Abstract
The goal of ulcerative colitis (UC) treatment has recently been shown to be “mucosal healing,” as no drug directly induces mucosal healing. Probiotics possess sufficient safety, but their efficacy in the treatment of UC remains controversial because of the influence of intestinal conditions. It is believed that the identification of bioactive molecules produced by probiotics and their application will help to solve this issue. We therefore identified a probiotic‐derived long‐chain polyphosphate as a molecule enhancing the intestinal barrier function. This study demonstrated that long‐chain polyphosphate exhibited antiinflammatory effects in a human macrophage and interleukin‐10 knockout transfusion mouse model. The first‐in‐human trial showed that 7 of the 10 enrolled patients acquired clinical remission, 4 of whom achieved endoscopic remission despite a history of treatment with anti–tumor necrosis factor (TNF)–α agents. No adverse reactions were observed. Long‐chain polyphosphate might be useful for the treatment of refractory UC, even in patients with failure or intolerance to anti‐TNF‐α therapy.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ More than half of ulcerative colitis (UC) patients fail to achieve mucosal healing with conventional drug therapy.
☑ The efficacy of probiotics in the treatment of UC remains controversial.
☑ We previously identified a long‐chain polyphosphate from Lactobacillus brevis SB88 and suggested that it was effective for improving the intestinal barrier function and inflammation.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ All drugs that are clinically available for the treatment of UC are antiinflammatory or immunomodulatory agents; none directly improve the intestinal barrier function.
☑ The safety and efficacy of oral enteric capsules containing long‐chain polyphosphates in humans remain unclear.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ No adverse reactions occurred in association with long‐chain polyphosphate treatment in our first‐in‐human trial.
☑ Among the 10 enrolled patients for whom conventional drug therapy had failed, 7 acquired a clinical response, 4 of whom achieved endoscopic remission.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Because of their high safety and efficacy, long‐chain polyphosphates are strongly believed to be useful for the treatment of refractory ulcerative colitis.
Ulcerative colitis (UC) is a condition of chronic intestinal inflammation, the pathogenesis of which is largely unknown. While steroids and mesalamine are the standard treatments for relieving symptoms, a treatment strategy for curing the disease has not yet been established. Most patients experience relapses repeatedly and suffer from abdominal pain, diarrhea, and hematochezia for more than 50 years. “Mucosal healing,” which is defined by a sufficient intestinal barrier function, leads to a significantly long‐term remission;1 however, more than half of UC patients fail to achieve mucosal healing, even when using recently developed anti–tumor necrosis factor (TNF)–α treatments.2
Probiotics are live microorganisms that confer a health benefit on the host when administered in adequate amounts3 and are used as functional foods as well as drugs for the treatment of acute intestinal inflammation and necrotizing enterocolitis.4, 5, 6 Probiotics possess sufficient safety to be used for a long time; however, their efficacy as a treatment for inflammatory bowel diseases remains controversial7 because the function of live bacteria is influenced by the intestinal conditions in individual patients. To solve this weak point of probiotic treatment, we have considered using the bioactive molecules produced by probiotics, which enhance the intestinal barrier function regardless of the intestinal conditions, as do drugs.
A method for identifying bioactive molecules8 was used to identify the probiotic‐derived molecule responsible for improving the intestinal barrier function. Briefly, fraction‐inducing HSP27, which is known to increase the intestinal barrier function, in human Caco2/BBE cells was separated from the conditioned media of Lactobacillus brevis SBC8803 using spin columns equipped with several molecular weight cutoff membranes, diethylaminoethyl anion‐exchange chromatography, and size‐exclusion chromatography. The amino acid composition and peptidoglycan content of the HSP27‐inducible fractions were shown to be extremely low; however, the fraction contained a large amount of phosphorus (90% weight for weight) and oxygen. Based on these results, we assumed that the HSP27‐inducible fraction was a polyphosphate. Synthesized polyphosphate poly P significantly induced HSP27 in Caco2/BBE cells, and the HSP27 induction by the culture supernatant of L. brevis was diminished by the degradation of polyphosphate using polyphosphate kinase, indicating that poly P was the molecule responsible for the induction of HSP27.9 We proposed that the administration of long‐chain polyphosphate led to the localization of cell‐adherent molecules along the tight junction in intestinal epithelia and thereby improved the intestinal barrier function and inflammation in vitro and in chemical‐induced colitis mice through the epithelial endocytosis of long‐chain polyphosphate‐integrin β1‐caveolin‐1 complex (patent nos. JP5660508, EP2559437).10, 11, 12 This was the first study to show that long‐chain polyphosphate was able to improve the intestinal barrier function and ameliorate intestinal injury under inflammatory conditions, although short‐polyphosphate is known to enhance the proliferation of human fibroblasts by stabilizing fibroblast growth factors13 and to exert antibacterial activity against many bacteria and fungi.14, 15, 16, 17 A subsequent study also showed that polyphosphate produced by a marine cyanobacterium, Synechococcus, exerted an antiinflammatory function.18
The present study identified the most suitable chain length of polyphosphate for the enhancement of the intestinal barrier function and confirmed the efficacy of long‐chain polyphosphate in inflammation models (including a genetically induced mouse colitis model and inflammation‐induced human macrophages). Furthermore, the safety of long‐chain polyphosphate was confirmed by preclinical tests, and we conducted the first‐in‐human trial to determine the safety and efficacy of long‐chain polyphosphate in patients with refractory UC.
