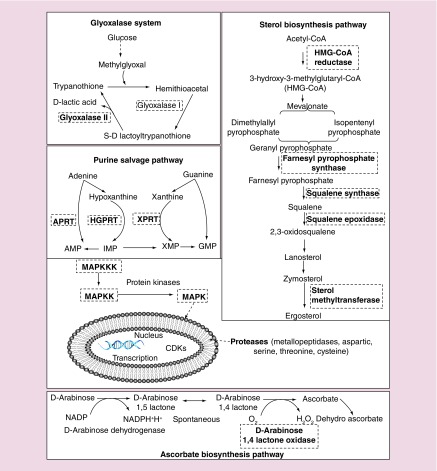
Leishmania adapts itself to the macrophage environment by adaptive differentiation and tweaks some important macrophage functions by modulating host metabolic pathways for their further survival and growth through some ecto-proteins, which eventually affects the host immunity [70]. Although, most of the research has been focused on unearthing some novel antileishmanial drugs commodities for the elimination of leishmaniasis, not much success has been achieved due their failures at several levels of drug testing. Several past studies have corroborated that the few metabolic pathways of Leishmania parasite and mammalian host are structurally and functionally different. Hence, a systematic and focused approach targeting them by biological or chemical means would be effectively to curb parasite growth. Some of the important and possible drug targets that may affect Leishmania include sterol biosynthesis pathway, purine salvage pathway, ascorbic acid pathway, polyamine and trypanothione metabolism, glyoxalase system, protein kinases and proteases (Figure 4) [1,71]. Baiocco et al. have focused in this direction by using silver NPs, which has inhibitory effect on trypanothione reductase activity reducing the L. infantum proliferation [72]. Additionally, more efforts have to be done by targeting other metabolic pathways in order to yield some fruitful results for the treatment of leishmaniasis. Studies targeting these metabolic pathways along with drug resistance using mannose receptor-targeted NPs-tagged drugs would be an alternative approach for elimination of the parasite. While the search for the true antileishmanial drug has been a prime focus for many years, some fault lines lie in approaching a single molecular target rather than multiple check points for the Leishmania parasite killing.
Figure 4. . An overview of several possible drug targets (bold inside the dotted box) in Leishmania species, which can be targeted through nanoformulations.
Glyoxalase system, sterol biosynthesis pathway, ascorbate biosynthesis pathway, purine salvage pathway, protein kinases and proteases are some of the important and possible drug targets that may affect the parasite.
APRT: Adenine phosphoribosyltransferase; CDK: Cyclin-dependent kinase; HGPRT: Hypoxanthineguanine phosphoribosyltransferase; MAPKK: MAPK kinase, MAPKKK: MAPK kinase kinase; XPRT: Xanthine phosphoribosyltransferase.
Probable histone post-translational modifications assisted by histone-modifying enzymes (histone acetyltransferases, histone deacetylases and histone methyltransferases) may also add up a layer alongside the multifactorial events during VL pathogenesis [70]. Although not much progress has been made in this direction for the study on pathogenesis front in host genome by pathogen Histone modifying enzymes (HMEs) during leishmaniasis. A recent study on histone acetyltransferase-2-mediated histone (H4K4) acetylation of chromatin in L. donovani may help us in better understanding of epigenetic determinants in the parasite that would finally help in designing some novel therapeutic strategies using nonmetal and biocompatible NPs for VL elimination [73].
Nanomedicine for macrophage receptor & ABC transporters
Based on the different characteristics (size, shape, surface charge, solubility, extent of agglomeration and chemical properties) of NPs, which determine their individual effects on biological systems and human health, may add up the options for possible alternative drug delivery system. As in the near future, there seems no additions to the present therapeutic commodities to be certified for use against leishmaniasis. Accordingly, several efforts are being made for modulating effective transportation of classical antileishmanial drugs and their respective delivery ways to the body. In view of these limitations, nanomedicine with different characteristics has emerged as safe, affordable, efficacious endocytic delivery of age-old antileishmanial drugs that is deprived of resistance. Previous studies have showed that enhanced expression of ABC transporters (MRPA, MDR1, PRP1) are responsible for the efflux of drugs from the macrophage infected with parasite, which eventually helps in evading the adverse action of the classical Leismhmania drugs [1]. However, using thiolated surface modified NPs with AmpB have shown 9.6-fold internalization due to inhibition of AmpB efflux in L. donovoni-resistant strain [40]. Further, macrophage receptor (mannose/scavenger)-based AmpB drug delivery through thiolated NPs carrier may act as an excellent strategy to inhibit Leishmania replication due to effective concentrations, which may finally help in eradication of parasite.
