Abstract
Background:
Alcohol-related mortality rates in the U.S. have risen since 2000, though how trends vary across socio-economic status is unclear.
Methods:
This analysis combines data from vital statistics and the National Health Interview Survey (NHIS) to estimate alcohol-related mortality rates at four levels of educational attainment (less than high school, high school/GED, some college/associate’s degree, four-year degree or more) over the period 2000–2017. The analysis includes a comprehensive set of 48 alcohol-related causes of death, including causes which are indirectly influenced by alcohol use. I consider period and cohort patterns in inequality using the relative index of inequality (RII).
Results:
Mortality rates increased over the study period, at all levels of educational attainment. Relative increases were larger for females than males at nearly all ages and levels of educational attainment, and were largest among 45–59 year-old women. Male and female members of the 1950–1959 birth cohort exhibited elevated rates of alcohol-related mortality relative to neighboring cohorts. Despite widespread increases in alcohol-related mortality, educational inequalities present at the beginning of the analysis persisted and exceeded those in all-cause mortality. Disparities were typically greatest among younger adults ages 30–44, though inequality in this age group declined over time. Inequality increased among females ages 60–74, as well as among males ages 45–74.
Implications:
While interventions targeting these groups may reduce educational disparities, care should also be taken to stem the increasing prevalence of alcohol-related deaths at all levels of educational attainment.
The Centers for Disease Control and Prevention (CDC) estimates that alcohol-related mortality rates increased 36% over the first 17 years of the 21st century (Miniño et al. 2002; Xu et al. 2018). Unfavorable trends in alcohol-related mortality were accompanied by growth in high risk drinking behaviors, especially among women, older adults, ethnic/racial minorities, and groups of lower socio-economic status (Grant et al. 2017). Although increases in alcohol-related mortality rates have often been considered a leading contributor to growing divides in mortality patterns by educational attainment (see, e.g., Case and Deaton 2015; 2017), trends in alcohol-related mortality by education are commonly examined only in combination with drug-overdose and suicide mortality. The mechanisms resulting in alcohol-related deaths, and their underlying trends, however, may differ from those resulting in drug-related or suicide deaths.
This study examines alcohol-related mortality rates by educational attainment, independent of drug-overdose and suicide mortality, and is guided by the following questions:
What are the recent trends in alcohol-related mortality rates by sex and level of educational attainment in the U.S.?
-
How has inequality in alcohol-related mortality by educational attainment changed over time?
2a. What are the period and cohort trends in inequality?
To answer these questions, I combine data from vital statistics and from the National Health Interview Survey. While past analyses have often been limited to a small set of alcohol-related causes, this is the first study to consider educational disparities in all 48 of the adult causes of death which have been consistently linked to the consumption of alcohol. The analysis is the first study of alcohol-related mortality to address bias associated with misreporting of decedents’ educational attainment on death certificates.
Background
Alcohol-related causes of death
To estimate the annual alcohol-related mortality rates cited above, the CDC limits alcohol-related causes of death to those which are 100% attributable to alcohol use. These are deaths which cannot occur without the consumption of alcohol, and include conditions such as alcoholic liver diseases and alcohol poisoning. In addition to these 100%-attributable causes, however, alcohol consumption can also influence mortality risks for other causes, including accidents, cardiovascular diseases, and cancers (Baan et al. 2007; Breslow and Graubard 2008; Rehm et al. 2010). The CDC’s Alcohol-Related Disease Impact (ARDI) application compiles empirical evidence linking consumption to cause-specific mortality risks in the form of alcohol-attributable fractions (AAF). Fractions range from .01 for certain conditions such as chronic hepatitis or hypertension to 1 for entirely alcohol-attributable causes. While fractions are small for many indirect causes, some indirect causes contribute considerably to the overall landscape of alcohol-related mortality. Notably, fatal motor vehicle accidents involving alcohol comprised 14% of alcohol-related deaths between 2006 and 2010, second only to alcoholic liver disease at 17% (ARDI). Using these fractions, Stahre et al. (2014) estimate that 1 in 10 deaths of working age adults between 2006 and 2010 were attributable to alcohol (age-standardized death rate of 27.9 per 100,000).
One mechanism through which alcohol use may influence mortality risks is through interaction with other risk factors. Heavy alcohol consumption reduces diet quality (Breslow et al. 2010; Lieber 2003) and can suppress immune function (Watzl and Watson 1992), consequences which may speed the development of fatal health conditions. Probst et al. (2014) hypothesize that these factors may be especially salient for lower status groups, whose lower likelihood to purchase healthy foods may decrease the body’s ability to process alcohol. Similarly, the combination of alcohol and cigarette use is associated with increased mortality risks from aero-digestive cancers, and possibly from traffic and fire injuries (Taylor and Rehm 2006). Given the negative relationship between cigarette smoking and educational attainment (Jamal et al. 2015), this interaction may disproportionately affect less educated groups. Heavy drinking is also associated with a greater risk of developing colorectal cancer, but only among obese individuals (Zhao et al. 2012). Since individuals of lower levels of educational attainment are more likely to have ever been obese (Vierboom 2017), this interaction too may disproportionately affect lower status groups.
Trends and differentials in alcohol consumption
Binge drinking (consuming 5 or more alcoholic drinks per sitting for men and 4 or more drinks for women) was responsible for more than half of all alcohol-related deaths between 2006 and 2010 (ARDI). While research suggests that moderate alcohol consumption may be protective for certain conditions (Bell et al. 2017), Roerecke and Rehm (2010) find that any beneficial effects for heart disease disappear when “light to moderate drinking is mixed with irregular heavy drinking occasions.” Although richer and more educated groups are more likely to binge drink, the frequency and intensity of binge drinking among binge drinkers is highest for less educated groups (CDC 2012; Kanny et al. 2018). Such differences in self-reported long-term drinking behaviors have been estimated to account for roughly 18% of differences in all-cause mortality between less-than-high-school and college graduates (Mehta et al. 2015).
Social factors are important mediators on the pathway between problematic alcohol use and death. Research by Rogers et al. (2015) suggests that the social harms associated with problematic drinking, such as a spouse threatening to leave, may have larger implications for mortality than the possible physical harms of drinking. The threshold for incurring social harm may vary across groups. People with lower levels of educational attainment are more likely to report higher levels of perceived stigma when it comes to their drinking behavior—a trait that is associated with a lower likelihood of seeking treatment for an alcohol disorder (Keyes et al. 2010). An individual with relatively little formal education may therefore face greater perceived social harm and be less likely to seek treatment than others, even at the same levels of alcohol consumption.
The prevalence of alcohol-related mortality and/or problematic drinking varies across cohorts, age groups, and period. Cohort-specific life course factors may initiate long-term “biological or psychological chains of risk” (Kuh et al. 2003) that create cohort patterns in alcohol consumption (Lui et al. 2018; Virtanen et al. 2018). Extended durations of poverty or involuntary unemployment in early adulthood, for example, significantly predict heavy alcohol use in adulthood (Caldwell et al. 2008; Mossakowski 2008). As with many health behaviors, the prevalence, frequency, and intensity of problematic drinking additionally is not evenly distributed across the life course, often peaking in young- to mid-adulthood (Kanny et al. 2018). An extensive body of research has also debated the existence of period effects in alcohol-related mortality and consumption, especially in response to recessions or fluctuations in the price of alcohol (Herttua et al. 2011; Herttua et al. 2017; Makela et al. 2015; Zaridze et al. 2009).
Methods & Data
Overview
I examine trends in alcohol-related mortality rates between 2000–2017 and consider educational disparities in those rates using the relative index of inequality (RII). I consider trends in four age groups (30+, 30–44, 45–59, and 60–74) and across six ten-year birth cohorts (1920’s-1960’s). To estimate period rates, I pool annual data across three 6-year time periods: 2000–05, 2006–11, and 2012–17. For cohort calculations, data is pooled across 10-year birth cohorts and 10-year age intervals. To avoid relying on small sample sizes of deaths, cohort analyses begin at age 40.
Educational attainment is treated in four levels: less than high school, high school degree or equivalent, some college or associate’s degree, and bachelor’s degree or higher.
All estimates were performed using Stata Version 15.
