Abstract
Abamectin is a common biocide used to control agricultural insect pests. However, the water insolubility of abamectin may result in extra organic solvent introduced in the environment. To solve this issue, it is desirable to develop nanoformulations to encapsulate abamectin with environment-friendly polymers. In this study, two polylactic acid based abamectin nanoformulations were prepared. The average particle sizes, measured by dynamic light scattering and transmission electron microscope, were 240 nm and 150 nm, respectively. The insecticidal activity of these nano-formulated abamectin was examined in the laboratory on the pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae). The acute toxicity of nano-formulated abamectin on non-target aphid predator Adalia bipunctata (Coleoptera: Coccinellidae) was also evaluated by topical, residual and oral exposure. The two nano-formulated abamectin had comparable insecticidal effect with commercial abamectin formulation against the pea aphid. Taking median lethal concentration (LC50) as the toxicological endpoint, nanoformulations had higher contact toxicity and lower oral toxicity to first-instar larvae of the predator A. bipunctata. These results are expected to contribute to the application of solvent-free nano-formulated pesticides that comply with the integrated pest management (IPM) strategies.
Introduction
One of the global challenges faced by the agriculture industry is the sustainable food production for the rapidly growing human population, reaching 9.7 billion of individuals by 2050 [1–2]. Therefore, plant protection products and fertilizers are required to maximize the agricultural productivity [3]. In order to avoid the well-documented deleterious effects of pesticides, the efforts of agrochemical industry are not only focused on looking for new active substances, but also in new pesticide formulations [4].
During the last two decades, nanotechnology has been considered to have the potential to lead revolution in agricultural practices, especially in agrochemicals [5–6]. The development of nanotechnologies applied to pesticide formulations has facilitated the safe application of conventional pesticides by achieving precise and targeted delivery [7–9]. Besides, nanopesticide formulations may decrease the use of organic solvent and improve the biological efficacy of pesticides owing to the increasing dispersity, wettability, and penetration properties [6, 10]. Among all the nanopesticides, polymer-based nanoformulations are regarded as having the greatest potential for further development and practical application, due to their biocompatibility, biodegradability, modifiability and miscibility properties [10–13].
Avermectins represent a class of macrocyclic lactones that are produced by the soil dwelling actinomycete, Streptomyces avermitilis [14]. There are eight structural components, and abamectin is a mixture of avermectin B1a and avermectin B1b. With a broad spectrum of activity, abamectin is one of most used biocides worldwide to control agricultural pests for its insecticide and acaricide activities [15]. However, the water insolubility of abamectin may result in extra organic solvent introduced in the environment [16], and abamectin is also susceptible to ultraviolet and strong acidic or alkaline conditions, which may cause premature degradation [17]. A great deal of efforts has been made to provide protection and sustainable release of abamectin, among which developing nanoformulations to encapsulate abamectin with environment-friendly polymers is an effective strategy [18].
Polylactic acid (PLA) is a USFDA-approved polymeric material that is widely employed as drug or cell carrier in the medical area for its biodegradable and mechanical properties that can be modified [19]. Active ingredients (AI) could be protected from photodegradation after encapsulation by PLA nanoparticles [15, 20]. Tannic acid (TA) is a plant polyphenol that exhibits antioxidant, antibacterial, antimicrobial, antimutagenic, and anticarcinogenic properties [21]. The unique structural properties of TA facilitate the interaction with other materials via electrostatic, hydrogen bonding, and hydrophobic interactions [21]. Due to the adhesive properties, TA-modified compounds were employed as coating materials and used for control of bacterial and mammalian cell adhesion, radical scavenging and marine fouling [22–24]. Therefore, TA modified nanopesticide could improve the adhesion of AI on target crops and pests. Besides, TA could also enhance the dispersibility and biocompatibility of nanomaterials [25], which may result in better efficacy of TA-modified nanopesticides.
Naturally-occurring biological control agents, such as arthropod predators, could control pests such as aphids in a wide range of cropping systems. However, the application of insecticide may disrupt the biological services through lethal and sublethal effects, including suppressing the population growth, extending the pre-adult period, and altering the mRNA expression in the progeny generation [26–29]. Nanopesticides may behave differently from conventional pesticides, so it is necessary to evaluate the effects on pest and non-target organisms [30].
In this study laboratory bioassays were conducted to identify the insecticidal effect of two nano-formulated abamectin on a major aphid species, Acyrthosiphon pisum (Hemiptera: Aphididae). Acyrthosiphon pisum is a worldwide distributed pest and a vector of more than 30 virus diseases [31]. In addition, as the protection of natural enemies of aphids in agricultural habitats remains an imperative issue, potential adverse effects on the non-target aphid predators Adalia bipunctata (Coleoptera: Coccinellidae) were also evaluated.