Immunomodulatory nanomedicine
The Leishmania parasite has developed several strategies, which can inhibit Th1 response by diverting the dendritic cells to a state that finally helps the parasite-infected macrophages toward anti-inflammatory Th2 response. These diversions by the phagocytic cells may lead to the alleviated production of reactive oxygen and reactive nitrogen species besides several proinflammatory cytokines such as TNF-α, and IFN-γ that limits the Th1-effector cells leading to pathogenesis in leishmaniasis [1]. Additionally, these early controls in Th1 response may help in the early control of innate immunity that eventually leads to compromised adaptive immunity characterized by reduced proliferation of CD4+ and CD8+ T cells that eventually leads to enhanced Th2 response through anti-inflammatory cytokines, such as TGF-β, IL-4 and IL-10. In the recent times, several studies have shown that priming of co-inhibitory ligands, such as CTLA-4, PD-L1, CD200 and Tim-3 has led to the compromised Th1 response and making the shift toward Th2 anti-inflammatory response [1]. Hence, any molecules that shift the balance back to Th1 response would be great area of interest for the parasite elimination strategies. To serve this purpose, immunomodulators tagged with NPs fit the bill, although not much progress has been achieved in this direction with only preclinical studies using PLGA encapsulating CpG-rich oligonucleotide [74] and cysteine peptidase A, B [75], nanocurcumin, miltefosine in combination with nanocurcumin [76] have shown some encouraging results. Hence, macrophage-specific NPs tagged to some natural products (with antileishmanial activity) may act as a potential combination for treatment of leishmaniasis by modulating its immune response shifting toward Th1 proinflammatory response. Although we can sense some encouraging results with NPs using some immunomodulators, exclusive studies should be done extensively in order to establish the exact effect of these NPs on the immune system as the human immune system is quite complex.
Additionally, Leishmania has evolved strategies for their survival by altering the transcriptional profile and protein content of the host-infected macrophages [70]. Marr et al. study on Leishmania has highlighted how the pathogen targets host chromatin for their growth and survival by alleviating the immune response [77]. Targeting these exosomal proteins probably control the host macrophage epigenome by altering the phenotype that favors Leishmania through prolonged immune suppression and necessary metabolic changes for their extensive growth and survival inside the host. Hence targeting Leishmania-dependent epigenetic host cell reprogramming through NPs would be an excellent addition in enhancing the host immunity.
Nanotoxicity: biodegradability & biopersistence
Nanomedicine and nanotoxicity are the two sides of the same coin, where intentional enhancement with theranostic nanomedicine (therapeutic and diagnostic) or unintentional nanotoxicity, due to their similar mechanism, is targeting identical metabolic pathways [78]. The NPs’ certain shapes, materials and surface treatments also make a huge difference adding the unintentional nanotoxicity. As described in earlier parts, NPs-based leishmanial research has covered the drug delivery system with a very little or no focus on pharmacokinetics and nanotoxicity in VL. Studies based on NPs to assess the pharmacokinetics, pharmacodynamics, immunogenicity and potential long-term nanotoxicity in vivo are essential to monitor their effects on patient populations [79]. The NPs accumulation, storage and slow clearance in tissues can generate free radicals besides phagocytes in the mononuclear phagocyte system can cause oxidative stress condition mainly in liver and spleen [78]. Some metallic nanocarriers and quantum dots (QDs), which are nonbiodegradable may further aggravate the oxidative/nitrosative stress. Hence more research has to be focused to change these NPs through additional biodegradable shapes, materials and surface treatments. Additionally, oxidative degradation of carbon nanotubes and several other types of carbonaceous NPs by oxidative enzymes (myeloperoxidase, eosinophil peroxidase, lacto-peroxidase, hemoglobin and xanthine oxidase) of inflammatory cells should be done to minimize asbestos like condition in the lung tissue [80]. Though, most of these studies on biodegradability and biopersistence of NPs are based on cancer, which should be translated in case of preclinical in vivo studies on leishmaniasis. Beside the oxidative degradation of NPs, biodegradable NPs have been a focus at the present research in leishmaniasis, which should be translated to the preclinical drug development.