The dual data source bias
Typically, mortality rates are calculated by dividing the number of deaths by person-years of exposure. In simplified notation, the alcohol-related mortality rate, , is estimated as:
| (1) |
where is the number of alcohol-related deaths and PYL is the number of person-years lived. In the U.S., these rates are often calculated using death certificate data from vital statistics in the numerator and person-year estimates from the Census or the Current Population Survey (CPS) in the denominator. Calculating age-specific mortality rates by educational attainment, however, requires having reliable information on educational attainment in both the numerator and the denominator. Unlike in surveys, educational attainment on death certificates is not self-reported, but reported by funeral directors, sometimes with help of surviving kin (Rostron et al. 2010). The CDC estimates that educational attainment on death certificates is incompatible with self-reported educational attainment in the CPS in 28% of cases (Rostron et al. 2010). Termed the dual data source bias, this numerator-denominator mismatch can significantly bias estimates of education-specific mortality rates (Hendi 2017; Rostron et al. 2010).
To avoid combining two sources of education reporting in a single fraction, I use a method outlined in Ho’s (2017) study of educational disparities in drug overdose deaths. Equation (1) above can be expanded to:
| (2) |
where is the number of deaths from all causes. Equation (2) can then be rewritten as:
| (3) |
where is the proportion of all deaths attributable to alcohol and is the mortality rate from all causes. Although and must still be estimated using survey and vital statistics data, each can be estimated using a single source and without combining different sources of education reporting in the same fraction.
Calculating the proportion of deaths attributable to alcohol (Ralc)
To estimate the proportion of deaths attributable to alcohol within a demographic group, I use public-use multiple cause-of-death files for years 2000–2017, downloaded from the National Bureau of Economic Research (NBER 2019). The records, which include all death certificates filed in a given year, list a decedent’s age, sex, educational attainment, underlying cause of death, and any contributing causes of death. I classify deaths as alcohol-related according to the classification system developed by the CDC’s Alcohol-Related Disease Impact (ARDI) application. The ARDI application groups alcohol-related causes of death into three categories, explained below and listed in Appendix Table 1. A more thorough explanation of the CDC’s methods is available on the ARDI website (cdc.gov/ARDI).
The first category is a set of 10 causes which are 100% attributable to alcohol use, such as alcohol abuse and alcoholic liver disease. In the present analysis, I classify a death as alcohol-related if any cause on the death certificate, either underlying or contributing, is one of these 10 causes.
The second classification of causes in the ARDI database are causes for which previous studies have directly observed the relationship between alcohol use and a given underlying cause of death. In some studies, investigators measured the proportion of persons dying from a particular cause with blood alcohol concentrations above a certain level. In others, follow-up studies obtained information from medical records or next-of-kin interviews to determine “a decedent’s pattern of alcohol consumption” (see ARDI methods section). Both types of studies provide a direct estimate of the proportion of deaths from a particular cause that are associated with alcohol use (termed the alcohol attributable fraction, or AAF). Appendix Table 1 lists the ICD-10 code(s) and AAF assigned to each cause.
The final set of ARDI causes include those for which CDC researchers combined data on the prevalence of alcohol consumption and data from meta-analyses on the association between cause-specific mortality risks and consumption to indirectly measure the alcohol-attributable fraction. Indirectly-measured fractions are based on cut-points for low, medium, and high levels of consumption. In this analysis, I assign all groups the fractions associated with a medium level of consumption. The fractions are often smallest at this level of consumption, thus minimizing possible bias associated with mis-assigning consumption levels. Under the assumption of medium consumption, these causes comprised between 4.4% and 9.0% of all alcohol-related deaths between 2000 and 2017 (Appendix Figure 1).
To estimate the total number of alcohol-related deaths in a given demographic group, I sum the number of deaths with 100% alcohol-attributable (underlying or contributing) causes of death with the number of deaths from (underlying) causes with directly- or indirectly-measured AAFs, each having been multiplied by their respective fraction. To avoid over-counting, deaths with an underlying AAF cause of death and a 100% alcohol-attributable contributing cause are excluded from the sum of AAF causes (since these deaths have already been classified as alcohol-related). In the case of a negative AAF, which indicates a protective effect of consumption, I subtract the number of deaths averted from the total of alcohol-related deaths. Appendix Figure 1 illustrates the distribution of the ARDI components of alcohol-related deaths over the period.
Calculating all-cause mortality rates by educational attainment (Mall)
To estimate all-cause mortality rates by educational attainment, I use data from the 1990–2009 public-use waves of the National Health Interview Survey (NHIS), downloaded from IPUMS (Blewett et al. 2018). NHIS, administered annually by the National Center for Health Statistics (NCHS), is a cross-sectional health survey that is nationally-representative of the non-institutionalized civilian population. Each wave has been linked to the National Death Index through December 2011, allowing for mortality follow-up. Although the NHIS includes decedents’ causes of death, the survey cannot be used to estimate alcohol-related mortality on its own due to the limited set of causes of death and small sample sizes of cause-specific deaths. More detailed information on survey design and sampling procedures are available on the IPUMS website (nhis.ipums.org).
I use mortality follow-up information to construct a person-year file in which respondents are censored upon contributing 10 years of follow-up, death, or at the end of 2011; whichever comes first. I restrict the study population to adults who were eligible for mortality follow-up and who were at least 30 years old at time of follow-up. Eligible respondents who were younger than 30 at baseline, but at least 25 (when formal schooling up to a college degree has likely been completed), can age into the sample upon reaching age 30. Although the analysis begins in 2000, I allow surviving respondents from waves as early as 1990 to enter the sample in 2000.
Table 1 shows the number of deaths and person-years lived in the person-year file. Although the counts in Table 1 are unweighted to show sample size, all calculations use the NCHS-recommended sampling weights to adjust for respondents who were ineligible for mortality follow-up (mortwt in IPUMS). To estimate age-specific mortality rates from all causes by period, I divide the weighted count of deaths by the count of person-years in each demographic group (sex, level of educational attainment, 5-year age interval), either across years (2000–2005 and 2006–2011) or 10-year birth cohort.
Table 1.
Sample sizes in NHIS person-year file used to estimate all-cause mortality rates (Mall).
| Period & education | Deaths | Person-years lived | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Age | ||||||||
| 30–44 | 45–59 | 60–74 | All 30+ | 30–44 | 45–59 | 60–74 | All 30+ | ||
| Males | 2000–2005 | ||||||||
| LHS | 265 | 765 | 2,046 | 7,575 | 89,205 | 80,113 | 72,503 | 292,152 | |
| HS | 408 | 1,148 | 2,196 | 7,033 | 206,406 | 182,559 | 100,507 | 533,029 | |
| SC | 250 | 819 | 1,093 | 3,765 | 144,537 | 142,210 | 55,525 | 364,562 | |
| BA | 83 | 357 | 529 | 1,869 | 96,627 | 94,467 | 37,450 | 243,495 | |
| 2006–2011 | |||||||||
| LHS | 185 | 669 | 1,418 | 5,208 | 72,190 | 69,390 | 50,540 | 227,242 | |
| HS | 292 | 1,077 | 1,709 | 5,771 | 143,091 | 164,099 | 87,161 | 432,585 | |
| SC | 204 | 747 | 1,036 | 3,516 | 119,747 | 134,969 | 65,596 | 343,072 | |
| BA | 68 | 279 | 436 | 1,683 | 82,784 | 87,843 | 43,351 | 229,501 | |
| Females | 2000–2005 | ||||||||
| LHS | 187 | 618 | 1,710 | 8,634 | 91,103 | 87,853 | 87,084 | 351,022 | |
| HS | 304 | 898 | 1,997 | 8,000 | 212,841 | 224,248 | 146,327 | 671,485 | |
| SC | 191 | 507 | 801 | 3,382 | 183,121 | 166,559 | 68,573 | 455,004 | |
| BA | 62 | 195 | 272 | 1,282 | 112,074 | 90,562 | 31,082 | 248,514 | |
| 2006–2011 | |||||||||
| LHS | 133 | 511 | 1,089 | 5,523 | 71,957 | 72,886 | 60,479 | 262,974 | |
| HS | 187 | 772 | 1,415 | 6,408 | 137,266 | 178,867 | 118,383 | 508,354 | |
| SC | 153 | 580 | 795 | 3,358 | 149,269 | 166,628 | 79,588 | 432,161 | |
| BA | 56 | 200 | 297 | 1,193 | 97,734 | 95,156 | 38,579 | 246,255 | |
Source: NHIS 1990–2009. Note: Unweighted sample sizes shown; all-cause mortality rates estimated using survey weights. LHS: less than high school. HS: High school diploma or GED. SC: Some college or associate’s degree. BA: Bachelor’s degree or more.
