Abstract
Risankizumab, an anti‐interleukin‐23 monoclonal antibody, achieved significantly (P < 0.001) greater Psoriasis Area and Severity Index (PASI) and static Physician Global Assessment (sPGA) clear or almost clear (0/1) responses than adalimumab in a phase III trial in patients with moderate‐to‐severe psoriasis. Meta‐analyses of the PASI 50, PASI 75, PASI 90, PASI 100, and sPGA0/1 responses after 16 weeks of treatment from eight (three for risankizumab and five for adalimumab) randomized, placebo‐controlled trials were conducted to estimate the efficacy difference between risankizumab and adalimumab. For PASI 75, PASI 90, PASI 100, and sPGA0/1 responses, the estimated effect differences (95% confidence interval) between risankizumab and adalimumab were 15.2% (10.1%, 20.4%), 23.7% (15.7%, 31.2%), 20.8% (13.0%, 28.7%), and 20.1% (13.7%, 26.1%), respectively. These results were consistent with the observed efficacy difference from the head‐to‐head phase III trial, which was not included in the meta‐analyses, providing independent, confirmatory evidence of the superior efficacy of risankizumab compared with adalimumab for treatment of moderate‐to‐severe psoriasis.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Risankizumab, an anti‐interleukin‐23 monoclonal antibody, achieved significantly (P < 0.001) greater Psoriasis Area and Severity Index (PASI) and static Physician Global Assessment (sPGA) clear or almost clear (0/1) responses than adalimumab in a phase III trial in patients with moderate‐to‐severe psoriasis.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This study excluded the head‐to‐head trial of risankizumab vs. adalimumab (IMMvent) in order to provide an independent assessment of the comparative efficacy of risankizumab and adalimumab through meta‐analyses of efficacy in placebo‐controlled trials.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ These efficacy responses of risankizumab relative to adalimumab estimated from the meta‐analyses were consistent with an independent head‐to‐head phase III trial of both drugs, providing confirmatory evidence of superior efficacy of risankizumab relative to adalimumab in treatment of chronic plaque psoriasis.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The approach utilized in this work can be used to provide supportive model‐based replication of evidence of efficacy comparisons, utilizing the totality of data across clinical trials, to further contextualize the results from head‐to‐head comparisons.
Risankizumab, a fully humanized immunoglobulin G1 monoclonal antibody, is specific for the p19 subunit of interleukin (IL)‐23.1 IL‐23 has been shown to have a key role in autoimmunity and inflammation through its induction of Th17 cells, which have been implicated in the pathogenesis of several immune‐mediated inflammatory diseases, such as psoriasis, psoriatic arthritis, Crohn's disease, and ulcerative colitis.2, 3 By blocking IL‐23 from binding to its receptor, risankizumab inhibits IL‐23‐dependent cell signaling and the release of proinflammatory cytokines.
In phase II trials, risankizumab was shown to be safe and efficacious in patients with moderate‐to‐severe chronic plaque psoriasis,4 psoriatic arthritis,5 and Crohn's disease.6 Four phase III trials of risankizumab were conducted in patients with moderate‐to‐severe chronic plaque psoriasis evaluating a risankizumab dose of 150 mg administered subcutaneously (SC) at week 0, week 4, and every 12 weeks (q12w) thereafter. Among these, three of the trials were placebo‐controlled (IMMhance (NCT02672852),7 UltIMMa‐1 (NCT02684370),8 and UltIMMa‐2 (NCT02684357)9), and two of the placebo‐controlled trials also included ustekinumab as an active comparator (UltIMMa‐1 and UltIMMa‐2).10 The fourth phase III trial was a study with adalimumab as the active comparator IMMvent (NCT02694523).11 The results from these phase III trials showed that risankizumab achieved significantly greater (P < 0.001) rates of skin responses (84–88% of patients with clear or almost clear skin) than ustekinumab (62–63% of patients), adalimumab (60% of patients), and placebo (7%) after 16 weeks of treatment.12, 13
The objective of the current meta‐analyses was to provide an independent estimate of the comparative efficacy of risankizumab and adalimumab by means of an indirect comparison using placebo as a common comparator. The primary end points of interest were Psoriasis Area and Severity Index (PASI) 90 and static Physician Global Assessment (sPGA) clear or almost clear (0/1) responses after 16 weeks of treatment with risankizumab (administered as 150 mg SC at week 0, week 4, and q12w thereafter) or adalimumab (administered as a 40 mg every two weeks (q2w) SC dosing regimen starting 1 week after an initial loading dose of 80 mg at week 0). The secondary end points of interest were PASI 50, PASI 75, and PASI 100 responses after 16 weeks of treatment with risankizumab or adalimumab. The analyses excluded the head‐to‐head trial of risankizumab vs. adalimumab (IMMvent) in order to provide an independent assessment of the relative efficacy.
Results
Patient enrollment and characteristics
Eight randomized, placebo‐controlled trials were included in the meta‐analyses: three for risankizumab and five for adalimumab. The data set for PASI and sPGA analyses included efficacy data from a total of 3,767 patients: 1,547 were treated with adalimumab, 1,005 were treated with risankizumab, and 1,215 were treated with placebo. A summary of patient characteristics by study and treatment group in the analysis data set is shown in Table 1.
Table 1.
