Abstract
Dual antiplatelet therapy (DAPT) is standard in acute coronary heart disease but confers a bleeding risk. To compare the effects of ticagrelor‐monotherapy with ticagrelor‐based DAPT on hemostatic system activation, we conducted a randomized controlled trial in 44 volunteers using a loading‐dose regimen and measured platelet‐aggregometry triggered by adenosine diphosphate (multiple electrode aggregometry (MEA)‐ADP) and arachidonic acid (MEA‐AA), the vasodilator‐stimulated phosphoprotein (VASP), prothrombin fragment 1.2 (f1.2), and d‐Dimer. Ticagrelor‐based DAPT and ticagrelor‐monotherapy significantly decreased MEA‐ADP (Δmean: −51.4 (−56.9; −45.8) and −46.2 (−51.7; −40.7)) and VASP (Δmean: −70.3 (−76.2; −64.4) and −69.6 (−75.5; −63.7)) at 2 hours and over 24 hours. MEA‐AA was reduced significantly by both treatments (Δmean: −72.9 (−80.6; −65.3) and −25.7 (−33.3; −18.0)) at 2 hours, and stronger by ticagrelor‐based DAPT over 24 hours. Both treatments decreased f1.2 (geometric mean ratio (GMR): 0.92 (0.84; 1.01) and 0.88 (0.80; 0.96)) and d‐Dimer (GMR: 0.89 (0.86; 0.92) and 0.91 (0.88; 0.94)) at 2 hours and d‐Dimer over 24 hours. Ticagrelor‐monotherapy and ticagrelor‐based DAPT comparably affect hemostatic system activation.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Dual antiplatelet therapy (DAPT) is effective in the prevention of thrombotic complications in coronary heart disease, but confers a high risk of bleeding. Ticagrelor‐monotherapy following 1 month of ticagrelor‐based DAPT after stenting is noninferior when compared with clopidogrel‐based or ticagrelor‐based DAPT.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ What is the difference in the effect on platelet reactivity and coagulation activation between ticagrelor‐based DAPT and ticagrelor monotherapy?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Evidence demonstrating the comparability of the impact of ticagrelor‐monotherapy and ticagrelor‐based DAPT on platelet reactivity and coagulation activation in healthy male volunteers.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The findings of our study support aspirin‐free treatment concepts in cardiovascular disease.
Coronary heart disease (CHD) is the most frequent cause of death in Western countries1, 2 and is associated with considerable morbidity and high hospitalization rates.3, 4, 5 Revascularization therapy with percutaneous coronary intervention and stent implantation in combination with inhibition of platelet and coagulation activation is the main treatment strategy in acute CHD. The backbone of antithrombotic CHD regimens is the inhibition of platelet function. Aspirin together with a P2Y12 inhibitor represents the standard antiplatelet therapy in acute CHD.6, 7, 8, 9, 10 High on‐treatment platelet reactivity is a substantial problem in the management of acute CHD and is responsible for increased rates of early in‐stent re‐thrombosis and subsequent treatment complications.11 Dual antiplatelet therapy (DAPT) with the reversible P2Y12 inhibitor ticagrelor together with aspirin has significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke, as well as the incidence of stent thrombosis when compared with clopidogrel‐based DAPT in acute coronary syndromes.12, 13
DAPT in acute CHD is highly effective in preventing thrombotic complications, but confers a considerable risk of bleeding.12, 14, 15, 16 Novel potent P2Y12 inhibitors as antiplatelet monotherapy may confer a decreased risk of bleeding without losing antithrombotic efficacy compared with conventional DAPT. To test this hypothesis, we studied the effects of monotherapy with the P2Y12 inhibitor ticagrelor vs. DAPT (ticagrelor plus aspirin) on markers of hemostatic system activation at the site of plug formation in blood emerging from the microvasculature after a standardized injury (made by creating minimal skin incisions). We did not find a difference in the inhibition of platelet activation or coagulation activation between mono‐antiplatelet therapy with a P2Y12 inhibitor and DAPT.17 Notably, ticagrelor with or without aspirin reduced coagulation activation as measured by prothrombin fragment 1.2 (f1.2), whereas antiplatelet therapy consisting of clopidogrel with or without aspirin lacked this effect. Further, we observed a significant decrease in thromboxane B2 (TxB2) generation by ticagrelor even in the absence of aspirin.17
In the present study, we sought to re‐evaluate the effects of ticagrelor without aspirin and ticagrelor‐based DAPT on platelets by using assay systems specifically designed to assess platelet reactivity in venous blood. Multiple electrode aggregometry (MEA) detects the electrical impedance change due to the adhesion and aggregation of platelets on two independent electrode‐set surfaces.18 Flow cytometric evaluation of the phosphorylation state of vasodilator‐stimulated phosphoprotein (VASP) provides a measure of the activity of the platelet adenosine diphosphate (ADP) receptor P2Y12. Both methods are commonly used for the rapid assessment of on‐treatment platelet reactivity in patients with CHD.19, 20, 21 Finally, we aimed to reassess the effects of ticagrelor with and without aspirin on coagulation activation by measuring the levels of f1.2 and d‐Dimer also in venous blood.
Results
Subjects
Of the 44 volunteers (median age 24 (25th; 75th percentile: 23; 27) years), 22 were randomized to ticagrelor together with aspirin and 22 were randomized to ticagrelor together with placebo.
The two groups did not differ in terms of blood counts and global coagulation tests at the start of treatment. No severe adverse event occurred in any of the volunteers.