Results
The reinforcement of the intestinal barrier by polyphosphate was dependent on the chain length
To determine the chain lengths of polyphosphate that might enhance the intestinal barrier function, an ex vivo 3H‐mannitol study was performed using 12.5, 65, 300, 450, and 700‐mer polyphosphates. The barrier function was enhanced by the polyphosphates in a concentration‐dependent manner (Figure S1 ). The grade of the barrier function when using the 450‐mer polyphosphate did not differ from that when using the 700‐mer polyphosphate (Figure 1 a). A transepithelial electric resistance (TER) test was performed to assess the effect of polyphosphates on the epithelial barrier function. The permeability of the Caco2 cell layer was increased by TNF‐α treatment, and both preventive and simultaneous treatments with the 450‐mer and 700‐mer polyphosphates improved the TER at 7.5 hours (Figure 1 b), indicating that treatment with the 450‐mer polyphosphate sufficiently increased the intestinal barrier function in comparison with longer polyphosphates. Thus, the 450‐mer polyphosphate was used as the long‐chain polyphosphate for the subsequent studies. The incubation of long‐chain polyphosphate under acidic conditions (pH 1.2) for 2 hours failed to reduce 3H‐mannitol leakage (Figure 1 c). A high‐performance liquid chromatography spectrum analysis revealed that the retention time of polyphosphate had shifted from 16.701 to 18.826 minutes, indicating that fragmentation of polyphosphate occurred under acidic conditions (Table 1). These findings suggest that the protection of long‐chain polyphosphates from gastric juice in the stomach, which has a low pH, is necessary for them to effectively enhance the intestinal barrier function.
Figure 1.
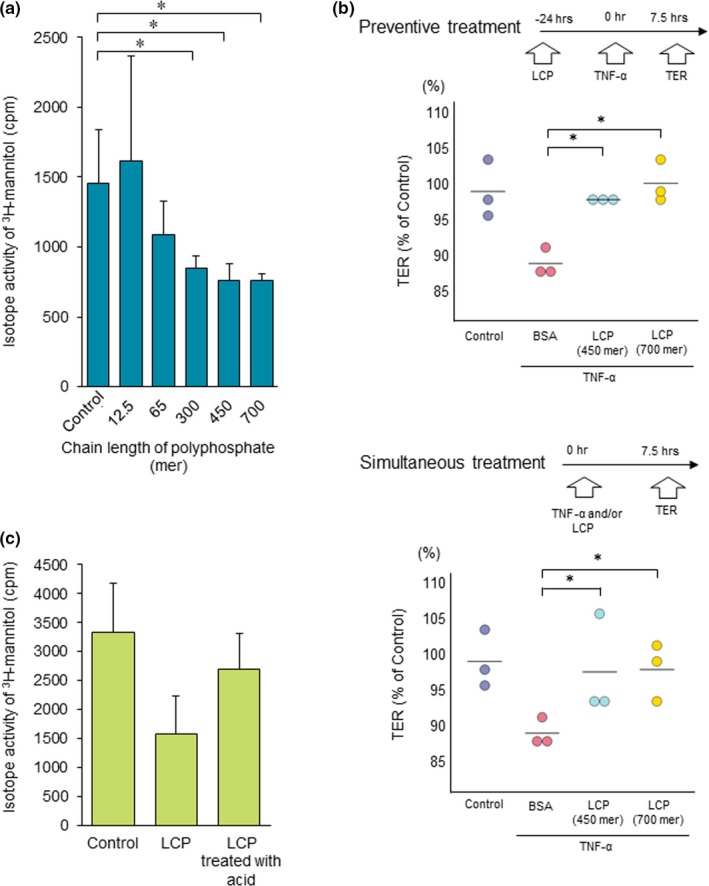
Polyphosphate enhanced the intestinal barrier function in a chain length‐dependent manner. (a) An ex vivo study revealed that the intestinal permeability induced by oxidative stress was significantly attenuated by treatment with 300‐mer, 450‐mer, and 700‐mer polyphosphates (n = 5). (b) The TER test is a method of assessing the resistance between the upper and lower sides of the transwell in vitro. The TER test showed that the permeability induced by TNF‐α treatment was reduced by long‐chain polyphosphate treatment (n = 3). (c) Long‐chain polyphosphates incubated under acidic conditions (pH 1.2) for 2 hours did not enhance the intestinal barrier function in an ex vivo study. *P < 0.05 by Student's t‐test. The error bars show the standard deviation. BSA, bovine serum albumin; LCP, long‐chain polyphosphate; NT, no treatment; TER, transepithelial electrical resistance; TNF‐α, tumor‐necrosis factor alpha. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Shortening of polyphosphates under acidic conditions
| R. Time (minutes) | LCP/BSA (R. Time) | 50%‐width (minutes) | 10%‐width (minutes) | |
|---|---|---|---|---|
| LCP | 16.701 | 0.971 | 1.358 | 2.948 |
| LCP treated with acid | 18.826 | 1.095 | 2.107 | 3.163 |
| BSA | 17.195 | — | — | — |
10%‐width, width of the wave 10% from the bottom of the wave; 50%‐width, width of the wave 50% from the bottom of the wave; BSA, bovine serum albumin; LCP, long‐chain polyphosphate; R. Time, retention time.
Long‐chain polyphosphate exerted an antiinflammatory effect in human‐derived M1 macrophages, an IL‐10 KO transfusion mouse model, and a DSS‐induced colitis rat model
To clarify the antiinflammatory effects in active macrophages, M1 macrophages were treated with the polyphosphate and Lipopolysaccharide (LPS). The LPS‐inducible production of TNF‐α in M1 macrophages was inhibited by treatment with 0.1 μm long‐chain to 3.0 μm long‐chain polyphosphates (≥450 mer) but not short‐chain polyphosphates (≤65 mer) ( Figure S2 ), suggesting that long‐chain polyphosphate exerted an antiinflammatory effect as well as enhanced the intestinal barrier function.