Summary of patient characteristics by study and treatment group
| Characteristic | CHAMPION | REVEAL | VOYAGE 1 | VOYAGE 2 | X‐PLORE | UltIMMa‐1 | UltIMMa‐2 | IMMhance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ada | Plc | Ada | Plc | Ada | Plc | Ada | Plc | Ada | Plc | RZB | Plc | RZB | Plc | RZB | Plc | |
| Agea, y | 42.9 | 40.7 | 44.1 | 45.4 | 42.9 | 44.9 | 43.2 | 43.3 | 47.5 | 45 | 48.3 | 49.3 | 46.2 | 46.3 | 49.6 | 47.9 |
| Weighta, kg | 81.7 | 82.6 | 92.3 | 94.1 | 90.5 | 88 | 87.6 | 88.6 | 91.6 | 93.6 | 87.8 | 88.8 | 92.2 | 92.2 | 92.2 | 91.1 |
| Males, % | 64.8 | 66 | 67.1 | 64.6 | 74.6 | 68.4 | 68.5 | 69.8 | 69.8 | 66.7 | 69.7 | 77.5 | 69.0 | 68.4 | 69.5 | 73 |
| Patients with PsAb, % | 21.3 | 20.8 | 27.5 | 28.4 | 18.6 | 17.2 | 17.7 | 18.5 | 25.6 | 28.6 | 28 | 35.3 | 25.1 | 32.6 | 34.9 | 34.0 |
| Disease durationa, y | 17.9 | 18.8 | 18.1 | 18.4 | 17 | 17.6 | 17.6 | 17.9 | 19.3 | 18 | NA | NA | NA | NA | NA | NA |
| Race: white, % | 95.4 | 92.5 | 91.2 | 90.2 | 82.9 | 83.3 | 80.6 | 83.1 | 90.7 | 92.9 | 65.8 | 69.6 | 86.7 | 88.8 | 78.6 | 82 |
| Race: black, % | 1.9 | 1.9 | 3.3 | 5 | 2.4 | 1.7 | 2 | 3.2 | 2.3 | 2.4 | 3.3 | 1.0 | 3.4 | 2.0 | 4.4 | 2.0 |
| Race: Asian, % | 2.8 | 3.8 | 2.6 | 1.8 | 14.1 | 13.2 | 14.9 | 10.9 | 7 | 4.8 | 28.3 | 27.5 | 8.5 | 7.1 | 15.7 | 15.0 |
| Affected BSAa, % | 33.6 (19.9) | 28.4 (16.1) | 25.8 (15.5) | 25.6 (14.8) | 28.6 (16.7) | 25.8 (15.9) | 29.1 (16.7) | 28.0 (16.5) | 26.8 (16.8) | 27.5 (19.3) | 26.2 (15.3) | 27.9 (17.2) | 26.2 (15.9) | 23.9 (15.7) | 25.6 (17.0) | 28.3 (19.1) |
| Baseline PASI scorea | 20.2 (7.5) | 19.2 (6.9) | 19.0 (7.1) | 18.8 (7.1) | 22.4 (9.0) | 20.4 (8.7) | 21.7 (9.0) | 21.5 (8.0) | 20.2 (7.6) | 21.8 (10.0) | 20.6 (7.7) | 20.5 (6.7) | 20.5 (7.8) | 18.9 (7.3) | 19.9 (7.9) | 21.2 (8.7) |
| Baseline sPGA scorea | 3.59 | 3.66 | 3.55 | 3.50 | 3.26 | 3.25 | 3.21 | 3.23 | 3.49 | 3.50 | 3.16 | 3.20 | 3.22 | 3.20 | 3.21 | 3.20 |
| sPGA end point | 6‐point PGAc | 6‐point PGAc | 6‐point PGAd | 6‐point PGAd | 5‐point IGAe | 5‐point IGAe | 5‐point IGAe | 5‐point IGAe | 6‐point PGAf | 6‐point PGAf | 5‐point PGAg | 5‐point PGAg | 5‐point PGAg | 5‐point PGAg | 5‐point PGAg | 5‐point PGAg |
| Prior biologic treatment (%) | 0h | 0h | 11.9 | 13.3 | 21.0 | 19.5 | 19.8 | 21.8 | 60.5 | 35.7 | 34.2 | 39.2 | 40.1 | 42.9 | 56.5 | 51.0 |
PGA and IGA end points were collectively analyzed as sPGA. Ada, adalimumab; BSA, body surface area; IGA, Investigator's Global Assessment; NA, not applicable; PASI, Psoriasis Area and Severity Index; Plc, placebo; PsA, psoriatic arthritis; RZB, risankizumab; sPGA, static Physician Global Assessment; y, year.
aMean (SD as applicable). bFor UltIMMa‐1, UltIMMa‐2, and IMMhance, PsA was either diagnosed or suspected. c0: clear, 1: minimal, 2: mild, 3: moderate, 4: moderate‐to‐severe, 5: very severe. d0: clear, 1: minimal, 2: mild, 3: moderate, 4: severe, 5: very severe. e0: clear, 1: minimal, 2: mild, 3: moderate, 4: severe. f0: clear, 1: minimal, 2: mild, 3: moderate, 4: marked, 5: severe. g0: clear, 1: almost clear, 2: mild, 3: moderate, 4: severe. hPrior biologic was not reported for the CHAMPION trial. Instead, prior tumor necrosis factor treatment is shown.
The observed PASI responses (PASI 50, PASI 75, PASI 90, and PASI 100) and sPGA 0/1 responses after 16 weeks of treatment in the eight trials included in the analysis data set are shown in Figures 1 and 2. The observed PASI 50, PASI 75, PASI 90, PASI 100, and sPGA 0/1 responses by trial are shown in Table 2.
Figure 1.
