Abstract
Background and Aims
Endo-sponge [Braun Medical] assisted early surgical closure [ESC] is an effective treatment to control pelvic sepsis after ileal pouch-anal anastomosis [IPAA] leakage, and became standard treatment in our centre from 2010 onwards. The aim of this cohort study was to assess the long-term pouch function of ulcerative colitis [UC] patients treated with ESC or conventional management [CM] for anastomotic leakage after IPAA.
Methods
Consecutive patients who underwent an IPAA for UC between 2002 and 2017 were included. Patients treated with ESC [2010–2017] or CM [2002–2009] for anastomotic leakage were compared with control patients without anastomotic leakage of the corresponding time period. Main endpoints were long-term pouch function on a 3-point scale and pouch failure, as measured with the validated pouch dysfunction score questionnaire.
Results
Some 280 of 334 patients [84%] returned the pouch dysfunction questionnaire, of whom 18 were treated with ESC and 22 with CM for anastomotic leakage. Control cohorts included 133 [2010–2017] and 107 patients [2002–2009]. Between ESC-treated patients and control patients, pouch function [p = 0.647] and pouch failure rates [0/18 versus 5/133, p >0.99] were similar. CM resulted in worse pouch function [p = 0.016] and a higher pouch failure rate [5/22 versus 5/107, p = 0.013] compared with control patients.
Conclusions
ESC, in contrast to CM, for IPAA leakage in UC patients is associated with preservation of pouch function and preclusion of pouch failure, probably due to early and effective treatment of pelvic sepsis.
Keywords: Ileal pouch-anal anastomosis, anastomotic leakage, ulcerative colitis
1. Introduction
Despite improvements of medical treatment strategies, a colectomy is still required in up to 20% of ulcerative colitis [UC] patients.1,2 For these patients, restorative proctocolectomy with ileal pouch-anal anastomosis [IPAA] is the treatment of choice. Anastomotic leakage occurs in up to 15% of these patients.3–5 Inadequately managed pelvic sepsis considerably affects long-term pouch function, due to postponement of stoma reversal, pouch fistulas, and pouch fibrosis, all of which are associated with pouch failure. Consequently, anastomotic leakage is the main cause of pouch failure [31%].5–7
The conventional management [CM] of anastomotic leakage entails a passive approach by diversion with ileostomy and occasional drainage of the presacral abscess cavity. Subsequently, a wait-and-see approach is adopted. However, the healing process can take up to months, possibly affecting functional outcomes.8 Aiming at a quick and efficient control of pelvic sepsis, active management of anastomotic leakage by Endo-sponge [Braun Medical, Melsungen, Germany] assisted early surgical closure [ESC] was implemented in our centre in 2010. ESC entails a short course of transanally inserted Endo-sponge [ Braun Medical] therapy to clean the presacral cavity and to facilitate early surgical closure of the anastomotic defect.9 The short-term results of this approach were very promising, revealing a 100% successful closure rate after a median of 7 weeks compared with 52% at 6 months after CM without significant differences in direct medical costs.10
Thus far, long-term results of ESC have not been reported. It is expected that the active ESC strategy, in contrast to the passive CM approach, preserves long-term pouch function due to effective control of pelvic sepsis. The aim of this study was to compare the long-term pouch function and pouch failure rate after ESC versus CM in UC patients with anastomotic leakage after IPAA.
2. Material and Methods
2.1. Design and patients
Consecutive patients who underwent IPAA in the Amsterdam UMC between January 2002 and October 2017 were prospectively maintained in the institutional IPAA database. Anastomotic leakage was confirmed either by radiological imaging or during surgical exploration within 90 days following IPAA surgery.11 From January 2010 onwards, patients with an anastomotic leakage after IPAA were managed with ESC. Patients treated with CM for anastomotic leakage between January 2002 and December 2009 were retrospectively identified. Adult UC and inflammatory bowel disease unclassified [IBDU] patients who underwent IPAA were screened for eligibility. Exclusion criteria were: patients with an indication for IPAA due to familial adenomatour polyposis (FAP), Crohn's disease, or colorectal cancer, postoperative diagnosis of Crohn's disease in the pouch, redo-pouch surgery only in the study period, anastomotic leakage detected later than 3 months after IPAA surgery, leakage treatment strategies not in accordance with the ESC or CM principles, a functioning IPAA of less than 1 year, cognitive inability to reply to the questionnaire, deceased during follow-up, and non-responders to the questionnaire. This study was waived from review of the medical ethics boards on March 9, 2016, since the prospective data collection, as well as the questionnaire, did not interfere with the psychological integrity of the patients. Reporting of the data adheres to the STROBE Statement.12 All participants provided written informed consent.