Materials and methods
Chemicals
Abamectin at a purity of 95.6% (pure technical grade) was purchased from Qilu Pharmaceutical Company, Ltd., China. PLA (molecular weight of 100,000) was purchased from Daigang Biomaterial Company, China. Poly(vinyl alcohol) (PVA) and agar were purchased from Sigma Aldrich. Dichloromethane (CH2Cl2, 99.8%), TA (95%), poly(ethylene glycol) (PEG, molecular weight of 10,000) were purchased from Bailingwei Technology Company, Ltd., China. A commercial emulsifiable concentrate (EC) containing 18 g/L of abamectin (Vertimec) was obtained from Syngenta, Belgium.
Preparation of nano-formulated abamectin
PLA-based nano-formulated abamectin was synthesized according to a previously reported emulsion solvent evaporation method (O/W) [32]. PLA (40 mg/mL) and abamectin (40 mg/mL) were dissolved in CH2Cl2 by magnetic stirring to form an organic phase. PVA was dissolved in ultrapure water to form water phase (10 mg/mL). The organic phase was added dropwise over 10 min into the water phase under high shear emulsification (C25, ATS Engineering Ltd., Vancouver, Canada). CH2Cl2 was eliminated from the nanosuspension by evaporation at room temperature with magnetic stirring (1,000 rpm) overnight. The abamectin PLA nanospheres (Abam-PLA-NS) were collected by centrifuging the nanosuspension at 15,000 rpm for 10 min at 4 °C and the deposition was redispersed in deionized water; this process was repeated three times to remove as much surfactant as possible.
TA modified nanospheres were formulated by a self-assembly method. PEG (12 mg/mL) was added to the nanosuspension mentioned above, and followed by dropping TA (12 mg/mL). After stirring for 1h, the TA modified abamectin PLA nanospheres (Abam-PLA-Tannin-NS) were collected via centrifugation at 15,000 rpm for 10 min at 4 °C and was washed three times with deionized water.
Characterization of nano-formulated abamectin
The hydrodynamic particle size and polydispersity index (PDI) of nanospheres were investigated at 25 °C by dynamic light scattering (DLS; Zetasizer Nano-ZS90, Malvern, Worcestershire, UK). The morphology of the nano-formulated abamectin was observed via transmission electron microscope (TEM, HT7700, Hitachi Ltd., Tokyo, Japan). About 6 μl of the dispersed nanospheres was dropped on the surface of a cleaned copper grid. The TEM images were performed at 80 kV and 10 mA after the nanospheres were completely dried. The abamectin loading efficiency of nanospheres was investigated by high performance liquid chromatography (HPLC, 1260 Infinity, Agilent Company, California, USA). In brief, an appropriate aliquot of nanospheres was dispersed in CH2Cl2 (5ml) and sonicated for 5min, followed by evaporation of the organic solvent at room temperature. Then abamectin was diluted to an appropriate volume with methanol. For the HPLC analysis, a C18 column (5 mm, 4.6 mm × 150 mm, Agilent Technologies; Santa Clara, CA, USA) was used to separate the target compound from others at room temperature. Methanol/water (90:10, v/v) was used as mobile phase at a flow rate of 1.0 mL/min. The wavelength of UV detector was 245 nm. Loading efficiency (%) = (weight of pesticide in nanospheres/weight of nanospheres) ×100%.
Biological materials
For aphid rearing, broad beans (Vicia faba L.) were used as host plants. The seeds were sown in 30 cm × 20 cm boxes, which contained a 1:1 mixture of vermiculite and perlite. The plants were infested with aphids at two-leaf stage. Aphids A. pisum were collected from Gembloux, Belgium, and they had been reared in the laboratory for several years. Aphids were kept under controlled conditions (22 ± 2 °C, 70 ± 10% R.H. and 16L:8D of photoperiod).
First instars of lady beetles A. bipunctata were purchased from Biobest Group NV, Belgium. Larvae were reared on a diet of frozen Ephestia kuehniella (Lepidoptera, Pyralidae) eggs and water until pupation or death. All studies were conducted at 22 ± 1 °C, 30 ± 5% R.H., and a photoperiod of 16L:8D of photoperiod.
Insecticidal effect of nano-formulated abamectin on the aphid A. pisum
An agar solution was prepared to perform the insecticidal assay on Petri dishes (diam. 3.5 cm) containing a broad bean leaf and aphids. Agar powder was mixed with distilled water (1%, w/w), heated until boiling and then allowed cooling while constantly mixing. After cooling for approximately 10 minutes, warm agar was poured into each Petri dish to a depth that was at least 3–4 mm. A round piece of leaf of 33 mm in diameter was cut using a sharpened metal tube, and put on the agar gel with abaxial surface facing skywards. Ten apterous aphid individuals were transferred onto each of the leaf discs using a fine brush.