To clarify the treatment effect of long‐chain polyphosphate in vivo, an interleukin (IL)‐10 knockout (KO) transfusion mouse model was constructed. The diarrhea score in IL‐10 KO transfusion mice transanally treated with long‐chain polyphosphate showed a significant improvement, and the colon weight in the IL‐10 KO transfusion group was significantly increased in comparison with the no‐transfusion group but was significantly reduced by the transanal treatment with long‐chain polyphosphate (Figure 2 a). The diarrhea score, but not the colon weight, of IL‐10 KO transfusion mice that received oral long‐chain polyphosphate recovered in comparison with the phosphate‐buffered saline (PBS) group (Figure 2 b). These data suggested that, in order to achieve sufficient efficacy, it was important to prevent the reduction of the activity of polyphosphates under acidic conditions. To overcome this issue, an enteric‐coated capsule containing long‐chain polyphosphate was generated, and the treatment effect of the capsule was analyzed in a dextran sodium sulfate (DSS)‐induced colitis rat model. The DSS‐treated rats showed shortening of the colon, and recovered following treatment with capsules in a dose‐dependent manner (Figure 2 c). A reverse transcription polymerase chain reaction revealed that the messenger RNA (mRNA) expression of proinflammatory cytokines, including TNF‐α and IL‐1β, and the histopathological scores of intestinal damage were reduced by the administration of the capsules (Figure 2 d,e). These findings suggest that an enteric‐coated capsule containing long‐chain polyphosphate may be useful for the treatment of intestinal inflammation.
Figure 2.
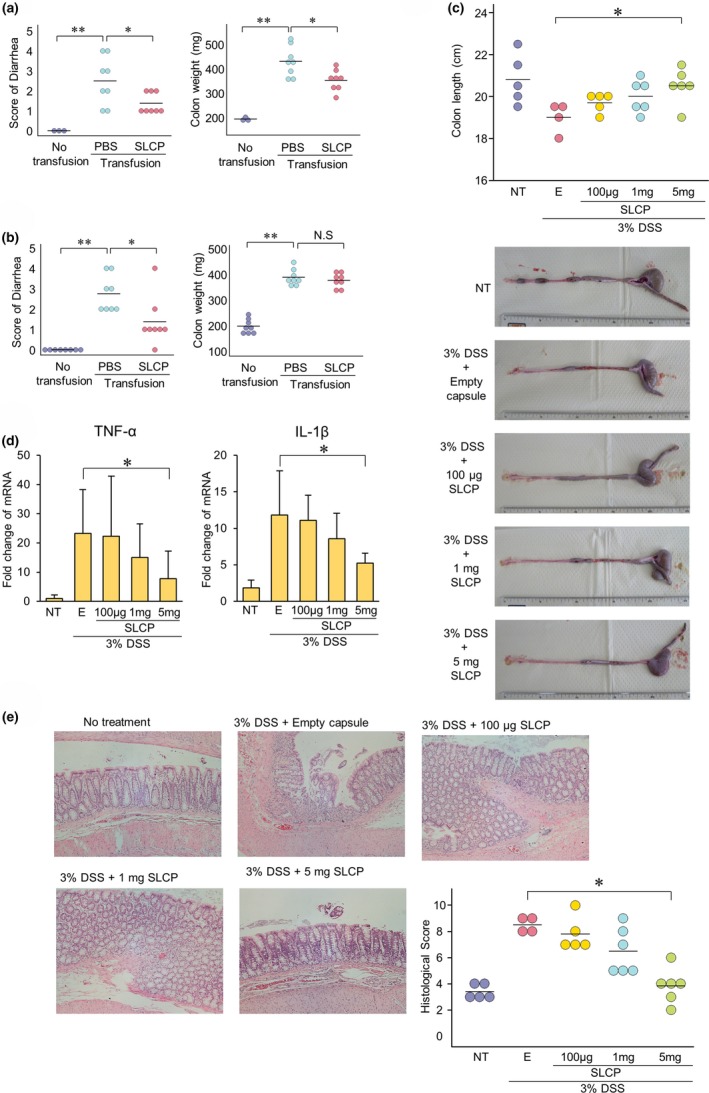
Long‐chain polyphosphate exerted treatment effects against inflammation both in vitro and in vivo. (a) The diarrhea score and colon weight of interleukin (IL)‐10 knockout (KO) transfusion mice recovered with transanal long‐chain polyphosphate treatment in comparison with the phosphate‐buffered saline (PBS) group (n = 6‐8). (b) The diarrhea score, but not the colon weight, of IL‐10 KO transfusion mice recovered with oral long‐chain polyphosphate treatment in comparison with the PBS group (n = 8). (c) To avoid exposure to acidic conditions and shortening of long‐chain polyphosphate, the polyphosphate was enclosed in gelatin capsules, and then enteric coating was performed. In the DSS‐induced colitis rat model, the colon length significantly recovered with the oral administration of enteric‐coated capsules containing long‐chain polyphosphate in the 5 mg/body polyphosphate group (n = 5). (d) The reverse transcription polymerase chain reaction revealed that in the DSS‐induced colitis rat model, the expression levels of TNF‐α and IL‐1β were significantly reduced by the administration of enteric‐coated polyphosphate capsules in the 5 mg/body polyphosphate group (n = 5). (e) In the DSS‐induced colitis rat model, the histopathological score recovered with the oral administration of enteric‐coated capsules containing long‐chain polyphosphate (n = 5). *P < 0.05, **P < 0.01 by Student's t‐test. The error bars show the standard deviation. DSS, dextran sodium sulfate; E, empty capsule; IL, interleukin; KO, knockout; LCP, long‐chain polyphosphate; NT, no treatment; PBS, phosphate‐buffered saline; TNF‐α, tumor‐necrosis factor alpha; Transfusion, IL‐10 KO‐derived T‐lymphocyte transfusion.
Preclinical tests
A nonrodent (beagle) repeated‐dose toxicity study using long‐chain polyphosphate (328 and 2,296 mg/body/day) for 4 weeks, a rodent single‐dose toxicity study using long‐chain polyphosphate (40, 200, and 1,000 mg/kg/day) for 2 weeks, a rodent single‐dose toxicity study using long‐chain polyphosphate (10 and 90 mg/body/day), a mutagenicity test, an in vitro micronucleus test of long‐chain polyphosphate in TK6 cells, and safety pharmacology studies to investigate the effects of long‐chain polyphosphate on the central nervous and respiratory systems in rats revealed no adverse events (details are shown in the study protocol).