2.2. Procedures
2.2.1. Ileal pouch-anal anastomosis
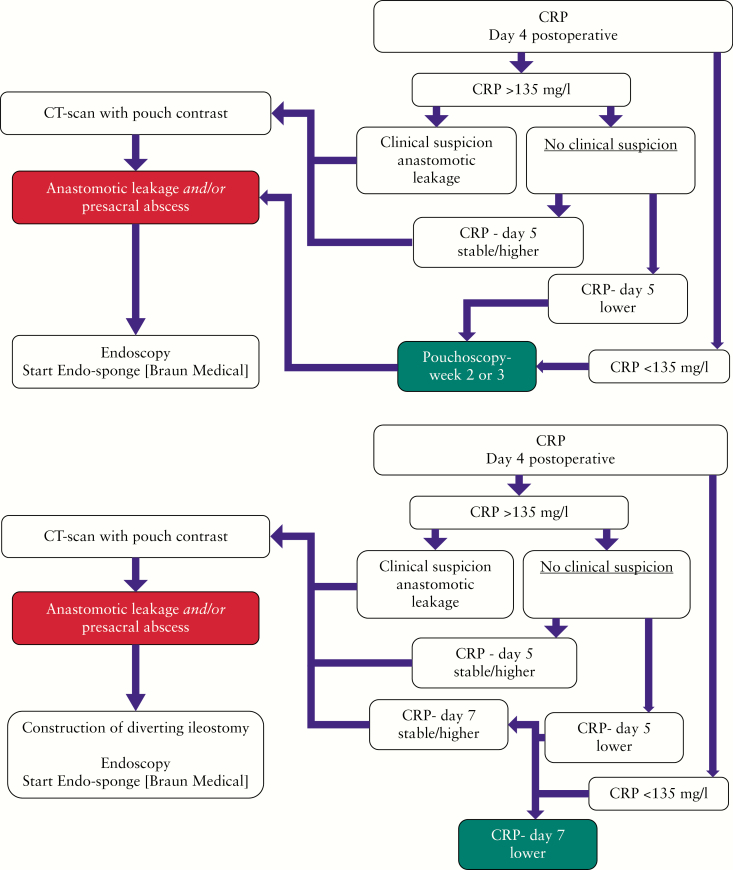
The IPAA was created during initial proctocolectomy, or at the time of completion proctectomy. The IPAA was not routinely defunctioned. Patients had an intraluminal pouch drain decompressing the pouch, which was removed the sixth day after surgery. Anastomotic leakage was diagnosed with C-reactive protein [CRP] levels at day 4 and 7 after pouch creation and with contrast enhanced CT imaging for any suspicion of a leak [see flowchart, Figure 1a and b]. Patients who developed anastomotic leakage underwent immediate pouch defunctioning if not done primarily. During the study period, the laparoscopic approach, as the modified two-stage procedure, became standard of care [stage 1: subtotal colectomy with end-ileostomy in order to improve clinical condition before restorative surgery, e.g. by discontinuing medication and optimising nutrition status, and stage 2: completion proctectomy with IPAA without diverting ileostomy].
Figure 1.
a] Postoperative management algorithm of early detection of anastomotic leakage in the diverted pouch. b] Postoperative management algorithm of early detection of anastomotic leakage in the non-diverted pouch. CRP, C-reactive protein; CT, computed tomography. *Pouch drain is removed at Day 6.
2.2.2. Endo-sponge [Braun Medical] assisted early surgical closure
ESC has been described previously.9,10 In short; in addition to the diversion, an Endo-sponge [Braun Medical] was inserted endoscopically and exchanged under light sedation every 3 to 4 days at the endoscopy room. Admission was not required for Endo-sponge [Braun Medical] therapy. After discharge, outpatient appointments were made to change the Endo-sponge [Braun Medical]. When the cavity was clean without significant proximal pouch retraction, transanal suture closure was performed under general anaesthesia in a short hospital admittance. Anastomotic integrity was assessed endoscopically 2 weeks after surgical closure. Subsequently, CT with intraluminal contrast was used to exclude presacral fluid collections. If closure failed, ESC was repeated.
2.2.3. Conventional management
CM of IPAA leakage consisted of diversion combined with transabdominal, transgluteal, or transanal drainage of the presacral abscess cavity. A wait-and-see policy was adopted and progress of anastomotic healing was regularly checked by either contrast enema X-ray or endoscopy. Removal of the drain and reversal of the ileostomy was planned when complete healing was confirmed.
2.3. Outcomes
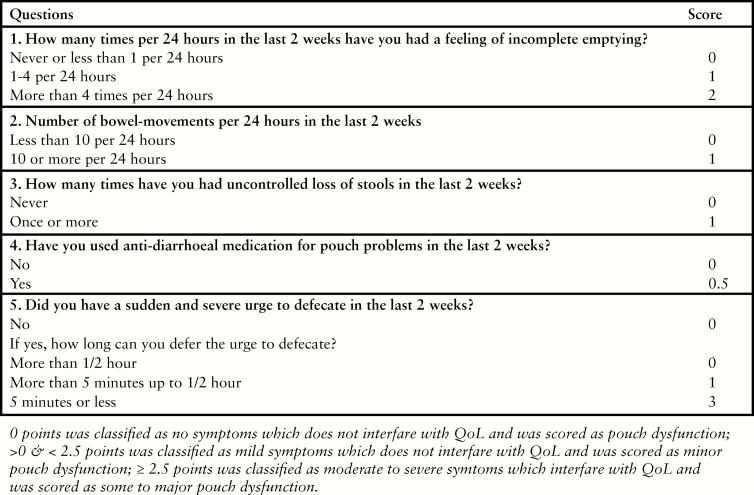
The primary outcome was pouch function which was measured with the validated pouch dysfunction questionnaire [Figure 2].13 Eligible patients were sent an invitation to participate in the study, together with information on the study and the questionnaire. Patients who did not initially respond, were contacted by telephone to encourage return of the questionnaire. Pouch function contained three categories: ‘none to minor’ dysfunction, ‘some to major’ dysfunction, and ‘pouch failure’. Pouch failure was defined as the requirement of a permanent stoma with or without pouch excision. Patients were asked if they had a stoma. If not, the questionnaire assessed incomplete emptying, number of bowel movement/24 h, major incontinence, use of antidiarrhoeal medication, and urgency, as these factors have a significant impact on quality of life [QoL] [score of 0 to 7.5 points]. Based on the derived scores, patients were categorised into: ‘none to minor’ dysfunction [0–<2.5 points], ‘some to major’ dysfunction [≥2.5 points], or pouch failure.
