Abstract
Native liquid extraction surface analysis (LESA) mass spectrometry allows direct analysis of folded proteins and protein complexes from biological substrates, such as dried blood spots and thin tissue sections, by use of native-like extraction/ionization solvents. Previously, we have demonstrated native LESA mass spectrometry of folded proteins up to 16 kDa as well as the 64 kDa hemoglobin tetramer, from mouse tissues. With denaturing LESA solvents, the highest mass protein detected in tissue to date is ∼37 kDa. Here, we demonstrate native LESA mass spectrometry by use of a Q Exactive UHMR Hybrid Quadrupole-Orbitrap (QE-UHMR) mass spectrometer, pushing the upper mass limit of proteins detected in tissue to >70 kDa. Moreover, a protein trimer of 42 kDa was detected and its stoichiometry confirmed by higher energy collision dissociation (HCD). The benefits of inclusion of detergents in the LESA sampling solvent are also demonstrated.
Native mass spectrometry is emerging as a powerful tool for the analysis of protein complexes and assemblies, providing structural and stoichiometric insights.1 Intra- and intermolecular noncovalent interactions are maintained by use of nondenaturing electrospray buffers (typically ammonium acetate) such that tertiary and quaternary structure is retained in the gas-phase. Retention of the protein fold leads to fewer available protonation sites, thus native MS is characterized by low charge states and high mass-to-charge ratios (m/z). Traditionally, native MS has made use of time-of-flight mass analyzers as they are not limited in the upper m/z range. More recently, higher mass resolving power mass spectrometers, including Orbitrap2 and Fourier transform ion cyclotron resonance3 analyzers, have been applied to native MS. Belov et al. coupled a high m/z quadrupole mass analyzer with an Orbitrap to achieve isolation and fragmentation of protein assemblies.4 Similar instrumental setups have been applied to the study of antibody drug conjugates5 and protein–lipid complexes,6 and a version has recently been commercialized as the Q-Exactive UHMR.
Liquid extraction surface analysis (LESA)7 mass spectrometry is a technique which enables direct analysis of analytes from solid substrates. We have shown that LESA MS is particularly suited to the in situ analysis of intact proteins from substrates including thin tissue sections,8 dried blood spots,9 and bacterial colonies growing on agar.10 The majority of work to date has made use of denaturing solvents, and typically proteins up to mass 20 kDa are detected, although proteins up to 37 kDa have been observed when high-field asymmetric waveform ion mobility (FAIMS) has been employed11. Recently, we have demonstrated native LESA mass spectrometry, in which folded proteins and protein assemblies are extracted directly from their substrate, on a Q-TOF mass spectrometer.12,13 Initial work focused on protein standards dried onto glass substrates. Native LESA MS of large protein assemblies (up to 800 kDa) and membrane protein assemblies was demonstrated.14 Moreover, we have shown native LESA MS imaging of protein and protein complexes in thin tissue sections of mouse brain and liver on a Q-TOF instrument.15
In this work, we present native LESA MS of thin tissue sections of rat brain and rat kidney on an QE-UHMR mass spectrometer, pushing the upper mass limit of proteins detected from tissue to >70 kDa. In addition, a 42 kDa protein trimer was detected in the kidney tissue and its stoichiometry confirmed by high-energy collision dissociation (HCD). We also show the benefits of inclusion of tetraethylene glycol monooctyl ether (C8E4) detergent in the LESA sampling/electrospray solvent.
Methods
Samples
The rat tissue was a kind gift of Dr. Richard Goodwin (AstraZeneca). Brain and kidney tissues were obtained from control (vehicle-dosed) adult male Hans-Wistar rats. HCD of the 28.4 kDa protein was performed on kidney tissue obtained from an adult male Hans-Wistar rat which had been orally dosed with olanzapine, terfenadine, nelfinavir, and moxifloxacin. Animals were euthanized by cardiac puncture under isofluorane anesthetic 2 h post dose. All tissue dissection was performed by trained AstraZeneca staff (project license 40/3484, procedure number 10). Tissues were snap frozen in dry ice chilled isopentane and stored at −80 °C. Tissues were subsequently cryosectioned at a thickness of 10 μm using a CM1810 Cryostat (Leica Microsystmes, Wetzlar, Germany) and thaw mounted onto glass slides.