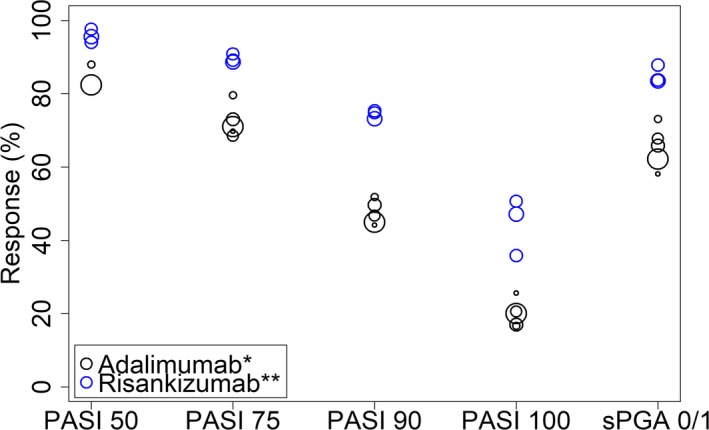
Observed Psoriasis Area and Severity Index (PASI) 50, PASI 75, PASI 90, PASI 100, and static Physician Global Assessment (sPGA) 0/1 responses after 16 weeks of treatment with adalimumab or risankizumab in the trials included in the analysis data set. Symbol size is proportional to sample size. *80 mg SC (subcutaneous) at week 0 followed by 40 mg q2w (every two weeks) starting week 1. **150 mg at week 0, week 4, and every 12 weeks thereafter.
Figure 2.
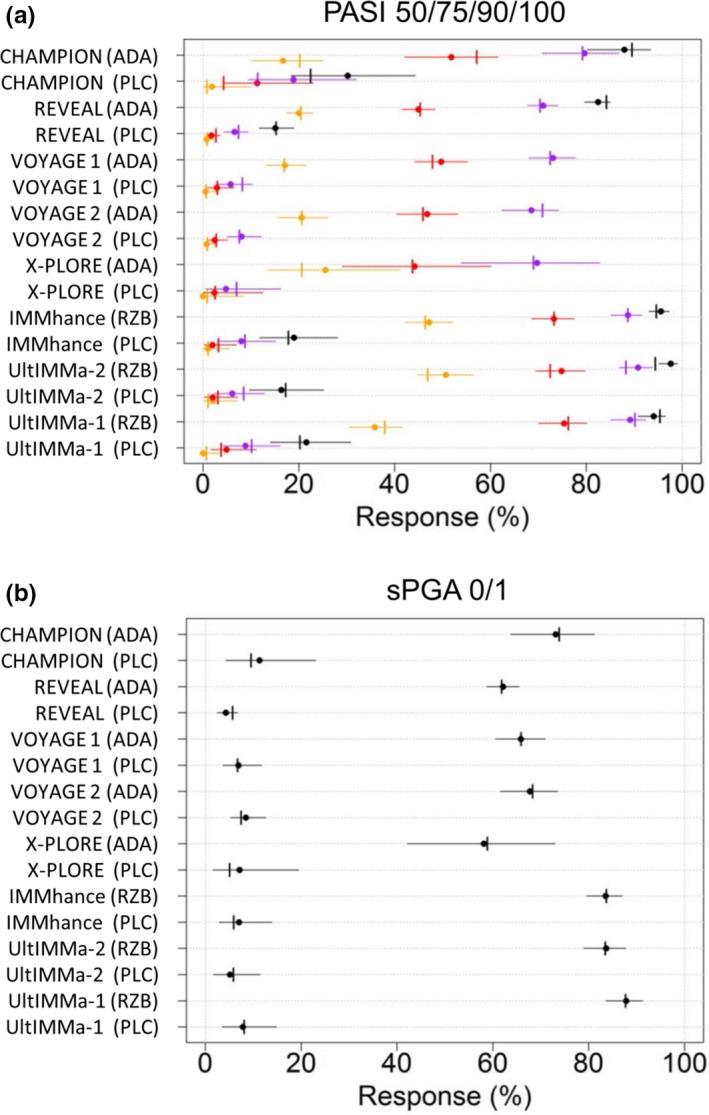
Observed and meta‐analyses estimated absolute (a) PASI and (b) sPGA responses following treatment with adalimumab, risankizumab, or placebo. Circles: a observed PASI 50 (black), PASI 75 (purple), PASI 90 (red), and PASI 100 (orange) responses or b sPGA 0/1 responses. Horizontal error bars: 95% confidence intervals of a observed PASI or b sPGA 0/1 responses. Vertical pins: meta‐analyses estimated a PASI or b sPGA 0/1 responses. ADA, adalimumab; PASI, Psoriasis Area and Severity Index; PLC, placebo; RZB, risankizumab; sPGA, static Physician Global Assessment.
Table 2.
Observed PASI 50, PASI 75, PASI 90, PASI 100, and sPGA 0/1 responses (n responders/N total (%)) by trial
| End point | CHAMPION | REVEAL | VOYAGE 1 | VOYAGE 2 | X‐PLORE | UltIMMa‐1 | UltIMMa‐2 | IMMhance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ada | Plc | Ada | Plc | Ada | Plc | Ada | Plc | Ada | Plc | RZB | Plc | RZB | Plc | RZB | Plc | |
| PASI 50 | 95/108 (88%) | 95/108 (88%) | 671/814 (82.4%) | 60/398 (15.1%) | NA | NA | NA | NA | NA | NA | 286/304 (94.1%) | 22/102 (21.6%) | 287/294 (97.6%) | 16/98 (16.3%) | 389/407 (95.6%) | 19/100 (19%) |
| PASI 75 | 86/108 (79.6%) | 86/108 (79.6%) | 578/814 (71%) | 26/398 (6.53%) | 244/334 (73.1%) | 10/174 (5.75%) | 170/248 (68.5%) | 20/248 (8.06%) | 30/43 (69.8%) | 2/42 (4.76%) | 271/304 (89.1%) | 9/102 (8.82%) | 267/294 (90.8%) | 6/98 (6.12%) | 361/407 (88.7%) | 8/100 (8%) |
| PASI 90 | 56/108 (51.9%) | 56/108 (51.9%) | 366/814 (45%) | 7/398 (1.76%) | 166/334 (49.7%) | 5/174 (2.87%) | 116/248 (46.8%) | 6/248 (2.42%) | 19/43 (44.2%) | 1/42 (2.38%) | 229/304 (75.3%) | 5/102 (4.9%) | 220/294 (74.8%) | 2/98 (2.04%) | 298/407 (73.2%) | 2/100 (2%) |
| PASI 100 | 18/108 (16.7%) | 18/108 (16.7%) | 163/814 (20%) | 3/398 (0.754%) | 57/334 (17.1%) | 1/174 (0.575%) | 51/248 (20.6%) | 2/248 (0.806%) | 11/43 (25.6%) | 0/42 (0%) | 109/304 (35.9%) | 0/102 (0%) | 149/294 (50.7%) | 2/98 (2.04%) | 192/407 (47.2%) | 1/100 (1%) |
| PGA ≤1 | 79/108 (73.1%) | 79/108 (73.1%) | 506/814 (62.2%) | 17/398 (4.27%) | 220/334 (65.9%) | 12/174 (6.9%) | 168/248 (67.7%) | 21/248 (8.47%) | 25/43 (58.1%) | 3/42 (7.14%) | 267/304 (87.8%) | 8/102 (7.84%) | 246/294 (83.7%) | 5/98 (5.1%) | 340/407 (83.5%) | 7/100 (7%) |
Ada, adalimumab; NA, not applicable; PASI, Psoriasis Area and Severity Index; PGA, Physician Global Assessment; Plc, placebo; RZB, risankizumab; sPGA, static Physician Global Assessment.