Combining Ralc and Mall
After multiplying all-cause mortality rates from the NHIS by the fraction of deaths associated with alcohol in vital statistics (Equation 3), I age-standardize period rates using the 2012 U.S. age distribution. To minimize cohort differences in age distributions across 10-year age intervals owing to differential exposure times, I standardize rates within each age interval (assuming a standard of 0.5 in both of the 5-year age intervals). To capitalize on the most recent vital statistics data, I estimate alcohol-related mortality rates for 2012–2017 under the assumption that all-cause mortality rates remained constant at 2006–2011 levels (while allowing Ralc to vary).
The Relative Index of Inequality (RII)
To calculate the relative index of inequality (Mackenbach and Kunst 1997; Schalick et al. 2000), I regress age-standardized death rates against the midpoints of the cumulative proportion of the four levels of educational attainment in the U.S. population, ranked lowest to highest. For period analyses, I use the sex-specific distribution of educational attainment in the CPS for the given age range in the first year of each period. For 30–44 year-olds, for example, I use the distribution of educational attainment among 30–44 year olds in years 2000, 2006, and 2012. For cohorts, I use the sex-specific distributions among 30–39 year-olds in the year when cohort members were 30–39 years old (for the 1940’s cohort, for example, I use the distribution among 30–39 year-olds in the 1980 CPS). I use weighted least squares regressions weighted by the relative size of each level of educational attainment.
The RII, commonly expressed as the ratio of the predicted mortality rate for the lowest status group against the predicted rate for the highest status group (Mackenbach and Kunst 1997; Schalick et al. 2000), can be interpreted as the relative disadvantage associated with belonging to the 0th vs. 100th percentile of educational attainment. The measure controls for changes in the distribution of educational attainment in the population over time, while additionally accounting for patterns throughout the distribution.
Results
Table 2 shows the percentage of all deaths attributable to alcohol (Ralc * 100) in vital statistics, by educational attainment. Regardless of sex or educational attainment, the percentage of deaths attributable to alcohol was greatest at ages 30–34 (hovering around 25% for males and 15% for females) and diminished with age (shrinking to less than 2% above age 80). Remarkably, the percentage of deaths attributable to alcohol increased between 2000–05 and 2012–17 for each of the 72 age, sex, and education groups in Table 2. Three of the four largest absolute increases were recorded among 30–34 year-old women with at least a high school degree (increases of 4.3–5.0 percentage points), while the four largest percent increases occurred among 55–64 year-old women without a college degree (increases of 68–85%). The negative association between Ralc and educational attainment, which is strong at young- and mid-adult ages, weakened with age and at some point was reversed. At ages 55 and above, the fraction of deaths attributable to alcohol was higher among women with college degrees than among women who did not graduate from high school.
Table 2.
Percentage of deaths in vital statistics attributable to alcohol (Ralc * 100).
| Period & education | Age | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80–84 | 85+ | |||
| Males | 2000–2005 | LHS | 26.88 | 23.73 | 21.77 | 18.03 | 13.75 | 8.94 | 6.10 | 4.07 | 2.67 | 1.79 | 1.30 | 0.93 |
| HS | 24.70 | 21.63 | 19.45 | 16.79 | 12.94 | 8.59 | 5.80 | 3.76 | 2.53 | 1.77 | 1.30 | 0.96 | ||
| SC | 23.02 | 20.03 | 18.14 | 15.40 | 12.37 | 8.30 | 5.56 | 3.71 | 2.60 | 1.81 | 1.36 | 1.03 | ||
| BA | 19.28 | 16.74 | 15.05 | 12.83 | 10.49 | 7.65 | 5.42 | 3.67 | 2.62 | 1.88 | 1.44 | 1.04 | ||
| 2006–2011 | LHS | 27.15 | 24.11 | 21.13 | 18.20 | 14.79 | 10.57 | 6.91 | 4.50 | 2.94 | 2.02 | 1.47 | 1.04 | |
| HS | 25.54 | 22.14 | 19.99 | 17.52 | 14.60 | 10.92 | 7.13 | 4.33 | 2.87 | 2.01 | 1.48 | 1.08 | ||
| SC | 24.30 | 21.13 | 19.16 | 16.68 | 13.79 | 10.34 | 7.08 | 4.45 | 2.86 | 2.08 | 1.55 | 1.16 | ||
| BA | 20.21 | 18.84 | 17.30 | 14.89 | 12.00 | 9.19 | 6.63 | 4.35 | 3.10 | 2.16 | 1.66 | 1.18 | ||
| 2012–2017 | LHS | 27.97 | 25.12 | 22.43 | 19.76 | 16.83 | 12.69 | 8.97 | 5.50 | 3.38 | 2.31 | 1.69 | 1.22 | |
| HS | 27.07 | 24.16 | 21.04 | 18.61 | 16.11 | 12.66 | 9.31 | 5.71 | 3.46 | 2.38 | 1.73 | 1.27 | ||
| SC | 26.22 | 23.94 | 21.05 | 18.29 | 15.30 | 11.82 | 8.96 | 5.61 | 3.48 | 2.41 | 1.82 | 1.32 | ||
| BA | 23.53 | 21.57 | 19.10 | 16.67 | 14.19 | 10.75 | 7.95 | 5.36 | 3.67 | 2.56 | 1.90 | 1.40 | ||
| Females | 2000–2005 | LHS | 15.33 | 14.58 | 12.81 | 9.81 | 6.35 | 3.79 | 2.50 | 1.75 | 1.31 | 0.99 | 0.76 | 0.54 |
| HS | 14.02 | 13.58 | 12.09 | 9.55 | 6.26 | 3.94 | 2.63 | 1.86 | 1.40 | 1.05 | 0.83 | 0.59 | ||
| SC | 14.44 | 13.02 | 11.41 | 9.07 | 6.54 | 4.19 | 3.10 | 2.16 | 1.58 | 1.19 | 0.92 | 0.64 | ||
| BA | 11.85 | 10.05 | 9.30 | 7.56 | 5.93 | 4.30 | 3.19 | 2.44 | 1.79 | 1.30 | 0.97 | 0.66 | ||
| 2006–2011 | LHS | 15.70 | 13.98 | 13.03 | 11.06 | 7.80 | 4.77 | 2.90 | 1.97 | 1.47 | 1.14 | 0.91 | 0.68 | |
| HS | 15.03 | 13.77 | 12.87 | 11.40 | 8.43 | 5.33 | 3.22 | 2.10 | 1.56 | 1.19 | 0.97 | 0.75 | ||
| SC | 15.98 | 14.00 | 12.70 | 10.78 | 8.48 | 5.75 | 3.68 | 2.43 | 1.75 | 1.38 | 1.05 | 0.83 | ||
| BA | 12.98 | 11.18 | 10.27 | 9.10 | 7.36 | 5.40 | 3.84 | 2.58 | 1.93 | 1.46 | 1.16 | 0.84 | ||
| 2012–2017 | LHS | 18.11 | 15.41 | 13.28 | 11.60 | 9.48 | 6.38 | 3.92 | 2.42 | 1.73 | 1.33 | 1.05 | 0.82 | |
| HS | 18.43 | 16.16 | 13.94 | 12.45 | 10.37 | 7.28 | 4.55 | 2.68 | 1.82 | 1.40 | 1.15 | 0.92 | ||
| SC | 19.39 | 16.90 | 14.36 | 12.35 | 10.26 | 7.43 | 4.95 | 2.99 | 2.06 | 1.55 | 1.24 | 1.01 | ||
| BA | 16.13 | 12.67 | 11.41 | 10.50 | 8.92 | 6.61 | 4.71 | 3.23 | 2.30 | 1.65 | 1.33 | 1.02 | ||
Source: Vital statistics 2000–2017. Note: The number of deaths in each cell (not shown) range from 735 to 33,299 deaths per 100,000, with an approximate mean of 5,600 deaths per 100,000. LHS: less than high school. HS: High school diploma or GED. SC: Some college or associate’s degree. BA: Bachelor’s degree or more.