Effects of ticagrelor combined with aspirin vs. ticagrelor combined with placebo on ADP‐induced platelet aggregation evaluated with MEA‐ADP
The reduction of ADP‐induced platelet aggregation at 2 hours (baseline vs. 2 hours) did not differ between groups and was 71% in volunteers who received ticagrelor combined with aspirin and 64% in volunteers who received ticagrelor combined with placebo (Table 1 and Figure 1). In line with this result, there was no difference between the two treatment groups with respect to inhibition of ADP‐induced platelet aggregation at 24 hours when compared with the 2‐hour levels (2 hours vs. 24 hours; Table S1 , Figure 1). A slight but significant difference between the two treatment groups in the inhibition of ADP‐induced platelet aggregation was observed at 24 hours when compared with the baseline levels (baseline vs. 24 hours; Table S1 , Figure 1).
Table 1.
Effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on platelet reactivity evaluated with MEA‐ADP, MEA‐AA, and VASP in venous blood from baseline to 2 hours in 44 healthy male volunteers
| MEA‐ADP (U) | MEA‐AA (U) | VASP (PRI) | ||||
|---|---|---|---|---|---|---|
| Ticagrelor + aspirin | Ticagrelor + placebo | Ticagrelor + aspirin | Ticagrelor + placebo | Ticagrelor + aspirin | Ticagrelor + placebo | |
| Baseline | 70 (63−79) | 67 (62−74) | 84.5 (78−91) | 83.5 (79−94) | 85 (82−87.6) | 85.6 (81.8−87.1) |
| 2 hours | 20 (16−24) | 24 (20−27) | 11 (7−16) | 60 (48−72) | 10.1 (4.9−16) | 12.1 (6.8−18.2) |
| P valuea | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| P valueb | 0.19 | < 0.0001 | 0.86 | |||
| Δmeana | −51.4 (−56.9 to −45.8) | −46.2 (−51.7 to −40.7) | −72.9 (−80.6 to −65.3) | −25.7 (−33.3 to −18.0) | −70.3 (−76.2 to −64.4) | −69.6 (−75.5 to −63.7) |
| Δmeanb | −5.2 (−13.0 to 2.6) | −47.2 (−58.1 to −36.4) | −0.7 (−9.1 to 7.7) | |||
Values are given as median (quartiles) or mean difference (Δmean) (95% confidence interval) for multiple electrode aggregometry ADPtest (MEA‐ADP), multiple electrode aggregometry ASPItest (MEA‐AA), and VASP. AA, arachidonic acid; ADP, adenosine diphosphate; MEA, multiple electrode aggregometry; PRI, platelet reactivity index; U, units; VASP, vasodilator‐stimulated phosphoprotein.
From baseline to 2 hours.
Differences between treatment groups.
Figure 1.
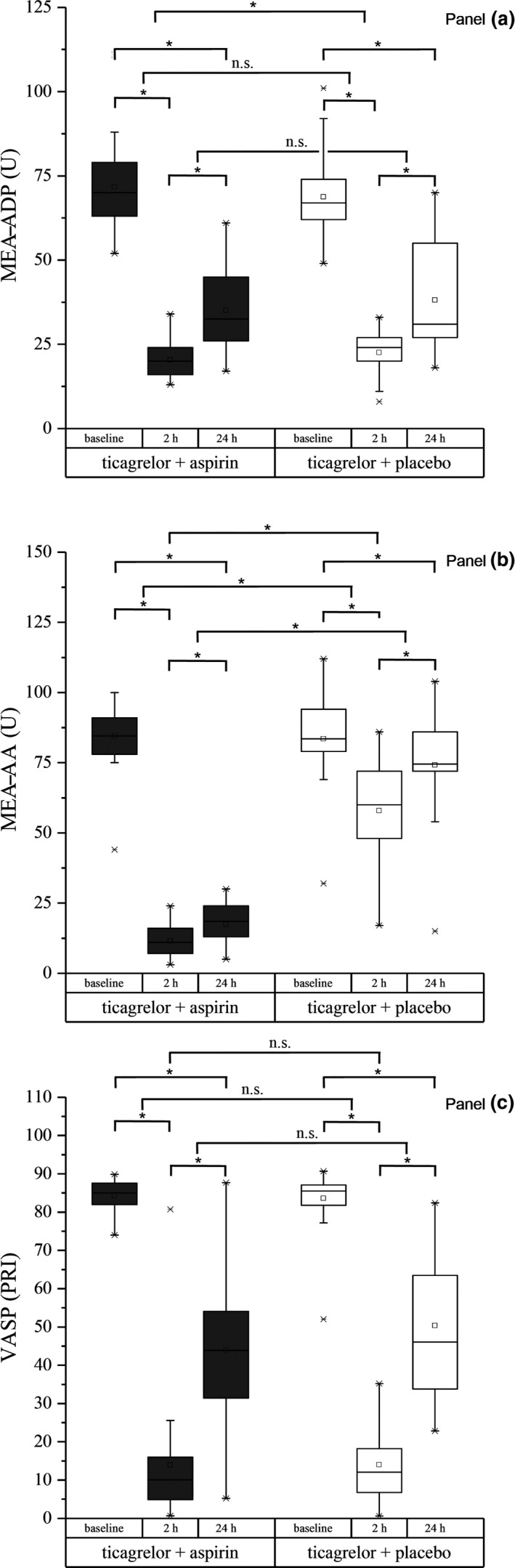
Boxplots of the effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on adenosine diphosphate‐triggered platelet aggregation (multiple electrode aggregometry (MEA)‐ADP) (a), arachidonic acid‐triggered aggregation (MEA‐AA) (b), and vasodilator‐stimulated phosphoprotein (VASP) levels (c) in venous blood at different time points over the observation period compared with baseline levels. *P < 0.05 for changes from baseline to 2 hours, from baseline to 24 hours, from 2 to 24 hours, and between treatment groups. n.s., nonsignificant; PRI, platelet reactivity index; U, units.