PASI and sPGA meta‐analyses
A joint analysis was conducted to describe the proportion of patients with PASI 50, PASI 75, PASI 90, and PASI 100 responses following treatment with placebo, risankizumab, or adalimumab. The correlation between different levels of PASI responses (i.e., PASI 50, PASI 75, PASI 90, and PASI 100) within each arm in a given trial was accounted for by assuming a compound symmetry correlation structure. Potential between‐trial heterogeneity in treatment effect was accounted for in the analysis by the between trial random effects. A similar separate analysis was performed for sPGA 0/1 response.
The parameter estimates for the PASI and sPGA meta‐analyses are provided in Table 3. In the PASI analyses, the PASI response‐level specific drug effects for adalimumab and risankizumab were not statistically significant compared with a constant drug effect across end points based on predefined statistical criteria (P value < 0.05), and the final model included a single estimate of log odds ratio (OR) for each drug.
Table 3.
Parameter estimates from the final PASI model and sPGA 0/1 model
| Parameter | PASI | sPGA 0/1 |
|---|---|---|
| Estimate (95% CI) on the logit scale | ||
| θoPASI 50 | 1.86 (1.75–1.97) | – |
| θoPASI 75 | 1.05 (0.97–1.13) | – |
| θoPASI 100 | −1.32 (−1.52 to −1.11) | – |
| EAdalimumab | 3.39 (3.17–3.61) | 3.29 (3.05–3.52) |
| ERisankizumab | 4.40 (4.08–4.72) | 4.40 (3.98–4.82) |
| ωoPASI 100 | 0.26 | – |
| ρ | 0.62 | – |
θoPASI 50, θoPASI 75, and θoPASI 100 represent the mean shift in placebo response on the logit scale between PASI 90 and PASI 50, PASI 75 and PASI 100, respectively. EAda and ERis represent the estimated constant drug effect across the PASI response levels, or the estimated drug effect on sPGA 0/1 response, for adalimumab and risankizumab, respectively. ωoPASI 100 represents the between‐trial variability (heterogeneity) represented as standard deviation for the difference between PASI 100 and PASI 90 placebo response; between‐trial variability could not be estimated for other PASI response levels. ρ represents the correlation between end points. A trial‐specific random effect on the drug effect (ω) for sPGA 0/1 was estimated to be very small; hence, not included in the model. CI, confidence interval; PASI, Psoriasis Area and Severity Index; sPGA, static Physician Global Assessment.
The estimated drug effects (log OR (95% confidence interval (CI))) of risankizumab vs. placebo were higher than that of adalimumab vs. placebo for both PASI and sPGA responses: 4.40 (4.08; 4.72) vs. 3.39 (3.17; 3.61) for PASI responses and 4.40 (3.98; 4.82) vs. 3.29 (3.05; 3.52) for sPGA 0/1 response. The higher drug effect estimate for risankizumab was in line with the higher observed PASI response levels and sPGA 0/1 response for risankizumab vs. adalimumab in Figure 1.
Observed and meta‐analyses estimated absolute PASI 50, PASI 75, PASI 90, and PASI 100 responses and sPGA 0/1 responses are shown in Figures 2 a and b, respectively. The observed and meta‐analyses estimated absolute responses were similar as shown by the inclusion of estimated responses within the 95% CI of the observed responses, except for the risankizumab PASI 50 response in the UltIMMa‐2 trial where the meta‐analyses estimated response was lower than the 95% CI of observed effect.
Estimated treatment effect difference between risankizumab and adalimumab
Table 4 shows the estimated treatment effect differences between risankizumab and adalimumab for the primary (PASI 90 and sPGA 0/1) and secondary efficacy parameters (PASI 50, PASI 75, and PASI 100) based on the meta‐analyses of PASI and sPGA 0/1 responses, together with the observed differences from a direct comparison between risankizumab and adalimumab in the IMMvent trial.11 For the primary efficacy parameters, the estimated treatment effect differences (95% CI) in PASI 90 response and sPGA 0/1 response between risankizumab and adalimumab were 23.7% (15.7%, 31.2%) and 20.1% (13.7%, 26.1%), respectively, based on placebo responses of 3.06% for PASI 90 and 6.40% for sPGA 0/1, as defined in the Materials and Methods section. For the secondary efficacy parameters, the estimated treatment effect difference values were 8.57% (5.59%, 11.8%), 15.2% (10.1%, 20.4%), and 20.8% (13.0%, 28.7%) for PASI 50, PASI 75, and PASI 100, respectively.