Although much has to be addressed for the elimination of VL, nanomedicine and its recent advancement for diagnosis, treatment and prophylaxis must be taken into account to fill the gaps for better elimination strategies of Leishmania parasite and its vector.
Conclusion: the coming decade
There is an urgent need for highly sensitive diagnostics and more efficacious and less toxic therapies for patients suffering from chronic infectious diseases, including VL. Despite many challenges, substantial progress has been made in the field of nanotheranostics. Extensive translational researches are needed in the coming years for developing effective diagnostics and therapeutic means for VL control program. VL affects almost exclusively and marginalized populations, and reducing the burden of disease will have a direct impact on poverty as it will reduce time away from work caring for the sick, paying for medical care and enabling all to continue to go to work and school. This will diminish the cycle of poverty in the community and promote economic growth. The message is clear – this is a war that must be won in order to improve the lives of millions affected by this disease.
Future perspective
With no new drug candidates and diagnostics under the human trial for leishmaniasis beside the side effect of conventional drugs, more focus has to shift toward NPs-based theranostics, which would help in early detection and clearance of the parasite with lower dose of the purified drug with reduced cost and toxicity. The nanomedicine and their applicability in VL has reached many considerable milestones both in diagnostics and drug delivery, but lot has to be done making it 100% applicable in the clinical settings. Although the age of nanotechnology has been making new innovation for the betterment of human mankind, a lot has been focused on their associated aftermaths in nanomedicine such as nanotoxicity, biodistribution and post exposure biopersistence beside their economic sustainability to the weaker sections of the society.
Executive summary.
Background
Visceral leishmaniasis (VL) remains the global public health concern, which primarily affects impoverished people of the developing world.
Current challenges in diagnostic & treatment options in leishmaniasis
The current available diagnostics demonstrate several limitations ranging from sensitivity, specificity, inability in discrimination between treated and nontreated beside their poor correlation between conventional and molecular methods in asymptomatic and post kala-azar dermal leishmaniasis individuals making them inappropiate in clinical settings.
Additionally, drug regimes available are not true antileishmanial (excluding antimonial compounds) beside their long list of limitations such as being expensive, resistant and their mode of admistration and repercussions like nephrotoxicity, infusion reactions, myocarditis, and even death.
How nanomedicine can improve the diagnostics & treatment & aid VL elimination program
The results of nano research is keeping the hope for better diagnostic tools and drugs regimes in cutting their limitations, with conventional being far from satisfactory. Nanoparticles (NPs)-based combinational drug delivery, receptor-mediated targeted drug delivery and bi-functionalized nanocarrier system may overcome the difficulties faced by the conventional drugs but a very limited pharmacokinetics, nanotoxicity and their biodistribution in vitro and in vivo in VL.
Lessons learned from preclinical in vivo studies: common pitfalls in nanotechnology
VL nanodiagnostic platforms seem to be dependable with limitations such as detection accuracy and sensitivity beside the false-negative results in a clinical setting.
Although, many groups have made long strides toward NPs-based drug delivery, a very primal nanotoxicity studies beside no studies on biodistribution and post exposure biopersistence have made these studies limited for future translational medicine in case of VL.
Technical issues & possible way forward: from science to policy
Though a lot has been explored with NPs in VL, future prospects and challenges for theranostic nanomedicine include nanodiagnosis development (with high accuracy and sensitivity), NPs for oxidative and nitrosative burst, NPs toward molecular targets and metabolic pathways, beside immunomodulatory nanomedicine and their economic sustainability in case of VL for better elimination strategies of parasite in order to translate down to the human trials.
Footnotes
Financial & competing interests disclosure
This work was supported by Department of Science & Technology (SR/NM/NS-57/2016), New Delhi (under nano-mission), and in part by the Extramural Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) as part of Tropical Medicine Research Centre award (Grant number U19AI074321). SLM is supported by the Department of Science and Technology (DST), Govt. of India (ECR/2016/00097).The funders had no role in design, decision to publish or preparation of the report. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Tiwari N, Gedda MR, Tiwari VK, Singh SP, Singh RK. Limitations of current therapeutic options, possible drug targets and scope of natural products in control of leishmaniasis. Mini Rev. Med. Chem. 18(1), 26–41 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Sundar S, Singh OP. Molecular diagnosis of visceral leishmaniasis. Mol. Diagn. Ther. 22(4), 443–457 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Expert opinion on visceral leishmaniasis (VL) diagnostics.