Effects of ticagrelor combined with aspirin vs. ticagrelor combined with placebo on arachidonic acid‐induced platelet aggregation evaluated with MEA‐arachidonic acid
The inhibition of arachidonic acid (AA)‐induced platelet aggregation at 2 hours (baseline vs. 2 hours) was more pronounced in the ticagrelor combined with aspirin group than in the ticagrelor combined with the placebo group (87% vs. 29%; Table 1 and Figure 1). Moreover, a significant difference in the extent of AA‐induced platelet aggregation was detectable between the groups at 24 hours when compared with the baseline and 2‐hour levels (baseline vs. 24 hours and 2 hours vs. 24 hours, respectively; Table S1 , Figure 1).
Effects of ticagrelor combined with aspirin vs. ticagrelor combined with placebo on VASP
Compared with baseline, at 2 hours after the first intake of the study drug, a significant and comparable decrease in platelet reactivity was found in the ticagrelor combined with aspirin group and in the ticagrelor combined with placebo group, as represented by a reduction in VASP (88% vs. 86%; Table 1 and Figure 1). No significant differences in platelet reactivity between the groups were found in terms of platelet inhibition as evaluated with VASP at 24 hours when compared with the baseline and 2‐hour levels after the first intake of the study drug (baseline vs. 24 hours and 2 hours vs. 24 hours, respectively; Table S1 , Figure 1).
Effects of ticagrelor combined with aspirin vs. ticagrelor combined with placebo on TxB2 formation
Ticagrelor combined with aspirin almost completely eliminated the TxB2 generation 24 hours after the first intake of the study drug when compared with baseline (P < 0.0001). Ticagrelor and placebo had no significant effect on TxB2 generation when the baseline levels were compared with the levels at 24 hours after the first ticagrelor intake (P = 0.85). The decrease in TxB2 generation at 24 hours was significantly stronger (P < 0.0001) in the ticagrelor plus aspirin group than in the ticagrelor plus placebo group (Table S3 , Figure 2).
Figure 2.
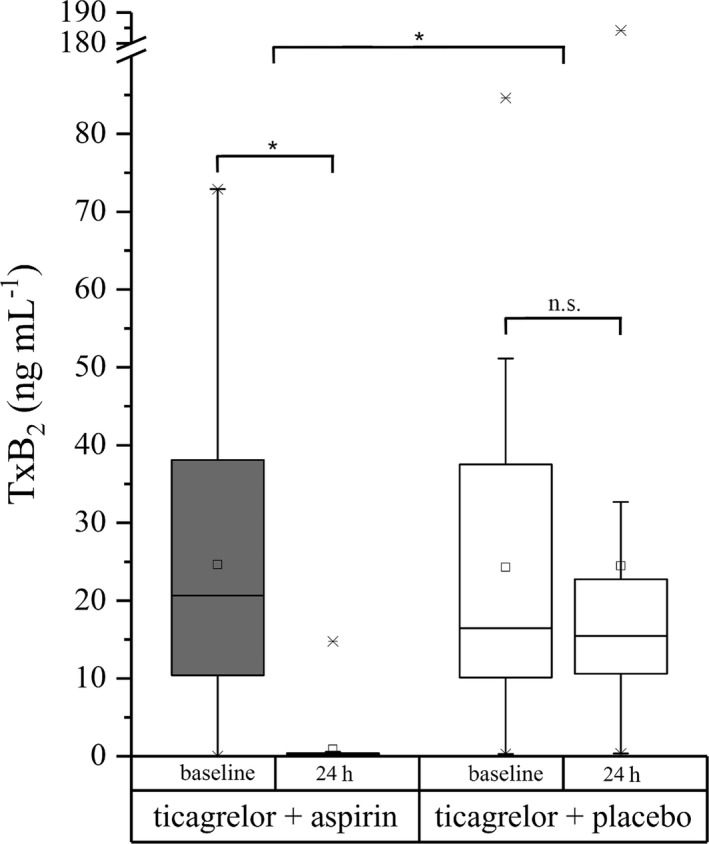
Boxplot of the effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on thromboxane B2 (TxB2) levels in venous blood at baseline and 24 hours after study treatment. *P < 0.05 for changes from baseline to 24 hours and between treatment groups. n.s., nonsignificant.
Effects of ticagrelor combined with aspirin vs. ticagrelor combined with placebo on coagulation activation
Compared with baseline, at 2 hours after the first intake of the study drug, a decrease in coagulation activation represented by levels of f1.2 and d‐Dimer was found in both the ticagrelor combined with aspirin group and the ticagrelor plus placebo group (Table 2 and Figure 3). With respect to d‐Dimer, the decrease recorded from baseline to 2 hours was significant, whereas there was a trend toward significance in the decrease in f1.2.
Table 2.
Effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on coagulation activation evaluated with f1.2 and d‐Dimer in venous blood from baseline to 2 hours in 44 healthy male volunteers
| f1.2 (nmol/L) | d‐Dimer (μg/mL) | |||
|---|---|---|---|---|
| Ticagrelor + aspirin | Ticagrelor + placebo | Ticagrelor + aspirin | Ticagrelor + placebo | |
| Baseline | 0.094 (0.084−0.126) | 0.095 (0.079−0.159) | 0.104 (0.085−0.166) | 0.142 (0.089−0.230) |
| 2 hours | 0.080 (0.076−0.116) | 0.091 (0.070−0.141) | 0.098 (0.080−0.142) | 0.138 (0.089−0.213) |
| P valuea | 0.082 | 0.005 | < 0.0001 | < 0.0001 |
| P valueb | 0.42 | 0.35 | ||
| GMRa | 0.92 (0.84−1.01) | 0.88 (0.80−0.96) | 0.89 (0.86−0.92) | 0.91 (0.88−0.94) |
| GMRb | 1.05 (0.93−1.20) | 0.98 (0.93−1.03) | ||
Values are given as median (quartiles) and GMR (95% confidence interval) for prothrombin fragment f1.2 (f1.2) and D‐dimer, respectively. GMR, geometric mean ratio.
From baseline to 2 hours.
Differences between treatment groups.
Figure 3.
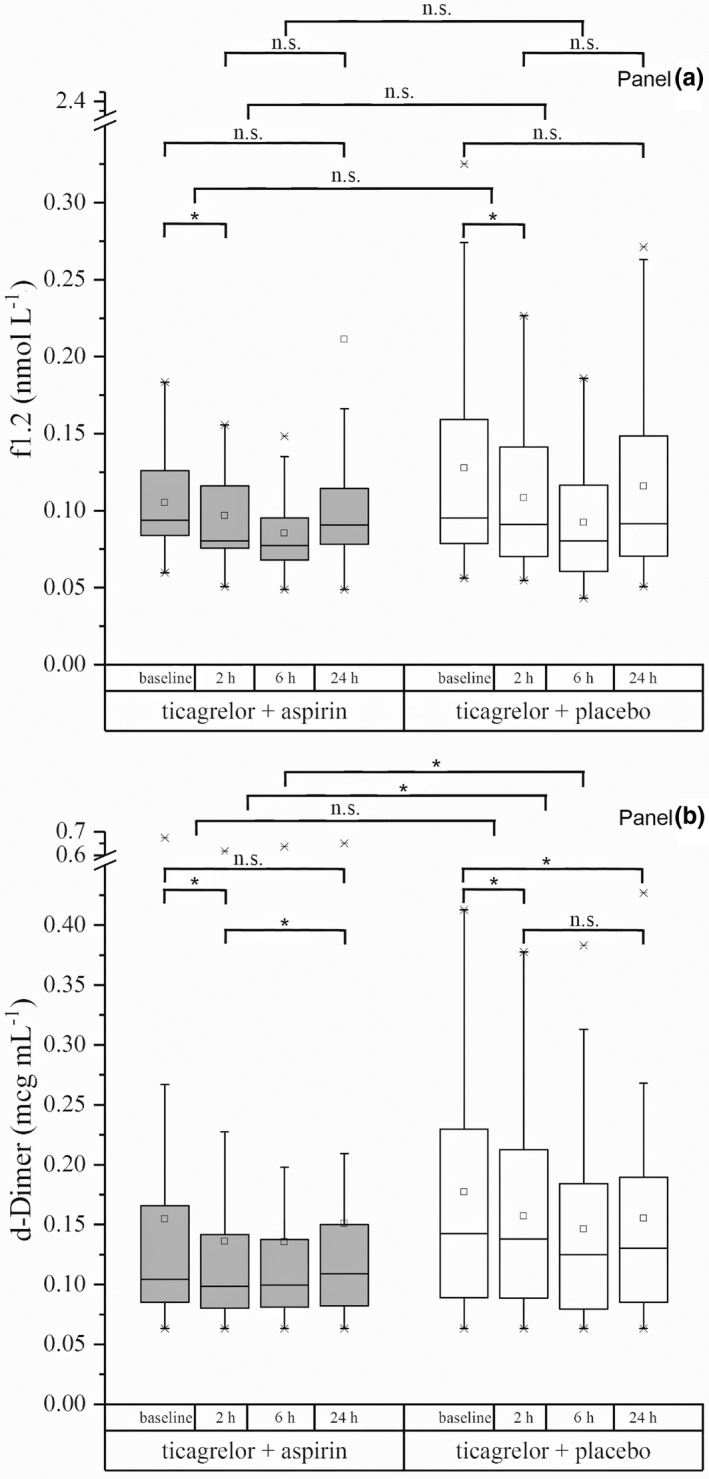
Boxplots of the effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on prothrombin fragment 1.2 (f1.2) (a) and d‐Dimer (b) levels in venous blood at different time points over the observation period compared with baseline levels. *P < 0.05 for changes from baseline to 2 hours, from baseline to 24 hours, from 2 to 24 hours, and between treatment groups. n.s., nonsignificant.
No significant difference of f1.2 between groups was detected when the levels at 24 hours after the start of treatment were compared with the baseline and 2‐hour levels (Table S2 , Figure 3). Concerning changes in d‐Dimer levels for these comparisons (baseline vs. 24 hours and 2 hours vs. 24 hours), there was a significant difference between the two groups (Table S2 , Figure 3).
Discussion
In this study, by using MEA‐ADP and VASP assays, we showed that ticagrelor with or without aspirin has a strong antiplatelet effect mediated by the inhibition of the ADP receptor. In healthy male volunteers, the MEA‐ADP and VASP levels were substantially reduced at 2 hours and 24 hours after ticagrelor intake with or without aspirin. The results of our study agree well with our previous findings in blood emerging from the microvasculature sampled directly at the site of thrombus formation.17 Ticagrelor without aspirin inhibited platelet activation, as represented by β‐thromboglobulin levels in the microcirculation, to a similar extent as ticagrelor combined with aspirin.