Table 4.
PASI and sPGA treatment effect difference between risankizumab and adalimumab reported in IMMvent compared with the independent estimates from the meta‐analyses that excluded IMMvent
| Parameter | Estimated treatment effect difference (95% CI) data set excluding IMMvent | Treatment effect difference (95% CI) observed in IMMvent |
|---|---|---|
| Primary | ||
| PASI 90, % | 23.7 (15.7–31.2)a | 25.1 (17.5–32.6) |
| sPGA 0/1, % | 20.1 (13.7–26.1)b | 23.5 (16.6–30.4) |
| Secondary | ||
| PASI 50, % | 8.57 (5.59–11.8)a | 13.5 (8.9–18.0) |
| PASI 75, % | 15.2 (10.1–20.4)a | 19.0 (13.0–25.0) |
| PASI 100, % | 20.8 (13.0–28.7)a | 16.8 (9.6–24.1) |
PASI, Psoriasis Area and Severity Index; sPGA, static Physician Global Assessment.
aBased on a typical PASI 90 placebo response of 3.06%. The corresponding typical placebo responses for PASI 50, PASI 75, and PASI 100 were 16.9%, 8.30%, and 0.84%, respectively. bBased on a typical sPGA 0/1 placebo response of 6.40%.
As shown in Table 4, the lower bound of the 95% CI of the meta‐analyses–based treatment effect difference was greater than zero for all five end points indicating that the PASI and sPGA responses for risankizumab were significantly higher compared with adalimumab.
The meta‐analyses–based differences in PASI and sPGA 0/1 responses were comparable to those obtained from an independent direct comparison between risankizumab and adalimumab in the IMMvent trial. The meta‐analyses–based efficacy difference for risankizumab over adalimumab were within the 95% CI of the observed difference in response in the IMMvent trial for all end points except PASI 50, where the estimated difference was slightly below the confidence interval of the observed difference in response.
Discussion
The objective of the analyses summarized in this manuscript was to compare PASI and sPGA 0/1 responses after 16 weeks of treatment with risankizumab administered as 150 mg SC at week 0, week 4, and q12w thereafter vs. adalimumab administered as 80 mg SC at week 0 followed by 40 mg q2w starting week 1. Two independent meta‐analyses were conducted to describe the efficacy reported in eight placebo‐controlled trials of adalimumab and risankizumab. One of these analyses described PASI 50, PASI 75, PASI 90, and PASI 100 responses in a combined model. The other analysis described the sPGA response of clear or almost clear (whether measured using 6‐point PGA (0–5), 5‐point (0–4) PGA, or 5‐point (0–4) Investigators Global Assessment scales).
PASI and sPGA are the recommended end points, usually used in conjunction, for evaluating the efficacy of psoriasis treatments in clinical trials.14, 15, 16, 17, 18, 19 PASI assessments are scored on a scale from 0 to 72.20 sPGA assessments, on the other hand, can be scored on a 5‐point, 6‐point, or 7‐point scale.21 In the current analyses, it was assumed that the treatment effect measured on the 5‐point PGA, 6‐point PGA, and 5‐point Investigators Global Assessment scales would be similar for clear and almost clear responses. This is because across all these scales, the response categories of 0 (clear) or 1 (minimal or almost clear) are consistently defined; the primary difference is at the upper end of the scale with the response category of 4 (in a 5‐point scale) or 4 and 5 (in the 6‐point scale). For a 5‐point scale, the response category of 4 includes subjects with severe skin symptoms, while for a 6‐point scale further gradation is included for severe response as moderate‐to‐severe/severe/marked (response category 4) or severe/very severe (response category 5). Hence, the differences in scales affected the categorization of subjects in different response categories upon entry in the trial but was assumed to have no impact on assessment of clear or clear almost response at week 16. Additionally, given the consistency of response across the trials, the assumption that these differences had no impact on the treatment effect was considered appropriate. Trial differences were accounted for in the analysis by an unstructured placebo model and between‐trial heterogeneity in treatment effect was handled by inclusion of random effects, which is a typical approach in network meta‐analysis.
Higher PASI and sPGA responses for the risankizumab clinical regimen (150 mg SC dose at week 0, week 4, and q12w thereafter) compared with the approved regimen of adalimumab (80 mg SC at week 0 followed by 40 mg q2w starting week 1) in adult patients with moderate‐to‐severe chronic plaque psoriasis were estimated for all efficacy end points evaluated in these meta‐analyses, consistent with the efficacy results observed in the clinical trials.10, 13 For the primary efficacy parameters, the estimated treatment effect difference (95% CI) in PASI 90 response between risankizumab and adalimumab was 23.7% (15.7–31.2%). The corresponding treatment effect difference in sPGA 0/1 response was 20.1% (13.7–26.1%). For the secondary efficacy parameters, the estimated treatment effect difference values were 8.57% (5.59–11.8%), 15.2% (10.1–20.4%), and 20.8% (13.0–28.7%) for PASI 50, PASI 75, and PASI 100, respectively, based on a placebo response of 3.06% for PASI 90 and 6.40% for sPGA 0/1 calculated using weighted (by sample size) mean of the placebo responses from the trials included in the analyses. The 95% CI of the estimated difference was greater than zero for all five end points, indicating that the PASI and sPGA responses for risankizumab were significantly higher compared with adalimumab, supporting the superiority of risankizumab over adalimumab in treatment of plaque psoriasis at week 16.