Figure 2.
Pouch dysfunction score.
Additionally, in supplementary analysis of this study, the reliability of the pouch dysfunction questionnaire was investigated. Therefore, along with the questionnaire, patients were also asked to report the impact of pouch dysfunction on QoL on a 4-point scale [none, minor, some, or major impact on QoL].
Secondary outcomes were pouch failure, treatment-specific details, and short-term results of ESC and CM. Treatment specific details included type of CM drainage, the number of Endo-sponge [Braun Medical] changes [during and after discharge], the number of Endo-sponges [Braun Medical] used, and duration of Endo-sponge [Braun Medical] treatment. Short-term results were time from IPAA to anastomotic leakage diagnosis, time from diagnosis to starting treatment, anastomotic closure at 6 months [chronic pelvic sepsis], time from diagnosis to observed closure on imaging, complications of anastomotic leakage treatment within 90 days, and time to ileostomy reversal.
2.4. Statistical analysis
Descriptive data were reported as mean ± standard deviation [SD] or median with interquartile range [IQR] according to the distribution. Normally distributed numerical data were analysed with unpaired t test for two subgroups or one-way analysis of variance [ANOVA] for three subgroups. Not normally distributed numerical data were analysed with the Mann–Whitney U test for two subgroups or Kruskal-Wallis for three subgroups. Categorical data were analysed with the chi-square or Fisher’s exact test, as appropriate. The functional outcomes of the ESC treated patients were compared with control patients without anastomotic leakage within the same study period [2010–2017]. CM treated patients were compared with control patients within the same study period [2002–2009]. Pouch function was assessed with the chi-square test for trend. Pouch failure over time was analysed using Kaplan-Meier analysis and compared with log-rank test.
To test the relation between the pouch dysfunction score and QoL, patients’ reported QoL was compared with the pouch dysfunction derived from the pouch dysfunction score [0–7.5], using one-way ANOVA and unpaired t-test as appropriate; p <0.05 was considered statistically significant. For statistical analyses, SPSS Statistics, version 24 [IBM Corp., Armonk, New York, USA] was used.
3. Results
3.1. Patients
Some 334 patients out of 493 patients who underwent IPAA surgery between January 2002 and October 2017 were eligible. The main exclusion criterion was FAP [n = 108, Supplementary Figure 1, available as Supplementary data at ECCO-JCC online]. Of the eligible patients, 280 returned the pouch dysfunction questionnaire [84% response rate]. Two of the 54 patients who did not return the questionnaire had anastomotic leakage after IPAA, both treated conventionally before 2010. Baseline characteristics between responders and the non-responders were not significantly different [Supplementary Table 1, available as Supplementary data at ECCO-JCC online].
The mean age of the 280 included patients was 38 years [SD 13] and 53% were male. Forty patients [14%] had anastomotic leakage after IPAA. Eighteen were treated with ESC [2010–2017] and 22 patients with CM [2002–2009]. The corresponding control cohorts included 133 patients [2010–2017] and 107 patients [2002–2009]. In 70 IPAA patients [25%] a primary diverting ileostomy was constructed during IPAA surgery. At baseline, patients treated with ESC compared with CM, were more often operated according to the modified two-stage and less often received immunosuppressive medication within 3 months before surgery [Table 1]. When comparing baseline characteristics of ESC and CM patients with the corresponding control patients of the same time period, no difference in baseline characteristics or treatment characteristics remained [Table 1].
Table 1.