The bioassay was conducted using Potter Precision Laboratory Spray Tower (Burkard Scientific, Uxbridge, UK) at a spray pressure 0.70 kg/cm2 (69 kPa; 10 psi) [33]. The biocidal efficacies of Abam-PLA-NS, Abam-PLA-Tannin-NS and the commercial EC were evaluated. Water was used as the untreated control. Based on preliminary experiments to establish the range of concentrations to be tested, six concentrations, 3.125 mg/L, 6.25 mg/L, 12.5 mg/L, 25 mg/L, 50 mg/L, and 100 mg/L were tested for each formulation. Each aphid-containing Petri dish was sprayed with 1 mL of the tested solution, representing a deposit of 27.9 ± 2.1 mg on the leaf-disc. Then, all dishes were sealed with a close-fitting, ventilated lids. Two days after the application, the number of alive aphids was counted in each dish. An aphid was considered dead if it failed to react when touched by the brush. There were 3 replicates in each treatment (formulation × concentration) and for the water control.
Non-target effect of nano-formulated abamectin on coccinellid predator A. bipunctata
Abamectin was relatively safe to adult lady beetles [34–35], so first instars were selected as the objects in this study. Larvae were treated with Abam-PLA-NS, Abam-PLA-Tannin-NS and the commercial EC of abamectin, using the same six concentrations in the aphid bioassay. Controls were maintained using water alone. Abamectin acts on glutamate-gated chloride ion channels in arthropods to produce long-term, high-intensity inhibitory effects, causing insects to die. Abamectin has oral and contact toxicity. Therefore, three groups of insecticidal assays were conducted on lady beetles or aphids with Potter spray tower:
(a) Topical exposure: Ten larvae were transferred to a Petri dish and then 1 mL of the tested solution was sprayed. Larvae were then individually transferred to clean plastic Petri dishes and checked for mortality daily.
(b) Residual exposure: Petri dishes were sprayed with 1mL of the tested solution (n = 10 for each concentration of a tested solution). A single larva was then individually transferred to a Petri dish. After 24 h of contact, they were transferred to clean plastic Petri dishes and checked for mortality daily.
(c) Oral exposure: 20 aphids (A. pisum) were sprayed with 1mL of the tested solution. After air dry, they were transferred to a clean plastic pot where a lady beetle larva was introduced (n = 10 for each concentration of a tested solution). Frozen Ephestia kuehniella eggs and water were offered after all the aphids died. Larvae were checked for mortality daily.
Statistical analysis
Insect mortality was corrected by Abbott’s formula [36], taking into account the natural mortality observed on the control. The mortality of insect exposure to different formulations was analyzed by one-way ANOVA. Probit analysis was performed in order to estimate the LC50 [37], in which, a Chi-square Goodness-of-fit test was used to analyze the mortality data. The dose-mortality relationships were considered valid when the observed data and the expected data did not diverge significantly (P<0.05). Data analysis was carried out on SPSS Statistics V.17 (IBM).
Results and discussions
Characterization of nano-formulated abamectin
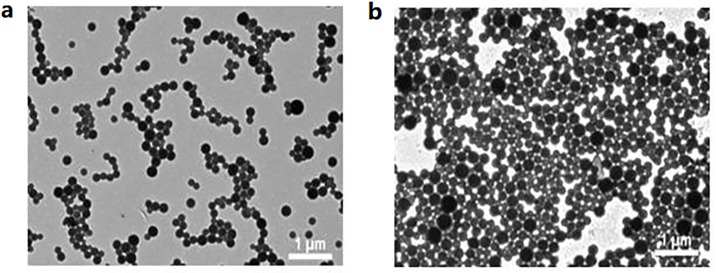
The hydrodynamic size of Abam-PLA-NS measured by DLS was 240.7±1.9 nm, and it increased to 243.6±1.2 nm for the Abam-PLA-Tannin-NS. The PDIs were 0.03±0.02 and 0.02±0.01 for Abam-PLA-NS and Abam-PLA-Tannin-NS, respectively. The low PDIs implied a narrow size distribution and monodispersion, which was also confirmed by TEM characterization results. TEM imaging (Fig 1) indicated that these nanoparticles exhibited nearly uniform spheres, and the statistical average size of 100 nanoparticles from the TEM images was 150.7±2.2 nm for Abam-PLA-NS, and it increased to 156.5±2.4 nm for the Abam-PLA-Tannin-NS. These results suggested that the surface of Abam-PLA-NS was capped with TA to form Abam-PLA-Tannin-NS. According to the HPLC analytical results, the abamectin loading efficiency of Abam-PLA-NS was 46.9%, and it decreased to 38.9% for Abam-PLA-Tannin-NS.
Fig 1. TEM images of Abam-PLA nanoparticles (a) and Abam-PLA-Tannin nanoparticles (b).
Insecticidal effect of nano-formulated abamectin on the aphid A. pisum
The bioassay results of nano-formulations and commercial EC on A. pisum were showed in Table 1. The data fitted the linear model (no statistically significant deviation of data from the regression equation). LC50 values were 33.3, 10.1 and 13.1 mg/L for Abam-PLA-NS, Abam-PLA-Tannin-NS, and abamectin EC, respectively. However, there was no significant difference among the three formulations, because their 95% confidence limits overlapped. And the statistical results of mortality also confirmed this (F = 0.949, P = 0.409).