A further finding of this study is that MEA‐ADP and VASP levels measured after 24 hours remained lower than the baseline levels. This observation must be interpreted by considering the comparatively short half‐life of ticagrelor (7–9 hours) and the ticagrelor loading dose regimen of 180 mg in our study rather than the twice‐daily dosing in long‐term routine use. In contrast to clopidogrel, which is a prodrug and an irreversible platelet function inhibitor, ticagrelor is a reversible P2Y12 inhibitor.22, 23, 24 This means that ticagrelor, after its release from initially inhibited platelets, may occupy P2Y12 receptors on previously unaffected platelets,25, 26, 27 which may, at least in part, explain the prolonged effect on platelet activity assessed using MEA‐ADP and VASP. Moreover, as the P2Y12 inhibitory effect of ticagrelor is independent of cytochrome P450 amplifications to generate full antiplatelet potential, an active metabolite is generated in the process of metabolization. This metabolite exhibits an up to 30% stronger potency in P2Y12 inhibition and has an even longer half‐life (8–12 hours) than unmetabolized ticagrelor.28
As expected, AA‐induced platelet aggregation was substantially inhibited in individuals receiving ticagrelor combined with aspirin reflecting the cyclooxygenase dependency of the assay system. Importantly, ticagrelor without aspirin also inhibited AA‐induced platelet aggregation albeit to a much lesser extent. The mechanisms explaining this observation could be intracellular cross‐linked signaling pathways leading to a reduced reactivity to AA stimulation caused by an antiplatelet effect mediated by potent P2Y12 receptor inhibition via ticagrelor. This consideration is in line with our findings that the levels of TxB2, which is generated via cyclooxygenase and, thus, is directly inhibited by the cyclooxygenase inhibitor aspirin, were not affected by ticagrelor without aspirin. Thus, it seems that ticagrelor also displays a moderate effect on AA‐induced platelet aggregation, partially mimicking an aspirin effect. It has been postulated that ticagrelor can blunt thromboxane A2 (TxA2) receptor signaling in platelets and reduce platelet production of TxA2.29 Our study confirms the findings of previous studies showing that the P2Y12 receptors are critical to the generation of irreversible aggregation through the TxA2‐dependent pathway.30 Therefore, the antiplatelet effects of aspirin and ticagrelor seem to be separately additive, indicating an overlap in effects. As a result, strong P2Y12 receptor blockade, as with ticagrelor without aspirin, causes the inhibition of AA‐induced platelet aggregation albeit to a lesser extent than with DAPT. These findings might offer an explanation for the recent results of the large‐scale Global Leaders trial.31 In that trial, patients undergoing percutaneous coronary intervention and stenting for stable coronary artery disease or acute coronary syndromes were randomly assigned to either ticagrelor‐monotherapy for 23 months or to DAPT (clopidogrel or ticagrelor based) for 11 months, followed by aspirin monotherapy for 12 months after a common first month of dual platelet function inhibition. There was no difference with respect to major cardiovascular events in the two groups.
Another noteworthy finding of our study is the lack of any influence of the 300 mg aspirin loading dose on ADP‐induced platelet aggregation. This result does not support the hypothesis that the higher aspirin dose routinely used in North America (300 mg daily vs. 100 mg daily in Europe) could explain the results of the PLATO trial.32 In that trial, the North American cohort, in contrast to the European cohort, failed to demonstrate the advantage of ticagrelor‐based DAPT over clopidogrel‐based DAPT.12 A prostanoid mechanism has been proposed as an explanation for this “North American ticagrelor‐aspirin phenomenon”: the addition of aspirin at higher doses could cause significant reductions in the production of prostanoids (e.g., prostaglandin I2 from the blood vessel wall), leading to a loss of possible synergistic antiplatelet effects of high endogenous prostacyclin and ticagrelor.33, 34, 35 Therefore, when combined with a high level of P2Y12 inhibition with ticagrelor, the net effect of aspirin doses > 300 mg could cause the removal of antithrombotic and vasodilating prostanoids, resulting in a reduction of the antiplatelet effectiveness of the aspirin treatment.29
Ticagrelor with and without aspirin affected not only the platelet function but also the activation state of the coagulation system. The levels of f1.2 and d‐Dimer in venous blood were diminished 2 hours after the first administration of the study drug independently of concomitant aspirin. These results confirm our previous findings in microvascular blood, in which ticagrelor combined with aspirin and ticagrelor combined with placebo reduced the levels of f1.2 by a geometric mean ratio of 0.58 (95% confidence interval 0.45−0.75) and 0.55 (95% confidence interval 0.38−0.80), respectively.17 although the inhibitory effects of ticagrelor on ADP‐induced and AA‐induced platelet aggregation as well as on VASP were sustained over 24 hours, the inhibitory effects on parameters of coagulation activation only persisted for d‐Dimer in the ticagrelor‐monotherapy group. The f1.2 (in both groups) and d‐Dimer (only in the ticagrelor combined with aspirin group) levels returned to the baseline values at 24 hours after intake of the loading dose.
The specific strengths and limitations of this study need to be addressed. We evaluated the effects of ticagrelor with and without aspirin in an elaborate experimental in vivo setting in men, by performing a randomized, double‐blinded, placebo‐controlled, parallel‐group trial. To study the effects of ticagrelor, we applied commercially available, widely used point‐of‐care assays that provide results relevant in clinical practice. We included only healthy male volunteers rather than patients with CHD, yielding a homogenous, well‐characterized cohort. The lack of a clinical end point is an obvious disadvantage of this study design. Another limitation concerns the duration of treatment and, consequently, the lack of long‐term data. To ensure safety for the probands, we kept the on‐treatment time as short as possible and administered only 1 loading dose (180 mg) of ticagrelor (combined with either 300 mg aspirin or placebo). The used dosages of ticagrelor and aspirin were chosen in accordance with the recommendations published in current guidelines regarding loading regimens of antiplatelet therapy in acute coronary syndromes.8, 9, 10 The reported results represent the secondary outcome parameters of a previously reported study,17 and the number of subjects was powered to detect a difference in the main outcome parameter, β‐thromboglobulin levels in microvascular blood, in that study.