Treatment effect estimates for PASI and sPGA responses for risankizumab relative to adalimumab from the meta‐analyses were in good agreement with the observed values in the head‐to‐head phase III trial (IMMvent), in which superiority of efficacy of risankizumab over adalimumab was demonstrated in subjects with moderate‐to‐severe plaque psoriasis.12 The treatment effect differences for PASI and sPGA 0/1 responses based on meta‐analyses were within the 95% CI of the observed treatment effect difference for all end points except for PASI 50. The estimated treatment effect difference in PASI 50 response was smaller than for other end points since the absolute PASI 50 response was close to 100%. It is noteworthy that the meta‐analyses excluded the data from IMMvent in order to provide an independent indirect estimate of the relative efficacy between risankizumab and adalimumab using placebo as a common comparator. Overall, the model predictions independently confirmed the superiority of risankizumab clinical regimen, 150 mg SC at week 0, week 4, and q12w thereafter, relative to the approved clinical regimen of adalimumab (80 mg SC at week 0 followed by 40 mg q2w starting week 1).
One of the limitations of these analyses was that long‐term efficacy of risankizumab vs. adalimumab beyond week 16 was not compared. The focus of these analyses was to primarily compare the efficacy of risankizumab vs. adalimumab based on the coprimary end points (PASI 90 and sPGA 0/1 at week 16) in the phase III head‐to‐head trial comparing risankizumab vs. adalimumab. Additionally, the long‐term efficacy data were either not available from all trials included in the analysis due to shorter treatment duration or the long‐term data were available at different time points across different trials (e.g., week 40 through 48).
The results of the current analyses support the additional benefit of risankizumab beyond that achieved with some of the currently available therapies for chronic plaque psoriasis.
Materials and Methods
Trials included in the analyses data set
The summary level efficacy data from placebo‐controlled studies of risankizumab and adalimumab available at AbbVie were included in the analyses data set. Additionally, summary level clinical efficacy information from all randomized controlled trials in patients with moderate‐to‐severe chronic plaque psoriasis that were published in the medical literature, available from the US Food and Drug Administration and European Medicines Agency, http://ClinicalTrials.gov, or published abstracts or presentations at conferences as of October 25, 2017 were compiled.
A total of eight placebo‐controlled trials of adalimumab in patients with plaque psoriasis were identified for possible inclusion in the analyses: CHAMPION (NCT00235820),22 M02‐528 (NCT00645814),23 M04‐688 (NCT00338754),24 M13‐606 (NCT01646073),25, 26 REVEAL (NCT00237887),27 VOYAGE 1 (NCT02207231),28 VOYAGE 2 (NCT02207244),29 and X‐PLORE (NCT01483599).30 Two of these eight studies were in Asian patients (Study M04‐688 in Japanese patients and Study M13‐606 in Chinese patients) and were excluded from the analyses because no data from risankizumab trials in Asian patients were available at the time of these analyses. Study M02‐528 was also excluded because the response was assessed in a placebo‐controlled setting for up to 12 weeks and the primary time point of the direct comparison between risankizumab and adalimumab in the phase III trial was at week 16. All five remaining trials evaluated adalimumab (given as a 40 mg q2w dosing regimen starting 1 week after an initial loading dose of 80 mg) vs. placebo after 16 weeks of treatment and were included in the analyses. Each of the studies included in the meta‐analyses were approved by the corresponding IRB/ethics committees.
A total of three placebo‐controlled pivotal phase III studies of risankizumab were included in the analyses: IMMhance (NCT02672852),7 UltIMMa‐1 (NCT02684370),8 and UltIMMa‐2 (NCT02684357).9, 10 Data from the phase III trial that directly compared risankizumab with adalimumab (IMMvent, NCT02694523)11 were not included in the analyses in order to provide an independent estimate of the relative efficacy with the meta‐analyses.
Meta‐analysis of PASI and sPGA responses
A joint response model describing the proportion of patients with PASI 50, PASI 75, PASI 90, and PASI 100 responses after 16 weeks of treatment (model code available in the Supplementary Information ) was developed using the methodology previously described by others.31 The number of patients with PASIk response in treatment arm j of trial i (N response,ijk) was assumed to follow a binomial distribution with probability of response P(response)ijk and sample size N ijk (Eq. (1)).
| (1) |
The probability of response was described as the inverse logit (log‐odds) sum of an unstructured placebo response in trial i (Eoik) and a response in active treatment arm j of trial i (Edrugijk) as shown in Eq. (2).
| (2) |
Edrugijk = 0 for placebo arms. The trial‐specific placebo response accounted for the trial‐to‐trial variability in overall response based on the following structure:
| (3) |
Eoi was an unstructured placebo model defined by a fixed effect for every trial representing the logit of the PASI 90 placebo response and ηoik was a trial‐specific random effect with mean θok and variance describing the shift in placebo response from PASI 90 for PASI 50, PASI 75, and PASI 100, respectively. The variances, , were set to 0 if there was no significant between‐trial heterogeneity in the shift between PASI 50, PASI 75, PASI 90, and PASI 100 placebo responses based on the likelihood ratio test.
Edrugijk represented the log odds‐ratio (log OR) of PASIk response between treatment arm j in trial i and the corresponding placebo arm:
| (4) |
Edrugjk was the estimated treatment effect, i.e., the log OR between response in treatment arm j and placebo for each of the PASI response levels (k), and ηik was a trial‐specific random effect with mean 0 and variance representing between‐trial heterogeneity in treatment effect. Edrugjk was 0 for placebo arms. A different Edrug was estimated for adalimumab and risankizumab. End‐point‐specific Edrug was only estimated if statistically significant based on the likelihood ratio test. The variance was set to 0 if there was no significant between‐trial heterogeneity in treatment effect. The correlation between multiple PASI responses within one arm in a given trial (i.e., PASI 50, PASI 75, PASI 90, and PASI 100) was accounted for by assuming a compound symmetry correlation structure.
