Summary
Objectives:
Pulmonary mucormycosis (PMM) is an emerging, frequently lethal fungal infection in immunosuppressed cancer patients. We sought to characterize the histopathologic features of PMM in this population.
Methods:
We identified patients with PMM who underwent autopsy or lung biopsy between 1990 and 2007. Histopathology slides were blindly reviewed by a pathologist and findings were scored on standardized forms. Pathologic findings were correlated with demographic and clinical data abstracted from patient’s medical records.
Results:
Twenty patients with PMM were included in this study. Nineteen patients (95%) had hematologic malignancies. High frequencies of angioinvasion (100%), hemorrhagic infarction (90%), coagulative necrosis (85%), and intra-alveolar hemorrhage (85%) were observed, whereas inflammatory infiltrates were uncommon (30%). Neutropenic patients had more extensive angioinvasion compared with non-neutropenic patients (77% versus 29%, P = 0.06). Allogeneic hematopoietic stem cell transplantation (HSCT) recipients, all of whom had graft-versus-host disease, had more inflammatory cell infiltration but less intra-alveolar hemorrhage than non-HSCT patients (67% versus 14%, P = 0.04; 50% versus 100%, P = 0.02, respectively).
Conclusions:
MM in immunocompromised cancer patients is characterized by extensive angioinvasion and coagulative necrosis. The different histopathologic features of PMM in neutropenic, non-neutropnic, and HSCT patients may reflect differences in the pathobiology of PMM in these populations.
Keywords: Mucormycosis, Cancer, Histopathology
Introduction
Filamentous fungi of the order Mucorales have recently emerged as important pathogens in patients with hematologic malignancies.1,2 In many cancer centers, mucormycosis is now the second most common invasive mold infection in hematologic cancer patients; in this population, mucormycosis typically presents as a sinopulmonary infection, and is associated with a mortality rate of 66%.1,3
The clinical immunobiology of pulmonary mucormycosis (PMM) remains undefined. Recent studies of invasive pulmonary aspergillosis, a more common pulmonary mycosis, demonstrate that the pattern of tissue injury and immune response depend on the type of immunosuppression in the host.4–7 Specifically, patients with neutropenia induced by cytotoxic drugs have numerous hyphal elements and frequent angioinvasion but scant inflammatory infiltrates in their lungs.4–7 In contrast, non-neutropenic patients who are immunosuppressed with corticosteroids have a low pulmonary fungal burden and extensive tissue infiltration by polymorphonuclear leukocytes.4–7
To our knowledge, no studies have evaluated the clinicopathological characteristics of PMM in patients with different forms of immunosuppression. Herein, we correlate the patterns of tissue injury and pulmonary inflammatory responses with different immunologic backgrounds among cancer patients with mucormycosis.
Design and methods
Study design
We identified patients with proven PMM who had undergone pulmonary biopsies or autopsies at The University of Texas M. D. Anderson Cancer Center (Houston, TX) between January 1990 and December 2007. Hyphae were considered typical of Mucorales if they were broad (10–25 μm), non-septated or pauci-septated, and ribbon-like in appearance and/or displayed right-angle branching. Cases with evidence of pulmonary fungal or bacterial coinfection were excluded from the final analysis.
Slides of pulmonary lesion samples were retrieved and examined blindly by an experienced pathologist (M.L.). Findings were scored on standardized forms that included fields for hyphal morphologic characteristics, angioinvasion, perineural invasion, intravascular thrombosis, coagulative necrosis, hemorrhagic infarction, inflammatory necrosis, granulomas, intra-alveolar hemorrhage, and inflammatory cell exudates.
Patient demographics and clinical data were abstracted from medical charts. Neutropenia was defined as an absolute neutrophil count of <500 cells/μL at the time of tissue collection. Treatment with high-dose corticosteroids was defined as a prednisone-equivalent dose of ≥600 mg per week for at least 3 of the 4 weeks preceding tissue collection.
Histopathologic definitions
The following definitions8 were used: angioinvasion was defined as the presence of hyphae within the lumen of blood vessels; intravascular thrombosis as the presence of thrombi consisting of fibrin, platelets, and red blood cells within the lumen of blood vessels; coagulative necrosis as the presence of acidophilic, anucleate cells with preserved general tissue architecture; hemorrhagic infarction as vascular congestion and extravasation of red blood cells in an area of necrotic lung parenchyma; inflammatory necrosis as infiltration of the margins of a necrotic zone with inflammatory cells; granulomas as aggregates of macrophages with an enlarged epithelioid appearance, with or without caseous necrosis; intra-alveolar hemorrhage as the presence of red blood cells within contiguous alveoli (with or without hemosiderin-laden macrophages); and perineural invasion as the presence of fungal hyphae within the fascial sheaths of nerves (perineurium).9
Statistical analysis
Differences in the frequencies of histopathologic features were compared between patient groups with Fisher’s exact test. A 2-sided P value of <0.05 was considered statistically significant. Calculations were performed using SPSS software version 15.0 (SPSS, Chicago, IL).