The concept of aspirin sparing in cardiovascular disease is heavily debated.36 Balancing the potential benefits of DAPT regarding cardiovascular end points with the risk of bleeding remains a challenge and has been addressed in clinical trials.12, 14, 15, 16 Several trials compared the effects of ticagrelor or aspirin monotherapy with ticagrelor‐based DAPT. The aforementioned Global Leaders trial found no difference in the rates of bleeding.31 Zhao et al.37 postulated a positive effect of ticagrelor‐based DAPT on the patency of saphenous vein grafts in elective coronary artery bypass grafting when compared with either ticagrelor or aspirin monotherapy. DAPT resulted in markedly higher rates of noncoronary artery bypass grafting‐related bleeding compared with ticagrelor or aspirin monotherapy.
Conclusion
The findings of our study support the emerging aspirin‐free concepts in CHD by demonstrating that P2Y12 inhibition without aspirin inhibits AA‐induced platelet aggregation to a moderate level, as well as the coagulation activation to a comparable degree to DAPT. Therefore, our study confirms the results of previous ex vivo studies and supports the concept postulating that the antiplatelet effects of aspirin and ticagrelor might be separately additive. Additional studies in patients with CHD evaluating the concept of P2Y12 inhibitor monotherapy are warranted.
Methods
Study population
The study population consisted of young, healthy, nonsmoking, male volunteers. The exclusion criteria were history of bleeding or any known disorders associated with an increased bleeding risk, any history of systemic inflammatory or cardiovascular diseases, obesity, allergy or contraindication to any of the studied drugs, known gastrointestinal disease, any other significant findings in physical examination or laboratory workup, excessive consumption of alcohol, or intake of any medication within 2 weeks before the start of the study.
This study was conducted according to the Declaration of Helsinki (including current revisions) and the International Conference on Harmonization Good Clinical Practice guidelines. The study protocol was approved by the Ethics Committee of the Medical University of Vienna, Austria (EK 184/2010), and the trial is registered at https://clinicaltrials.gov (NCT02120092) and in the European clinical trials database (EudraCT 2010‐019643‐19). Written informed consent was obtained from all subjects before any study‐related procedures were conducted.
Study design
The study was conducted as a randomized, parallel‐group, double‐blind, placebo‐controlled trial from May until December 2013 at the Departments of Medicine I and Clinical Pharmacology, Medical University of Vienna, Vienna, Austria. Randomization was done using the permuted block method with a block size of four. A person not directly involved in study‐related procedures performed concealment of the respective drugs. Investigators involved in the study were not aware of the randomization codes, which were only broken after completion of the trial and of all laboratory analyses.
The trial was scheduled for two consecutive days. The study medication was administered in the fasting state once at day 1. Blood sampling was performed on day 1 before the first intake of the study medication and 2, 6, and 24 hours thereafter. The volunteers received 180 mg ticagrelor (Brilique/Brilinta; AstraZeneca, Södertälje, Sweden) together with 300 mg nonenteric coated aspirin (DAPT) or 180 mg ticagrelor together with placebo.
Blood sampling
Venous blood was collected from an antecubital vein after loosening the tourniquet by using a 21‐gauge needle or an 18‐gauge venous catheter. The first tube of collected blood was discarded. Venous blood samples for platelet and coagulation tests were collected in tubes containing a 3.8% (0.129 mol/L) solution of sodium citrate (Vacuette; Greiner Bio‐One, Kremsmünster, Austria), hirudin (Roche, Mannheim, Germany), or into serum barrier gel tubes (Vacuette; Greiner Bio‐One). Where required, the samples were centrifuged at 2,600 g for 15 minutes at 4°C. The clear supernatant was separated and stored at −80°C until further use.
Laboratory analyses
Multiple electrode aggregometry
MEA was performed using a bedside impedance aggregometer (Multiplate Analyzer; Roche).20, 21, 38, 39, 40, 41 Hirudin anticoagulated venous whole blood is used in this standardized assay. The ADP‐stimulated assay ADPtest (MEA‐ADP) was used to test for the antiplatelet effect of P2Y12 inhibitors. The AA‐stimulated assay ASPItest (MEA‐AA) was used to test for the antiplatelet effect of aspirin.
Vasodilator‐stimulated phosphoprotein
The platelet reactivity index was calculated using the results determined using flow cytometry with a commercially available assay (PLT/VASP P2Y12; Stago, Asnières sur Seine, France). Flow cytometric analysis of citrated whole blood was performed using a FACScan Cytometer with CellQuest 4.0.2 software (Becton Dickinson, Franklin Lakes, NJ).
TxB2 was determined in serum using a commercially available enzyme‐linked immunoassay kit (Thromboxane B2 EIA Kit; Cayman Chemical, Ann Arbor, MI).
The f1.2 and d‐Dimer levels were determined in citrated plasma using commercially available enzyme‐linked immunoassay kits (Enzygnost F1+2 (monoclonal); Siemens Healthcare Diagnostics Products, Erlangen, Germany; Asserachrom D‐Di; Stago).
Platelet and hemoglobin levels as well as prothrombin time, activated partial thromboplastin time, and fibrinogen level were determined using standard assays and procedures according to hospital routine.