- 3.Alvar J, Velez ID, Bern C. et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7(5), e35671 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Report on global incidence of leishmaniasis.
- 4.Sundar S, Chakravarty J. Recent advances in the diagnosis and treatment of kala-azar. Natl Med. J. India 25(2), 85 (2012). [PubMed] [Google Scholar]
- 5.WHO. WHO Expert Committee on specifications for pharmaceutical preparations. : World Health Organ Technical Report Series. Geneva, Switzerland: 1–387 (2014). [PubMed] [Google Scholar]
- 6.Karunaweera ND, Rajapaksa US. Is leishmaniasis in Sri Lanka benign and be ignored? J. Vector Borne Dis. 46(1), 13–17 (2009). [PubMed] [Google Scholar]
- 7.Yangzom T, Cruz I, Bern C. et al. Endemic transmission of visceral leishmaniasis in Bhutan. Am. J. Trop. Med. Hyg. 87(6), 1028–1037 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zenaishvili O, Gugushvili G, Chubabria G, Manjgaladze M, Kokaia N. New data on epidemiology of visceral leishmaniasis in Georgia. Georgian Med. News 172-173, 76–80 (2009). [PubMed] [Google Scholar]
- 9.Ponirovskii EN, Kondrashin AV, Erokhin PI, Annacharyeva D. Milestones and major results of studies on leishmaniasis and sand fly fevers in Turkmenistan. Med. Parazitol. (Mosk) (4), 29–34 (2011). [PubMed] [Google Scholar]
- 10.Albuquerque PL, Silva Junior GB, Freire CC. et al. Urbanization of visceral leishmaniasis (kala-azar) in Fortaleza, Ceara, Brazil. Rev. Panam. Salud. Publica. 26(4), 330–333 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Uniting To Combat Neglected Tropical Diseases. London declaration on neglected tropical diseases. (2012). http://unitingtocombatntds.org/london-declaration-neglected-tropical-diseases/
- 12.WHO. Control of the leishmaniases. World Health Organisation Techchnical Report Series (2011). http://apps.who.int/iris/handle/10665/44412 [Google Scholar]
- 13.Bhattacharyya T, Bowes DE, El-Safi S. et al. Significantly lower anti-leishmania IgG responses in Sudanese versus Indian visceral leishmaniasis. PLoS Negl. Trop. Dis. 8(2), e2675 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh OP, Singh B, Chakravarty J, Sundar S. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infect. Dis. Poverty 5, 19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundar S, Singh A, Rai M. et al. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin. Infect. Dis. 55(4), 543–550 (2012). [DOI] [PubMed] [Google Scholar]; • First report of therapeutic efficacy of Miltefosine in endemic areas after a decade of use.
- 16.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N. Engl. J. Med. 362(6), 504–512 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Sundar S, Sinha PK, Rai M. et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet 377(9764), 477–486 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Singh OP, Hasker E, Boelaert M, Sundar S. Elimination of visceral leishmaniasis on the Indian subcontinent. Lancet Infect. Dis. 12, e304–e309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Expert opinion on VL elimination strategies.
- 19.Ficai D, Grumezescu AM. Nanostructures for Novel Therapy: Synthesis, Characterization and Applications Elsevier Science, Amsterdam, The Netherlands: (2017). [Google Scholar]
- 20.Bruni N, Stella B, Giraudo L, Della Pepa C, Gastaldi D, Dosio F. Nanostructured delivery systems with improved leishmanicidal activity: a critical review. Int. J. Nanomedicine 12, 5289 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutiérrez V, Seabra AB, Reguera RM, Khandare J, Calderón M. New approaches from nanomedicine for treating leishmaniasis. Chem. Soc. Rev. 45(1), 152–168 (2016). [DOI] [PubMed] [Google Scholar]; •• Expert opinion on nanomedicine for treating leishmaniasis.