First‐in‐human trial
After confirming the effects and safety of long‐chain polyphosphate with preclinical studies, a first‐in‐human trial was conducted. In this study, no adverse reactions associated with the intake of long‐chain polyphosphate were observed on any electrocardiograms, urinary tests, or blood examinations (including abnormal serum levels of phosphates).
In step 1, the partial Mayo scores of four of five patients were decreased. Two of the patients achieved a clinical remission (partial Mayo score ≤2) as well as endoscopic remission (Mayo endoscopic subscore ≤1, corresponding to mucosal healing). These two patients who achieved remission had shown either treatment failure or intolerance to anti‐TNF‐α agents. In step 2, the partial Mayo scores of three of five patients were decreased, including two patients with failure or intolerance of anti‐TNF‐α agents. Among the 10 patients in the study population, long‐chain polyphosphate relieved the disease activity in 7 patients, including 4 who achieved endoscopic remission at the end of the 4‐week drug administration period. Long‐chain polyphosphate was able to induce clinical and endoscopic remission in patients with refractory UC regardless of the failure or intolerance of anti‐TNF‐α agents (Table 2). The mRNA expression of inflammatory cytokines decreased in six of seven patients, thereby relieving their disease activity. In the three patients who did not respond to long‐chain polyphosphate therapy, the partial Mayo score at one week after starting the treatment did not improve, and the mRNA expression of inflammatory cytokines did not decrease (data not shown).
Table 2.
The prestudy evaluation and Mayo scores before and after long‐chain polyphosphate treatment
| Step | Case | Age | Sex | Type | Treatment history | Before treatment | After treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid | 5‐ASAe | Anti‐TNF | CI | TMS | PMS | MES | TMS | PMS | MES | CR | MH | |||||
| 1 | 1 | 58 | M | Left side | D | + | − | − | 8 | 5 | 3 | 5 | 3 | 2 | + | − |
| 2 | 72 | M | Proctitis | D | + | − | − | 5 | 3 | 2 | 5 | 3 | 2 | − | − | |
| 3 | 39 | F | Total | D | + | + | + | 6 | 4 | 2 | 3 | 2 | 1 | + | + | |
| 4 | 36 | F | Left side | D | + | + | − | 6 | 4 | 2 | 2 | 1 | 1 | + | + | |
| 5 | 55 | F | Left side | R | + | − | − | 7 | 5 | 2 | 5 | 3 | 2 | + | − | |
| 2 | 1 | 69 | M | Left side | D | + | + | + | 8 | 6 | 2 | 5 | 3 | 2 | + | − |
| 2 | 53 | F | Left side | D | + | + | − | 6 | 4 | 2 | 3 | 2 | 1 | + | + | |
| 3 | 19 | F | Total | D | + | + | − | 7 | 5 | 2 | 10 | 7 | 3 | − | − | |
| 4 | 54 | F | Total | D | + | + | − | 7 | 5 | 2 | 5 | 4 | 1 | + | + | |
| 5 | 44 | F | Left side | R | + | − | − | 7 | 5 | 2 | 10 | 7 | 3 | − | − | |
5‐ASA, aminosalicylic acid (mesalamine); Anti‐TNF, anti‐TNF‐α agents; CI, calcineurin inhibitor; CR, clinical response; D, steroid‐dependent; MES, Mayo endoscopic subscore; MH, mucosal healing; PMS, partial Mayo score; R, steroid‐resistant; TMS, total Mayo score.
Case presentation (Case 4 in step 1)
A 36‐year‐old woman with an onset of UC at 22 years of age visited our hospital because of recurrence with a high frequency of bowel movements and hematochezia. Despite receiving 12 subcutaneous injections of adalimumab, she failed to achieve clinical remission. Before the administration of long‐chain polyphosphate, her partial Mayo score and Mayo endoscopic subscore had been 4 and 2, respectively. After 4 weeks of long‐chain polyphosphate treatment (300 mg, daily) her partial Mayo score and Mayo endoscopic subscore decreased to 2 and 1, respectively (Figure 3 a). Colonoscopy revealed an edematous mucosa and multiple small depressions in the sigmoid colon and rectum before long‐chain polyphosphate treatment. These findings disappeared after the administration of the drug (Figure 3 b). A clinical remission has been maintained for more than one year since the treatment.
Figure 3.
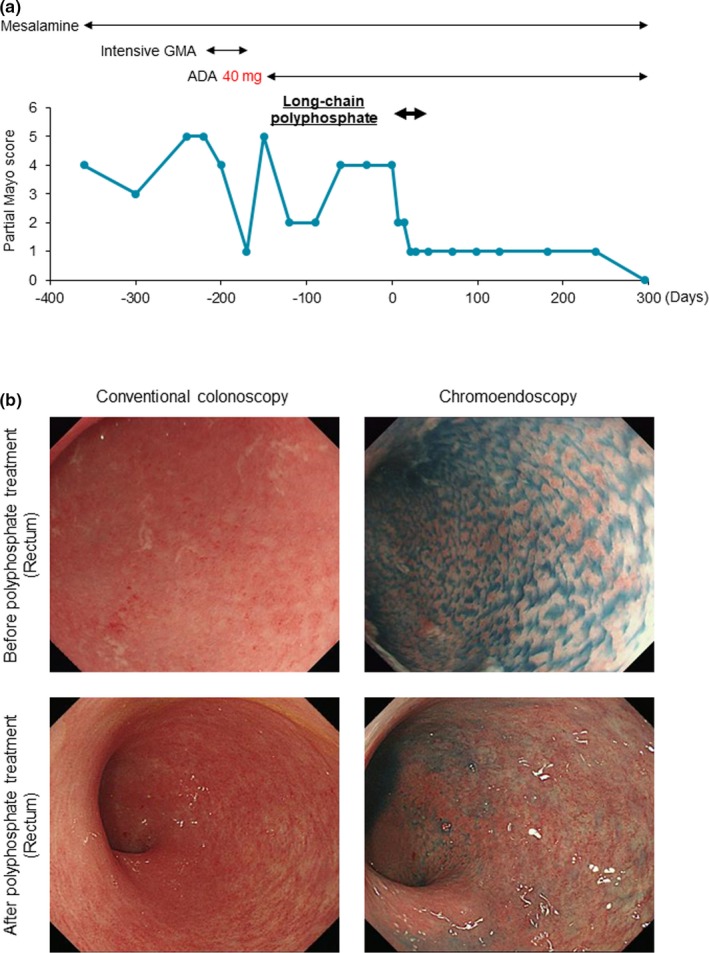
The treatment history and clinical course in one patient at 1 year before and after the administration of long‐chain polyphosphates. (a) One year before the administration of long‐chain polyphosphates, the patient experienced relapse three times. Just before undergoing long‐chain polyphosphate treatment, her partial Mayo score was 4. After 4 weeks of treatment with long‐chain polyphosphate (300 mg), her partial Mayo score decreased to 1, and a clinical remission has been maintained for more than 1 year since treatment. (b) Conventional endoscopy revealed an edematous mucosa with an indistinct vascular pattern (upper left), while chromoendoscopy detected multiple small depressions (upper right) in the sigmoid colon and rectum before the administration of long‐chain polyphosphate. The edematous mucosa disappeared (lower left), and a vascular pattern was visualized on conventional colonoscopy, with no further small depressions detected by chromoendoscopy (lower right) after drug administration. 5‐ASA, 5‐aminosalicylic Acid; ADA, adalimumab; GMA, granulocyte and monocyte adsorption apheresis.