Statistical methods
We analyzed the parameters of platelet reactivity and coagulation activation according to the protocol‐predefined secondary end points of a recently published study.17 The number of subjects was powered to detect a difference in the primary outcome variable (β‐thromboglobulin levels) in microvascular blood at baseline and 2 hours after the first intake of the study drug and was set to a final sample size of 22 volunteers per group following a predefined interim analysis regarding the sample size.
Data are given as median and quartiles (25th and 75th percentiles), unless stated otherwise. Owing to their skewed distributions, some of the continuous outcome variables (TxB2, f1.2, and d‐Dimer) were log‐transformed before statistical analyses.
To assess the short‐term and long‐term effects (by comparing baseline values with measurements at 2 and 24 hours after the first intake of the study drug), and to compare these changes between the two treatment groups (with or without aspirin), repeated‐measures analysis of variance models were used. Within these analysis of variance models, the time point was considered as the repeated within‐subject factor and the treatment group as the between‐subject factor. In untransformed variables, effect sizes are described by the mean difference (Δmean (95% confidence interval)). In log‐transformed variables, effects are described by the geometric mean ratio (95% confidence interval) obtained by retransforming mean differences from the logarithmic to the original scale.
All P values were the results of two‐sided tests, and P values < 0.05 were considered statistically significant.
Funding
The study was supported by the Medizinisch‐Wissenschaftlicher Fonds des Bürgermeisters der Bundeshauptstadt Wien (Project number: 12059).
Conflict of Interest
Jolanta M. Siller‐Matula reports lecture or consultant fees from Bayer, Astra Zeneca, Eli Lilly, Daiichi‐Sankyo, and Roche. All other authors declared no competing interests for this work.
Author Contributions
L.T., S.E., J.M.S.‐M., and P.A.K. wrote the manuscript. L.T. and P.A.K. designed the research. L.T., M.K., and M.W. performed the research. L.T., A.K., S.E., P.A.K., and J.M.S.‐M. analyzed the data. M.W. and M.F.W. contributed new reagents/analytical tools.
Supporting information
Table S1. Effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on platelet reactivity evaluated with MEA‐ADP, MEA‐AA, and VASP in venous blood from baseline to 24 hours and from 2 to 24 hours in 44 healthy male volunteers.
Table S2. Effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on coagulation activation evaluated with f1.2 and d‐Dimer in venous blood from baseline to 24 hours and from 2 to 24 hours in 44 healthy male volunteers.
Table S3. Effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on platelet activity evaluated with TxB2 in 44 healthy male volunteers.
References
- 1. Go, A.S. et al Heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation 129, e28–e292 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nichols, M. , Townsend, N. , Scarborough, P. & Rayner, M. Cardiovascular disease in Europe 2014: epidemiological update. Eur. Heart J. 35, 2950–2959 (2014). [DOI] [PubMed] [Google Scholar]
- 3. Alpert, J.S. , Thygesen, K. , Antman, E. & Bassand, J.P. Myocardial infarction redefined – a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J. Am. Coll. Cardiol. 36, 959–969 (2000). [DOI] [PubMed] [Google Scholar]
- 4. Widimsky, P. et al Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur. Heart J. 31, 943–957 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reed, G.W. , Rossi, J.E. & Cannon, C.P. Acute myocardial infarction. Lancet 389, 197–210 (2016). [DOI] [PubMed] [Google Scholar]
- 6. Roffi, M. et al 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 37, 267–315 (2016). [DOI] [PubMed] [Google Scholar]
- 7. O'Gara, P.T. et al 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127, e362–e425 (2013). [DOI] [PubMed] [Google Scholar]
- 8. Neumann, F.J. et al 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 40, 87–165 (2019). [DOI] [PubMed] [Google Scholar]
- 9. Ibanez, B. et al 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39, 119–177 (2017). [DOI] [PubMed] [Google Scholar]
- 10. Amsterdam, E.A. et al 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 130, 2354–2394 (2014). [DOI] [PubMed] [Google Scholar]
- 11. Bonello, L. et al Consensus and future directions on the definition of high on‐treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 56, 919–933 (2010). [DOI] [PubMed] [Google Scholar]
- 12. Wallentin, L. et al Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361, 1045–1057 (2009). [DOI] [PubMed] [Google Scholar]
- 13. Steg, P.G. et al Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective, randomized PLATO trial. Circulation 128, 1055–1065 (2013). [DOI] [PubMed] [Google Scholar]
- 14. Sabatine, M.S. et al Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST‐segment elevation. N. Engl. J. Med. 352, 1179–1189 (2005). [DOI] [PubMed] [Google Scholar]
- 15. Steinhubl, S.R. et al Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 288, 2411–2420 (2002). [DOI] [PubMed] [Google Scholar]
- 16. Wiviott, S.D. et al Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357, 2001–2015 (2007). [DOI] [PubMed] [Google Scholar]
- 17. Traby, L. , Kollars, M. , Kaider, A. , Eichinger, S. , Wolzt, M. & Kyrle, P.A. Effects of P2Y12 receptor inhibition with or without aspirin on hemostatic system activation: a randomized trial in healthy subjects. J. Thromb. Haemost. 14, 273–281 (2016). [DOI] [PubMed] [Google Scholar]
