Abstract
Our primary objective was to describe demographic characteristics and enrollment patterns in a unique eleven-year full sample of adult Wisconsin Medicaid beneficiaries with identified ASD or ID. We obtained de-identified Medicaid claims data for adults with a recorded ASD or intellectual disability (ID) diagnosis aged 21 and older with any Medicaid fee-for-service claims between January 1, 2008 and December 31, 2018. We assessed enrollment, age, number of visits and paid amount per year using generalized linear models with a random intercept for each beneficiary. We identified claims for 4,775 autistic adults without ID, 2,738 autistic adults with ID, 14,945 adults with ID, and 3,484 adults with Down syndrome. The age distribution of the diagnostic group with ASD diagnoses was right skewed with a majority of beneficiaries less than age 30. The ASD without ID diagnostic group had the least visits and paid amount per person per year compared to other groups. In each age category, the ASD with ID diagnostic group had the most paid amount per person per year compared to other groups. It is urgent that we identify the health and health service needs of autistic adults from young adulthood through old age. Our findings have implications for ensuring adequate health coverage across the lifespan and highlight the importance of a strong and accessible health care system for autistic people.
Keywords: Autism spectrum disorder, Medicaid, longitudinal, enrollment, intellectual disability, health service
Lay Abstract
Medicaid provides health insurance to disabled people who meet income requirements. We assessed patterns of enrollment and service use among autistic adults and adults with developmental disabilities in Wisconsin Medicaid. We found a consistent influx of new young autistic adults without intellectual disability into the Medicaid system, with fewer visits and lower paid amounts compared to other developmental disability groups. The changing population of autistic people using Medicaid has implications for providing health care to autistic adults in the future.
Introduction
Over the past two decades the identified prevalence of autism spectrum disorder (ASD) in children has increased by more than 200% (Baio et al., 2018). The ‘autism boom,’ or drastic increase in identified prevalence of ASD, may partially be attributable to diagnostic change and increased awareness rather than a true ‘autism epidemic’ (Coo et al., 2007; Roetzer, Schwarzbraun, Obenauf, Hauser, & Speicher, 2010). However, the fact remains that more and more children are in need of, and are accessing, autism-related diagnostic and treatment services. The increase in identified prevalence impacts the service system, often resulting in barriers to care including delayed diagnosis and long wait lists for services (Durkin et al., 2017; Monz, Houghton, Law, & Loss, 2019; Sheldrick et al., 2019). Service providers and the autism research community are recognizing that children on the spectrum become adults on the spectrum (Frazier et al., 2018) but there is comparatively little information about the health service needs and utilization of adults. In adulthood, needed health services may change from a focus on autism-related diagnostic and behaviorally-oriented treatment services to medical services that treat and prevent health conditions that co-occur, are caused by, or are associated with ASD (Mandell, 2018; Rubenstein & Bishop-Fitzpatrick, 2018). As the population of people diagnosed with ASD ages (who we refer to interchangeably as ‘people / adults on the spectrum’ and ‘autistic adults’), it is crucial to understand how increasingly large cohorts of adults access and use health services. Understanding service use is a first step to ensuring that people on the autism spectrum receive adequate care over the life course.
Assessing health service use in adults on the autism spectrum offers insight into how aging and age-related trends impact health at a population level. Similar to the general population, health greatly impacts an autistic person’s well-being and imparts individual and system-level cost. While we expect to see increased service needs and the development of chronic health conditions as a person ages, a growing body of research finds excess morbidity in adults with diagnosed ASD. Studies find that individuals with ASD claims in their medical records are at increased risk of diabetes, cardiovascular disease, sleep disorders, and gastrointestinal disorders (Bishop-Fitzpatrick et al., 2018; Bishop-Fitzpatrick & Rubenstein, 2019; Croen et al., 2015; Zerbo et al., 2018). While excess morbidity is evident in cross sectional studies, more work is needed to assess health trajectories across the age range. Improving our knowledge about demography and health care use of autistic adults receiving services will improve our ability to serve and prepare for a growing and aging population of autistic adults with increasingly complex medical needs.
While the level of awareness and public health action around ASD has corresponded to clear patterns in diagnostic change for children on the spectrum (Baio et al., 2018) we do not know effects of these changes downstream into adulthood. Diagnostic substitution, where someone receives an ASD diagnosis after having an incorrect prior diagnosis, is often evident in epidemiologic data on children on the autism spectrum (Coo et al., 2007; Rubenstein, Daniels, et al., 2018). For example, we frequently see clear decreases in intellectual disability (ID) diagnoses aligned with an increase in ASD diagnosis (Baio et al., 2018). Additionally, changes in diagnostic accretion, where a child receives an ASD diagnoses while retaining an original diagnosis, may explain differences over time in the prevalence of co-occurring ID among children on the autism spectrum (Rubenstein, Schieve, et al., 2018; Van Naarden Braun et al., 2015). Taken together, a phenotypically different cohort of people with identified ASD may be entering the adult service system as compared to past cohorts. While phenotypically different, these younger cohorts also have difference in service use during childhood (Benevides, Carretta, Ivey, & Lane, 2017; Rubenstein, Daniels, et al., 2018) that may continue into adulthood. Without understanding temporal changes, our preparation for and allocation of resources to fund future services may be misguided.
The first large cohorts of children identified in ASD-surveillance in the 1990’s and 2000’s prevalence studies (Autism and Developmental Disabilities Monitoring Network Principal Investigators, 2007; Van Naarden Braun et al., 2015) are now adults and are in need of services appropriate for adulthood. We believe that it is time to examine adult services using a longitudinal framework that accounts for the changing demography of the population on the spectrum. Without such understanding, estimates of health in the population of people on the autism spectrum are likely confounded by age and length of enrollment in service systems.