Surface Sampling
“Contact” LESA21 was carried out by use of the Triversa Nanomate (Advion Biosciences, Ithaca, NY) controlled by the advanced user interface (AUI) of the Chipsoft software. The extraction/ionization solvent comprised ammonium acetate (10 mM, 99.999% metal free) (Aldrich, St. Louis, MO) with either 5% methanol (Fisher Chemical, Optima LC/MS grade, Waltham, MA) or 0.125% C8E4 (0.5 CMC) (Sigma, St. Louis, MO) as indicated in the text. During extraction, 10 μL of solvent was aspirated from the solvent well before relocating to a defined position on the tissue. A volume of 5 μL of solvent was dispensed and held in contact for 60 s before 6 μL of solvent was reaspirated and introduced to the mass spectrometer by electrospray (gas pressure of 0.15 psi and voltage of 2.0 kV). For HCD of m/z 4265, the tissue was sampled as above and extracts were collected in a well and directly infused using a 1.2 mm o.d. borosilicate capillary (ES387, Thermo Scientific, Waltham, MA). Capillary voltages were set at 1.8 kV in positive mode.
Mass Spectrometry
All mass spectrometry experiments were performed on a Thermo Scientific Q Exactive UHMR Hybrid Quadrupole-Orbitrap mass spectrometer. Full scan data were acquired at resolving power 6250 at m/z 400 in the m/z range 400–20 000 (brain) and 2 000–20 000 (kidney). Mass spectra presented are comprised of ∼120–150 scans. For the kidney samples, high-resolution mass spectra were also recorded at a resolving power 200 000 at m/z 400 in the m/z range 500–6000 or 2 000–20 000. In HCD, precursor ions were selected for fragmentation in isolation windows of 20 m/z. MS/MS spectra were recorded at a resolving power of 200 000 at m/z 400 in the m/z range 500–6 000. For HCD of ions with m/z 4265, the HCD energy was stepped between 150, 180, and 200%. The HCD mass spectrum presented is comprised of ∼180 scans. For HCD of m/z 5687, the HCD energy was 80% and the mass spectrum presented is comprised of ∼50 scans. The automatic gain control (AGC) mode was fixed. Ion transfer optics and voltage gradients throughout the instrument were tuned specifically for every analyte as described previously.22
Data were analyzed by use of Xcalibur software and BioPharma Finder 3.1 (both Thermo Fisher Scientific) as described in the text. High-resolution mass spectra were deconvoluted by use of the Xtract function in Xcalibur to obtain monoisotopic masses. Mass spectra were processed with a signal-to-noise threshold of 2.
Results and Discussion
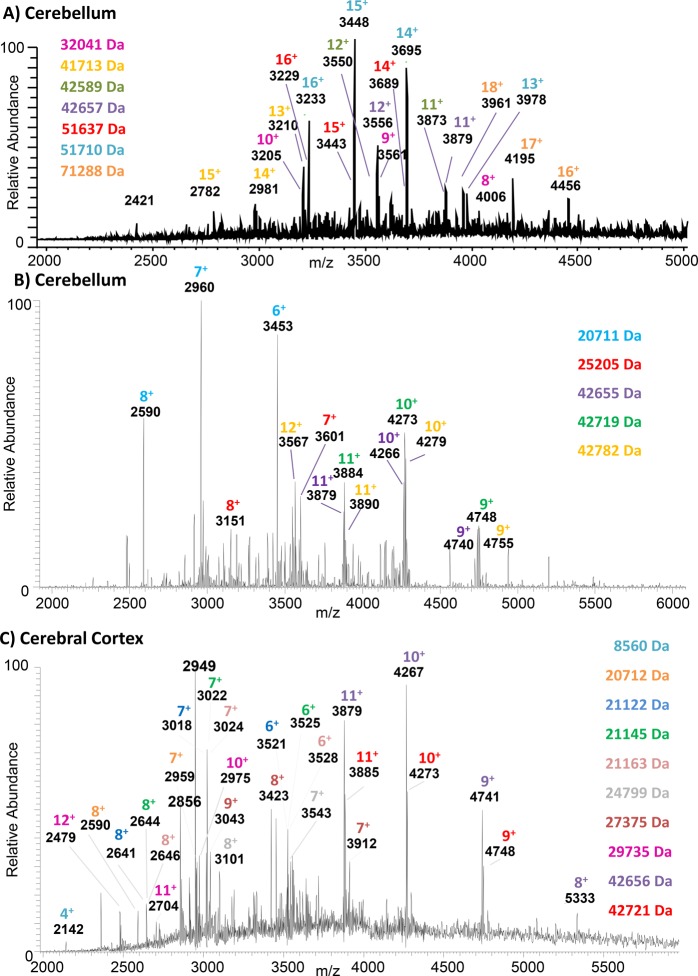
Figure 1A shows the native LESA mass spectrum observed following sampling of a location within the cerebellum with 10 mM ammonium acetate, 5% MeOH. The mass spectrum was analyzed by use of the ReSpect deconvolution algorithm in Biopharma Finder software (Thermo Fisher Scientific). Search parameters and outputs are shown in Supplemental File 1. Twelve proteins were assigned in the mass range 32–80 kDa. Charge states and molecular weights of the more abundant ions are indicated. The results obtained following sampling of a second location within the cerebellum with the same solvents are shown in Supplemental File 2, and a comparison of the deconvoluted mass spectra is shown in Supplemental Figure 1A. Two of the proteins (MW ∼51 710 and 42 657 Da) were detected at both locations; the remainder differed between the two locations. None of these species have been reported via LESA previously and are much larger than the 37 kDa protein species previously reported in LESA FAIMS experiments with denaturing solvents;11 moreover, this mass range surpasses the 64 kDa hemoglobin tetramer complex reported in a vasculature feature of liver.15 Proteins of similar molecular weight have been detected by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry following pretreatment of the tissue with hexafluoroisopropanol.18 The benefit of the native LESA approach is that the protein species detected are in their (native) folded conformations, and noncovalent complexes can be probed (see below).