Baseline characteristics
| Anastomotic leakage | p-value | No leakage | p-value | No leakage | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n = 280 | Conventional | ESC | between | Control | Conventional vs | Control | ESC vs | |||||
| 2002–2009 | 2010–2017 | leakage | 2002–2009 | control | 2010–2017 | control | ||||||
| n = 22 | 7.9% | n = 18 | 6.4% | groups | n = 107 | 38.2% | n = 133 | 47.5% | ||||
| Gender [M] | 11 | 50.0% | 12 | 66.7% | 0.35 | 57 | 53.3 | 0.82 | 68 | 51.1% | 0.32 | |
| Age at IPAA surgery, mean [SD] | 34.68 | [12.98] | 40.56 | [14.48] | 0.19 | 38.95 | 10.83 | 0.16 | 36.8 | [13.55] | 0.31 | |
| Diagnosisa | UC | 18 | 81.8% | 17 | 94.4% | 0.36 | 92 | 86.0 | 0.74 | 128 | 96.2% | 0.54 |
| IBDU | 4 | 18.2% | 1 | 5.6% | 15 | 14.0 | 5 | 3.8% | ||||
| ASA score | 1 | 7 | 31.8% | 4 | 22.2% | 0.60 | 31 | 29.0 | 0.92 | 25 | 19.0% | 0.86 |
| 2 | 14 | 63.6% | 14 | 77.8% | 70 | 65.4 | 103 | 78.0% | ||||
| 3 | 1 | 4.5% | 0 | 0.0% | 6 | 5.6 | 4 | 3.0% | ||||
| BMI [kg/m2] | Mean [SD] | 23.95 | [3.86] | 25.36 | [4.58] | 0.34 | 23.64 | [3.52] | 0.76 | 23.56 | [4.65] | 0.16 |
| Smoking | Yes | 2 | 9.1% | 5 | 27.8% | 0.37 | 7 | 6.5 | 0.35 | 18 | 13.5% | 0.27 |
| No | 16 | 72.7% | 11 | 61.1% | 90 | 84.1 | 100 | 75.2% | ||||
| Previously | 3 | 13.6% | 2 | 11.1% | 7 | 6.5 | 15 | 11.3% | ||||
| Unknown | 1 | 4.5% | 0 | 0.0% | 3 | 2.8 | 0 | 0.0% | ||||
| Preoperative | None | 7 | 33.3% | 16 | 88.9% | 0.001 | 41 | 41.1 | 0.78 | 107 | 81.7% | 0.66 |
| medicationb | Steroids | 6 | 28.6% | 1 | 5.6% | 23 | 23.0 | 7 | 5.3% | |||
| AZA/6MP/MTX | 7 | 33.3% | 0 | 0.0% | 33 | 33.0 | 11 | 8.4% | ||||
| Anti-TNF | 1 | 4.8% | 1 | 5.6% | 3 | 3.0 | 6 | 4.6% | ||||
| IPAA stages | 1-stage | 14 | 63.6% | 4 | 22.2% | 0.001 | 38 | 35.5 | 0.14 | 7 | 5.3% | 0.08 |
| 2-stage | 3 | 13.6% | 0 | 0.0% | 27 | 25.2 | 9 | 6.8% | ||||
| Modified 2-stage | 4 | 18.2% | 13 | 72.2% | 28 | 26.2 | 103 | 77.4% | ||||
| 3-stage | 1 | 4.5% | 1 | 5.6% | 14 | 13.1 | 14 | 10.5% | ||||
| IPAA | Open | 9 | 40.9% | 3 | 16.7% | 0.22 | 52 | 49.5 | 0.32 | 35 | 26.7% | 0.62 |
| Procedurec | Laparoscopic | 4 | 18.2% | 5 | 27.8% | 5 | 4.8 | 41 | 31.3% | |||
| Hand-assisted | 9 | 40.9% | 10 | 55.6% | 48 | 45.7 | 55 | 42.0% | ||||
| J-pouch design | 22 | 100% | 18 | 100% | n/a | 103 | 97.2 | >0.99 | 127 | 96.9% | >0.99 | |
| Primary diversion | 4 | 18.2% | 1 | 5.6% | 0.36 | 41 | 38.3 | 0.09 | 24 | 18.2% | 0.31 | |
| Abscess size [cm3], mean [SD]d | 177.0 | [151.40] | 116.0 | [106.2] | 0.22 | n/a | n/a |
ESC, early surgical closure; M, male; IPAA, ileal pouch-anal anastomosis; SD, standard deviation; UC, ulcerative colitis; IBDU, inflammatory bowel disease unclassified; ASA, American Society of Anesthesiologists; BMI, body mass index.
aPreoperative diagnosis of UC or IBDU was based on results from colonoscopy and pathology reports.
bImmunosuppressive drug usage was defined as such when patients used steroids, immunomodulators (azathioprine [AZA], 6-mercaptopurine [6MP], methotrexate [MTX]), or anti-tumour necrosis factor-alpha [anti-TNF] within 12 weeks before IPAA, considering the anti-TNF half-life.14 In case of steroids, patients had to use more than 20 mg/day.5
cThe approach of the colectomy was considered laparoscopic in case of a single port, multiport, or hand-assisted approach, and open if the colectomy was performed via a median laparotomy or Pfannenstiel incision without the use of any ports.
cInitial abscess size [cm3] associated with the anastomotic leakage was measurement at maximum size by an abdominal CT scan; the length was measured on the sagittal or axial plane, the height on the sagittal or axial plane, the width of the cavity on the coronal or axial plane.