Our primary objective was to describe the demographic characteristics and enrollment patterns in a unique eleven-year full sample of all Wisconsin Medicaid beneficiaries with identified ASD or ID. Describing enrollment patterns has the potential to provide key information about the changing diagnostic distribution of Medicaid beneficiaries in Wisconsin. We aimed to compare trends in enrollment, beneficiary age, visits, and paid amount between enrollees with ASD without co-occurring ID (‘ASD’ diagnostic group), ASD with co-occurring ID (‘ASD+ID’ diagnostic group), intellectual disability without ASD and excluding Down Syndrome (‘ID-only’ diagnostic group), and Down syndrome without ASD (‘DS’ diagnostic group) between 2008–2018. We hypothesized that we would see an influx of young autistic adults without ID in the Medicaid service system with less service use and expense, but similar patterns of continuous enrollment compared to other diagnostic groups.
Methods
Data Source
We obtained de-identified Medicaid claims data for adults with a recorded ASD or ID diagnosis (including DS; see below) aged 21 and older with any Medicaid fee-for-service claims between January 1, 2008 and December 31, 2018. These Medicaid data were obtained under a limited data use agreement directly from the Wisconsin Department of Health Services. We had access to: demographic information (age, race/ethnicity, sex, county of residence); all medical claims (dental claims, home health claims, long term care claims, pharmacy claims and professional, inpatient, and outpatient claims and crossover claims; hospital-based acute care claims not included) and their corresponding ICD-9 or ICD-10 and their month and year; and paid amount for each claim.
In Wisconsin, an individual with an intellectual or developmental disability may be eligible for Medicaid as an adult (21+) for three general reasons: (1) they receive Supplemental Security Income or Social Security Disability Insurance benefits because their disability precludes them from working (Social Security Administration Disability Determination); (2) because they purchase Medicaid health benefits through a Medicaid Purchase Plan; or (3) because of poverty, potentially resulting from well-established low rates of employment and high rates of underemployment in adults on the spectrum. For those that receive benefits solely because of income, they are in a program in Wisconsin called BadgerCare Plus (Wisconsin’s Medicaid program for people of low income). Therefore, our Medicaid sample captures people on the spectrum or ID who receive services for ASD or ID but are enrolled either due to disability or have low income.
Developmental Disability Identification
Individuals were included in these data if they had two Medicaid claims for a developmental disability on two different days during their entire lifetime period of Medicaid enrollment. Participants were included if they had ICD-9 or ICD-10 codes for autism, autistic disorder, Asperger syndrome, pervasive developmental disorder not otherwise specified, or ASD (299.0x, 299.8x, 299.9x, F84.0, F84.5, or F84.9), ID (317.x, 318.x, 319.x, F70, F71, F73, F78, or F79), or DS (758.0x, Q90, or Q90.x). Using two claims rather than one ensures that the developmental disability claim is not a rule out diagnosis (i.e., a diagnosis recorded for a suspected health condition but that is not a primary or final diagnosis; Rector et al., 2004) and is consistent with past work examining autism in claims and electronic health records data in adults (Bishop-Fitzpatrick & Rubenstein, 2019; Croen et al., 2015; Maddox, Kang-Yi, Brodkin, & Mandell, 2018).
Each individual beneficiary was classified into one diagnostic group: ASD; ASD+ID; ID-only; and DS. The ASD diagnostic group included beneficiaries with claims for ASD without claims for ID and ID-only group included individuals with ID without any claims for ASD or DS. Intellectual and developmental disabilities present with many associated health conditions (Bishop-Fitzpatrick & Rubenstein, 2019; McCarron et al., 2013; Rubenstein & Bishop-Fitzpatrick, 2019), but when we refer to the ‘ASD’ or ‘ID-only’ diagnostic group it is in reference to lack of claims for the other intellectual and developmental disabilities we assessed, not all other health conditions. We elected to analyze groups of adults with DS and ID-only separately because the known etiologic origin of DS allows us to compare ASD and ID-only to a condition not affected by time-varying diagnostic practice (Seltzer et al., 2004). If an individual had claims for ASD, ID, and DS (N=135, 0.5%), we placed them in the ASD+ID diagnostic group, since DS is a subset of ID. We ran sensitivity analyses excluding the 135 individuals with claims for ASD, DS, and ID.
Years of Enrollment (Years Person Time)
Because beneficiaries could vary in their length of enrollment, calendar years of enrollment, and age, we elected to use years of person time (YPT), a measure of years of enrollment, as our denominator when assessing service use and paid amount. With YPT, we analyze individual years a person was enrolled rather than analyzing individuals. Therefore, a person enrolled from 2008–2018 adds 11 YPT whereas a person enrolled for just 2018 adds 1 YPT. Additionally, measuring YPT allows us to assess how many years’ worth of coverage the Medicaid system provided for diagnostic groups, and for diagnostic groups within an age bracket. Because of our data structure, a person year corresponded to a single calendar year. Our use of person year assumes that if a beneficiary had a claim in a year, they were enrolled for the full calendar year. If they did not have a claim in a year, they did not contribute to YPT for that year.
Covariates
The Wisconsin Department of Health Service provided the beneficiary’s sex, race/ethnicity, and county of residence. Demographic data were self-reported. We categorized county by urbanicity (rural or urban) based on county designations from the Wisconsin Office of Rural Health Policy (Blank, 2016). When assessing number of visits, we did not have data on specific date of visit (just month and year) due to the potentially identifiable nature of specific visit dates. We estimated unique visits by summing each block of claims with the same claim ID (each of which was composed of multiple claims with different claim detail numbers) for each beneficiary in each year. Paid amount was the cost that Medicaid paid for each unique claim detail number, summed over all of an individual’s YPT and transformed to constant 2018 dollars (Bureau of Labor Statistics, US Department of Labor, 2019).