Results
Twenty patients with PMM were identified. The median patient age was 52 years (range, 15 to 79 years); 14 patients (70%) were male. All but 1 patient (95%) had hematologic malignancies. Thirteen patients (65%) were neutropenic at the time of diagnosis, and 14 (70%) had received high-dose corticosteroids. Six patients (30%) were allogeneic hematopoietic stem cell transplantation (HSCT) recipients; all HSCT recipients had graft-versus-host disease (GVHD) and were receiving calcineurin inhibitors. Five HSCT recipients had received high-dose corticosteroids and 3 were neutropenic. Six patients had diabetes mellitus.
Cultures were positive in 9 patients (45%) and included Rhizopus species (3 patients), Mucor species (2 patients), Cunninghamella species (2 patients), and Rhizomucor and Syncephalastrum species (1 patient each). The organisms were recovered from tissue obtained at autopsy in 7 patients and from bronchoalveolar lavage samples in 2 patients.
The most common histopathologic findings were angioinvasion, hemorrhagic infarction, coagulative necrosis, and intra-alveolar hemorrhage (Table 1, Fig. 1). Angioinvasion was seen in all 20 patients and was extensive (involving >50% of vessels) in 12 (60%). In 16 patients (80%), angioinvasion was accompanied by intravascular thrombosis. Hemorrhagic infarction was seen in 18 patients (90%), and intra-alveolar hemorrhage and coagulative necrosis were each observed in 17 patients (85%). In contrast, inflammatory necrosis was observed in only 6 patients (30%); when present, inflammatory cell infiltrates consisted predominantly of mononuclear cells. Granulomas were seen in only 2 patients. Perineural invasion by fungal hyphae was not observed.
Table 1.
Histopathologic findings according to immunologic background in 20 cases of pulmonary mucormycosis.
| Total (N = 20) | Neutropenic (N = 13) | Non-neutropenic (N = 7)a | Allogeneic HSCT (N = 6)b | |
|---|---|---|---|---|
| Angioinvasion | 20 (100) | 13 (100) | 7 (100) | 6 (100) |
| Angioinvasion in >50% of vessels | 12 (60) | 10 (77) | 2 (29)c | 4 (67) |
| Intravascular thrombosis | 16 (80) | 12 (92) | 4 (57) | 5 (83) |
| Intra-alveolar hemorrhage | 17 (85) | 12 (92) | 5 (71) | 3 (50)d |
| Hemorrhagic infarction | 18 (90) | 12 (92) | 6 (86) | 5 (83) |
| Coagulative necrosis | 17 (85) | 10 (77) | 7 (100) | 6 (100) |
| Inflammatory necrosis | 6 (30) | 4 (31) | 2 (29) | 4 (67)d |
| Granuloma | 2 (10) | 1 (8) | 1 (14) | 0 (0) |
Five non-neutropenic patients had been treated with high-dose corticosteroids.
All allogeneic HSCT recipients had significant GVHD, 3 were neutropenic, and 5 had been treated with high-dose corticosteroids.
P = 0.06 for the comparison of neutropenic and non-neutropenic patients.
P < 0.05 for the comparison of allogeneic HSCT recipients with non-allogeneic HSCT recipients.
Figure 1.