3.2. Long-term pouch function and failure
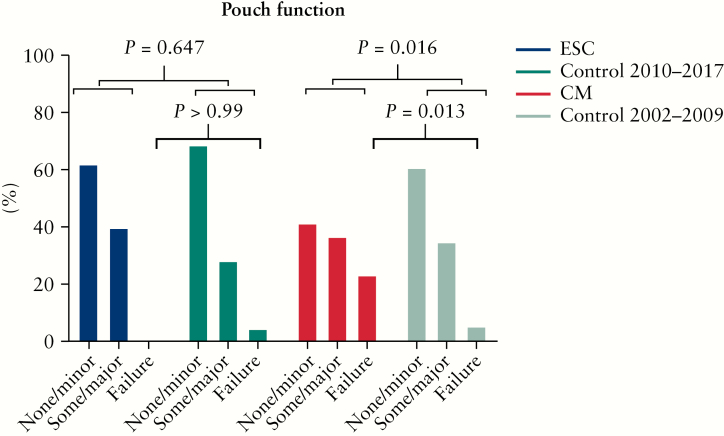
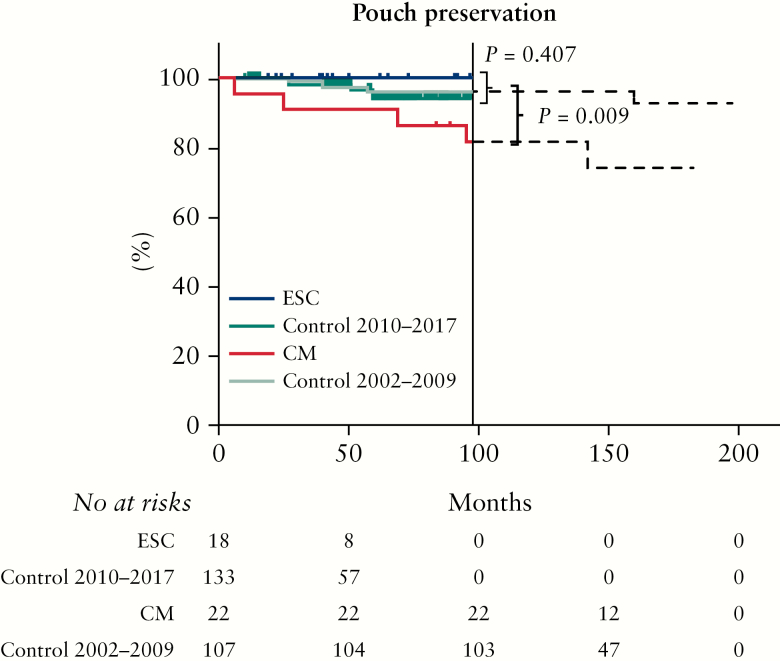
The overall median time of follow-up was 8 years [IQR 4–12]. Median follow-up time was significantly shorter after ESC compared with CM (4 years [IQR 3–6] and 13 years [IQR 10–15], p <0.001). When comparing both treatment strategies with the corresponding control groups, the follow-up time was similar (ESC 4 years [IQR 3–6] versus control [2010–2017] 4 years [IQR 2–6, p = 0.664) and (CM 13 years [IQR 10–15] versus control [2002–2009] 12 years [IQR 10–14], p = 0.673). Overall, 175 patients [62.5%] had ‘none to minor’ pouch dysfunction, 90 patients [32.1%] had ‘some to major’ pouch dysfunction, and 15 patients [5.4%] had pouch failure. Long-term pouch function is shown in Figure 3. When comparing the 18 ESC-treated patients with 133 controls, no difference in pouch function could be observed [p = 0.647]. In contrast, the 22 CM-treated patients had significantly worse pouch function compared with the 107 controls [p = 0.016]. Regarding pouch failure, no difference was observed between ESC-treated patients and control patients [0/18, 0.0% versus 5/133, 3.8%, p >0.99], whereas CM-treated patients had a significant higher pouch failure rate compared with controls [5/22, 22.7% versus 5/107, 4.7%, p = 0.013]. This significant association of pouch failure with CM compared with controls remained after Kaplan-Meier analysis [pouch preservation of 81% versus 96%, p = 0.009, respectively Figure 4].
Figure 3.
Long-term pouch function.
Figure 4.
Pouch failure over time.
3.3. Secondary outcomes
Treatment details and short-term results are shown in Table 2. Time to diagnosis and time to starting treatment were comparable between ESC- and CM-treated patients. All anastomoses after ESC were successfully closed at 6 months [Figure 5]. One ESC-treated patient required a second course of ESC due to a failed anastomotic closure, as demonstrated on the 2 week post-ESC endoscopy. In comparison with CM, ESC resulted in significantly more anastomotic closures in a shorter period of time: 100% closure after a median of 30 days versus 67% closure after a median of 76 days. Treatment-related complications occurred in two patients, both treated with CM by transgluteal drainage. In one patient, a recurrent abscess developed 6 months after initial drain placement. The other patient developed a fistula in the former drain tract, which remained symptomatic for 2 years. Median time to stoma reversal was 4 months in both the ESC [IQR 3–6] and the CM group [IQR 3–13]. This was for both treatment strategies [significantly] later compared with the corresponding control patients [2010–2017, n = 27 and 2002–2009, n = 38] who received a defunctioning ileostomy at IPAA surgery in the absence of anastomotic leakage (both control groups median 3 months [IQR 2–4], control versus ESC; p = 0.052 and control versus CM; p = 0.018).
Table 2.