Table 3 shows trends in age-standardized all-cause and alcohol-related mortality rates by educational attainment for adults 30+. Between 2000–05 and 2006–11, all-cause mortality rates decreased 6%−16% for all groups except less-than-high-school graduates. Rates among this group showed little change for men (an increase of 0.5%) and a small decrease for women (3%). In contrast to widespread improvements in all-cause mortality, death rates from alcohol-related causes increased at all levels of educational attainment over the same period, a trend that continued into 2012–17. Although absolute increases over the 18-year study period were largest for less-than-high-school graduates (increases of 28.3 additional alcohol-related deaths per 100,000 among men and 12.8 among women), relative increases were largest for high school graduates (30% for males and 45% for females). Males exhibited a higher incidence of alcohol-related mortality than women, though relative increases were larger among the latter at all levels of educational attainment (11–30% vs. 27–45%).
Table 3.
Age-standardized mortality rate (per 100,000) and relative index of inequality in all-cause and alcohol-related mortality by period, adults 30+.
| Cause & education | Period | % change (2000–05) – (2012–17) |
|||
|---|---|---|---|---|---|
| 2000–2005 | 2006–2011 | 2012–2017 | |||
| Males | All causes | ||||
| Relative index of ineq. | 1.74 | 2.03 | -- | ||
| Mortality rates | |||||
| LHS | 1712.0 | 1720.2 | -- | -- | |
| HS | 1341.2 | 1255.9 | -- | -- | |
| SC | 1249.8 | 1111.1 | -- | -- | |
| BA+ | 941.6 | 796.1 | -- | -- | |
| Alcohol-related causes | |||||
| Relative index of ineq. | 2.61 | 3.00 | 2.93 | ||
| Mortality rates | |||||
| LHS | 100.5 | 111.4 | 128.8 | 28.16 | |
| HS | 67.1 | 75.0 | 86.8 | 29.36 | |
| SC | 59.1 | 61.6 | 71.4 | 20.81 | |
| BA+ | 36.5 | 34.6 | 40.5 | 10.96 | |
| Females | All causes | ||||
| Relative index of ineq. | 1.96 | 2.04 | -- | ||
| Mortality rates | |||||
| LHS | 1222.6 | 1186.8 | -- | -- | |
| HS | 862.8 | 810.6 | -- | -- | |
| SC | 746.0 | 696.2 | -- | -- | |
| BA+ | 616.5 | 547.5 | -- | -- | |
| Alcohol-related causes | |||||
| Relative index of ineq. | 2.52 | 2.54 | 2.50 | ||
| Mortality rates | |||||
| LHS | 36.1 | 41.3 | 48.9 | 35.46 | |
| HS | 23.0 | 27.2 | 33.3 | 44.78 | |
| SC | 19.7 | 23.0 | 28.0 | 42.13 | |
| BA+ | 14.0 | 15.0 | 17.8 | 27.14 | |
Source: NHIS 1990–2009 and vital statistics 2000–2017. Note: Mortality rates age-standardized using the 2012 U.S. population distribution. LHS: less than high school. HS: High school diploma or GED. SC: Some college or associate’s degree. BA: Bachelor’s degree or more.
Table 3 suggests that well-documented educational inequalities in all-cause mortality were eclipsed by those in alcohol-related mortality. In 2000–05, non-high-school graduate males were 1.8 times more likely to die from all causes than college graduates, but 2.8 times more likely to die from alcohol-related causes (2.0 vs. 2.6 among women). Among males, inequality in alcohol-related mortality as measured by the RII grew over the 18-year study period, peaking at 3.00 in the middle of the period. In contrast, increases in alcohol-related mortality rates among women were accompanied by little change in the RII (stable around 2.5).
Appendix Table 2 shows the RII for 100% alcohol-attributable causes only. Throughout the study period, educational inequality was highest for the combination of 100%-attributable underlying and contributing causes, and lowest for the comprehensive set of alcohol-related causes used by the main analysis. Among males, inequality increased for 100% alcohol-attributable deaths over the period. Among females, inequality in 100%-attributable causes declined over time, driven by especially speedy rate increases at higher levels of educational attainment (not shown).
To illuminate age patterns underlying trends in Table 3, Table 4 shows age-standardized alcohol-related mortality rates and RIIs for three 15-year age intervals (30–44, 45–59, and 60–74). Over the period, alcohol-related mortality rates increased at least 11% for all groups except college-educated males ages 60–74 (3.1% increase), 30–44 year-old females without a high school degree (0.8% increase), and 30–44 year-old males without a high school degree (a decline of 8.1%). No change or even declines in rates for the least educated group at ages 30–44 is reflected in significant declines in the RII over time. Nevertheless, inequality among women in this age group remained higher than at any other age throughout the period. Among males, accelerating inequality among 45–59 year-olds surpassed inequality at younger ages by the middle of the period.
Table 4.
Age-standardized mortality rate (per 100,000) and relative index of inequality in alcohol-related mortality, by age and period.
| Age & education | Period | % change (2000–05) – (2012–17) |
|||
|---|---|---|---|---|---|
| 2000–2005 | 2006–2011 | 2012–2017 | |||
| Males | 30–44 | ||||
| Relative index of ineq. | 4.29 | 3.55 | 3.28 | ||
| Mortality rates | |||||
| LHS | 68.5 | 60.1 | 63.0 | −8.1 | |
| HS | 39.3 | 43.9 | 46.8 | 19.0 | |
| SC | 33.0 | 33.1 | 36.6 | 11.0 | |
| BA+ | 14.7 | 15.7 | 17.8 | 21.2 | |
| 45–59 | |||||
| Relative index of ineq. | 3.09 | 3.82 | 3.71 | ||
| Mortality rates | |||||
| LHS | 122.8 | 144.0 | 165.5 | 34.8 | |
| HS | 77.1 | 90.3 | 100.6 | 30.4 | |
| SC | 66.6 | 69.9 | 78.2 | 17.4 | |
| BA+ | 36.7 | 34.3 | 39.9 | 8.9 | |
| 60–74 | |||||
| Relative index of ineq. | 2.05 | 2.87 | 2.91 | ||
| Mortality rates | |||||
| LHS | 109.0 | 133.6 | 166.1 | 52.5 | |
| HS | 81.7 | 88.6 | 114.2 | 39.7 | |
| SC | 73.3 | 77.3 | 96.8 | 32.1 | |
| BA+ | 51.1 | 43.8 | 52.8 | 3.1 | |
| Females | 30–44 | ||||
| Relative index of ineq. | 4.91 | 4.14 | 3.94 | ||
| Mortality rates | |||||
| LHS | 27.7 | 26.1 | 28.0 | 0.8 | |
| HS | 16.9 | 17.7 | 20.4 | 20.5 | |
| SC | 12.2 | 13.1 | 15.3 | 25.7 | |
| BA+ | 5.5 | 5.8 | 6.6 | 20.3 | |
| 45–59 | |||||
| Relative index of ineq. | 3.28 | 3.32 | 3.27 | ||
| Mortality rates | |||||
| LHS | 42.9 | 55.2 | 66.2 | 54.3 | |
| HS | 24.2 | 33.5 | 41.2 | 70.5 | |
| SC | 18.8 | 26.7 | 32.6 | 73.2 | |
| BA+ | 13.0 | 14.7 | 17.6 | 35.9 | |
| 60–74 | |||||
| Relative index of ineq. | 1.70 | 1.84 | 1.84 | ||
| Mortality rates | |||||
| LHS | 34.6 | 39.2 | 49.9 | 44.3 | |
| HS | 24.1 | 25.8 | 33.9 | 40.5 | |
| SC | 25.4 | 24.9 | 31.5 | 24.2 | |
| BA+ | 19.1 | 20.2 | 24.8 | 29.8 | |
Source: NHIS 1990–2009 and Vital Statistics 2000–17. Note: Mortality rates age-standardized using the 2012 U.S. population distribution. LHS: less than high school. HS: High school diploma or GED. SC: Some college or associate’s degree. BA: Bachelor’s degree or more.