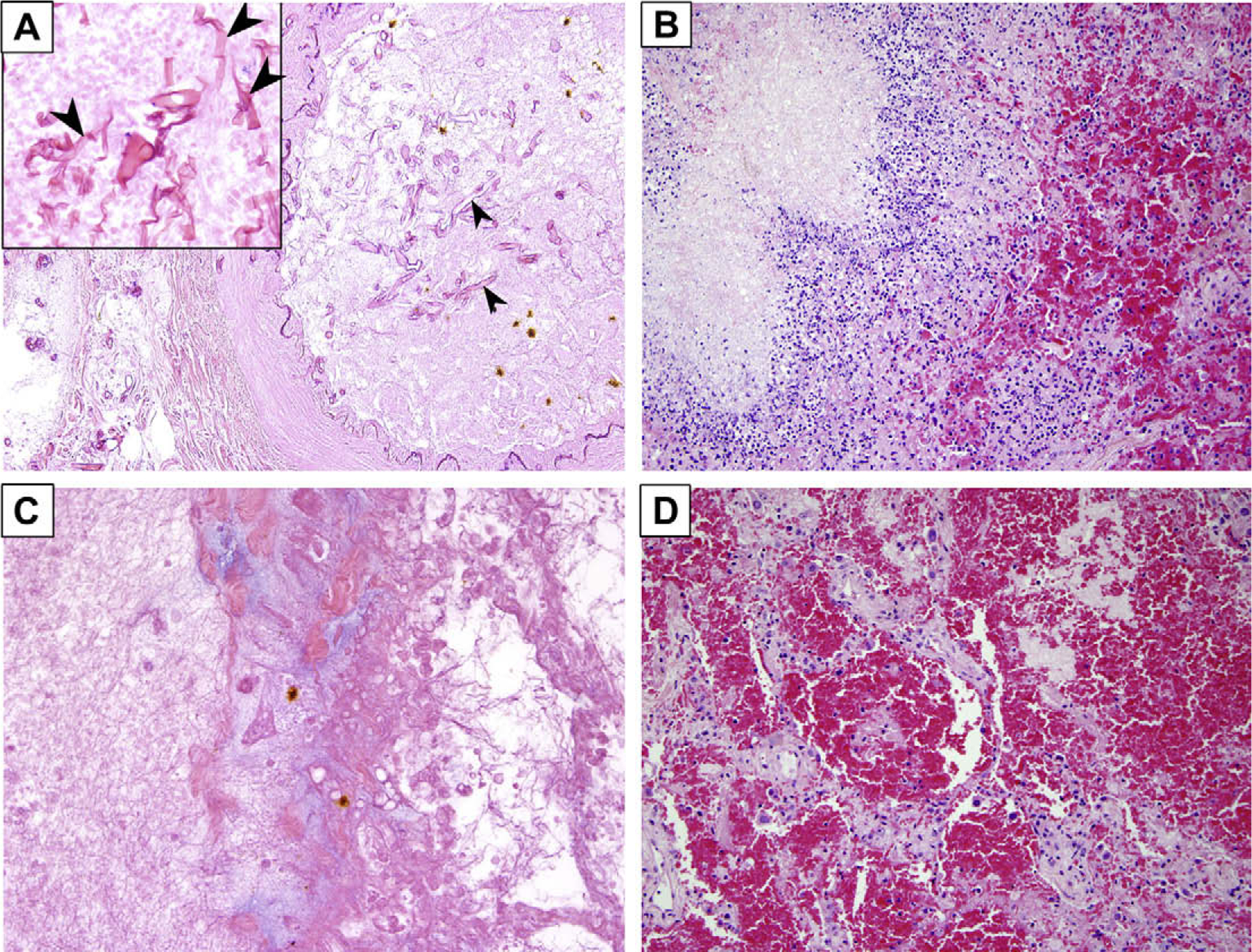
Histopathologic features of pulmonary mucormycosis in cancer patients. (A) Hyphae invading a blood vessel lumen (arrowheads). Inset: short pleomorphic, non-septated hyphae typical of Mucorales species (hematoxylin-and-eosin [H&E] stain ×400). (B) Inflammatory necrosis (H&E stain ×400). (C) Coagulative necrosis with no significant inflammatory infiltration (H&E stain ×400). (D) Intra-alveolar hemorrhage (H&E stain ×400).
A comparison between patients with different immunologic backgrounds revealed differences in the patterns of tissue damage and inflammatory response. Neutropenic patients tended to have more extensive angioinvasion by fungal hyphae than did non-neutropenic patients (10 of 13 [77%] versus 2 of 7 [29%], respectively; P = 0.06]. Compared with patients who had not received HSCT, allogeneic HSCT recipients had a higher frequency of inflammatory necrosis (2 of 14 [14%] versus 4 of 6 [67%], respectively; P = 0.04) and a lower frequency of intra-alveolar hemorrhage (14 of 14 [100%] versus 3 of 6 [50%], respectively; P = 0.02) (Table 1).