Treatment details
| CM n = 22 | ESC n = 18 | p-value | |
|---|---|---|---|
| Treatment-specific details | |||
| Transabdominal drain, n | 14 | ||
| Transgluteal drain, n | 4 | ||
| Transanal drain, n | 4 | ||
| No Endo-sponge [Braun Medical] changes p.p., mean [SD] | 2.7 [1.4] | ||
| No Endo-sponge [Braun Medical] changes after discharge, n [%] | 23 / 48 [47.9 %] | ||
| No Endo-sponge [Braun Medical] used p.p., mean [SD] | 3.2 [1.7] | ||
| Time to Endo-sponge [Braun Medical] treatment [days], median [IQR] | 11 [5–15] | ||
| Complications of anastomotic leakage treatment, n [%] | 2 [9.1 %] | 0 [0.0 %] | n/a |
| Time to diagnosis [days], median [IQR] | 8 [6–17] | 9 [7–13] | 0.87 |
| Anastomotic closure at 6 months, n [%] | 14 [66.7 %]a | 18 [100.0 %] | 0.01 |
| Time till anastomotic closure [days], median[ IQR] | 76 [49–339]b | 30 [17–40] | <0.001 |
| Time to stoma reversal [months], median [IQR] | 4 [3–13]b | 4 [3–6] | 0.43 |
CM, conventional management; ESC, Endo-sponge [Braun Medical] assisted early surgical closure; p.p., per patient; SD, standard deviation; IQR, interquartile range; n/a, not applicable.
aOne patient in the CM group was excluded from this analysis, as leakage follow-up was stopped after 3 months since an end-ileostomy was created due to pouch failure. At last check-up for leakage at 3 months, leakage still persisted.
bThree patients in the CM group were excluded from this analysis since leakage follow-up was stopped after a persistent stoma was created. The same three patients were excluded from the time to stoma reversal analysis, as the stoma was never reversed due to persistent leakage problems. Time to starting treatment [days] was comparable between CM and ESC, as treatment started in all patients within 24 h after diagnosis.
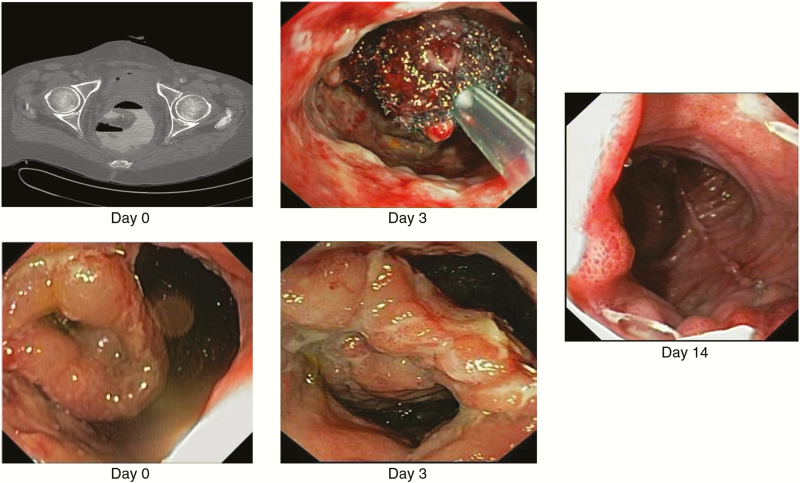
Figure 5.
Endo-sponge [Braun Medical] assisted early surgical closure. Day 0: anastomotic leakage. Day 3: after first Endo-sponge change. Day 14: after surgical closure.
4. Discussion
For the treatment of anastomotic leakage after IPAA surgery in UC patients, ESC is associated with the preservation of pouch function and the pouch, whereas CM is associated with significantly worse pouch function and a higher pouch failure rate compared with controls. Moreover, ESC preserves pouch function despite anastomotic leakage. The present study is the first report on long-term outcomes of ESC treatment for anastomotic leakage after IPAA.
Anastomotic leakage is the main cause of pouch dysfunction and pouch failure [33%].15–19 The impact is probably even bigger, as silent chronic leaks are responsible for one-third of therapy-refractory chronic pouchitis.20 A pro-active treatment strategy of anastomotic leakage using ESC, resulted in a quicker restoration of the anastomotic integrity in all patients. In contrast, after the passive CM approach, one-third of the leaks persisted. Time to diagnosis and time to starting treatment were similar between ESC- and CM-treated patients. Consequently, the effectiveness of the ESC strategy in controlling pelvic sepsis in the short-term is presumably the basis of the improved pouch function in the long-term. Following these study results, it remains unknown which factor resulted in the preservation of pouch function [e.g. Endo-sponge (Braun Medical) or early surgical closure]. However the whole strategy, in which Endo-sponge [Braun Medical] therapy facilitates early surgical closure, seems promising as it reduces time of pelvic sepsis. Therefore, this strategy should become standard care for the treatment of anastomotic leakage.
To increase the results of ESC in daily clinical practice, early diagnosis of the anastomotic leakage is essential. Late initiation [>3–6 weeks] of ESC is less successful, because the chronic sepsis may have already affected the pouch compliance, causing retraction of anastomotic edges precluding surgical closure. As such, a strict postoperative algorithm to monitor the integrity of the anastomosis has been designed using the negative predictive value of CRP [see flowchart, Figure 1a and b].21 Although literature is conflicting,22 most studies point towards the direction that it safe to omit pouch diversion [modified two-stage].23–26 The 14% leak rate in this study represents the total leak rate and not the generally used 30 days and in-hospital leak rates in diverted pouches. These rates should not be compared with each other, since a considerable percentage of leaks are diagnosed late and are not included in the reported leak rate. According to literature looking at leaks rates at 1 year after IPAA surgery, the number is between 15% and 20%.3,4,27 Therefore, leak rates depend on the time frame chosen to report outcomes, explaining the discrepancy between studies. These study results, following the early diagnosis of the leaks using CRP, CT scanning, and pouchoscopy [Figure 1b of the manuscript] in combination with the ‘back-up plan’ ESC, support the policy to primarily refrain from diverting ileostomies, as the pelvic sepsis can be controlled in a timely manner.