Percent increases in alcohol-related mortality rates were larger for females than males in 10 out of 12 age and education comparisons. The top three largest increases in Table 4 occurred for women ages 45–59. Increases here were led by women with some college or an associate’s degree (73.2% increase), followed by women with a terminal high school degree (70.5% increase). As a result of these sizable rate increases in the middle of the education distribution, the female RII in the middle ages remained constant over the period. In contrast, inequality grew for females 60–74, as well as for males 45–74.
Tables 5a and 5b consider trends in alcohol-related mortality rates and inequality across 10-year birth cohorts at comparable 10-year age intervals, for males (Table 5a) and females (Table 5b). For both sexes, the 1950’s cohort stands out for its unusual patterns. It is the only cohort for which mortality rates at all levels of educational attainment consistently exceeded rates in a neighboring cohort (relative to the 1940’s cohort for females, and relative to both the 1940’s and 1960’s cohorts for males). Within-cohort rate increases between ages 40–49 and 50–59 in this cohort were also notably large, especially for college graduates (for whom rates nearly doubled with age). Males born between 1950–1959 experienced greater levels of educational inequality in alcohol-related mortality than did neighboring cohorts. For females in this cohort, inequality at ages 40–49 was greater than in any subgroup in any other table (RII of 5.69). By ages 50–59, however, the RII for females in this cohort declined considerably to 2.90, driven by rapid increases in alcohol mortality among the most educated groups.
Table 5a.
Relative index of inequality in alcohol-related mortality, by age & cohort. Males.
| Cohort & Education | Age | |||
|---|---|---|---|---|
| 40–49 | 50–59 | 60–69 | 70–79 | |
| 1920–1929 | ||||
| Relative index of inequality | 1.67 | |||
| Mortality rates | ||||
| LHS | 106.5 | |||
| HS | 82.9 | |||
| SC | 78.2 | |||
| BA | 59.8 | |||
| 1930–1939 | ||||
| Relative index of inequality | 2.06 | 1.70 | ||
| Mortality rates | ||||
| LHS | 111.5 | 110.5 | ||
| HS | 79.4 | 80.3 | ||
| SC | 70.9 | 78.8 | ||
| BA | 53.5 | 64.9 | ||
| 1940–1949 | ||||
| Relative index of inequality | 2.80 | 2.91 | ||
| Mortality rates | ||||
| LHS | 121.1 | 126.3 | ||
| HS | 80.9 | 86.4 | ||
| SC | 70.0 | 74.6 | ||
| BA | 40.0 | 39.7 | ||
| 1950–1959 | ||||
| Relative index of inequality | 4.90 | 3.55 | ||
| Mortality rates | ||||
| LHS | 113.7 | 164.3 | ||
| HS | 60.6 | 100.2 | ||
| SC | 46.9 | 78.0 | ||
| BA | 21.6 | 42.6 | ||
| 1960–1969 | ||||
| Relative index of inequality | 3.97 | |||
| Mortality rates | ||||
| LHS | 78.3 | |||
| HS | 58.2 | |||
| SC | 46.2 | |||
| BA | 16.4 | |||
Source: NHIS 1990–2009 and vital statistics 2000–2017. Note: To minimize cohort differences in age distributions due to different exposure times, rates are age-standardized within each 10-year age interval (assuming a standard of 0.5 in each 5-year age interval). LHS: less than high school. HS: High school diploma or GED. SC: Some college or associate’s degree. BA: Bachelor’s degree or more.
Table 5b.
Relative index of inequality in alcohol-related mortality, by age & cohort. Females.
| Cohort & Education | Age | |||
|---|---|---|---|---|
| 40–49 | 50–59 | 60–69 | 70–79 | |
| 1920–1929 | ||||
| Relative index of inequality | 1.23 | |||
| Mortality rates | ||||
| LHS | 36.4 | |||
| HS | 30.9 | |||
| SC | 33.0 | |||
| BA | 29.7 | |||
| 1930–1939 | ||||
| Relative index of inequality | 1.76 | 1.40 | ||
| Mortality rates | ||||
| LHS | 33.2 | 37.5 | ||
| HS | 21.7 | 29.7 | ||
| SC | 25.8 | 30.9 | ||
| BA | 18.4 | 26.1 | ||
| 1940–1949 | ||||
| Relative index of inequality | 3.24 | 2.02 | ||
| Mortality rates | ||||
| LHS | 41.4 | 37.6 | ||
| HS | 21.9 | 23.5 | ||
| SC | 18.0 | 22.9 | ||
| BA | 13.2 | 17.1 | ||
| 1950–1959 | ||||
| Relative index of inequality | 5.69 | 2.80 | ||
| Mortality rates | ||||
| LHS | 49.0 | 50.5 | ||
| HS | 23.6 | 32.8 | ||
| SC | 17.0 | 27.7 | ||
| BA | 8.7 | 15.7 | ||
| 1960–1969 | ||||
| Relative index of inequality | 4.72 | |||
| Mortality rates | ||||
| LHS | 44.7 | |||
| HS | 26.3 | |||
| SC | 18.9 | |||
| BA | 8.9 | |||
See notes for Table 5a.
Discussion
Findings in context
This paper examined trends in inequalities in alcohol-related mortality by educational attainment in the U.S. over the period 2000–2017. It is the first study to document universal increases in alcohol-related mortality at ages 30+ for both sexes and at all levels of educational attainment, with increases ranging from 11–45% (Table 3). Large educational disparities in alcohol-related mortality that existed at the beginning of the study widened among men ages 45–74, as well as among women ages 60–74. Inequality at ages 30–44 declined for both sexes, driven by declining or stable rates among less-than-high-school graduates and rising rates at higher levels of educational attainment. Shifting patterns in this age group are consistent with work by Lui et al. (2018) who find that, in cohorts born after 1975, heavy drinking is more common among groups with higher levels of educational attainment.
Relative to neighboring cohorts, the 1950’s birth cohort experienced elevated alcohol-related mortality rates. Educational inequalities were especially high in this cohort, particularly among 40–49 year-old women. Disparities are consistent with work documenting notably high levels of heavy drinking among less educated women in the 1956–1960 birth cohort (Lui et al. 2018). That pattern may have weakened with age, however. By ages 50–59, considerable increases in alcohol-related mortality for well-educated women led to significant reductions in inequality.
How does the U.S. compare to other high-income countries? In an analysis of alcohol-related mortality in several European countries, Mackenbach et al. (2015) document a distinction between Western and Southern European countries on one hand (where alcohol-mortality rates changed little over recent decades), and Eastern and Northern countries on the other (where rates rose for all socio-economic groups over the period). The patterns documented in this analysis suggest that the U.S. trajectory is similar to the one observed in Eastern and Northern Europe. Widespread increases in these countries were particularly rapid for lower SES groups, considerably widening social disparities. This is also true for U.S. males, though U.S. females do not consistently fit this pattern, given particularly rapid increases in alcohol-related mortality among women with a terminal high school degree or some college experience.
Despite similar trends, the magnitude of alcohol mortality rates among males in Eastern and Northern European countries far exceeds that documented among U.S. males in the present analysis. Herttua et al.’s (2007) analysis of alcohol-related mortality at low, medium, and high levels of educational attainment in Finland estimated that male rates in 2002–03 ranged from roughly 62 deaths per 100,000 at the high level of educational attainment to 195 deaths per 100,000 at the low level; nearly twice as high as the 2000–05 range in the present analysis (37 to 101 deaths per 100,000). Surprisingly, rates among females were remarkably similar between these two studies, ranging from roughly 14 to 40 deaths per 100,000 for the high and low levels of educational attainment in Herttua et al.’s study and from 14 to 36 deaths per 100,000 in the present analysis. Compared to sex differences in European countries, these findings suggest that alcohol-related mortality levels may be unusually high among U.S. women (or unusually low among U.S. men). Declining sex differences in alcohol-related mortality in the U.S. are consistent with declining sex differences in high risk drinking and alcohol-related emergency room admissions (Grant et al. 2017; White et al. 2018).