- 22.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382(6592), 607–609 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Torres-Sangiao E, Holban AM, Gestal MC. Advanced nanobiomaterials: vaccines, diagnosis and treatment of infectious diseases. Molecules 21(7), 867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anfossi L, Di Nardo F, Profiti M. et al. A versatile and sensitive lateral flow immunoassay for the rapid diagnosis of visceral leishmaniasis. Anal. Bioanal. Chem. 410(17), 4123–4134 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Souto DE, Fonseca AM, Barragan JT. et al. SPR analysis of the interaction between a recombinant protein of unknown function in Leishmania infantum immobilised on dendrimers and antibodies of the visceral leishmaniasis: a potential use in immunodiagnosis. Biosens. Bioelectron. 70, 275–281 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Perinoto AC, Maki RM, Colhone MC. et al. Biosensors for efficient diagnosis of leishmaniasis: innovations in bioanalytics for a neglected disease. Anal. Chem. 82(23), 9763–9768 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Andreadou M, Liandris E, Gazouli M. et al. Detection of Leishmania-specific DNA and surface antigens using a combination of functionalized magnetic beads and cadmium selenite quantum dots. J. Microbiol. Methods 123, 62–67 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Heli H, Sattarahmady N, Hatam G, Reisi F, Vais RD. An electrochemical genosensor for Leishmania major detection based on dual effect of immobilization and electrocatalysis of cobalt-zinc ferrite quantum dots. Talanta 156, 172–179 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Bose PP, Kumar P, Munagala N. Concurrent visual diagnosis and susceptibility profiling of the first line drug against visceral leishmaniasis by plasmonic detection of PCR amplified genetic biomarker. Acta Trop. 152, 208–214 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Andreadou M, Liandris E, Gazouli M. et al. A novel non-amplification assay for the detection of Leishmania spp. in clinical samples using gold nanoparticles. J. Microbiol. Methods 96, 56–61 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Toubanaki DK, Athanasiou E, Karagouni E. Gold nanoparticle-based lateral flow biosensor for rapid visual detection of Leishmania-specific DNA amplification products. J. Microbiol. Methods 127, 51–58 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Bose PP, Kumar P. Visual assessment of parasitic burden in infected macrophage by plasmonic detection of Leishmania specific marker RNA. Biochem. Biophys. Res. Commun. 480(1), 81–86 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Emeje MO, Obidike IC, Akpabio EI, Ofoefule SI. Nanotechnology in drug delivery. : Recent Advances in Novel Drug Carrier Systems. Intech, Rijeka: 69–106 (2012). [Google Scholar]
- 34.Sundar S, Singh A. Recent developments and future prospects in the treatment of visceral leishmaniasis. Ther. Adv. Infect. Dis. 3(3-4), 98–109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akbari M, Oryan A, Hatam G. Application of nanotechnology in treatment of leishmaniasis: a review. Acta Trop. 172, 86–90 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Prajapati VK, Awasthi K, Gautam S. et al. Targeted killing of Leishmania donovani in vivo and in vitro with amphotericin B attached to functionalized carbon nanotubes. J. Antimicrob. Chemother. 66(4), 874–879 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mudavath SL, Talat M, Rai M, Srivastava ON, Sundar S. Characterization and evaluation of amine-modified graphene amphotericin B for the treatment of visceral leishmaniasis: in vivo and in vitro studies. Drug Des. Devel. Ther. 8, 1235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prajapati VK, Awasthi K, Yadav TP, Rai M, Srivastava ON, Sundar S. An oral formulation of amphotericin B attached to functionalized carbon nanotubes is an effective treatment for experimental visceral leishmaniasis. J. Infect. Dis. 205(2), 333–336 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Report on efficacy of oral nanomedicine in experimental VL therapy.