Statistical Analysis
We calculated descriptive statistics for demographic variables and aggregate enrollment data by diagnostic group. We then graphed the percentage of beneficiaries at each age in each study year by diagnostic group. Next, we compared distribution of visits per YPT and cost per YPT across age category and diagnostic group. Both visits per YPT and paid amount per YPT had exponential distributions, so we log transformed the variables in order to run generalized linear models. We ran a model for each outcome (log visits per YPT and log costs per YPT) with beneficiary as a random intercept, age category, diagnostic group, year of service (to adjust for slight changes in Wisconsin Medicaid practice between years, as Wisconsin did not expand Medicaid during this period), member gender, and an interaction between age category and diagnostic group. We tested whether there were differences between age categories, controlling for diagnostic group, year of service, and member gender, and whether there were differences for diagnostic group, controlling for age category, year of service, and member gender. For ease of interpretation, we presented exponentiated mean estimates and their corresponding 95% confidence intervals for all diagnostic groups and age categories.
Results
Demographic Characteristics
We identified claims for 25,942 Medicaid beneficiaries with developmental disabilities between January 1, 2008 and December 31, 2018, including 4,775 adults with ASD diagnoses alone, 2,738 adults with ASD and ID diagnoses, 14,945 adults with ID-only diagnoses, and 3,484 adults with DS diagnoses. Consistent with epidemiological studies, both ASD diagnostic groups were predominantly male (ASD: 73.3% male, ASD+ID: 68.5% male) while the ID-only and DS diagnostic groups had similar numbers of males and females (ID only: 51.5% male, DS: 50.6% male; Table 1). While race/ethnicity was missing for a at least a quarter of beneficiaries in each diagnostic group, white race exceeded 80% in all diagnostic groups when race/ethnicity was known; in the US 2010 census, 86.2% of Wisconsin residents were white. Greater than 75% of beneficiaries in each diagnostic group resided in urban counties; in the US 2010 census, 70% of Wisconsin residents lived in urban areas (U.S. Census Bureau, 2012).
Table 1.
Demographic and enrollment descriptive statistics for adults 18+ with intellectual and developmental disabilities enrolled in Wisconsin Medicaid, 2008–2018
| ASD | ASD+ID | ID-only | DS | |||||
|---|---|---|---|---|---|---|---|---|
| N=4,775 | N=2,738 | N=14,945 | N=3,484 | |||||
| N | % | N | % | N | % | N | % | |
| Sex | ||||||||
| Male | 3,500 | 73.3 | 1,675 | 68.5 | 7,694 | 51.5 | 1,763 | 50.6 |
| Female | 1,275 | 26.7 | 863 | 31.5 | 7,251 | 48.5 | 1,721 | 49.4 |
| Race | ||||||||
| White | 2,858 | 86.8 | 1,667 | 83.8 | 9,329 | 82.2 | 2,298 | 90.5 |
| Black | 249 | 7.6 | 228 | 11.5 | 1,350 | 11.9 | 116 | 4.6 |
| Hispanic | 108 | 3.3 | 56 | 2.8 | 347 | 3.1 | 76 | 3.0 |
| Asian/Pacific Islander/Native American | 76 | 2.3 | 38 | 1.9 | 328 | 2.9 | 49 | 1.9 |
| Other/Unknown | 1,484 | 749 | 3,591 | 945 | ||||
| County DesignationA | ||||||||
| Rural | 949 | 19.9 | 505 | 18.4 | 3,376 | 22.6 | 797 | 22.9 |
| Urban | 3,826 | 80.1 | 2,233 | 81.6 | 11,569 | 77.4 | 2,687 | 77.1 |
| Follow up time | ||||||||
| Enrollment in 2008 | 1,308 | 27.4 | 1,874 | 68.4 | 11,486 | 76.9 | 2,486 | 71.3 |
| Enrollment in 2018 | 3,572 | 74.8 | 2,075 | 75.8 | 11,312 | 74.7 | 2,625 | 75.3 |
| Enrolled consistently 2008–2018 | 968 | 20.3 | 1,441 | 52.6 | 8,699 | 58.2 | 1,876 | 53.9 |
| Total YPT | 29,533 | 24,592 | 130,682 | 27,886 | ||||
| Service use | ||||||||
| Median visits per YPT, IQR | 11 | 22 | 17 | 26 | 20 | 31 | 12 | 21 |
| Median paid amount YPT ($), IQR | 1,895.0 | 6,921 | 9,153.8 | 8,376 | 6,473.0 | 32,691 | 2,878.5 | 10,204 |
ASD: autism spectrum disorder; ID: Intellectual disability; DS: Down Syndrome; YPT: Year person time; IQR: Interquartile range; ID-only: claims for ID without claims for ASD or DS
Rural/urban county designation taken from Office of Rural Health
In the ASD diagnostic group, 20.3% were enrolled for all eleven years of follow up and 72.6% entered Medicaid after 2008. Fifty percent of beneficiaries in the ASD diagnostic group were 21 during follow-up, explaining the low rate of being enrolled from 2008 to 2018. More than 50% of beneficiaries in the other diagnostic groups were enrolled continuously from 2008 to 2018. In each diagnostic group, approximately one quarter of beneficiaries were not enrolled in 2018. Ninety percent of beneficiaries had no gaps in claims (a claim in every year from the year of first claim to the year of last claim).
From 2008 to 2018, Wisconsin Medicaid covered 212,693 years’ worth of claims for beneficiaries with ASD, ID, or DS claims. The ASD+ID diagnostic group had the fewest YPT (24,592 YPT) and the ID-only diagnostic group had the most (130,682 YPT). The median visits per YPT was lowest in the ASD diagnostic group (11 visits per YPT) and highest in the ID-only diagnostic group (20 visits per YPT). As a post-hoc sensitivity analysis, we examined whether YPT changed if we assessed time from first claim to last claim, rather than just years in which there was a claim. We found minimal difference in total YPT increases for the ID (2.0%), DS (3.0%), ASD+ID (1.2%), and ASD (3.5%) groups. Median paid amount per YPT was $2,015.8 in the ASD diagnostic group, $9,770.8 in the ASD+ID diagnostic group, $6,924.9 in the ID-only diagnostic group, and $3,078.0 in the DS diagnostic group.