The limitations of this study include the small number of patients with anastomotic leakage. Nevertheless, the differences in pouch function and failure between ESC and CM cannot be ignored. Furthermore, the intuitive logic of improved long-term results based on improved leakage control may also be a prominent factor advocating for ESC. The inevitable difference in time period between ESC and CM limits the study, as with time the approach concerning diversion of the IPAA, preoperative medication, and laparoscopy changed. Additionally, likely unknown or unmeasured confounders changed over time. It would be inappropriate to build a multivariable model because of the small leakage numbers. Therefore, it was decided to not directly compare ESC with CM. Instead, we analysed the results of both strategies in comparison with the control patients of the same study period. Between both strategies and their controls, none of these baseline differences occurred any more. However, since the ESC and the corresponding controls had a shorter follow-up time compared with CM and the corresponding control group, the pouch failure rates in these groups might be underestimated. Yet, it is questionable if a longer follow-up would also lead to a significant increase of pouch failures, as a persistent leak seems prevented with ESC. Furthermore, the analyses seems justified, as the pouch failure rates of both control groups were comparable. Moreover, the significant association of CM with pouch failure also remained after the Kaplan-Meier analyses for 7 years [i.e., the maximum follow-up of the ESC and corresponding control group]. As treatment allocation for pouch leakage [ESC or CM] was only dependent on time [standard treatment before or after 2010], it is unlikely that selection bias has occurred. The generalisability of the results is limited, as it is a single-centre study. Inversely, as ESC was only performed in an expert centre, the influence of a learning curve on the results is restricted.
The response rate to the questionnaire was greater than 80% to the validated questionnaire, without baseline differences between non-responders and responders, ensuring a high external validity. Furthermore, the reliability of the novel pouch dysfunction questionnaire has been endorsed.13 Pouch dysfunction is a key patient-reported outcome. Following the supplementary analyses, the pouch dysfunction score is highly associated with the patient-reported dysfunction on QoL [i.e. a higher pouch dysfunction score represents an increased patient-reported impact on QoL, Supplementary Table 2, available as Supplementary data at ECCO-JCC online]. However, ‘some’ negative impact on QoL could not be distinguished from ‘major’ negative impact on QoL. This was also seen in the validation study. Likely, this group was underpowered, since only 21 [8%] patients reported a major negative impact on QoL due to pouch dysfunction symptoms. It is postulated that the majority of patients with such severe dysfunction actually already had pouch failure.
In conclusion, in contrast to CM, the ESC approach is associated with the preservation of pouch function and preclusion of pouch failure. This observation is likely related to the quick resolution of anastomotic leakage, precluding chronic pelvic sepsis.
Funding
This work was supported by the company B. Braun Medical B.V. grant number 22225, without having any influence on the trial design, data collection and analysis, interpretation of results, or manuscript development.
Conflict of Interest
RH, PJT, and WAB have received speaker’s fees from the company B. Braun. For the remaining authors, no conflict was declared.
Author Contributions
KW, MR, MS, take responsibility for sending out all the questionnaires; KW had full access to all data in the study and takes responsibility for the integrity of the data; KW, CB, and WB were responsible for the study concept and design; KW and WB were responsible for the analysis and interpretation of data and drafting the manuscript; MR, MS, CB, RH, PT, and CP were responsible for critical revision of the manuscript.
Supplementary Material
Acknowledgment
T. Mackay, MD, for English editing.
Congress presentations: this study was presented as an oral presentation at the Digestive Disease Days Veldhoven, The Netherlands, October 2018 and at the Dutch Surgical Days, Veldhoven, The Netherlands, May 2018.