Discussion
Probiotics possess sufficient safety to be used for a long time, but their efficacy in UC treatment remains controversial7 due to the diversity of intestinal conditions among patients. To resolve the issue and develop a drug that is effective against UC, regardless of intestinal conditions, we developed a method for identifying bioactive molecules from probiotic‐conditioned media8 and isolated the bioactive long‐chain polyphosphate molecule from media conditioned with L. brevis SB88.9 Our findings suggested that long‐chain polyphosphate treatment enhanced the intestinal barrier function and relieved the intestinal injury under inflammatory conditions both in vitro and in an IL‐10 KO transfusion mouse model. The function of polyphosphates is known to depend on their chain length. Short‐chain‐length polyphosphates have been shown to enhance the proliferation of human fibroblasts by stabilizing fibroblast growth factors13 and exert antibacterial effects against microorganisms.14, 15, 16, 17 The present study showed that long‐chain (chain length ≥ 450) polyphosphates improved the intestinal barrier function under conditions of oxidative stress, while short‐chain polyphosphates (chain length ≤ 65) exerted no significant effect on the intestinal barrier function. We therefore developed a procedure for fashioning long‐chain polyphosphates for clinical trials and conducted the first‐in‐human trial to assess the outcomes of long‐chain polyphosphate therapy for refractory UC in 10 patients who responded poorly to currently available treatments. Seven patients achieved a clinical response, and four achieved endoscopic remission after 4 weeks of treatment with long‐chain polyphosphate. The response and remission induction rates were almost the same as those in previous studies using anti‐TNF‐α agents2 or recently developed biological drugs, including Janus kinase inhibitors and vedolizumab.19, 20 Because our human trial was a single‐arm study, it is difficult to compare the effects of long‐chain polyphosphate therapy with a control group, such as the achievement of spontaneous remission. However, Travis, et al.21 showed in a phase III study of budesonide that the remission rates at 8 weeks were 4.5% among patients in the placebo group. In a phase III study of budesonide, Sandborn, et al.22 showed that the remission rates at 8 weeks were 6.2% among patients in the placebo group. These recent studies suggested that the spontaneous remission rate was quite low. Thus, the remission rate of 40% in our study is thought to be higher in comparison with the general spontaneous remission rate.
It is notable that efficacy was observed in patients with failure or intolerance to anti‐TNF‐α agent therapy, as well as in naïve patients. Our ex vivo intestinal loop study and in vitro TER test showed that long‐chain polyphosphate improved the intestinal barrier function impaired by not only TNF‐α but also monochloramine (NH2CL), suggesting that long‐chain polyphosphate does not directly target TNF‐α. Thus, long‐chain polyphosphate therapy has a function that is distinct from that of anti‐TNF‐α agents and is a feasible alternative to costly biological drugs for treating refractory UC.
The drugs that are currently available for the treatment of UC in the clinical setting are all antiinflammatory or immunomodulating agents, and none have directly improved the intestinal barrier function. We previously reported the mechanisms of action of long‐chain polyphosphates on the improvement of intestinal injuries. Briefly, long‐chain polyphosphates bind to the integrin β1 expressed on the intestinal epithelia,9 forming a complex with caveolin‐1 (phosphate‐integrin β1‐caveolli‐1 complex). Thereafter, the complex is endocytosed into the cytosol of the intestinal epithelia and p38 mitogen‐activated protein kinase is activated. Then, cell‐adhesion‐associated molecules are strongly upregulated and the intestinal barrier function is increased.11 Based on our results, the effect of long‐chain polyphosphate on the intestinal barrier function was thought to be due to the upregulation of cell‐adhesion‐associated molecules. The impairment of the intestinal barrier function is known to lead to the translocation of bacteria and virulence, thereby aggravating the disease status of intestinal disorders, including inflammatory bowel diseases. A recent study showed that mesalamine targeted polyphosphate kinase in pathogenic bacteria but did not show that mesalamine enhanced the intestinal barrier function at all.23 Long‐chain polyphosphates may be the first drugs to help prevent the invasion of bacteria and virulence through the direct enhancement of the intestinal barrier function.