Age Distribution
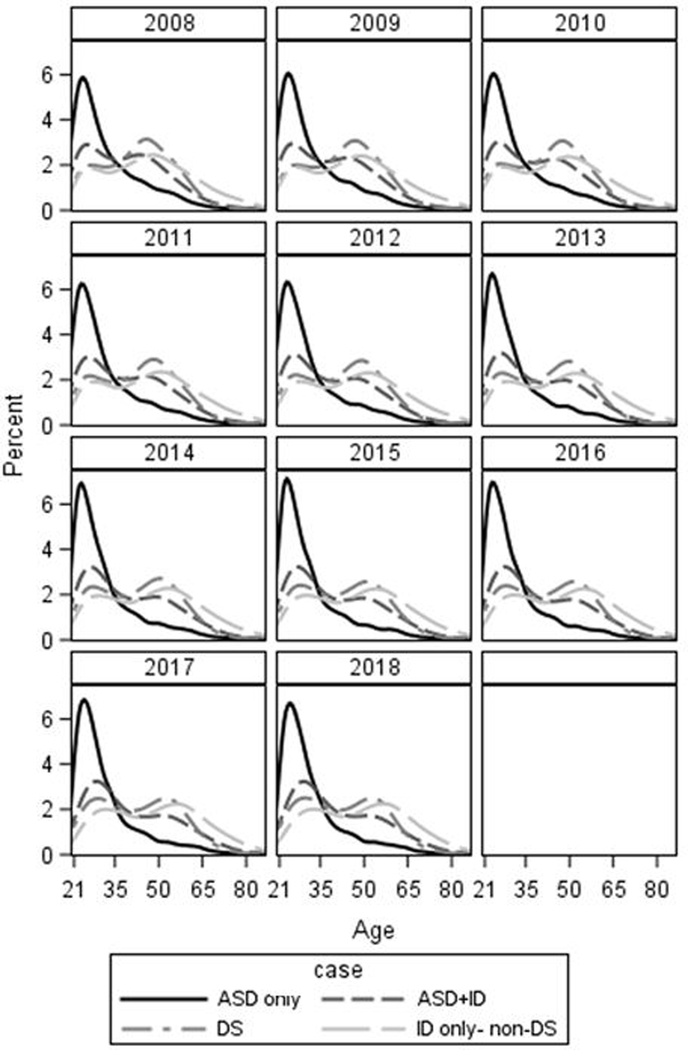
We present the age distribution for each diagnostic group in each year in Figure 1 (descriptive statistics in Supplement 1). Age distribution was qualitatively similar across the eleven years of follow up. The highest proportion of beneficiaries in the ASD diagnostic group were in their late twenties with a sharp decrease in proportion with increasing age; this corresponded to a median age between 27 and 28 and a mean of between 30.9 and 32.0 (depending on year of service). The highest proportion of the ASD+ID was in the late 20’s / early 30’s with a decrease in the proportion in that age range that was less steep compared to the ASD diagnostic group. The age distribution corresponded to medians between 36 and 37 and means 38.4 to 40.7 (depending on year of service). For both the ID-only and DS diagnostic group there were bimodal distributions with modes near 30 and 55. By year, median age in the ID-only diagnostic group ranged from 46 to 49 (depending on year) with a mean that ranged from 45.9 to 48.6. The DS diagnostic group had median age of 43 or 44 (depending on year) with means of 42.5 to 42.7.
Figure 1.
Age distribution of Medicaid enrollees with intellectual and developmental disability, by year and case type
ASD: Autism spectrum disorder; ASD+ID: Autism spectrum disorder with ID; ID: Intellectual disability; DS: Down syndrome
Visits
We found statistically significant effects of age category and diagnostic group on log number of visits (Table 2). After exponentiating estimates and 95% confidence intervals, the 21–29 age category had the least number of visits and the 60+ age category had the most visits for all diagnostic groups. The ASD and ASD+ID diagnostic groups only had overlapping confidence intervals in number of visits for the 60+ group, with the ASD+ID diagnostic group having more visits in all other age categories. The DS diagnostic group had the fewest number of visits in each age category. The amount of YPT we see in each diagnostic group is consistent with the age distribution we saw in Figure 1, with most YPT for the ASD and ASD+ID diagnostic groups in the younger age categories and a more even distribution over age categories for ID-only and DS.
Table 2.
Mean number of visits in which a claim was made per person year among adults with intellectual and developmental disability in Wisconsin Medicaid 2008–2018, by age category and case status
| ASD N=4,775 | ASD+ID N=2,738 | ID-only N=14,945 | DS N=3,484 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age category | YPT | Visits/YPT | 95% CI | YPT | Visits/YPT | 95% CI | YPT | Visits/YPT | 95% CI | YPT | Visits/YPT | 95% CI |
| 21–29 | 17,069 | 9.3 | 9.1, 9.4 | 7,641 | 14.4 | 14.1, 14.8 | 21,169 | 15.9 | 15.7, 16.2 | 5,985 | 8.1 | 7.9, 8.3 |
| 30–39 | 6,722 | 12.3 | 12.0, 12.6 | 5,597 | 16.0 | 15.5, 16.4 | 23,451 | 17.2 | 17.0 17.4 | 5,589 | 9.4 | 9.1, 9.7 |
| 40–49 | 2,952 | 15.2 | 14.6, 15.8 | 4,794 | 17.5 | 17.0, 18.1 | 25,554 | 17.8 | 17.5, 18.0 | 6,923 | 11.4 | 11.1, 11.7 |
| 50–59 | 1,684 | 17.3 | 16.4, 18.4 | 4,039 | 19.1 | 18.4, 19.7 | 28,927 | 19.8 | 19.6, 20.0 | 6,669 | 15.1 | 14.7, 15.5 |
| 60+ | 1,106 | 23.2 | 21.7, 24.8 | 2,521 | 23.2 | 22.2, 24.2 | 31,581 | 20.7 | 20.5, 21.0 | 2,720 | 19.1 | 18.3, 19.9 |
Group differences: Case, controlling for age; F= 1054.0, P<0.0001; DS<ASD<ASD+ID<ID only
Age category, controlling for case; F=898.6, P<0.0001; 21–29<30–39<40–49<50–59<60+
ASD: Autism spectrum disorder; ID: Intellectual disability; DS: Down syndrome; YPT: Years person time; ID-only: claims for ID without claims for ASD or DS
Estimates calculated using generalized linear regression with random intercept for subject and log number of visits as outcome. Model includes categorical age, case status, year of service, member gender, and age*case interaction term. Data presented are back-transformed.