Discussion
Clinicopathologic studies may offer insights into the pathobiology of invasive mycoses. Such studies have helped elucidate the host–pathogen interactions of invasive pulmonary aspergillosis by demonstrating divergent patterns of tissue damage and inflammation in neutropenic versus non-neutropenic hosts.4,5 However, histopathologic data about PMM in patients with different forms of immunosuppression is lacking. We studied the histopathologic features of PMM in 20 cancer patients. Angioinvasion, intravascular thrombosis, coagulative necrosis, and pulmonary hemorrhage were present in almost all cases; however, more extensive angioinvasion was observed in neutropenic patients compared with non-neutropenic patients. These findings differ from those of the aforementioned studies of pulmonary aspergillosis. Specifically, we did not observe a paradoxical hyperinflammatory response in the lungs of non-neutropenic patients with mucormycosis. Further, Stergiopoulou et al.4 and Shaukat et al.10 reported that the histopathologic findings in allogeneic HSCT recipients with pulmonary aspergillosis resembled those of neutropenic patients, possibly because of impaired neutrophil trafficking. In contrast, in our study, HSCT recipients had significantly more inflammatory exudates and inflammatory necrosis in their lungs and less intra-alveolar hemorrhage than did non-HSCT recipients.
Our findings in HSCT recipients may reflect the complex local and systemic immunologic milieu in these patients. All HSCT recipients in our study developed mucormycosis ≥100 days after transplantation, had concomitant chronic GVHD, and were treated with multiple immunosuppressive agents. Both acute and late-onset lung injury syndromes have been associated with GVHD.11 Because these syndromes may reflect an inflammatory response to occult infections and other undefined stimuli, we speculate that the inflammatory response to Mucorales may be dysregulated in the presence of allogeneic HSCT and active GVHD.
Recent observations from in vitro studies and animal models have uncovered significant differences in the inflammatory response to Aspergillus species and Mucorales.Wholegenome profiling in Drosophila melanogaster after infection with Rhizopus oryzae revealed downregulation of the expression of multiple genes involved in host defense and immunity, whereas a higher proportion of genes were upregulated in flies infected with Aspergillus fumigatus.12 Consistent observations were derived from Balb/c mice infected with R. oryzae and A. fumigatus13;the transcription of genes encoding for proinflammatory cytokines, such as tumor necrosis factor α and interleukin 1, was significantly upregulated after infection with A. fumigatus in corticosteroid-treated mice. However, differential expression of genes that encode for proinflammatory cytokines was not observed in mice infected with R. oryzae, regardless of the mode of immunosuppression.13 Significant differences in gene expression profiles of normal human elutriated monocytes were also observed for responses to A. fumigatus and R.oryzae.14 Thus, our observations in clinical histopathologic specimens are consistent with those of animal studies that demonstrate a muted host inflammatory response in experimental mucormycosis.
A recent study by Frater et al.9 summarized the histopathologic findings in 20 cases of mucormycosis, 6 of which were pulmonary. Similar to our findings, angioinvasion and tissue infarction were described in all pulmonary specimens. However, the authors also observed neutrophilic infiltrates in all cases and granulomatous inflammation in 3 of the 6 pulmonary cases. These differences may be a result of the different patient populations represented in these studies. Although comorbidities were not reported by Frater et al., their study may have included patients with less immunosuppression than ours. Perineural invasion was noted predominantly in cases of rhinocerebral mucormycosis, whereas we found no cases of pulmonary perineural invasion in our study.
A limitation of our study is that the diagnosis of mucormycosis was confirmed by culture in 45% of cases, reflecting the relative rarity of culture-positive PMM, even in a tertiary cancer center such as ours. Although morphological examination may occasionally lead to misclassification errors,15 studies have shown that the finding of broad, non-septate hyphae in tissue sections has high positive predictive value (PPV) for the diagnosis of mucormycosis. For example, in a study of percutaneous lung biopsies performed in immunocompromised patients, 100% of specimens with non-septate hyphae were identified as Mucorales species by culture or polymerase chain reaction (PCR), whereas no specimen with septate hyphae was identified as belonging to this order.16 Additional studies have demonstrated that the PPV of histopathlogy compares favorably with PCR analysis (96–100% with PCR as a reference)15,17 and in situ hybridization (100% versus 86%, with culture as a gold standard).18
Establishing the diagnosis of PMM and differentiating it from pulmonary aspergillosis has important therapeutic implications, because the Mucorales are resistant to some of the antifungal drugs used against Aspergillus spp., such as voriconazole and the echinocandins. Knowledge of the histopathological features of PMM in cancer patients could aid in the diagnosis of this life-threatening opportunistic infection. Furthermore, we found that while PMM was generally associated with a muted inflammatory response, inflammatory necrosis was prominent in the lungs of allogeneic HSCT recipients with PMM. These variations in histopathology may reflect differences in the immunobiology of PMM in different patient groups.
Acknowledgements
We dedicate this work to the memory of Dr. Mario Luna, a pioneer in the study of the histopathologic characteristics of fungal infections in cancer patients.