Strengths and limitations
This analysis has several strengths. It is the first to examine trends in alcohol-related mortality by educational attainment in the United States using a comprehensive set of alcohol-related causes of death, including causes which are not entirely attributable to alcohol use. Its method of mortality rate estimation aims to reduce bias introduced by education misreporting on death certificates. Inequality is summarized using the relative index of inequality, which accounts for changes in the distribution of educational attainment over time and adjusts for trends throughout the distribution.
This study also has several limitations, four of which are discussed below. First, while the analysis includes all adult causes of death which research has rigorously linked to alcohol consumption, other causes continue to be identified and are not included here (Rehm et al. 2010). Second, the alcohol-attributable fractions in the ARDI application are assumed to apply across population subgroups (the sex- and age-specific fractions for motor vehicle fatalities are a notable exception). Assuming a constant contribution of alcohol across levels of educational attainment likely underestimates disparities in alcohol-related mortality. Evidence from abroad suggests that lower status groups bear a disproportionately larger burden of alcohol-related harm, even at similar levels of alcohol consumption (Makela and Paljarvi 2008; Probst et al. 2014).
Third, while the estimation approach for calculating alcohol-related mortality rates reduces some of the bias associated with education misreporting on death certificates, it cannot avoid the bias entirely. Education reporting on death certificates must still be relied on to estimate the fraction of deaths attributable to alcohol. If education misreporting operates in different directions, or at different magnitudes, for alcohol-related vs. all-cause mortality, the fraction of deaths associated with alcohol will be biased. However, it is difficult to hypothesize the direction of such a bias. Finally, the analysis assumes that all-cause mortality rates in 2012–17 remained constant over time. The assumption is necessary to study the most recent trends in the rapidly expanding proportion of deaths associated with alcohol in vital statistics (Table 2). Case and Deaton’s (2017) finding of a sustained deterioration in mortality conditions among non-Hispanic white college dropouts tentatively suggests that the growing divide in all-cause mortality documented between 2000–05 and 2006–11 in the present analysis may have continued into the final period. If so, estimates of educational disparities in alcohol mortality in the final period are conservative.
Conclusion
The prevalence of high risk drinking, alcohol use disorders, and alcohol-related emergency room visits has increased in recent years (Grant et al. 2017; White et al. 2018). This analysis documented that these trends were accompanied by near-universal increases in alcohol mortality across sex, age, and education subgroups (rising between 11% and 45% for adults ages 30+). Relative increases were consistently larger for females than males at all levels of educational attainment.
Future research should more clearly situate the U.S. among its peer countries, paying careful attention to differences between the U.S. and countries in which alcohol mortality has not increased. Additionally, research on age- and cohort-specific patterns of alcohol consumption by socio-economic status should aim to establish a link between changes in behavior and alcohol-related mortality.
Age- and cohort-patterns suggest the importance of targeted interventions across the life-course. Educational disparities in alcohol-related mortality increased among males ages 45–74 and females ages 60–74. Although inequality declined at ages 30–44, considerable disparities in this age group remained at the end of the study. Given the concentration of binge drinking at these ages (Kanny et al. 2018), persistent inequality at ages 30–44 may be amenable to interventions focused on binge drinking. Interventions aimed at groups with lower levels of educational attainment in this and other age groups may reduce educational disparities, yet care should also be taken to stem the increasing prevalence of alcohol-related deaths at all levels of educational attainment.
Appendix
Appendix Figure 1.
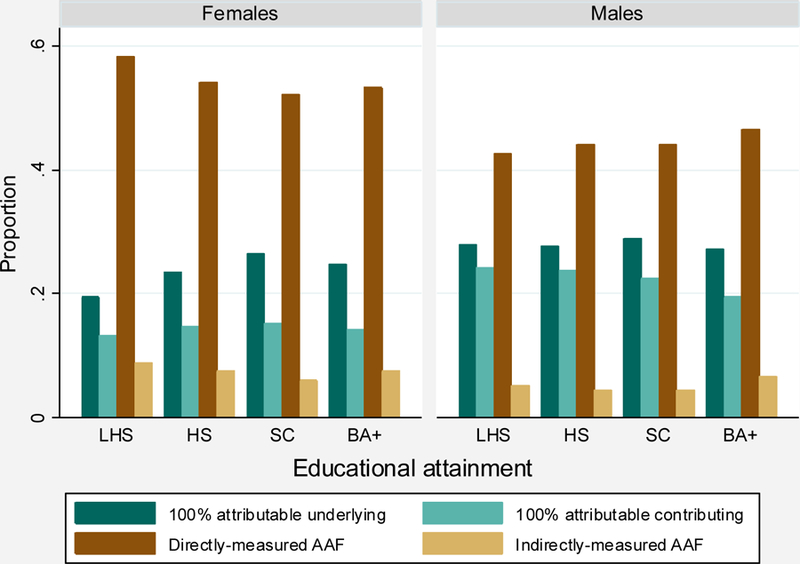
Distribution of alcohol-related cause-of-death components between 2000 and 2017.
Source: Vital statistics 2000–2017.
The following 4 categories are mutually exclusive and exhaustive (also see Appendix Table 1):
100% attributable underlying: Deaths with 100% alcohol-attributable underlying causes.
100% attributable contributing: Deaths with 100% alcohol-attributable contributing causes.
Directly-measured AAF: Deaths with an underlying cause with a directly-measured alcohol-attributable fraction.
Indirectly-measured AAF: Deaths with an underlying cause with an indirectly-measured alcohol-attributable fraction.
Appendix Table 1.
Alcohol-attributable fractions (AAF) and ICD-10 codes for alcohol-related adult causes of death.
| Cause of death | Sexa | AAF | Indirect AAF by consumption level | ICD-10 Codes | ||
|---|---|---|---|---|---|---|
| Low | Med | High | ||||
| CHRONIC CAUSES | ||||||
| 100% attributable | ||||||
| Alcoholic psychosis | 1 | -- | -- | -- | F10.3-F10.9 | |
| Alcohol abuse | 1 | -- | -- | -- | F10.0-F10.1 | |
| Alcohol dependence syndrome | 1 | -- | -- | -- | F10.2 | |
| Alcohol polyneuropathy | 1 | -- | -- | -- | G62.1 | |
| Degeneration of nervous system due to alcohol | 1 | -- | -- | -- | G31.2 | |
| Alcoholic myopathy | 1 | -- | -- | -- | G72.1 | |
| Alcohol cardiomyopathy | 1 | -- | -- | -- | I42.6 | |