- 39.Mudavath SL, Talat M, Rai M, Srivastava ON, Sundar S. An oral formulation of amphotericin B for the treatment of visceral leishmaniasis: f-Gr-AmB. Int. J. Infect. Dis. 45, 367 (2016). [Google Scholar]
- 40.Shahnaz G, Edagwa BJ, McMillan J. et al. Development of mannose-anchored thiolated amphotericin B nanocarriers for treatment of visceral leishmaniasis. Nanomedicine 12(2), 99–115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khatik R, Dwivedi P, Khare P. et al. Development of targeted 1, 2-diacyl-sn-glycero-3-phospho-l-serine-coated gelatin nanoparticles loaded with amphotericin B for improved in vitro and in vivo effect in leishmaniasis. Expert Opin. Drug Deliv. 11(5), 633–646 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Asthana S, Gupta PK, Jaiswal AK, Dube A, Chourasia MK. Overexpressed macrophage mannose receptor targeted nanocapsules-mediated cargo delivery approach for eradication of resident parasite: in vitro and in vivo studies. Pharm. Res. 32(8), 2663–2677 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Asthana S, Gupta PK, Jaiswal AK, Dube A, Chourasia MK. Targeted chemotherapy of visceral leishmaniasis by lactoferrin-appended amphotericin B-loaded nanoreservoir: in vitro and in vivo studies. Nanomedicine 10(7), 1093–1109 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Manandhar KD, Yadav TP, Prajapati VK. et al. Antileishmanial activity of nano-amphotericin B deoxycholate. J. Antimicrob. Chemother. 62(2), 376–380 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Mohamed-Ahmed AH, Seifert K, Yardley V, Burrell-Saward H, Brocchini S, Croft SL. Antileishmanial activity, uptake, and biodistribution of an amphotericin B and poly (α-glutamic acid) complex. Antimicrob. Agents Chemother. 57(10), 4608–4614 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costa Lima SA, Resende M, Silvestre R. et al. Characterization and evaluation of BNIPDaoct-loaded PLGA nanoparticles for visceral leishmaniasis: in vitro and in vivo studies. Nanomedicine 7(12), 1839–1849 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Lima SAC, Silvestre R, Barros D. et al. Crucial CD8+ T-lymphocyte cytotoxic role in amphotericin B nanospheres efficacy against experimental visceral leishmaniasis. Nanomed. Nanotechnol. 10(5), e1021–e1030 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Pham T, Barratt G, Michel J, Loiseau P, Saint-Pierre-Chazalet M. Interactions of antileishmanial drugs with monolayers of lipids used in the development of amphotericin B–miltefosine-loaded nanocochleates. Colloids Surf. B Biointerfaces 106, 224–233 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Barros D, Costa Lima SA, Cordeiro-da-Silva A. Surface functionalization of polymeric nanospheres modulates macrophage activation: relevance in leishmaniasis therapy. Nanomedicine 10(3), 387–403 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Italia JL, Ravi Kumar M, Carter K. Evaluating the potential of polyester nanoparticles for per oral delivery of amphotericin B in treating visceral leishmaniasis. J. Biomed. Nanotechnol. 8(4), 695–702 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Chaubey P, Mishra B. Mannose-conjugated chitosan nanoparticles loaded with rifampicin for the treatment of visceral leishmaniasis. Carbohydr. Polym. 101, 1101–1108 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Tempone AG, Perez D, Rath S, Vilarinho AL, Mortara RA, de Andrade HF., Jr Targeting Leishmania (L.) chagasi amastigotes through macrophage scavenger receptors: the use of drugs entrapped in liposomes containing phosphatidylserine. J. Antimicrob. Chemother. 54(1), 60–68 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Kansal S, Tandon R, Dwivedi P. et al. Development of nanocapsules bearing doxorubicin for macrophage targeting through the phosphatidylserine ligand: a system for intervention in visceral leishmaniasis. J. Antimicrob. Chemother. 67(11), 2650–2660 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Nahar M, Dubey V, Mishra D, Mishra PK, Dube A, Jain NK. In vitro evaluation of surface functionalized gelatin nanoparticles for macrophage targeting in the therapy of visceral leishmaniasis. J. Drug Target. 18(2), 93–105 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Sinha J, Mukhopadhyay S, Das N, Basu MK. Targeting of liposomal andrographolide to L. donovani-infected macrophages in vivo. Drug Deliv. 7(4), 209–213 (2000). [DOI] [PubMed] [Google Scholar]