Footnotes
Conflict of interest
The authors have no conflict of interest.
References
- 1.Kontoyiannis DP, Lewis RE. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect Dis Clin North Am 2006;20:581–607. [DOI] [PubMed] [Google Scholar]
- 2.Pyrgos V, Shoham S, Walsh TJ. Pulmonary zygomycosis. Semin Respir Crit Care Med 2008;29:111–20. [DOI] [PubMed] [Google Scholar]
- 3.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634–53. [DOI] [PubMed] [Google Scholar]
- 4.Stergiopoulou T, Meletiadis J, Roilides E, Kleiner DE, Schaufele R, Roden M, et al. Host-dependent patterns of tissue injury in invasive pulmonary aspergillosis. Am J Clin Pathol 2007;127:349–55. [DOI] [PubMed] [Google Scholar]
- 5.Balloy V, Huerre M, Latge JP, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun 2005;73:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamilos G, Luna M, Lewis RE, Bodey GP, Chemaly R, Tarrand JJ, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period. Haematologica 1989–2003;2006(91):986–9. [PubMed] [Google Scholar]
- 7.Berenguer J, Allende MC, Lee JW, Garrett K, Lyman C, Ali NM, et al. Pathogenesis of pulmonary aspergillosis. Granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am J Respir Crit Care Med 1995;152:1079–86. [DOI] [PubMed] [Google Scholar]
- 8.Kumar V, Fausto N, Abbas A. Robbins & Cotran pathologic basis of disease. 7th ed Philadelphia: W.B. Saunders; 2004. [Google Scholar]
- 9.Frater JL, Hall GS, Procop GW. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch Pathol Lab Med 2001;125:375–8. [DOI] [PubMed] [Google Scholar]
- 10.Shaukat A, Bakri F, Young P, Hahn T, Ball D, Baer MR, et al. Invasive filamentous fungal infections in allogeneic hematopoietic stem cell transplant recipients after recovery from neutropenia: clinical, radiologic, and pathologic characteristics. Mycopathologia 2005;159:181–8. [DOI] [PubMed] [Google Scholar]
- 11.Watkins TR, Chien JW, Crawford SW. Graft versus host-associated pulmonary disease and other idiopathic pulmonary complications after hematopoietic stem cell transplant. Semin Respir Crit Care Med 2005;26:482–9. [DOI] [PubMed] [Google Scholar]
- 12.Chamilos G, Lewis RE, Hu J, Xiao L, Zal T, Gilliet M, et al. Drosophila melanogaster as a model host to dissect the immunopa-thogenesis of zygomycosis. Proc Natl Acad Sci USA 2008; 105: 9367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Ami R, Lewis RE, Kontoyiannis DP. Differences in expression of angiogenesis and inflammation-encoding genes in aspergillosis versus zygomycosis: the impact of mechanism of immune suppression in a pulmonary model of infection In: Proceedings of the 48th interscience conference on antimicrobial agents and chemotherapy, 2008, Washington, DC. Washington, DC: American Society for Microbiology; 2008. p. 684. [Google Scholar]
- 14.Cortez K, Lyman C, Lempicki R, Ren P, Yang J, Cotton C, et al. Comparative functional pathway analysis of the genomic profiles of human monocytes in response to Aspergillus fumigatus and Rhizopus oryzae In: Proceedings of the 47th interscience conference on antimicrobial agents and chemotherapy, 2007, Chicago, IL. Washington, DC: American Society for Microbiology; 2007. p. 456. [Google Scholar]
- 15.Rickerts V, Mousset S, Lambrecht E, Tintelnot K, Schwerdtfeger R, Presterl E, et al. Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin Infect Dis 2007;44:1078–83. [DOI] [PubMed] [Google Scholar]
- 16.Lass-Florl C, Resch G, Nachbaur D, Mayr A, Gastl G, Auberger J, et al. The value of computed tomography-guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin Infect Dis 2007;45:e101–4. [DOI] [PubMed] [Google Scholar]
- 17.Bialek R, Konrad F, Kern J, Aepinus C, Cecenas L, Gonzalez GM, et al. PCR based identification and discrimination of agents of mucormycosis and aspergillosis in paraffin wax embedded tissue. J Clin Pathol 2005;58:1180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden RT, Qian X, Procop GW, Roberts GD, Lloyd RV. In situ hybridization for the identification of filamentous fungi in tissue section. Diagn Mol Pathol 2002;11:119–26. [DOI] [PubMed] [Google Scholar]