We also confirmed that long‐chain polyphosphates reduced the expression of inflammatory cytokines, including TNF‐α and IL‐12, in human M1 macrophages and in the colon of DSS‐induced and IL‐10 KO transfusion mouse models. This suggests that long‐chain polyphosphate possesses an antiinflammatory function both in vitro and in vivo. Regarding the function in relation to macrophages, we previously showed that long‐chain polyphosphates reduced the expression of TNFα in LPS‐stimulated THP‐1 cells that were pretreated with macrophage colony‐stimulating factor and IFNγ. We also confirmed that long‐chain polyphosphate improved the intestinal injury in a 2,4,6‐Trinitrobenzenesulfonic acid–induced colitis model10 as well as a DSS‐induced colitis model. While the mechanisms of the antiinflammatory effect are still being explored, our previous study showed that long‐chain polyphosphates might target M1 macrophages. Based on these findings in experimental models, long‐chain polyphosphates are expected to exhibit an antiinflammatory effect in patients with inflammatory bowel diseases.
Regarding the safety of long‐chain polyphosphates, preclinical studies, including nonrodent repeated‐dose toxicity studies, rodent single‐dose toxicity studies, mutagenicity tests, and safety pharmacology tests, revealed no adverse events. In addition, the present study showed no adverse events in 10 UC patients with active inflammation. These data suggest that long‐chain polyphosphates are a safe drug.
In conclusion, long‐chain polyphosphate was safe and effective for the treatment of refractory UC in the first‐in‐human study. The next phase of this study has been prepared and will provide stronger evidence concerning the safety and efficacy of long‐chain polyphosphate in the treatment of UC, including appropriate targets for treatment.
Materials and Methods
Animals
Details are provided in the Supplementary Material .
Polyphosphate
Polyphosphates with a length of 12.5 (BENX‐PolyP‐S), 65 (BENX‐PolyP), and 700 (BENX‐EPolyP) mers were purchased from BIOENEX, Higashihiroshima, Japan, while 300‐mer and 450‐mer polyphosphates were obtained by enzymatic reactions.9
Calculating the chain length of the polyphosphates
Polyphosphates were incubated in reaction buffer containing 50 mM Tris‐HCl pH 7.5, 0.01% N,N‐Bis(4‐sulfobutyl)‐3‐methylaniline disodium salt, 0.01% 4‐aminoantipyrine, 2 mM adenosine diphosphate, 2 mM MgCl2, 10 mM glycerol, 10 U/mL glycero‐3‐P oxidase, 5 U/mL peroxidase, 1 U/mL glycerol kinase, and 0.1 mg/mL polyphosphate kinase at 37°C for 10 minutes. Two reaction volumes of 2% sodium dodecyl sulfate were then added, and detection was performed at an optical density of 550 nm. The chain length of the polyphosphates was calculated using a 700‐mer polyphosphate as the standard.
The HPLC spectrum analysis of the polyphosphates
The polyphosphates were separated using an HPLC system (Shimadzu Corporation, Kyoto, Japan) with a TSKgel GMPW XL (φ7.8 mm × 300 mm) column at 30°C and eluted with 10 mM phosphate buffer containing 0.5 M sodium chloride, at a flow rate of 0.5 mL/minute. The eluent was monitored by ultraviolet spectrophotometry at 260 nm. Bovine serum albumin was used as a standard.
TER test
Details are provided in the Supplementary Material .
Human peripheral blood‐derived macrophages
Details are provided in the Supplementary Material .
The ex vivo intestinal loop study
C57Bl/6 mice were euthanized, and the small intestine was divided into three pieces, with each end ligated with silk sutures and the loops filled with RPMI 1640 medium containing the sample. The loops were incubated for 2 hours at 37°C in a 5% CO2 incubator. To assess the permeability effects, the loops were filled with RPMI 1640 medium containing 1 mCi/mL [3H]‐mannitol with or without 0.3 mM freshly prepared NH2Cl. The loops were placed into the middle section of the organ culture dish in 4 mL of RPMI 1640 without NH2Cl. To assess the permeability, samples were taken at 5, 20, and 35 minutes to determine the flux of mannitol from the lumen to the media outside the loops. Radioactivity was measured using a scintillation spectrometer.
Isotope‐labeled polyphosphate
Details are provided in the Supplementary Material .
Pharmacokinetics study
Mice were treated with 32P‐labeled long‐chain polyphosphate (500 μg) orally or through the duodenum and then euthanized 2, 5, or 24 hours later. The organs, stool, urine, inferior vena cava blood, and portal blood were collected at euthanizing, and the radioactivity was measured using a scintillation spectrometer.
Enteric‐coated capsules
Details are provided in the Supplementary Material .
DSS‐induced colitis
Details are provided in the Supplementary Material .
The real‐time polymerase chain reaction
Details are provided in the Supplementary Material .
The IL‐10 KO‐derived T‐lymphocyte transfusion (IL‐10 KO transfusion) mouse model
CD4‐positive cells were purified from the spleen, mesenteric lymph node, and sacral lymph node of IL‐10 KO mice. A total of 5 × 106 CD4‐positive cells were suspended in 1 mL PBS, and 0.2 mL/mouse of cell suspension was transferred to SCID mice. Starting on day 1, long‐chain polyphosphate or PBS was transanally administered to the mice every day. The diarrhea score was assessed at day 15. The scores were determined as follows: score 0, solid stool that was not deformed when pushed by finger; score 1, solid stool that was deformed when pushed by finger; score 3, solid stool that was easily collapsed when pushed by finger; score 3, soft stool that was easily deformed and stuck to container; score 4, muddy or watery stool. The mice were euthanized on day 15 under isoflurane anesthesia, the colon was sampled, and the weight of the colon was measured.
First‐in‐human trial
We designed an investigator‐initiated, nonrandomized, open‐label pilot study of long‐chain polyphosphate (University Hospital Medical Information Network number, UMIN000021555) involving 10 adult patients with refractory UC. The study was approved by the ethics committee of Asahikawa Medical University (Number, 15189), and each patient provided their written informed consent.