Table 1. Laboratory bioassay results of abamectin formulations against aphids after 48h.
| Formulation | Toxicity regression equation | R2 | LC50 (mg/L) |
95% confidence limit | χ2 (df = 4) |
P |
|---|---|---|---|---|---|---|
| Abam-PLA-NS | y = 2.87+1.40x | 0.886 | 33.3 | 18.6–59.5 | 1.296 | 0.862 |
| Abam-PLA-Tannin-NS | y = 4.11+0.88x | 0.812 | 10.1 | 4.2–23.9 | 4.336 | 0.362 |
| Abmectin EC | y = 3.38+1.45x | 0.987 | 13.1 | 7.5–22.8 | 1.679 | 0.795 |
Nano-formulated pesticides are expected to improve the efficiency of pesticide and reduce environmental pollution [6, 10]. These two solvent-free nano-formulated abamectin exhibited similar biocidal efficacy on aphids, because nano-sized formulations can improve the adhesivity and penetrability of pesticides on surface of organisms [20, 38]. Abam-PLA-Tannin-NS had a lower LC50 than Abam-PLA-NS, which attributed to the enhancing contact of abamectin on the epidermis of aphids, due to the adhesive properties of TA.
Abamectin is categorized as highly toxic with acute oral and dermal toxicity with low LD50 concentrations for different organisms [39]. However, its popularity is growing due to the effective pest control. Usually, in the bioassay, the type, developmental stage and physiological condition of the target organism have a great influence on the efficacy of the pesticide. In addition, the environmental condition may also affect the response of the target to the agent. The recorded LC50 of abamectin for mustard aphid Lipaphis erysimi Kalt (Hemiptera: Aphididae) was 0.63 mg/L [40]. The recorded LC50 for peach aphid Myzus persicae Sulzer (Hemiptera: Aphididae) were 1.5 mg/L [41], and that was 5.5 mg/L for Anjum et al’s bioassay [42]. But results of our study could get support from our recent bioassay on M. persicae (LC50 of 17.38 mg/L and 10.68 mg/L for Abam-PLA-NS and Abam-PLA-Tanning-NS respctively) [32].
Non-target effect of nano-formulated abamectin on coccinellid predator A. bipunctata
Some studies have confirmed that nanoformulations were harmless to non-target organism, such as different cell lines and soil microorganisms [43], but only a few safety studies were carried out on natural predators [44].
The biocidal effects of all tested formulations against lady beetle larvae after 48 h and 120 h are presented in Tables 2, 3 and 4 for topical exposure, residual exposure and oral exposure, respectively. For all the trials, there was no statistically significant deviation of data from the regression equation. For each exposure group, overlapped 95% confidence limits indicated non-significant difference among three formulations. There was no significant difference of larvae mortality in each group as well (topical exposure, 48 h, F = 0.024, P = 0.976; 120 h, F = 0.118, P = 0.889; residual exposure 48 h, F = 0.658, P = 0.532; 120 h, F = 0.317, P = 0.733; oral exposure, 48 h, F = 0.139, P = 0.872; 120 h, F = 0.056, P = 0.946).
Table 2. Topical exposure for three abamectin formulations on lady beetle larvae.
| Time | Formulation | Toxicity regression equation | R2 | LC50 (mg/L) |
95% confidence limit | χ2 (df = 4) |
P |
|---|---|---|---|---|---|---|---|
| 48 h | Abam-PLA-NS | y = 3.79+1.10x | 0.871 | 12.5 | 6.2–25.2 | 5.817 | 0.121 |
| Abam-PLA-Tannin-NS | y = 3.44+1.26x | 0.789 | 16.6 | 8.9–31.0 | 9.005 | 0.061 | |
| Abmectin EC | y = 2.72+1.75x | 0.935 | 19.4 | 12.0–31.3 | 4.626 | 0.328 | |
| 120 h | Abam-PLA-NS | y = 3.75+1.44x | 0.844 | 7.4 | 4.2–13.2 | 1.174 | 0.882 |
| Abam-PLA-Tannin-NS | y = 3.92+1.37x | 0.831 | 6.0 | 3.3–11.0 | 1.945 | 0.746 | |
| Abmectin EC | y = 3.36+1.64x | 0.877 | 10.3 | 6.2–17.1 | 1.105 | 0.893 |
Table 3. Residual exposure for three abamectin formulations on lady beetle larvae.