- 18. Sibbing, D. et al Assessment of ADP‐induced platelet aggregation with light transmission aggregometry and multiple electrode platelet aggregometry before and after clopidogrel treatment. Thromb. Haemost. 99, 121–126 (2008). [DOI] [PubMed] [Google Scholar]
- 19. Siller‐Matula, J.M. , Panzer, S. & Jilma, B. Reproducibility and standardized reporting of the vasodilator‐stimulated phosphoprotein phosphorylation assay. Platelets 19, 551–554 (2008). [DOI] [PubMed] [Google Scholar]
- 20. Siller‐Matula, J.M. , Gouya, G. , Wolzt, M. & Jilma, B. Cross validation of the multiple electrode aggregometry. A prospective trial in healthy volunteers. Thromb. Haemost. 102, 397–403 (2009). [DOI] [PubMed] [Google Scholar]
- 21. Siller‐Matula, J.M. , Christ, G. , Lang, I.M. , Delle‐Karth, G. , Huber, K. & Jilma, B. Multiple electrode aggregometry predicts stent thrombosis better than the vasodilator‐stimulated phosphoprotein phosphorylation assay. J. Thromb. Haemost. 8, 351–359 (2010). [DOI] [PubMed] [Google Scholar]
- 22. Jj, V.A.N.G. et al Ticagrelor binds to human P2Y(12) independently from ADP but antagonizes ADP‐induced receptor signaling and platelet aggregation. J. Thromb. Haemost. 7, 1556–1565 (2009). [DOI] [PubMed] [Google Scholar]
- 23. Kubisa, M.J. , Jezewski, M.P. , Gasecka, A. , Siller‐Matula, J.M. & Postula, M. Ticagrelor – toward more efficient platelet inhibition and beyond. Ther. Clin. Risk Manag. 14, 129–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winter, M.P. et al Advocating cardiovascular precision medicine with P2Y12 receptor inhibitors. Eur. Heart J. Cardiovasc. Pharmacother. 3, 221–234 (2017). [DOI] [PubMed] [Google Scholar]
- 25. Teng, R. , Carlson, G.F. , Nylander, S. & Andersson, T.L. Effects of autologous platelet transfusion on platelet inhibition in ticagrelor‐treated and clopidogrel‐treated subjects. J. Thromb. Haemost. 14, 2342–2352 (2016). [DOI] [PubMed] [Google Scholar]
- 26. Niezgoda, P. et al Crushed sublingual versus oral ticagrelor administration strategies in patients with unstable angina. A pharmacokinetic/pharmacodynamic study. Thromb. Haemost. 117, 718–726 (2017). [DOI] [PubMed] [Google Scholar]
- 27. Siller‐Matula, J.M. & Jilma, B. Ticagrelor: from discovery to phase III clinical trial. Future Cardiol. 6, 753–764 (2010). [DOI] [PubMed] [Google Scholar]
- 28. Dobesh, P.P. & Oestreich, J.H. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy 34, 1077–1090 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Warner, T.D. , Nylander, S. & Whatling, C. Anti‐platelet therapy: cyclo‐oxygenase inhibition and the use of aspirin with particular regard to dual anti‐platelet therapy. Br. J. Clin. Pharmacol. 72, 619–633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armstrong, P.C. et al In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J. Thromb. Haemost. 9, 552–561 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vranckx, P. et al Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug‐eluting stent: a multicentre, open‐label, randomised superiority trial. Lancet 392, 940–949 (2018). [DOI] [PubMed] [Google Scholar]
- 32. Mahaffey, K.W. et al Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 124, 544–554 (2011). [DOI] [PubMed] [Google Scholar]
- 33. FitzGerald, G.A. et al Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J. Clin. Invest. 71, 676–688 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Storey, R.F. et al Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J. Am. Coll. Cardiol. 56, 1456–1462 (2010). [DOI] [PubMed] [Google Scholar]
- 35. Cattaneo, M. & Lecchi, A. Inhibition of the platelet P2Y12 receptor for adenosine diphosphate potentiates the antiplatelet effect of prostacyclin. J. Thromb. Haemost. 5, 577–582 (2007). [DOI] [PubMed] [Google Scholar]
- 36. Capodanno, D. et al Aspirin‐free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat. Rev. Cardiol. 15, 480–496 (2018). [DOI] [PubMed] [Google Scholar]
- 37. Zhao, Q. et al Effect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting: a randomized clinical trial. JAMA 319, 1677–1686 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sibbing, D. et al Platelet reactivity after clopidogrel treatment assessed with point‐of‐care analysis and early drug‐eluting stent thrombosis. J. Am. Coll. Cardiol. 53, 849–856 (2009). [DOI] [PubMed] [Google Scholar]
- 39. Connor, D.E. et al Effects of antiplatelet therapy on platelet extracellular vesicle release and procoagulant activity in health and in cardiovascular disease. Platelets 27, 805–811 (2016). [DOI] [PubMed] [Google Scholar]
- 40. Siller‐Matula, J.M. et al Distribution of clinical events across platelet aggregation values in all‐comers treated with prasugrel and ticagrelor. Vascul. Pharmacol. 79, 6–10 (2016). [DOI] [PubMed] [Google Scholar]
- 41. Siller‐Matula, J.M. et al Inter‐patient variability of platelet reactivity in patients treated with prasugrel and ticagrelor. Platelets 27, 373–377 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on platelet reactivity evaluated with MEA‐ADP, MEA‐AA, and VASP in venous blood from baseline to 24 hours and from 2 to 24 hours in 44 healthy male volunteers.
Table S2. Effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on coagulation activation evaluated with f1.2 and d‐Dimer in venous blood from baseline to 24 hours and from 2 to 24 hours in 44 healthy male volunteers.
Table S3. Effects of ticagrelor combined with aspirin and ticagrelor combined with placebo on platelet activity evaluated with TxB2 in 44 healthy male volunteers.