Paid Amount
Similar to visits, we saw increasing log paid amount with increasing age category in our generalized linear models (Table 3). Patterns in log paid amount by diagnostic group significantly differed when controlling for age and year of service; highest expenses were in the ASD+ID diagnostic group, followed by the ID-only diagnostic group, DS diagnostic group, and ASD diagnostic group. After exponentiation, age groups >40 for the ASD+ID diagnostic group had paid amounts four to seven times that of the ASD diagnostic group and two times that of the ID-only diagnostic group. The DS diagnostic group had lower paid amount compared to the ID-only diagnostic group in all age categories except the 60+ group. Our results did not meaningfully change when excluding beneficiaries with ASD and DS claims (data not shown).
Table 3.
Mean paid amount per person year among adults with intellectual and developmental disability in Wisconsin Medicaid 2008–2018, by age category and case status
| ASD N=4,775 | ASD+ID N=2,738 | ID-only N=14,945 | DS N=3,484 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age category | YPT | Paid ($) /YPT | 95% CI | YPT | Paid ($) /YPT | 95% CI | YPT | Paid ($) /YPT | 95% CI | YPT | Paid ($) /YPT | 95% CI |
| <30 | 17,069 | 713.23 | 649.9, 782.7 | 7,641 | 3,957.23 | 3,485.6, 4,492.7 | 21,169 | 3,276.73 | 3,016.8, 3,559.1 | 5,985 | 1,041.38 | 902.3, 1,201.9 |
| 30–39 | 6,722 | 2,237.24 | 1,960.6, 2,552.9 | 5,597 | 10,898.71 | 9,577.4, 12,402.4 | 23,451 | 6,574.15 | 6,111.0, 7,072.4 | 5,589 | 2,416.08 | 2,134.5, 2,734.8 |
| 40–49 | 2,952 | 2,288.38 | 1,893.8, 2,765.1 | 4,794 | 13,823.15 | 11,916.6, 16,034.7 | 25,554 | 7,226.43 | 6,761.9, 7,722.9 | 6,923 | 3,202.86 | 2,876.5, 3,566.3 |
| 50–59 | 1,684 | 2,800.91 | 2,191.4, 3,579.9 | 4,039 | 20,266.00 | 16,937.4, 24,248.7 | 28,927 | 9,120.68 | 8,579.7, 9,695.8 | 6,669 | 5,907.63 | 5,315.4, 6,565.9 |
| 60+ | 1,106 | 9,037.16 | 6,547.8, 12,421.8 | 2,521 | 27,906.08 | 21,748.2, 35,807.5 | 31,581 | 11,514.93 | 10,835.9, 12,236.5 | 2,720 | 11,667.94 | 9,570.5, 14,225.0 |
Group differences: Case, controlling for age, year of service; F= 4890.9, P<0.0001; ASD < DS < ID only < ASD+ID
Age category, controlling for case, year of service; F=2702.55, P<0.0001; 21–29 < 30–39 < 40–49 < 50–59 < 60+
ASD: Autism spectrum disorder; ID: Intellectual disability; DS: Down syndrome; YPT: Years person time; ID-only: claims for ID without claims for ASD or DS. Cost in US dollars adjusted for inflation.
Estimates calculated using a generalized linear regression with random intercept for subject and log number of visits as outcome. Model includes categorical age, year of service, member gender, case status and age*case interaction term. Data presented are back-transformed.
Discussion
Because of the poor health outcomes identified in recent cross-sectional studies, it is urgent that we identify the health and health service needs of autistic adults from young adulthood through old age. This concern is even more pressing given that has been an increase in identified ASD prevalence in children which will lead to a future with more adults with ASD diagnoses. Understanding changing enrollment patterns in adult service systems helps us understand service needs while accounting for the changing demographics of the population of autistic adults. We sought to examine enrollment patterns in adult Medicaid beneficiaries with ASD and other developmental disability claims in Wisconsin between 2008 and 2018. We believe that our study of eleven years of longitudinal Medicaid enrollment data of adult beneficiaries with identified ASD highlights the patterns of enrollment, visits, and service costs in a population that faces disparities in health outcomes (Bishop-Fitzpatrick & Kind, 2018; Rubenstein & Bishop-Fitzpatrick, 2019). The findings have implications for ensuring adequate health care coverage across the lifespan and highlight the importance of a strong and accessible health care system for people with intellectual and developmental disabilities.