The absolute and relative treatment effects of risankizumab vs. adalimumab were presented as the efficacy difference (ED) and log OR for each PASI response level as shown by Eqs. (5) and (6), respectively.
| (5) |
| (6) |
Eok was a typical placebo PASI response for end point k. The ED and log OR of risankizumab and adalimumab vs. placebo were derived as shown in Eqs. (7) and (8).
| (7) |
| (8) |
with Edrug = Erzb or Eada for risankizumab and adalimumab, respectively.
Meta‐analysis for sPGA 0/1 response
The number of patients with an sPGA 0/1 response in treatment arm j of trial i was described by a binary response model (model code available in the Supplementary Information ):
| (9) |
The probability of response was modeled as the inverse logit of the sum of a placebo response and a drug response:
| (10) |
Eoi was a fixed effect for every trial representing the logit of the sPGA 0/1 placebo response. Edrugij represented the log odds‐ratio of sPGA 0/1 between the treatment arm j in trial i and the corresponding placebo arm:
| (11) |
Edrugj was the estimated log odds‐ratio between response in treatment arm j and placebo (treatment effect) for sPGA 0/1 response and ηi was a trial‐specific random effect with mean 0 and variance ω2 representing between‐trial heterogeneity in treatment effect. Edrugj was 0 for placebo arms. A different Edrug was estimated for adalimumab and risankizumab. The variance ω2 was set to 0 if there was no significant between‐trial heterogeneity in treatment effect.
The absolute and relative treatment difference between risankizumab and adalimumab in sPGA 0/1 response were derived as follows:
| (12) |
| (13) |
Eok was a typical placebo sPGA 0/1 response. The ED and log OR of risankizumab and adalimumab vs. placebo were derived as shown in Eqs. (14) and (15), respectively.
| (14) |
| (15) |
A total of 10,000 sets of parameter values were sampled from the variance‐covariance matrix of the meta‐analysis models for PASI and sPGA 0/1 responses. PASI and sPGA 0/1 responder rates were predicted for each set of model parameters in order to generate the predictive distribution for each drug. The 95% CI was defined as the 2.5th to 97.5th percentile range across the 10,000 model‐predicted responder rates for each drug.
The observed and meta‐analyses–based estimates of PASI and sPGA responder rates for placebo, risankizumab, and adalimumab treatment by trial were shown graphically to confirm that meta‐analysis well described the observed data. These plots compared the observed response and their 95% confidence interval (CI) for each treatment arm in each trial with the meta‐analysis–based estimates of efficacy response.
The relative treatment effect of risankizumab vs. adalimumab were estimated in this analysis and presented as the treatment effect difference and log odds ratio (OR) for each PASI response level and sPGA 0/1 response. A typical placebo response of 3.06% for PASI 90 and 6.40% for sPGA 0/1 at week 16, based on the weighted (by sample size) mean of the trial‐specific placebo responses, was used in the calculation of the treatment effect difference.
The meta‐analyses were considered to provide evidence for superiority of efficacy of risankizumab over adalimumab if the lower bound of the 95% CI of the simulated treatment effect difference and log OR between risankizumab and adalimumab was greater than zero for both PASI 90 and sPGA 0/1.
These analyses were conducted using generalized least squares regression function (gnls) and the nonlinear mixed effect regression function (nlme) provided in the nlme package in R (version 3.3.2 or later, R Foundation for Statistical Computing, Vienna, Austria). The maximum likelihood estimates of the model parameters were obtained assuming a large sample size normal approximation to the binomial likelihood.31
Funding
This work was supported by AbbVie.
Conflict of Interest
A.A.O. is an employee and shareholder of AbbVie. A.K. is a former employee of AbbVie and may hold AbbVie stocks or stock options. J.M. is an employee and shareholder of Certara. H.W. and P.M.D. are employees of Certara. H.W., P.M.D., and J.M. provided consulting services to AbbVie.
Author Contributions
H.W., A.K., P.M.D., J.M., and A.A.O. wrote the manuscript; A.A.O. and J.M. designed the research; H.M., A.K., and P.M.D. performed the research; H.M., A.K., P.M.D., J.M., and A.A.O. analyzed the data.
Data Sharing Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial‐level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html
Supporting information
Supplementary Information.
Acknowledgments
The authors designed and conducted the meta‐analyses and AbbVie contributed to the writing, review, and approval of the publication. A.A.O. is an employee and shareholder of AbbVie. A.K. is a former employee of AbbVie and may hold AbbVie stocks or stock options. J.M. is an employee and shareholder of Certara. H.W. and P.M.D. are employees of Certara. H.W., P.M.D., and J.M. provided consulting services to AbbVie. Editorial support was provided by Allison Kitten and Wesley Wayman, employees of AbbVie.
References
- 1. Singh, S. et al Selective targeting of the IL23 pathway: generation and characterization of a novel high‐affinity humanized anti‐IL23A antibody. MAbs 7, 778–791 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaffen, S.L. , Jain, R. , Garg, A.V. & Cua, D.J. The IL‐23‐IL‐17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campa, M. , Mansouri, B. , Warren, R. & Menter, A. A review of biologic therapies targeting IL‐23 and IL‐17 for use in moderate‐to‐severe plaque psoriasis. Dermatol. Ther. (Heidelb) 6, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papp, K.A. et al Risankizumab versus ustekinumab for moderate‐to‐severe plaque psoriasis. N. Engl. J. Med. 376, 1551–1560 (2017). [DOI] [PubMed] [Google Scholar]
- 5. Mease, P.J. et al Efficacy and safety results from a phase 2 trial of risankizumab, a selective IL‐23 p19 inhibitor, in patients with active psoriatic arthritis [abstract]. Arthritis Rheum. 69 (Suppl. 10), (2017) <http://acrabstracts.org/abstract/efficacy-and-safety-results-from-a-phase-2-trial-of-risankizumab-a-selective-il-23p19-inhibitor-in-patients-with-active-psoriatic-arthritis/> Accessed January 5, 2019. [Google Scholar]
- 6. Feagan, B.G. et al Induction therapy with the selective interleukin‐23 inhibitor risankizumab in patients with moderate‐to‐severe Crohn's disease: a randomised, double‐blind, placebo‐controlled phase 2 study. Lancet 389, 1699–1709 (2017). [DOI] [PubMed] [Google Scholar]
- 7. AbbVie . BI 655066/ABBV‐066 (Risankizumab) in Moderate to Severe Plaque Psoriasis With Randomized Withdrawal and Re‐treatment [http://ClinicalTrials.gov identifier: NCT02672852].