References
- 1. Targownik LE, Singh H, Nugent Z, Bernstein CN. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am J Gastroenterol 2012;107:1228–35. [DOI] [PubMed] [Google Scholar]
- 2. Sahami S, Konté K, Buskens CJ, et al. Risk factors for proximal disease extension and colectomy in left-sided ulcerative colitis. United European Gastroenterol J 2017;5:554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sossenheimer PH, Glick LR, Dachman A, et al. Abnormal pouchogram predicts pouch failure even in asymptomatic patients. Dis Colon Rectum 2018;62:463–9. [DOI] [PubMed] [Google Scholar]
- 4. Santorelli C, Hollingshead J, Clark SK. Clinical value of pouchogram prior to ileostomy closure after ileal pouch anal anastomosis. Tech Coloproctol 2018;22:541–4. [DOI] [PubMed] [Google Scholar]
- 5. Sahami S, Bartels SAL, D’Hoore A, et al. A multicentre evaluation of risk factors for anastomotic leakage after restorative proctocolectomy with ileal pouch-anal anastomosis for inflammatory bowel disease. J Crohns Colitis 2016;10:773–8. [DOI] [PubMed] [Google Scholar]
- 6. de Buck van Overstraeten A, Mark-Christensen A, Wasmann KA, et al. Transanal versus transabdominal minimally invasive [completion] proctectomy with ileal pouch-anal anastomosis in ulcerative colitis: a comparative study. Ann Surg 2017;266:878–83.. [DOI] [PubMed] [Google Scholar]
- 7. Bartels SA, Gardenbroek TJ, Aarts M, et al. Short-term morbidity and quality of life from a randomized clinical trial of close rectal dissection and total mesorectal excision in ileal pouch-anal anastomosis. Br J Surg 2015;102:281–7. [DOI] [PubMed] [Google Scholar]
- 8. Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc 2008;22:1818–25. [DOI] [PubMed] [Google Scholar]
- 9. Verlaan T, Bartels SA, van Berge Henegouwen MI, Tanis PJ, Fockens P, Bemelman WA. Early, minimally invasive closure of anastomotic leaks: a new concept. Colorectal Dis 2011;13[Suppl 7]: 18–22. [DOI] [PubMed] [Google Scholar]
- 10. Gardenbroek TJ, Musters GD, Buskens CJ, et al. Early reconstruction of the leaking ileal pouch-anal anastomosis: a novel solution to an old problem. Colorectal Dis 2015;17:426–32. [DOI] [PubMed] [Google Scholar]
- 11. Caulfield H, Hyman NH. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg 2013;148:177–82. [DOI] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative The strengthening the reporting of observational studies in epidemiology [STROBE] statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 13. Brandsborg S, Nicholls RJ, Mortensen LS, Laurberg S. Restorative proctocolectomy for ulcerative colitis: development and validation of a new scoring system for pouch dysfunction and quality of life. Colorectal Dis 2013;15:e719–25. [DOI] [PubMed] [Google Scholar]
- 14. Ternant D, Aubourg A, Magdelaine-Beuzelin C, et al. Infliximab pharmacokinetics in inflammatory bowel disease patients. Ther Drug Monit 2008;30:523–9. [DOI] [PubMed] [Google Scholar]
- 15. Forbes SS, O’Connor BI, Victor JC, Cohen Z, McLeod RS. Sepsis is a major predictor of failure after ileal pouch-anal anastomosis. Dis Colon Rectum 2009;52:1975–81. [DOI] [PubMed] [Google Scholar]
- 16. Fazio VW, Tekkis PP, Remzi F, et al. Quantification of risk for pouch failure after ileal pouch anal anastomosis surgery. Ann Surg 2003;238:605–14; discussion 614–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tulchinsky H, Hawley PR, Nicholls J. Long-term failure after restorative proctocolectomy for ulcerative colitis. Ann Surg 2003;238:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sagap I, Remzi FH, Hammel JP, Fazio VW. Factors associated with failure in managing pelvic sepsis after ileal pouch-anal anastomosis [IPAA] – a multivariate analysis. Surgery 2006;140:691–703; discussion 703–4. [DOI] [PubMed] [Google Scholar]
- 19. Lightner AL, Dattani S, Dozois EJ, Moncrief SB, Pemberton JH, Mathis KL. Pouch excision: indications and outcomes. Colorectal Dis 2017;19:912–6. [DOI] [PubMed] [Google Scholar]
- 20. van der Ploeg VA, Maeda Y, Faiz OD, Hart AL, Clark SK. The prevalence of chronic peri-pouch sepsis in patients treated for antibiotic-dependent or refractory primary idiopathic pouchitis. Colorectal Dis 2017;19:827–31. [DOI] [PubMed] [Google Scholar]
- 21. Warschkow R, Beutner U, Steffen T, et al. Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg 2012;256:245–50. [DOI] [PubMed] [Google Scholar]
- 22. Mark-Christensen A, Erichsen R, Brandsborg S, et al. Pouch failures following ileal pouch-anal anastomosis for ulcerative colitis. Colorectal Dis 2018;20:44–52. [DOI] [PubMed] [Google Scholar]
- 23. Weston-Petrides GK, Lovegrove RE, Tilney HS, et al. Comparison of outcomes after restorative proctocolectomy with or without defunctioning ileostomy. Arch Surg 2008;143:406–12. [DOI] [PubMed] [Google Scholar]
- 24. Mennigen R, Senninger N, Bruewer M, Rijcken E. Pouch function and quality of life after successful management of pouch-related septic complications in patients with ulcerative colitis. Langenbecks Arch Surg 2012;397:37–44. [DOI] [PubMed] [Google Scholar]
- 25. Sahami S, Buskens CJ, Fadok TY, et al. Defunctioning ileostomy is not associated with reduced leakage in proctocolectomy and ileal pouch anastomosis surgeries for IBD. J Crohns Colitis 2016;10:779–85. [DOI] [PubMed] [Google Scholar]
- 26. Mark-Christensen A, Erichsen R, Brandsborg S, et al. Pouch failures following ileal pouch-anal anastomosis for ulcerative colitis. Colorectal Dis 2018;20:44–52. [DOI] [PubMed] [Google Scholar]
- 27. Widmar M, Munger JA, Mui A, et al. Diverted versus undiverted restorative proctocolectomy for chronic ulcerative colitis: an analysis of long-term outcomes after pouch leak short title: outcomes after pouch leak. Int J Colorectal Dis 2019;34:691–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.