| Time | Formulation | Toxicity regression equation | R2 | LC50 (mg/L) |
95% confidence limit | χ2 (df = 4) |
P |
|---|---|---|---|---|---|---|---|
| 48 h | Abam-PLA-NS | y = 2.47+2.26x | 0.976 | 13.2 | 8.8–19.7 | 4.808 | 0.308 |
| Abam-PLA-Tannin-NS | y = 3.52+1.27x | 0.983 | 14.7 | 7.9–27.3 | 3.395 | 0.494 | |
| Abmectin EC | y = 3.72+0.66x | 0.846 | 83.9 | 23.5–299.8 | 3.162 | 0.531 | |
| 120 h | Abam-PLA-NS | y = 3.03+2.01x | 0.920 | 9.7 | 6.2–15.0 | 7.926 | 0.094 |
| Abam-PLA-Tannin-NS | y = 3.40+1.76x | 0.960 | 8.1 | 5.0–13.6 | 4.303 | 0.367 | |
| Abmectin EC | y = 3.14+1.58x | 0.805 | 15.3 | 9.1–25.6 | 2.376 | 0.667 |
Table 4. Oral exposure for three abamectin formulations on lady beetle larvae.
| Time | Formulation | Toxicity regression equation | R2 | LC50 (mg/L) |
95% confidence limit | χ2 (df = 4) |
P |
|---|---|---|---|---|---|---|---|
| 48 h | Abam-PLA-NS | y = 2.57+1.16x | 0.884 | 110.2 | 50.9–238.6 | 4.935 | 0.294 |
| Abam-PLA-Tannin-NS | y = 1.63+1.82x | 0.998 | 71.3 | 42.2–120.4 | 5.414 | 0.247 | |
| Abmectin EC | y = 1.85+1.95x | 0.806 | 41.9 | 26.4–66.4 | 2.770 | 0.597 | |
| 120 h | Abam-PLA-NS | y = 2.98+1.09x | 0.816 | 74.0 | 34.7–157.5 | 4.354 | 0.360 |
| Abam-PLA-Tannin-NS | y = 1.13+2.58x | 0.984 | 31.3 | 21.6–45.6 | 4.531 | 0.339 | |
| Abmectin EC | y = 3.01+1.46x | 0.762 | 22.5 | 13.0–39.2 | 4.899 | 0.298 |
Taking LC50 as the toxicological endpoint, two nanoformulations showed higher contact toxicity than EC in both topical and residual applications 48 h and 120 h after the spray. It resulted in a similar trend as compared to aphids. This suggested that the nano-formulated abamectin had no selectivity for either insect group. For the commercial formulation, free abamectin on the surface of insects and Petri dishes were rapidly degraded, thereby reducing exposure to toxic residues. Nanoformualtions could enhance the stability of the pesticide and inhibit the degradation of abamectin [32, 45], then, improved the toxicity to ladybeetles. While the oral exposure results showed totally opposite trends. Both nano-formulated abamectin showed higher LC50 than abamectin EC after feeding. It could be accounted by the fact that PLA prevented abamectin from contacting with the lady beetles after entering the digestive tract [46]. The existence of TA also increased the toxicity of abamectin nanospheres due to the improved adhesion.
When applied in crop protection, abamectin turns into a source of concern for non-targeted beneficial arthropods and evolving resistance in pests [47]. The effects of 13 agrochemicals used in grapevines on spider mite Panonychus ulmi (Acari: Tetranychidae) and the predatory mite Neoseiulus californicus (Acari: Phytoseiidae) were evaluated, and exposure to abamectin significantly reduced survival both of P. ulmi and of N. californicus [48]. A study proved that commercial abamectin was the most harmful substance (in term of lethal and sublethal effects) among 14 tested pesticides against Orius laevigatus Fieber (Hemiptera: Anthocoridae), a commonly used predator in biological control programs [49]. It was found that commercial abamectin EC at recommended concentration combined with 0.5% summer oil was highly toxic to Rhyzobius lophanthae Blaisdell (Coleoptera: Coccinellidae) adults and larvae in both direct applications and pesticide residue situations [50]. The recorded LC50 of abamectin for the first instar and adult of multicolored Asian lady beetles, Harmonia axyridis (Coleoptera: Coccinellidae) were <0.09 mg/L and 4.88 mg/L, respectively, with a topical method in laboratory condition [51]. Whereas abamectin commercial formulation of 18 mg/L could cause 14.66% mortality of H. axyridis in eggplant ecosystem [52] with the spray method. It is difficult to verify such contradictory results, which may partly attribute to the differences of both experimental methods and environmental conditions. Compared the topical exposure results with the recorded LC50, two solvent-free nanoformulations were more safety to ladybeetles.
Conclusions
In this study, two solvent-free nano-formulated abamectin were prepared, and their biocidal efficacy on aphids and on lady beetles was tested. Generally, there was no significant difference between nanoformulations and commercial EC in mortality of the acute toxicity. The environment-friendly compositions of nanoformulations make them more suitable as the plant protection products in IPM strategies. It is noticeable that the side effects on the non-target organisms at different sublethal concentrations, should also be considered for a complete understand of the nanoformulations [29, 53]. Meanwhile, further studies on the field trial would be certainly worthwhile.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported by the National Key Research and Development Program of China (2017YFD0200300 and 2016YFD0200500).