- 56.Chaubey P, Mishra B, Mudavath SL. et al. Mannose-conjugated curcumin-chitosan nanoparticles: efficacy and toxicity assessments against Leishmania donovani. Int. J. Biol. Macromol. 111, 109–120 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Duong AD, Sharma S, Peine KJ. et al. Electrospray encapsulation of Toll-like receptor agonist resiquimod in polymer microparticles for the treatment of visceral leishmaniasis. Mol. Pharm. 10(3), 1045–1055 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Want MY, Islamuddin M, Chouhan G. et al. Therapeutic efficacy of artemisinin-loaded nanoparticles in experimental visceral leishmaniasis. Colloids Surf. B Biointerfaces. 130, 215–221 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Want MY, Islammudin M, Chouhan G. et al. Nanoliposomal artemisinin for the treatment of murine visceral leishmaniasis. Int. J. Nanomedicine 12, 2189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kunjachan S, Gupta S, Dwivedi AK, Dube A, Chourasia MK. Chitosan-based macrophage-mediated drug targeting for the treatment of experimental visceral leishmaniasis. J. Microencapsul. 28(4), 301–310 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Chaurasia M, Pawar VK, Jaiswal AK, Dube A, Chourasia MK. Chondroitin nanocapsules enhanced doxorubicin induced apoptosis against leishmaniasis via Th1 immune response. Int. J. Biol. Macromol. 79, 27–36 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Kansal S, Tandon R, Verma PRP, Dube A, Mishra PR. Development of doxorubicin loaded novel core shell structured nanocapsules for the intervention of visceral leishmaniasis. J. Microencapsul. 30(5), 441–450 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Yu L, Kong X, Sun L. Application of nanodiagnostics in point-of-care tests for infectious diseases. Int. J. Nanomedicine 12, 4789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong BSE, Hu Q, Baeg GH. Epigenetic modulations in nanoparticle-mediated toxicity. Food Chem. Toxicol. 109, 746–752 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Peine KJ, Gupta G, Brackman DJ. et al. Liposomal resiquimod for the treatment of Leishmania donovani infection. J. Antimicrob. Chemother. 69(1), 168–175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hobson DW, Roberts SM, Shvedova AA, Warheit DB, Hinkley GK, Guy RC. Applied nanotoxicology. Int. J. Toxicol. 35(1), 5–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crist RM, Grossman JH, Patri AK. et al. Common pitfalls in nanotechnology: lessons learned from NCI’s Nanotechnology Characterization Laboratory. Integr. Biol. 5(1), 66–73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan J, He N, He Q. et al. A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO. Nanoscale 7(47), 20055–20062 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarwar HS, Ashraf S, Akhtar S. et al. Mannosylated thiolated polyethylenimine nanoparticles for the enhanced efficacy of antimonial drug against leishmaniasis. Nanomedicine 13(1), 25–41 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Lamotte S, Späth GF, Rachidi N, Prina E. The enemy within: targeting host–parasite interaction for antileishmanial drug discovery. PLoS Negl. Trop. Dis. 11(6), e0005480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Strategies targeting leishmaniasis.
- 71.Jain V, Jain K. Molecular targets and pathways for the treatment of visceral leishmaniasis. Drug Discov. Today 1, 161–170 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Baiocco P, Ilari A, Ceci P. et al. Inhibitory effect of silver nanoparticles on trypanothione reductase activity and Leishmania infantum proliferation. ACS Med. Chem. Lett. 2(3), 230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jha PK, Khan MI, Mishra A, Das P, Sinha KK. HAT2 mediates histone H4K4 acetylation and affects micrococcal nuclease sensitivity of chromatin in Leishmania donovani. PLoS One 12(5), e0177372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siefert AL, Ehrlich A, Corral MJ, Goldsmith-Pestana K, McMahon-Pratt D, Fahmy TM. Immunomodulatory nanoparticles ameliorate disease in the Leishmania (Viannia) panamensis mouse model. Biomaterials 108, 168–176 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noormehr H, Hosseini AZ, Soudi S, Beyzay F. Enhancement of Th1 immune response against Leishmania cysteine peptidase A, B by PLGA nanoparticle. Int. J. Immunopharmacol. 59, 97–105 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Tiwari B, Pahuja R, Kumar P, Rath SK, Gupta KC, Goyal N. Nanotized curcumin and miltefosine, a potential combination for treatment of experimental visceral leishmaniasis. Antimicrob. Agents Chemother. 61(3), e01169–e011216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marr AK, MacIsaac JL, Jiang R, Airo AM, Kobor MS, McMaster WR. Leishmania donovani infection causes distinct epigenetic DNA methylation changes in host macrophages. PLoS Pathog. 10(10), e1004419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shvedova A, Pietroiusti A, Kagan V. Nanotoxicology ten years later: lights and shadows. Toxicol. Appl. Pharmacol. 299, 1–2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ventola CL. The nanomedicine revolution: part 1: emerging concepts. Pharm. Ther. 37(9), 512 (2012). [PMC free article] [PubMed] [Google Scholar]
- 80.Vlasova II, Kapralov AA, Michael ZP. et al. Enzymatic oxidative biodegradation of nanoparticles: mechanisms, significance and applications. Toxicol. Appl. Pharmacol. 299, 58–69 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]