In the Wisconsin Medicaid system from 2008 to 2018, the majority of autistic adults were less than 30 years of age, as evidenced by the age distributions and YPT. Not only were most service years provided to this younger age group, but there was a sharp decrease in the number of beneficiaries and the corresponding YPT with increasing age within the ASD cohorts. The pattern in distribution was consistent across study years, which implies that there is a consistent influx of new young adults on the spectrum without ID into the Medicaid system. There is also the possibility that a significant proportion of adults with ASD-only are able to enroll in employer sponsored healthcare plans; we cannot determine instances of enrollment in employer-sponsored health plans in these data. However, only one quarter of enrollees were not enrolled in our last year of assessment (similar to ID and DS). We saw a similar right skewed distribution, albeit with a wider age range and a more gradual decline in proportion over age, for beneficiaries with ASD+ID diagnoses. The older age of ASD+ID diagnostic group compared to the ASD diagnostic group may be an effect of the decreasing ratio of ASD+ ID to ASD without ID (Baio et al., 2018; Van Naarden Braun et al., 2015). In early epidemiologic studies, the estimated prevalence of ID within ASD exceeded 70% (Fombonne, 2002). Now, estimates are closer to 30% (Baio et al., 2018). The more recent change may be demonstrated in our data by a larger proportion of beneficiaries with ASD+ID diagnoses being old enough to be enrolled in earlier study years compared to beneficiaries with ASD diagnoses alone. Could this be an effect of diagnostic substitution or accretion? It is hard to determine using solely administrative data, but from 2008 to 2018 we see less YPT for young adults with ID compared to older adults with ID. Besides the hypothesis that the young adults with ID are now being classified as ASD+ID, it is possible that there is a meaningful decrease in ID in the population unrelated to ASD increase. It is also possible that traumatic brain injuries and other conditions that can cause ID to occur later in life and incident ID shifts the age distribution. DS had a more stable rate of YPT, with slight increases across age categories up to up to 50–59; this trend is likely an illustration of the stable DS prevalence plus the decreased life expectancy attributable to the condition (de Graaf, Buckley, Dever, & Skotko, 2017).
Not surprisingly, we saw increased number of visits in older age categories across all diagnostic groups but did see clear differences in number of visits comparing ASD to ASD+ID diagnostic groups in the younger three age categories. It is possible that the ASD+ID diagnostic group has more complicated health needs that require more services. For instance, Cashin et al. (2018) suggested that autistic adults with ID have more physical health conditions compared to autistic adults without ID, while we found few differences in physical and mental health in autistic adults based on ID status in a separate Medicaid sample (Bishop-Fitzpatrick & Rubenstein, 2019). Alternatively, we cannot rule out that a reason for more visits is the difficulty in communicating one’s health needs for individuals with autism-related diagnostic differences in social communication abilities leading to extra visits and unnecessary services (Doody & Doody, 2012). When aged 60+, diagnostic groups were more similar, likely an effect of increasing health care needs with age. The lower rates of visits in the DS diagnostic group is curious and may be an effect of the well-known course of DS or lesser communication impairment in DS compared to ID.
Similar to visits, we see differences in paid amount between diagnostic groups with increasing costs with increasing age. The ASD+ID diagnostic group had paid amounts at least twice that of the ASD group at every age category. Again, this may be an effect of a more complex phenotype with more associated health conditions, although the literature is mixed. While costs for the ASD group was less than the ID-only or DS diagnostic groups, the majority of years served were to individuals in the younger age category. If we were to assume similar paid amount in each age category over time and minor decrease in person time due to death, the individuals that contributed to 17,000 YPT <30 would contribute a similar amount of YPT when they are 60+. The aging of this group would lead to 16 times more YPT than the current 60+ cohort who have paid amounts almost thirteen times that of the <30 cohort. While this extrapolation may be overly simplistic, we are confident based on our data and the existing literature that young adults on the spectrum already enrolled in Medicaid will likely stay enrolled and have increased costs that correspond to increasing age. It is possible—and in some cases certain—that past generations of adults with ASD were not identified as having ASD but still enrolled in Medicaid (diagnostic substitution). While we cannot account for these temporal changes related to diagnostic substitution in our data, the large increase in identified ASD better enables us to anticipate ASD-specific service needs and prevent health conditions that commonly present with ASD (Rubenstein & Bishop-Fitzpatrick, 2018) for a growing cohort of adult Medicaid beneficiaries with ASD.
When assessing these data, it is important to place results in the context of current estimates of ASD prevalence. In these Medicaid data, the most recent year of birth could be 1997 (21 years old in 2018). For the 1998 birth cohort, the ADDM Network prevalence estimate of ASD was 9 per 1000 (Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 & Principal Investigators; Centers for Disease Control and Prevention (CDC), 2009). Eight years later, the estimate for the 2006 birth cohort was 16.8 per 1000 (Baio et al., 2018). Children born past 1997 are not yet old enough to receive adult Medicaid services, but as time passes more and more children on the spectrum are becoming adults on the spectrum and the rightward skew of age in the population with ASD diagnoses will continue to impact the health service system. An additional wrinkle that impacts the meaning of these findings is the lack of a reliable way to provide incident diagnoses of ASD in adults (Lai & Baron-Cohen, 2015). Scientific consensus is that there is a ‘lost-generation’ of adults with autism without a diagnosis. If these individuals can be identified and served in the health service system, there may be a distributional shift in the age and need of the autistic population receiving health services in the future.
Based on our findings we believe that Medicaid will become even more vital as a payer of health care as cohorts of children on the spectrum age into adulthood, adults age into old age, and we better understand how to best serve this population. However, the program remains under threat as federal and state funding priorities change (Mandell & Barry, 2017). Medicaid is currently funded jointly by the federal government and by states yet administered by states. The two proposed policy solutions to reduce Medicaid costs – block grants (providing a fixed amount of federal Medicaid reimbursement to states regardless of enrollment) and per capita caps (providing a fixed federal reimbursement to states per enrollee) – would transfer the burden of funding Medicaid from the federal government to states (Mager-Mardeusz, Lenz, & Kominski, 2017). Greater reliance on state funding of Medicaid has already led to changes in who is eligible for Medicaid (work requirements), which health services Medicaid will cover, and the rates at which covered services will be reimbursed to providers (Carroll, 2018). Given that our findings suggest that both the distribution of enrollees across diagnostic groups and the actual number of enrollees will likely change in the coming years (based on what we know about intellectual and developmental disabilities epidemiology in children; Baio et al., 2018), it is possible that changes to Medicaid policy now could have even greater impacts on autistic adults, particularly in light of findings of excess morbidity in ASD. Importantly, we are hopeful that innovation in how health services are provided and paid for that broadly support population health (beyond preserving current levels of funding for social service programs like Medicaid) could improve and lengthen the lives of autistic people.