- 8. AbbVie . BI 655066 (Risankizumab) Compared to Placebo and Active Comparator (Ustekinumab) in Patients With Moderate to Severe Chronic Plaque Psoriasis [http://ClinicalTrials.gov identifier: NCT02684370].
- 9. AbbVie . BI 655066 compared to placebo & active comparator (ustekinumab) in patients with moderate to severe chronic plaque psoriasis [http://ClinicalTrials.gov identifier: NCT02684357].
- 10. Gordon, K.B. et al Efficacy and safety of risankizumab in moderate‐to‐severe plaque psoriasis (UltIMMa‐1 and UltIMMa‐2): results from two double‐blind, randomised, placebo‐controlled and ustekinumab‐controlled phase 3 trials. Lancet 392, 650–661 (2018). [DOI] [PubMed] [Google Scholar]
- 11. Reich, K. et al Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. The Lancet 394, 576–586 (2019). [DOI] [PubMed] [Google Scholar]
- 12. Risankizumab Meets All Co‐Primary and Ranked Secondary Endpoints, Achieving Significantly Greater Efficacy Versus Standard Biologic Therapies in Three Pivotal Phase 3 Psoriasis Studies [press release]. AbbVie; October 26, 2017 <https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm> (2017). Accessed June 4, 2018.
- 13. Risankizumab Meets All Primary Endpoints Reporting Positive Results in Fourth Pivotal Phase 3 Psoriasis Study [press release]. AbbVie; December 4, 2017 <https://news.abbvie.com/news/risankizumab-meets-all-primary-endpoints-reporting-positive-results-in-fourth-pivotal-phase-3-psoriasis-study.htm> (2017). Accessed June 4, 2018.
- 14. Reich, K. et al Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 390, 276–288 (2017). [DOI] [PubMed] [Google Scholar]
- 15. Thaci, D. et al Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J. Am. Acad. Dermatol. 73, 400–409 (2015). [DOI] [PubMed] [Google Scholar]
- 16. Gordon, K.B. et al Phase 3 trials of ixekizumab in moderate‐to‐severe plaque psoriasis. N. Engl. J. Med. 375, 345–356 (2016). [DOI] [PubMed] [Google Scholar]
- 17. Stelara(ustekinumab) [US prescribing information] (Janssen Biotech Inc, Horsham, PA, 2018). [Google Scholar]
- 18. Tremfya(guselkumab) [US prescribing information] (Janssen Biotech Inc, Horsham, PA, 2017). [Google Scholar]
- 19. Committee for Medicinal Products for Human Use (CHMP) . Guideline on clinical investigation of medicinal products indicated for the treatment of psoriasis. European Medicines Agency; <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003329.pdf> (2004). Accessed January 15, 2019. [Google Scholar]
- 20. Fredriksson, T. & Pettersson, U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica 157, 238–244 (1978). [DOI] [PubMed] [Google Scholar]
- 21. Chow, C. , Simpson, M.J. , Luger, T.A. , Chubb, H. & Ellis, C.N. Comparison of three methods for measuring psoriasis severity in clinical studies (Part 1 of 2): change during therapy in Psoriasis Area and Severity Index, Static Physician Global Assessment and Lattice System Physician Global Assessment. J. Eur. Acad. Dermatol. Venereol. 29, 1406–1414 (2015). [DOI] [PubMed] [Google Scholar]
- 22. Saurat, J.H. et al Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br. J. Dermatol. 158, 558–566 (2008). [DOI] [PubMed] [Google Scholar]
- 23. Gordon, K.B. et al Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double‐blind, randomized controlled trial and open‐label extension study. J. Am. Acad. Dermatol. 55, 598–606 (2006). [DOI] [PubMed] [Google Scholar]
- 24. Asahina, A. , Nakagawa, H. , Etoh, T. & Ohtsuki, M. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J. Dermatol. 37, 299–310 (2010). [DOI] [PubMed] [Google Scholar]
- 25. Cai, L. et al Efficacy and safety of adalimumab in Chinese patients with moderate‐to‐severe plaque psoriasis: results from a phase 3, randomized, placebo‐controlled, double‐blind study. J. Eur. Acad. Dermatol. Venereol. 31, 89–95 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang, J. et al Efficacy and safety of adalimumab in Chinese patients with moderate to severe plaque psoriasis: results from a phase 3, randomized, placebo‐controlled, double‐blind study. J. Am. Acad. Dermatol. 72, AB232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Menter, A. et al Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J. Am. Acad. Dermatol. 58, 106–115 (2008). [DOI] [PubMed] [Google Scholar]
- 28. Blauvelt, A. et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J. Am. Acad. Dermatol. 76, 405–417 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Reich, K. et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J. Am. Acad. Dermatol. 76, 418–431 (2017). [DOI] [PubMed] [Google Scholar]
- 30. Gordon, K.B. et al A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N. Engl. J. Med. 373, 136–144 (2015). [DOI] [PubMed] [Google Scholar]
- 31. Checchio, T. et al Quantitative evaluations of time‐course and treatment effects of systemic agents for psoriasis: a model‐based meta‐analysis. Clin. Pharmacol. Ther. 102, 1006–1016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information.