We developed a double‐capsule formulation using size 0 and 2 DRcaps capsules (Capsugel Japan, Tokyo, Japan) containing 100 mg of long‐chain polyphosphate in a capsule. Ten UC patients, including two steroid‐resistant and eight steroid‐dependent cases (refractory UC), who visited Asahikawa Medical University Hospital from May 2016 to December 2017 and who showed a disease activity with a Mayo score ranging from 3 to 8 with obvious hematochezia were enrolled in this study. The first five patients orally took 300 mg of long‐chain polyphosphate once daily for 4 weeks (step 1); the second five patients orally took 900 mg of long‐chain polyphosphate once daily for 4 weeks (step 2). Anti‐TNF‐α agent treatments were fixed at 20 weeks before taking the study drugs, and other medications were fixed 2 weeks before taking the study drugs. All treatments used before the enrollment were continued during the study. In the patients enrolled in the present study, biologics were fixed ≥20 weeks before taking the study drugs, and all other medications were fixed ≥4 weeks before taking the study drugs, except for antibiotics, which were fixed 2 weeks before taking the study drugs in one case (case 2 in step 2). Therefore, none of the enrolled patients were excluded due to concomitant medications.
The disease activity of the enrolled patients was assessed using the Mayo score (the calculation of the Mayo and partial mayo scores is described in the Supplementary Methods ). The partial Mayo score was assessed before starting the study and every week until the administration schedule was completed. The Mayo endoscopic subscore was assessed before and after the study. Before enrollment, pan‐colonoscopy was performed in all patients to determine the most severe inflammation site, which was set as the assessment area. The severity of the inflammation was then assessed based on the Mayo endoscopic subscore. After long‐chain polyphosphate treatment, the subscore of the assessment area evaluated by initial colonoscopy was reassessed. Pan‐colonoscopy was performed as second colonoscopy only if the endoscopist concluded that other sites needed to be evaluated.
The mRNA expression of inflammatory cytokines, including TNF‐α, IFN‐γ, and IL‐1β, in biopsy samples obtained from the rectosigmoid colon were measured before and after this study. The primary endpoint was the safety of the long‐chain polyphosphate, and the secondary endpoint was the efficacy of this drug in patients with refractory UC. Monitoring and inspection of this study were performed by a third‐party organization (MRS Planning, Tokyo, Japan).
Steroids, mesalamine, anti‐TNF‐α agents, and calcineurin inhibitors were used by 10, 10, 6, and 2 of the enrolled patients, respectively, before their entry in this study. In step 1, the disease activity was classified as follows: Mayo 5, n = 1; Mayo 6, n = 2 patients; Mayo 7, n = 1 patient; and Mayo 8, n = 1. In step 2, the activity at the beginning of this study was classified as Mayo 6, n = 1; Mayo 7, n = 3; and Mayo 8, n = 1 (Table 1). Blood examinations performed before starting this study revealed no abnormalities, with the exception of slight C‐reactive protein elevation in three patients.
Statistical analyses
Details are provided in study protocols of the first‐in human trial.
Funding
This study was partially supported by Ministry of Education, Culture, Sports, Science and Technology Grants‐in‐Aid for Scientific Research, No. 26460956 (M.F.), Translational Research Network Program of Japan Agency for Medical Research and Development, No. C40 (M.F.), and Development and Intractable Disease Health and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare. This study was partially supported as a joint research project of Asahikawa Medical University and EA Pharma Co., Ltd.
Conflict of Interest
M.F. reports grants from Japanese Grants‐in‐Aid for Scientific Research, grants from Translational Research Network Program of Japan Agency for Medical Research and Development, nonfinancial support from Development and Intractable Disease Health, and Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare. During the conduct of the study: grants and personal fees from Yakult Honsha Co., Ltd, grants and personal fees from Nippon Kayaku Co., Ltd., grants, personal fees, and nonfinancial support from EA Pharma Co., Ltd., personal fees from Novartis Pharmaceuticals, grants from Boehringer Ingelheim GmbH, personal fees from Technical Information Institute Co., Ltd, personal fees from Pfizer Inc., grants and personal fees from Takeda Pharmaceutical Company Limited, grants and personal fees from Daiichi Sankyo Company, Limited, grants from Shionogi & Co., Ltd, personal fees from Olympus Co., Ltd., personal fees from Janssen Pharmaceutical K.K., personal fees from Kyorin Pharmaceutical Co., Ltd., personal fees from Taisho Toyama Pharmaceutical Co., Ltd., grants from AstraZeneca, personal fees from Tomakomai Minpo Co., Ltd., personal fees from Meiji Seika Pharma Co., Ltd., grants and personal fees from Mochida Pharmaceutical Co., Ltd, grants from Astellas Pharma Inc., personal fees from Mitsubishi Tanabe Pharma Corporation, grants from Baxter International Inc, personal fees from Bristol‐Myers Company, grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants from MSD K.K., grants and personal fees from Otsuka Pharmaceutical Co., Ltd, grants and personal fees from Kyowa Hakko Kirin Co., Ltd., grants from Taiho Pharmaceutical Co., Ltd, personal fees from Zeria Pharmaceutical Co., Ltd., grants from Alexion Pharmaceutical Co., Ltd., grants, personal fees, and nonfinancial support from Ajinomoto Pharmaceutical Co., Ltd., personal fees from Asahi Kasei Corporation, grants and nonfinancial support from Sapporo Breweries Ltd., grants and personal fees from Eisai Co., Ltd., grants from GlaxoSmithKline K.K., grants from AbbVie Inc, grants from Sumitomo Dainippon Pharma Co., Ltd., grants from Asuka Pharmaceutical Co., Ltd., grants from Boston Scientific Corporation or its affiliates, grants and nonfinancial support from Kamui Pharma. Inc., outside the submitted work. In addition, M.F. has been issued patents for intestinal protectants (JP 5660508 and EP 2559437). The other authors declared no conflicts of interest in association with the present study.
Author Contributions
M.F. and N.U. wrote the manuscript; M.F. and N.U. designed the research; M.F, N.U., S.K., K.T, K.A., Ke.M., H.K., N.K., Y.T., Tom.O. and Ka.M. performed the research; M.F, N.U., A.S., M.T., and Tos.O. analyzed the data.