Administrative claims data are ideal for answering service-system level questions but have some inherent limitations. Specific to our study, we did not have the detail necessary to describe the phenotypic presentation of individual Medicaid beneficiaries which may be important when evaluating cohort effects. Additionally, data were frequently missing for individual-level race/ethnicity and we did not have information on individual education, income, or geographic location beside county.
For this study, we did not have data on whether a participant was dual enrolled with Medicare nor did we have claims for hospitalization, both of which may affect paid amount. In our analysis we assumed that enrollment was continuous over a full calendar year. Our assumption is supported by the 90% rate of continuous enrollment in the population of people with disabilities from the 2010 Medical Expenditure Panel Survey (Ku & Steinmetz, 2013) but may not necessarily hold in the intellectual and developmental disabilities subpopulation or to Wisconsin Medicaid; therefore, it is possible that some differences in visits and paid amount represent differences in enrollment across groups rather than differences in utilization. Further, if a beneficiary did not have a claim in a year, we considered this a gap in enrollment and did not count that year toward our total YPT; this assumption may be overly conservative as it is possible that the beneficiary just had no health care utilization, especially in young adults (Lau, Adams, Boscardin, & Irwin, 2014). We did not have information on whether an individual met Medicaid disability enrollment criteria. While these data would help inform our temporal trend analyses, this population is receiving services for IDD whether or not they meet the State disability criteria and these results have implications for how services are allocated. We assumed that beneficiaries were accurately identified and assigned to our diagnostic groups. Finally, the validity of identification of ASD in Medicaid claims data has not been specifically tested, although studies have found that claims-based identification of ASD in children tracks well with criterion standard assessment (Dodds et al., 2009) and diagnosis based on review of medical records (Fombonne et al., 2004).
The aggregate service needs of the full population of autistic people may be changing. Historically, autism has been perceived as a childhood condition, and research has focused primarily on that life stage. The average American spends more than three-quarters of their life in adulthood, and despite the possibility of reduced life expectancy in autistic people, we can assume that the majority of the life course is spent in adulthood. The patterns we see in Medicaid enrollment and use underscore the importance of developing strategies to ensure adequate health care coverage and a strong and accessible health care system for autistic adults. Indeed, the changing enrollment patterns that we identified in this study suggest that the boom in autism prevalence is heading for the adult service system. In the coming years it will be crucial that research and resources are directed towards identifying strategies to support the health and healthcare needs of the growing population of autistic adults.
Supplementary Material
Acknowledgements:
This study was supported by grants from the National Institute of Child Health and Human Development (U54HD090256; T32HD007489) and the National Center for Advancing Translational Sciences (UL1TR002373; KL2TR002374; KL2TR000428).
References
- Autism and Developmental Disabilities Monitoring Network Principal Investigators. (2007). Prevalence of autism spectrum disorders--autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR: Surveillance Summaries, 56(1), 1–11. [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators; Centers for Disease Control and Prevention (CDC). (2009). Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR: Surveillance Summaries, 58(10), 1–20. [PubMed] [Google Scholar]
- Baio J, Wiggins LD, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries, 67(6), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevides TW, Carretta HJ, Ivey CK, & Lane SJ (2017). Therapy access among children with autism spectrum disorder, cerebral palsy, and attention-deficit-hyperactivity disorder: a population-based study. Developmental Medicine and Child Neurology, 59(12), 1291–1298. [DOI] [PubMed] [Google Scholar]
- Bishop-Fitzpatrick L, & Kind AJ (2017). A scoping review of health disparities in autism spectrum disorder. Journal of Autism and Developmental Disorders, 47(11), 3380–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop‐Fitzpatrick L, Movaghar A, Greenberg JS, Page D, DaWalt LS, Brilliant MH, & Mailick MR (2018). Using machine learning to identify patterns of lifetime health problems in decedents with autism spectrum disorder. Autism Research, 11(8), 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop-Fitzpatrick L, & Rubenstein E (2019). The physical and mental health of middle aged and older adults on the autism spectrum and the impact of intellectual disability. Research in Autism Spectrum Disorders, 63, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank P (2016). Wisconsin Divided Six Ways: A Review of Rural-Urban Classification Systems. Retrieved from http://worh.org/sites/default/files/Wisconsin%20Divided%20Six%20Ways_1.pdf
- Bureau of Labor Statistics, US Department of Labor (2019) Archived consumer price index detailed report information. Available at: https://www.bls.gov/bls/news-release/cpi.htm (accessed 2 May 2019).