References
- 1.Department of Economic and Social Affairs of the United Nations Secretariat: World population prospects: the 2015 revision, key findings and advance tables. 2015. https://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf
- 2.Godfray HCJ, Garnett T. Food security and sustainable intensification. Philos T Roy Soc B. 2014; 369: 20120273 10.1098/rstb.2012.0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Oliveira JL, Campos EV, Bakshi M, Abhilash PC, Fraceto LF. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: prospects and promises. Biotechnol Adv. 2014; 32: 1550–1561. 10.1016/j.biotechadv.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 4.Villaverde JJ, Sevilla-Morán B, López-Goti C, Sandín-España P, Alonso-Prados JL. An overview of nanopesticides in the frame work of European legislation In: Grumezescu AM, editor. New Pesticides and Soil Sensors. London: Elsevier; 2017. pp 227–271. [Google Scholar]
- 5.Chen H, Yada R. Nanotechnologies in agriculture: New tools for sustainable development. Trends Food Sci Tech. 2011; 22: 585–594. 10.1016/j.tifs.2011.09.004 [DOI] [Google Scholar]
- 6.Kah M, Beulke S, Tiede K, Hofmann T. Nanopesticides: State of knowledge, environmental fate, and exposure modeling. Crit Rev Environ Sci Technol. 2013; 43: 1823–1867. 10.1080/10643389.2012.671750 [DOI] [Google Scholar]
- 7.Nuruzzaman M, Rahman MM, Liu Y, Naidu R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J Agric Food Chem. 2016; 64: 1447–1483. 10.1021/acs.jafc.5b05214 [DOI] [PubMed] [Google Scholar]
- 8.Khandelwal N, Barbole RS, Banerjee SS, Chate GP, Biradar AV, Khandare JJ, et al. Budding trends in integrated pest management using advanced micro- and nano-materials: Challenges and perspectives. J Environ Manage. 2016; 184: 157–169. 10.1016/j.jenvman.2016.09.071 [DOI] [PubMed] [Google Scholar]
- 9.Satehi AB, Ziaee M, Ashrafi A. Silica nanoparticles: a potential carrier of chlorpyrifos in slurries to control two insect pests of stored products. 2018; 37: 77–91. 10.1127/entomologia/2017/0406 [DOI] [Google Scholar]
- 10.Kah M, Hofmann T. Nanopesticide research: Current trends and future priorities. Environ Int. 2014; 63: 224–235. 10.1016/j.envint.2013.11.015 [DOI] [PubMed] [Google Scholar]
- 11.Campos EVR, de Oliveira JL, Fraceto LF, Singh B. Polysaccharides as safer release systems for agrochemicals. Agron Sustain Dev. 2015; 35: 47–66. 10.1007/s13593-014-0263-0 [DOI] [Google Scholar]
- 12.Roy A, Singh SK, Bajpai J, Bajpai AK. Controlled pesticide release from biodegradable polymers. Cent Eur J Chem. 2014; 12: 453–69. 10.2478/s11532-013-0405-2 [DOI] [Google Scholar]
- 13.Campolo O, Cherif A, Ricupero M, Siscaro G, Grissa-Lebdi K, Russo A, et al. Citrus peel essential oil nanoformulations to control the tomato borer, Tuta absoluta: chemical properties and biological activity. Sci Rep. 2017, 7: 13036 10.1038/s41598-017-13413-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burg RW, Miller BM, Baker EE, Birnbaum J, Currie SA, Hartman R, et al. Avermectins, new family of potent anthelmintic agents: Producing organism and fermentation. Antimicrob Agents Chemother. 1979: 15: 361–367. 10.1128/aac.15.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu M, Yao J, Liang J, Zeng Z, Cui B, Zhao X, et al. Development of functionalized abamectin poly(lactic acid) nanoparticles with regulatable adhesion to enhance foliar retention. RSC Adv. 2017; 7: 11271 10.1039/c6ra27345a [DOI] [Google Scholar]
- 16.Cui B, Wang C, Zhao X, Yao J, Zeng Z, Wang Y, et al. Characterization and evaluation of avermectin solid nanodispersion prepared by microprecipitation and lyophilisation techniques. PLoS ONE. 2018; 13: e0191742 10.1371/journal.pone.0191742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He S, Zhang WB, Li DG, Li PL, Zhu YC, Ao MM, et al. Preparation and characterization of double-shelled avermectin microcapsules based on copolymer matrix of silica–glutaraldehyde–chitosan. J Mater Chem B. 2013; 1: 1270–1278. 10.1039/C2TB00234E [DOI] [PubMed] [Google Scholar]
- 18.Li D, Liu B, Yang F, Wang X, Shen H, Wu D. Preparation of uniform starch microcapsules by premix membrane emulsion for controlled release of avermectin. Carbohydr Polym. 2016; 136: 341–349. 10.1016/j.carbpol.2015.09.050 [DOI] [PubMed] [Google Scholar]