| Alcoholic gastritis | 1 | -- | -- | -- | K29.2 | |
| Alcoholic liver disease | 1 | -- | -- | -- | K70-K70.4, K70.9 | |
| Alcohol-induced chronic pancreatitis | 1 | -- | -- | -- | K86.0 | |
| -- | -- | -- | ||||
| Causes with direct AAF estimates | -- | -- | -- | |||
| Acute pancreatitis | 0.24 | -- | -- | -- | K85 | |
| Chronic pancreatitis | 0.84 | -- | -- | -- | K86.1 | |
| Epilepsy | 0.15 | -- | -- | -- | G40, G41 | |
| Esophageal varices | 0.40 | -- | -- | -- | I85, I98.2 | |
| Gastroesophageal hemorrhage | 0.47 | -- | -- | -- | K22.6 | |
| Liver cirrhosis, unspecified | 0.40 | -- | -- | -- | K74.3-K74.6, K76.0, K76.9 | |
| Portal hypertension | 0.40 | -- | -- | -- | K76.6 | |
| Spontaneous abortion (females only) | 0.04 | -- | -- | -- | O03 | |
| Causes with indirect AAF estimates | ||||||
| Breast cancer (females only) | Females | -- | N/A | 0.01 | 0.00 | C50 |
| Cholelithiases | Males | -- | N/A | −0.01 | −0.01 | K80 |
| Females | -- | N/A | −0.01 | 0.00 | ||
| Chronic hepatitis | Males | -- | N/A | 0.01 | 0.02 | K73 |
| Females | -- | N/A | 0.01 | 0.01 | ||
| Esophageal cancer | Males | -- | N/A | 0.03 | 0.07 | C15 |
| Females | -- | N/A | 0.04 | 0.02 | ||
| Hypertension | Males | -- | N/A | 0.01 | 0.02 | I10-I15 |
| Females | -- | N/A | 0.01 | 0.01 | ||
| Ischemic heart disease | Males | -- | N/A | 0.00 | 0.00 | I20-I25 |
| Females | -- | N/A | 0.00 | 0.00 | ||
| Laryngeal cancer | Males | -- | N/A | 0.07 | 0.09 | C32 |
| Females | -- | N/A | 0.08 | 0.03 | ||
| Liver cancer | Males | -- | N/A | 0.05 | 0.06 | C22 |
| Females | -- | N/A | 0.05 | 0.02 | ||
| Oropharyngeal cancer | Males | -- | N/A | 0.02 | 0.10 | C01-C06, C09-C10, C12-C14 |
| Females | -- | N/A | 0.02 | 0.03 | ||
| Psoriasis | Males | -- | N/A | 0.02 | 0.03 | L40.0-L40.4, L40.8, L40.9 |
| Females | -- | N/A | 0.02 | 0.01 | ||
| Stroke, ischemic | Males | -- | N/A | 0.03 | 0.01 | G45, I63, I65-I67, I69.3 |
| Females | -- | N/A | 0.01 | 0.00 | ||
| Stroke, hemorrhagic | Males | -- | N/A | 0.07 | 0.11 | I60-I62, I69-I69.2 |
| Females | -- | N/A | 0.02 | 0.01 | ||
| Superventricular cardiac dysrhythmia | Males | -- | N/A | 0.03 | 0.03 | I47.1, I47.9, I48 |
| Females | -- | N/A | 0.03 | 0.01 | ||
| ACUTE CAUSES | ||||||
| 100% attributable | ||||||
| Alcohol poisoning | 1 | -- | -- | -- | X45, Y15, T51.0, T51.1, T51.9 | |
| Excessive blood alcohol level | 1 | -- | -- | -- | R78.0 | |
| Suicide by an exposure to alcohol | 1 | -- | -- | -- | X65 | |
| Causes with direct AAF estimates | ||||||
| Air-space transport | 0.18 | -- | -- | -- | V95-V97 | |
| Aspiration | 0.18 | -- | -- | -- | W78-W79 | |
| Drowning injuries | 0.34 | -- | -- | -- | W65-W74 | |
| Fall injuries | 0.32 | -- | -- | -- | W00-W19 | |
| Fire injuries | 0.42 | -- | -- | -- | X00-X09 | |
| Firearm injuries | 0.18 | -- | -- | -- | W32-W34 | |
| Homicide | 0.47 | -- | -- | -- | X85-Y09, Y87.1 | |
| Motor-vehicle non-traffic crashes | 0.18 | -- | -- | -- | V02.0, V03.0, V04.0, V09.0, V12-V14(.0-.2), V19.0-V19.3, V20-V28(.0-.2), V29.0-V29.3, V30-V39(.0-.3),V40-V49(.0-.3), V50-V59(.0-.3), V60-V69(.0-.3), V70-V79(.0-.3), V81.0, V82.0, V83-V86(.4-.9), V88.0-V88.8, V89.0 | |
| Motor-vehicle traffic crashes | Males | V02(.1, .9), V03(.1, .9), V04(.1, .9), V09.2, V12-V14(.3-.9), V19.4-V19.6, V20-V28(.3-.9), V29.4-V29.9, V30-V39(.4-.9), V40-V49(.4-.9), V50-V59(.4-.9), V60-V69(.4-.9), V70-V79(.4-.9), V80.3-V80.5, V81.1, V82.1, V83-V86(.0-.3), V87.0-V87.8, V89.2 | ||||
| 25–34 yrs | 0.49 | -- | -- | -- | ||
| 35–44 yrs | 0.47 | -- | -- | -- | ||
| 45–54 yrs | 0.41 | -- | -- | -- | ||
| 55–64 yrs | 0.28 | -- | -- | -- | ||
| 65+ yrs | 0.12 | -- | -- | -- | ||
| Females | ||||||
| 25–34 yrs | 0.37 | -- | -- | -- | ||
| 35–44 yrs | 0.34 | -- | -- | -- | ||
| 45–54 yrs | 0.28 | -- | -- | -- | ||
| 55–64 yrs | 0.16 | -- | -- | -- | ||
| 65+ yrs | 0.08 | -- | -- | -- | ||
| Occupational and machine injuries | 0.18 | -- | -- | -- | W24-W31, W45 | |
| Other road vehicle crashes | 0.18 | -- | -- | -- | V01, V05-V06, V09.1, V09.3, V09.9, V10-V11, V15-V18, V19.3, V19.8-V19.9, V80.0-V80.2, V80.6-V80.9, V81.2-V81.9, V82.2-V82.9, V87.9, V88.9, V89.1, V89.3, V89.9 | |
| Poisoning (not alcohol) | 0.29 | -- | -- | -- | X40-X49 (except X45) | |
| Suicide | 0.23 | -- | -- | -- | X60-X84 (except X65), Y87.0 | |
| Water transport | 0.18 | -- | -- | -- | V90-V94 | |
Applies to both males and females if not otherwise specified
Adapted from the CDC’s ADRI application (2019). This list includes all ARDI adult causes of death for the country as a whole between 2006–2010. Indirectly-measured AAFs were estimated for any consumption of alcohol.
Appendix Table 2.
Relative index of inequality in alcohol-related mortality by classification method of alcohol-related deaths and period, adults 30+.
| Classification method | Period | |||
|---|---|---|---|---|
| 2000–2005 | 2006–2011 | 2012–2017 | ||
| Males | 100% attributable (underlying) | 2.89 | 3.33 | 3.01 |
| 100% attributable | 3.20 | 3.61 | 3.32 | |
| Comprehensive | 2.61 | 3.00 | 2.93 | |
| Females | 100% attributable (underlying) | 2.85 | 2.70 | 2.49 |
| 100% attributable | 2.71 | 2.59 | 2.40 | |
| Comprehensive | 2.52 | 2.54 | 2.50 | |
Source: NHIS 1990–2009 and vital statistics 2000–2017.
100% attributable (underlying): Only deaths with 100% alcohol-attributable underlying causes (see Appendix Table 1) are considered alcohol-related.
100% attributable: Only deaths with 100% alcohol-attributable underlying or contributing causes are considered alcohol-related.
Comprehensive: Classification method of the main analysis. Deaths with 100% alcohol-attributable underlying or contributing causes, as well as underlying causes with alcohol-attributable fractions, are considered alcohol-related.
References
- Alcohol-Related Disease Impact (ARDI) database. Available at cdc.gov/ardi.
- Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Cogliano V, & WHO Int Agency Res Canc Monograph. (2007). Carcinogenicity of alcoholic beverages. Lancet Oncology, 8(4), 292–293. [DOI] [PubMed] [Google Scholar]
- Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, Casas JP, Dale CE, Denaxas S, Shah AD, & Hemingway H (2017). Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: Population based cohort study using linked health records. British Medical Journal, 356, j909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blewett L, Rivera Drew JA, Griffin R, King ML, & Williams KCW IPUMS Health Surveys: National Health Interview Survey, Version 6.2 Minneapolis: University of Minnesota, 2016. 10.18128/D070.V6.2 [DOI] [Google Scholar]
- Breslow RA, & Graubard BI (2008). Prospective study of alcohol consumption in the United States: Quantity, frequency, and cause-specific mortality. Alcoholism: Clinical and Experimental Research, 32(3), 513–521. [DOI] [PubMed] [Google Scholar]
- Breslow RA, Guenther PM, Juan W, & Graubard BI (2010). Alcoholic beverage consumption, nutrient intakes, and diet quality in the U.S. adult population, 1999–2006. Journal of the American Dietetic Association, 110(4), 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell TM, Rodgers B, Clark C, Jefferis BJMH, Stansfeld SA, & Power C (2008). Lifecourse socioeconomic predictors of midlife drinking patterns, problems and abstention: Findings from the 1958 British birth cohort study. Drug and Alcohol Dependence, 95(3), 269–278. [DOI] [PubMed] [Google Scholar]
- Case A, & Deaton A (2017). Mortality and morbidity in the 21st century. Brookings Papers on Economic Activity, 397–476. [DOI] [PMC free article] [PubMed]
- Case A, & Deaton A. (2015). Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proceedings of the National Academy of Sciences of the United States of America, 112(49), 15078–15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2012). Vital signs: Binge drinking prevalence, frequency, and intensity among adults: United States, 2010. Morbidity and Mortality Weekly Report, 61(1), 14–9. [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, & Hasin DS (2017). Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013 results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 74(9), 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendi AS (2017). Trends in education-specific life expectancy, data quality, and shifting education distributions: A note on recent research. Demography, 54(3), 1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herttua K, Mäkelä P, & Martikainen P (2014). Affordability of alcohol and educational disparities in alcohol-related mortality: A time-series analysis. International Journal of Behavioral Medicine, 21, S141–S141. [Google Scholar]
- Herttua K, Mäkelä P, & Martikainen P (2011). An evaluation of the impact of a large reduction in alcohol prices on alcohol-related and all-cause mortality: Time series analysis of a population-based natural experiment. International Journal of Epidemiology, 40(2), 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herttua K, Ostergren O, Lundberg O, & Martikainen P (2017). Influence of affordability of alcohol on educational disparities in alcohol-related mortality in Finland and Sweden: A time series analysis. Journal of Epidemiology and Community Health, 71(12), 1168–1176. [DOI] [PubMed] [Google Scholar]
- Ho JY (2017). The contribution of drug overdose to educational gradients in life expectancy in the United States, 1992–2011. Demography, 54(3), 1175–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Homa DM, O’Connor E, Babb SD, Caraballo RS, Singh T, Hu SS, & King BA (2015). Current cigarette smoking among adults: United States 2005–2014. Centers for Disease Control and Prevention Morbidity and Mortality Weekly Report, 64(44), 1233–40. [DOI] [PubMed] [Google Scholar]
- Kanny D, Naimi TS, Liu Y, Lu H, & Brewer RD (2018). Annual total binge drinks consumed by U.S. adults, 2015. American Journal of Preventive Medicine, 54(4), 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, McLaughlin KA, Link B, Olfson M, Grant BF, & Hasin D (2010). Stigma and treatment for alcohol disorders in the United States. American Journal of Epidemiology, 172(12), 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, & Power C (2003). Life course epidemiology. Journal of Epidemiology and Community Health, 57(10), 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C (2003). Relationships between nutrition, alcohol use, and liver disease. Alcohol Research & Health, 27(3), 220–231. [PMC free article] [PubMed] [Google Scholar]
- Lui CK, Kerr WC, Mulia N, & Ye Y (2018). Educational differences in alcohol consumption and heavy drinking: An age-period-cohort perspective. Drug and Alcohol Dependence, 186, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenbach JP, Kulhanova I, Bopp M, Borrell C, Deboosere P, Kovacs K, Looman CWN, Leinsalu M, Mäkelä P, Martikainen P, Menvielle G, Rodriguez-Sanz M, Rychtarikova J, & de Gelder R (2015). Inequalities in alcohol-related mortality in 17 European countries: A retrospective analysis of mortality registers. PLOS Medicine, 12(12), e1001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenbach J, & Kunst A (1997). Measuring the magnitude of socio-economic inequalities in health: An overview of available measures illustrated with two examples from Europe. Social Science & Medicine, 44(6), 757–771. [DOI] [PubMed] [Google Scholar]
- Mäkelä P, & Paljärvi T (2008). Do consequences of a given pattern of drinking vary by socioeconomic status? A mortality and hospitalisation follow-up for alcohol-related causes of the Finnish drinking habits surveys. Journal of Epidemiology and Community Health, 62(8), 728–733. [DOI] [PubMed] [Google Scholar]
- Mäkelä P, Herttua K, & Martikainen P (2015). The socioeconomic differences in alcohol-related harm and the effects of alcohol prices on them: A summary of evidence from Finland. Alcohol and Alcoholism, 50(6), 661–669. [DOI] [PubMed] [Google Scholar]
- Mehta NK, House JS, & Elliott MR (2015). Dynamics of health behaviours and socioeconomic differences in mortality in the USA. Journal of Epidemiology and Community Health, 69(5), 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniño AM, Arias E, Kochanek KD, Murphy SL, Smith BL (2002). Deaths: Final Data for 2000. National vital statistics reports; vol 50 no 15 Hyattsville, Maryland: National Center for Health Statistics. [PubMed] [Google Scholar]
- Mossakowski KN (2008). Is the duration of poverty and unemployment a risk factor for heavy drinking? Social Science & Medicine, 67(6), 947–955. [DOI] [PubMed] [Google Scholar]
- National Bureau of Economic Research (2019). Vital Statistics NCHS’ Multiple Cause of Death Data, 1959–2017 Cambridge, MA: https://www.nber.org/data/vital-statistics-mortality-data-multiple-cause-of-death.html [Google Scholar]
- Probst C, Roerecke M, Behrendt S, & Rehm J (2014). Socioeconomic differences in alcohol-attributable mortality compared with all-cause mortality: A systematic review and meta-analysis. International Journal of Epidemiology, 43(4), 1314–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GLG, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V, Roerecke M, Room R, Samokhvalov AV, & Taylor B (2010). The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction, 105(5), 817–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M, & Rehm J (2010). Irregular heavy drinking occasions and risk of ischemic heart disease: A systematic review and meta-analysis. American Journal of Epidemiology, 171(6), 633–644. [DOI] [PubMed] [Google Scholar]
- Rogers RG, Boardman JD, Pendergast PM, & Lawrence EM (2015). Drinking problems and mortality risk in the United States. Drug and Alcohol Dependence, 151, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostron BL, Boies JL, & Arias E (2010). Education reporting and classification on death certificates in the United States. National Center for Health Statistics. Vital Health Stat, 151(2). [PubMed] [Google Scholar]
- Schalick L, Hadden W, Pamuk E, Navarro V, & Pappas G (2000). The widening gap in death rates among income groups in the United States from 1967 to 1986. International Journal of Health Services, 30(1), 13–26. [DOI] [PubMed] [Google Scholar]
- Stahre M, Roeber J, Kanny D, Brewer RD, & Zhang X (2014). Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Preventing Chronic Disease, 11, 130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B, & Rehm J (2006). When risk factors combine: The interaction between alcohol and smoking for aero-digestive cancer, coronary heart disease, and traffic and fire injury. Addictive Behaviors, 31(9), 1522–1535. [DOI] [PubMed] [Google Scholar]
- Vierboom YC (2017). The contribution of differences in adiposity to educational disparities in mortality in the United States. Demographic Research, 37(54), 1735–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen S, Kaprio J, Viken R, Rose R, & Latvala A (2018). Birth cohort effects on the quantity and heritability of alcohol consumption in adulthood: A Finnish longitudinal twin study. Behavior Genetics, 48(6), 518–519. [DOI] [PubMed] [Google Scholar]
- Watzl B, & Watson R (1992). Role of alcohol-abuse in nutritional immunosuppression. Journal of Nutrition, 122(3), 733–737. [DOI] [PubMed] [Google Scholar]
- White AM, Slater ME, Ng G, Hingson R, & Breslow R (2018). Trends in alcohol-related emergency department visits in the United States: Results from the nationwide emergency department sample, 2006 to 2014. Alcoholism: Clinical and Experimental Research, 42(2), 352–359. [DOI] [PubMed] [Google Scholar]
- Xu JQ, Murphy SL, Kochanek KD, Bastian B, Arias E (2018). Deaths: Final data for 2016. National Vital Statistics Reports; vol 67 no 5 Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- Zaridze D, Brennan P, Boreham J, Boroda A, Karpov R, Lazarev A, Konobeevskaya I, Igitov V, Terechova T, Boffetta P, & Peto R (2009). Alcohol and cause-specific mortality in Russia: A retrospective case-control study of 48,557 adult deaths. Lancet, 373(9682), 2201–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhu Y, Wang PP, West R, Buehler S, Sun Z, Squires J, Roebothan B, McLaughlin JR, Campbell PT, & Parfrey PS (2012). Interaction between alcohol drinking and obesity in relation to colorectal cancer risk: A case-control study in Newfoundland and Labrador, Canada. BMC Public Health, 12(94). [DOI] [PMC free article] [PubMed] [Google Scholar]