- Carroll AE (2018). The Problem With Work Requirements for Medicaid JAMA Forum. JAMA, 319(7), 646–647. [DOI] [PubMed] [Google Scholar]
- Cashin A, Buckley T, Trollor JN, & Lennox N (2018). A scoping review of what is known of the physical health of adults with autism spectrum disorder. Journal of Intellectual Disability, 22(1), 96–108. [DOI] [PubMed] [Google Scholar]
- Coo H, Ouellette-Kuntz H, Lloyd JEV, Kasmara L, Holden JJA, & Lewis MES (2007). Trends in Autism Prevalence: Diagnostic Substitution Revisited. Journal of Autism and Developmental Disorders, 38(6), 1036–1046. [DOI] [PubMed] [Google Scholar]
- Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, & Kripke C (2015). The health status of adults on the autism spectrum. Autism, 19(7), 814–823. [DOI] [PubMed] [Google Scholar]
- de Graaf G, Buckley F, Dever J, & Skotko BG (2017). Estimation of live birth and population prevalence of Down syndrome in nine U.S. states. American Journal of Medical Genetics. Part A, 173(10), 2710–2719. [DOI] [PubMed] [Google Scholar]
- Dodds L, Spencer A, Shea S, Fell D, Armson BA, Allen AC, & Bryson S (2009). Validity of autism diagnoses using administrative health data. Chronic Diseases in Canada, 29(3), 102–107. [PMC free article] [PubMed] [Google Scholar]
- Doody CM, & Doody O (2012). Health promotion for people with intellectual disability and obesity. British Journal of Nursing, 21(8), 460–465. [DOI] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Baio J, Christensen D, Daniels J, Fitzgerald R, … Yeargin-Allsopp M (2017). Autism Spectrum Disorder Among US Children (2002–2010): Socioeconomic, Racial, and Ethnic Disparities. American Journal of Public Health, 107(11), 1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E (2002). Epidemiological trends in rates of autism. Molecular Psychiatry, 7(Suppl 2), S4–S6. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Heavey L, Smeeth L, Rodrigues LC, Cook C, Smith PG, … Hall AJ (2004). Validation of the diagnosis of autism in general practitioner records. BMC Public Health, 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Dawson G, Murray D, Shih A, Sachs JS, & Geiger A (2018). Brief Report: A Survey of Autism Research Priorities Across a Diverse Community of Stakeholders. Journal of Autism and Developmental Disorders, 48(11), 3965–3971. [DOI] [PubMed] [Google Scholar]
- Ku L, & Steinmetz E (2013). Bridging the Gap: Continuity and Quality of Coverage in Medicaid. Retrieved from https://ccf.georgetown.edu/wp-content/uploads/2013/09/GW-Continuity-Report-9-10-13.pdf
- Lai M-C, & Baron-Cohen S (2015). Identifying the lost generation of adults with autism spectrum conditions. The Lancet Psychiatry, 2(11), 1013–1027. [DOI] [PubMed] [Google Scholar]
- Lau JS, Adams SH, Boscardin WJ, & Irwin CE Jr. (2014). Young adults’ health care utilization and expenditures prior to the Affordable Care Act. Journal of Adolescent Health, 54(6), 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox BB, Kang-Yi CD, Brodkin ES, & Mandell DS (2018). Treatment Utilization by Adults with Autism and Co-Occurring Anxiety or Depression. Research in Autism Spectumr Disorders, 51, 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager-Mardeusz H, Lenz C, & Kominski GF (2017). A “Cap” on Medicaid: How Block Grants, Per Capita Caps, and Capped Allotments Might Fundamentally Change the Safety Net. Policy Brief UCLA Cent Health Policy Res(Pb2017–2), 1–10. [PubMed] [Google Scholar]
- Mandell DS (2018). Dying before their time: Addressing premature mortality among autistic people. Autism, 22(3), 234–235. [DOI] [PubMed] [Google Scholar]
- Mandell DS, & Barry CL (2017). Care for Autism and Other Disabilities - A Future in Jeopardy. New England Journal of Medicine, 376(10), e15. [DOI] [PubMed] [Google Scholar]
- McCarron M, Swinburne J, Burke E, McGlinchey E, Carroll R, & McCallion P (2013). Patterns of multimorbidity in an older population of persons with an intellectual disability: results from the intellectual disability supplement to the Irish longitudinal study on aging (IDS-TILDA). Research in Developmental Disabilities, 34(1), 521–527. [DOI] [PubMed] [Google Scholar]
- Monz BU, Houghton R, Law K, & Loss G (2019). Treatment patterns in children with autism in the United States. Autism Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector TS, Wickstrom SL, Shah M, Thomas Greeenlee N, Rheault P, Rogowski J, … Escarce JJ (2004). Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health Services Research, 39(6 Pt 1), 1839–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roetzer KM, Schwarzbraun T, Obenauf AC, Hauser E, & Speicher MR (2010). Further evidence for the pathogenicity of 15q24 microduplications distal to the minimal critical regions. American Journal of Medical Genetics, Part A, 152 A(12), 3173–3178. [DOI] [PubMed] [Google Scholar]
- Rubenstein E, & Bishop-Fitzpatrick L (2018). A Matter of Time: The Necessity of Temporal Language in Research on Health Conditions that Present with Autism Spectrum Disorder. Autism Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein E, Daniels J, Schieve LA, Christensen DL, Van Naarden Braun K, Rice CE, … Lee LC (2018). Trends in Special Education Eligibility Among Children With Autism Spectrum Disorder, 2002–2010. Public Health Reports, 133(1), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein E, Schieve L, Wiggins L, Rice C, Van Naarden Braun K, Christensen D, … Lee LC (2018). Trends in documented co-occurring conditions in children with autism spectrum disorder, 2002–2010. Research in Developmental Disabilities, 83, 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick RC, Frenette E, Vera JD, Mackie TI, Martinez-Pedraza F, Hoch N, … Carter AS (2019). What Drives Detection and Diagnosis of Autism Spectrum Disorder? Looking Under the Hood of a Multi-stage Screening Process in Early Intervention. Journal of Autism and Developmental Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Abbeduto L, Krauss MW, Greenberg J, Swe A (2004). Comparison groups in autism family research : down syndrome, fragile X syndrome, and schizophrenia. Journal of Autism and Developmental Disorders. 34 (1), 41–48. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2012). Wisconsin 2010: Population and Housing Unit Counts. Retrieved from US Government Printing Office, Washington DC: https://www.census.gov/prod/cen2010/cph-2-51.pdf [Google Scholar]
- Van Naarden Braun K, Christensen D, Doernberg N, Schieve L, Rice C, Wiggins L, … Yeargin-Allsopp M (2015). Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991–2010. PloS One, 10(4), e0124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Qian Y, Ray T, Sidney S, Rich S, Massolo M, & Croen LA (2018). Healthcare Service Utilization and Cost Among Adults with Autism Spectrum Disorders in a U.S. Integrated Healthcare System. Autism in Adulthood: Knowledge, Practice, and Policy, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.