- 19.Lee BK, Yun Y, Pakr K. PLA micro- and nano-particles. Adv Drug Deliver Rev. 2016; 107: 176–191. 10.1016/j.addr.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu B, Wang Y, Yang F, Wang X, Shen H, Cui H, et al. Construction of a controlled-release delivery system for pesticides using biodegradable PLA-based microcapsules. Colloid Surf B Biointerfaces. 2016; 144: 38–45. 10.1016/j.colsurfb.2016.03.084 [DOI] [PubMed] [Google Scholar]
- 21.Rahim MA, Ejima H, Cho KL, Kempe K, Mullner M, Best JP, et al. Coordination-driven multistep assembly of metal–polyphenol films and capsules. Chem Mater. 2014; 26: 1645–1653. 10.1021/cm403903m [DOI] [Google Scholar]
- 22.Ejima H, Richardson JJ, Liang K, Best JP, van Koeverden MP, Such GK, et al. One-step assembly of coordination complexes for versatile film and particle engineering. Science. 2013; 341: 154–157. 10.1126/science.1237265 [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Gim T, Kang SM. Versatile, tannic acid-mediated surface PEGylation for marine antifouling applications. ACS Appl Mater Interfaces. 2015; 7: 6412–6416. 10.1021/acsami.5b01304 [DOI] [PubMed] [Google Scholar]
- 24.Sileika TS, Barrett DG, Zhang R, Lau KHA, Messersmith P.B. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew Chem Int Edit. 2013; 52: 10766–10770. 10.1002/anie.201304922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Liu M, Zhang X, Deng F, Zhou C, Hui J, et al. Interaction of tannic acid with carbon nanotubes: enhancement of dispersibility and biocompatibility. Toxicol Res. 2015; 4: 160–1688. 10.1039/C4TX00066H [DOI] [Google Scholar]
- 26.Jam NA, Saber M. Sublethal effects of imidacloprid and pymetrozine on the functional response of the aphid parasitoid, Lysiphlebus fabarum. Entomol Gen. 2018; 38: 173–190. 10.1127/entomologia/2018/0734 [DOI] [Google Scholar]
- 27.Mohammed AAAH, Desneux N, Fan Y, Han P, Ali A, Song D, et al. Impact of imidacloprid and natural enemies on cereal aphids: Integration or ecosystem service disruption? Entomol Gen. 2018; 37: 47–61. 10.1127/entomologia/2017/0471 [DOI] [Google Scholar]
- 28.Ullah F, Gul H, Desneux N, Tariq K, Ali A, Gao X, et al. Clothianidin-induced sublethal effects and expression changes of vitellogenin and ecdysone receptors genes in the melon aphid, Aphis gossypii. Entomol Gen. 2019; 39: 137–149. 10.1127/entomologia/2019/0865 [DOI] [Google Scholar]
- 29.Desneux N, Decourtye A, Delpuech JM. The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol. 2007; 52: 81–106. 10.1146/annurev.ento.52.110405.091440 [DOI] [PubMed] [Google Scholar]
- 30.Kookana RS, Boxall ABA, Reeves PT, Ashauer R, Beulke S, Chaudhry Q, et al. Nanopesticides: guiding principles for regulatory evaluation of environmental risks. J Agric Food Chem. 2014; 62: 4227–4240. 10.1021/jf500232f [DOI] [PubMed] [Google Scholar]
- 31.Hullé M, Chaubet B, Turpeau E, Simon JC. Encyclop’Aphid: a website on aphids and their natural enemies. Entomol Gen. 2019. 10.1127/entomologia/2019/0867 [DOI] [Google Scholar]
- 32.Yu M, Sun C, Xue Y, Liu C, Qiu D, Cui B, et al. Tannic acid-based nanopesticides coating with highly improved foliage adhesion to enhance foliar retention. RSC Adv. 2019; 9: 27096 10.1039/c9ra05843e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao S, Wang G, Zhou Y, Wang M, Yang D, Yuan H, et al. Water-soluble food dye of allura red as a tracer to determine the spray deposition of pesticide on target crops. Pest Manag Sci. 2019; 75: 2592–2597. 10.1002/ps.5430 [DOI] [PubMed] [Google Scholar]
- 34.Mani M. Hundred and sixty years of Australian lady bird beetle Crypotolaemus montrouzieri Mulsant—a global view. Biocontrol Sci Tech. 2018; 28: 938–952. 10.1080/09583157.2018.1487029 [DOI] [Google Scholar]
- 35.Ozawa A, Uchiyama T. Effects of pesticides on adult ladybird beetle Serangium japonicum (Coleoptera: Coccinellidae), a potential predator of the tea spiny whitefly Aleurocanthus camelliae (Hemiptera: Aleyrodidae). JPN J Appl Entomol Z. 2016; 60: 45–49. [Google Scholar]
- 36.Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925; 18: 265–267. [Google Scholar]
- 37.Finney DJ. Probit analysis. 3rd ed Cambridge, Cambridge University Press; 1971. [Google Scholar]
- 38.Boehm ALL, Martinon I, Zerrouk R, Rump E, Fessi H. Nanoprecipitation technique for the encapsulation of agrochemical active ingredients. J Microencapsul. 2003; 20: 433–441. 10.1080/0265204021000058410 [DOI] [PubMed] [Google Scholar]
- 39.Lewis KA, Green A, Tzilivakis J, Warner D. The pesticide properties database (PPDB) developed by the Agriculture & Environment Research Unit (AERU). University of Hertfordshire; 2015, pp 2006e2015.