Supporting information
Figure S1. The effects of several concentrations of long‐chain polyphosphate on the improvement of the intestinal barrier function.
Figure S2. Long‐chain polyphosphate inhibited the anti–tumor necrosis factor–α production in Lipopolysaccharide‐stimulated M1 macrophages.
Supplementary Methods
Clinical Trial Protocol
Acknowledgments
We thank Miyuki Sato, Kuninori Iwayama, Naoya Kamiyama, Takashi Ono, and other members of the Department of Hospital Pharmacy and Pharmacology and the Clinical Research Support Center of Asahikawa Medical University, Professor Hiroshi Funakoshi, Professor Yasuaki Saijyo, and Professor Nobuyuki Sato, and Atsushi Yonezawa and Takayuki Nakagawa and other members of the Department of Clinical Pharmacology and Therapeutics of Kyoto University Hospital for their great support of this study.
References
- 1. Pineton de Chambrun, G. , Peyrin‐Biroulet, L. , Lémann, M. & Colombel, J.F. Clinical implications of mucosal healing for the management of IBD. Nat. Rev. Gastroenterol. Hepatol. 7, 15–29 (2010). [DOI] [PubMed] [Google Scholar]
- 2. Cholapranee, A. , Hazlewood, G.S. , Kaplan, G.G. , Peyrin‐Biroulet, L. & Ananthakrishnan, A.N. Systematic review with meta‐analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn's disease and ulcerative colitis controlled trials. Aliment. Pharmacol. Ther. 45, 1291–1302 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Food and Agriculture Organization and WHO . Guidelines for the Evaluation of Probiotics in Food. 1–11 (WHO, London, ON, 2002). [Google Scholar]
- 4. Neu, J. & Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sazawal, S. , Hiremath, G. , Dhingra, U. , Malik, P. , Deb, S. & Black, R.E. Efficacy of probiotics in prevention of acute diarrhoea: a meta‐analysis of masked, randomised, placebo‐controlled trials. Lancet Infect. Dis. 6, 374–382 (2006). [DOI] [PubMed] [Google Scholar]
- 6. Deshpande, G. , Rao, S. & Patole, S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet 369, 1614–1620 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Derwa, Y. , Gracie, D.J. , Hamlin, P.J. & Ford, A.C. Systematic review with meta‐analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 46, 389–400 (2017). [DOI] [PubMed] [Google Scholar]
- 8. Fujiya, M. et al The Bacillus subtilis quorum‐sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe 1, 299–308 (2007). [DOI] [PubMed] [Google Scholar]
- 9. Segawa, S. et al Probiotic‐derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin‐p38 MAPK pathway. PLoS ONE 6, e23278 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kashima, S. et al Polyphosphate, an active molecule derived from probiotic Lactobacillus brevis, improves the fibrosis in murine colitis. Transl. Res. 166, 163–175 (2015). [DOI] [PubMed] [Google Scholar]
- 11. Tanaka, K. et al Probiotic‐derived polyphosphate improves the intestinal barrier function through the caveolin‐dependent endocytic pathway. Biochem. Biophys. Res. Commun. 467, 541–548 (2015). [DOI] [PubMed] [Google Scholar]
- 12. Sakatani, A. et al Polyphosphate derived from Lactobacillus brevis inhibits colon cancer progression through induction of cell apoptosis. Anticancer Res. 36, 591–598 (2016). [PubMed] [Google Scholar]
- 13. Shiba, T. et al Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J. Biol. Chem. 278, 26788–26792 (2003). [DOI] [PubMed] [Google Scholar]
- 14. Post, F.J. , Krishnamurty, G.B. & Flanagan, M.D. Influence of sodium hexametaphosphate on selected bacteria. Appl. Microbiol. 11, 430–435 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knabel, S.J. , Walker, H.W. & Harman, P.A. Inhibition of Aspergillus flavus and selected gram‐positive bacteria by chelation of essential metal cations by polyphosphates. J. Food Prot. 54, 360–365 (1991). [DOI] [PubMed] [Google Scholar]
- 16. Rajkowski, K.T. , Calderone, S.M. & Jones, E. Effect of polyphosphate and sodium chloride on the growth of Listeria monocytogenes and Staphylococcus aureus in ultra‐high temperature milk. J. Dairy Sci. 77, 1503–1508 (1994). [DOI] [PubMed] [Google Scholar]
- 17. Maier, S.K. , Scherer, S. & Loessner, M.J. Long‐chain polyphosphate causes cell lysis and inhibits Bacillus cereus septum formation, which is dependent on divalent cations. Appl. Environ. Microbiol. 65, 3942–3949 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng, G. et al Biogenic polyphosphate nanoparticles from a marine cyanobacterium Synechococcus sp. PCC 7002: production, characterization, and antiinflammatory properties in vitro. Mar. Drugs. 16, 322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feagan, B.G. et al Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369, 699–710 (2013). [DOI] [PubMed] [Google Scholar]
- 20. Sandborn, W.J. et al Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376, 1723–1736 (2017). [DOI] [PubMed] [Google Scholar]
- 21. Travis, S.P. et al Once‐daily budesonide MMX in active, mild‐to‐moderate ulcerative colitis: results from the randomised CORE II study. Gut 63, 433–441 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandborn, W.J. et al Induction of clinical and colonoscopic remission of mild‐to‐moderate ulcerative colitis with budesonide MMX 9 mg: pooled analysis of two phase 3 studies. Aliment. Pharmacol. Ther. 41, 409–418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dahl, J.U. et al The antiinflammatory drug mesalamine targets bacterial polyphosphate accumulation. Nat. Microbiol. 2, 16267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The effects of several concentrations of long‐chain polyphosphate on the improvement of the intestinal barrier function.
Figure S2. Long‐chain polyphosphate inhibited the anti–tumor necrosis factor–α production in Lipopolysaccharide‐stimulated M1 macrophages.
Supplementary Methods
Clinical Trial Protocol