- 40.Ujjan AA, Khanzada M, Shahzad S. Insecticide and papaya leaf extract toxicity to mustard aphid (Lipaphis Erysimikal). J Agri-Food Appl Sci. 2014; 2: 45–48. [Google Scholar]
- 41.Al Antary TM, Abdel-Wali MI. Integration of biological and chemical control of Myzus persicae (Sulzer) (Hemiptera: Aphididae) under greenhouse conditions. Egypt J Biol Pest Co. 2016; 26: 533–537. [Google Scholar]
- 42.Anjum F, Wright D. Relative toxicity of insecticides to the crucifer pests Plutella xylostella and Myzus persicae and their natural enemies. Crop Prot. 2016; 88: 131–136. 10.1016/j.cropro.2016.06.002 [DOI] [Google Scholar]
- 43.Pasquoto-Stigliani T, Campos EVR, Oliveira JL, Silva CMG, Bilesky-José N, Guilger M, et al. Nanocapsules containing neem (Azadirachta Indica) oil: development, characterization, and toxicity evaluation. Sci Rep. 2017; 7: 5929 10.1038/s41598-017-06092-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papanikolaou NE, Kalaitzaki A, Karamaouna F, Michaelakis A, Papadimitriou V, Dourtoglou V, et al. Nano-formulation enhances insecticidal activity of natural pyrethrins against Aphis gossypii (Hemiptera: Aphididae) and retains their harmless effect to non-target predators. Environ Sci Pollut Res Int. 2018; 25: 10243–10249. 10.1007/s11356-017-8596-2 [DOI] [PubMed] [Google Scholar]
- 45.Wang A, Wang Y, Sun C, Wang C, Cui B, Zhao X, et al. Fabrication, characterization, and biological activity of avermectin nano-delivery systems with different particle sizes. Nanoscale Res Lett. 2018; 13: 2 10.1186/s11671-017-2405-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee BK, Yun Y, Park K. PLA micro- and nano-particles. Adv Drug Deliver Rev. 2016; 107: 176–191. 10.1016/j.addr.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai SH, Ogbourne S. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere. 2016; 154: 204–214. 10.1016/j.chemosphere.2016.03.113 [DOI] [PubMed] [Google Scholar]
- 48.Silva DE, do Nascimento JM, da Silva RTL, Juchem CF, Ruffatto K, da Silva GL, et al. Impact of vineyard agrochemicals against Panonychus ulmi (Acari: Tetranychidae) and its natural enemy, Neoseiulus californicus (Acari: Phytoseiidae) in Brazil. Crop Prot. 2019; 123: 5–11. 10.1016/j.cropro.2019.05.014 [DOI] [Google Scholar]
- 49.Biondi A, Desneux N, Siscaro G, Zappalà L. Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: Selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere. 2012; 87: 803–812. 10.1016/j.chemosphere.2011.12.082 [DOI] [PubMed] [Google Scholar]
- 50.Kaspi R, Madar R, Domeradzki S. Acaricides compatibility with the armored scale predator Rhyzobius lophanthae. Biol Control. 2019; 132: 42–48. 10.1016/j.biocontrol.2019.01.011 [DOI] [Google Scholar]
- 51.Youn YN, Seo MJ, Shin JG, Jang C, Yu YM. Toxicity of greenhouse pesticides to multicolored Asian lady beetles, Harmonia axyridis (Coleoptera: Coccinellidae). Biol Control. 2016; 28: 164–170. 10.1016/S1049-9644(03)00098-7 [DOI] [Google Scholar]
- 52.Islam T, Das G. Compatibility of selected biorational pesticides with the predatory arthropods in brinjal ecosystem. J Bangladesh Agril Univ. 2017; 15: 234–238. 10.3329/jbau.v15i2.35068 [DOI] [Google Scholar]
- 53.Ricupero M, Desneux N, Zappalà L, Biondi A. Target and non-target impact of systemic insecticides on a polyphagous aphid pest and its parasitoid. Chemosphere. 2020. 10.1016/j.chemosphere.2019.125728 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.