Figure 1.
Native LESA MS of rat brain tissue. A) Sampling location: cerebellum. LESA extraction/ionisation solvent: 10 mM ammonium acetate, 5% MeOH. B) Sampling location: cerebellum. LESA extraction/ionisation solvent: 10 mM ammonium acetate, 0.125% C8E4. C) Sampling location: cerebral cortex. LESA extraction/ionisation solvent: 10 mM ammonium acetate, 0.125% C8E4. Molecular weights of assigned protein are listed. Colors correlate with protein charge states indicated in the mass spectra.
In the majority of cases, sampling with LESA solvent comprising 10 mM ammonium acetate, 5% MeOH, resulted in unresolved noisy mass spectra. Sampling of the cerebellum with a solvent comprising 10 mM ammonium acetate with 0.125% C8E4 resulted in the mass spectrum shown in Figure 1B. Deconvolution of the data (see Supplemental File 3 for search parameters and outputs) revealed five proteins in the mass range 20–43 kDa. Three further locations were sampled with 10 mM ammonium acetate containing 0.125% C8E4 in the cerebellum, and in each case, proteins in the mass range 20–43 kDa were detected. Supplemental Figure 1B shows a comparison of the deconvoluted mass spectra for two separate cerebellum locations sampled with detergent-containing solvent, and Supplemental Figure 1C shows a comparison of deconvoluted mass spectra for two cerebellum locations, one sampled with MeOH-containing solvent and one with detergent-containing solvent. These mass spectra appear to suggest that sampling with detergent results in a lower mass range; however, it may simply be the result of sampling different locations, i.e., different proteins are present in the sample.
The challenges associated with MeOH were particularly noticeable when sampling tissue from the cerebral cortex region of the brain. Supplemental Figure 2 shows a native LESA mass spectrum obtained from the cerebral cortex region of the brain tissue. (Similar mass spectra were obtained from six separate sampling locations (three locations on two tissue sections analyzed on separate days; data not shown)). Sampling of this region with the solvent comprising 10 mM ammonium acetate with 0.125% C8E4 resulted in a mass spectrum containing multiple protein peaks, see Figure 1C. Previous work by Fournier and co-workers showed that inclusion of ionic detergents (SDS and CHAPS) in the LESA sampling solvent improve protein extraction as demonstrated by SDS PAGE of the LESA extract.19 Our results suggest that use of nonionic detergents may also improve protein extraction. Deconvolution of the data (see Supplemental File 4 for search parameters and outputs), together with manual analysis, revealed the presence of 10 proteins in the mass range 20–43 kDa. In addition, the peak at m/z 2142 corresponds to the 4+ charge state of ubiquitin which has been described previously.15 The 42.6 kDa protein and the 20.7 kDa protein were also detected in the cerebellum region. Supplemental Figure 1D shows a comparison of two deconvoluted mass spectra from each region (cerebellum and cerebral cortex).
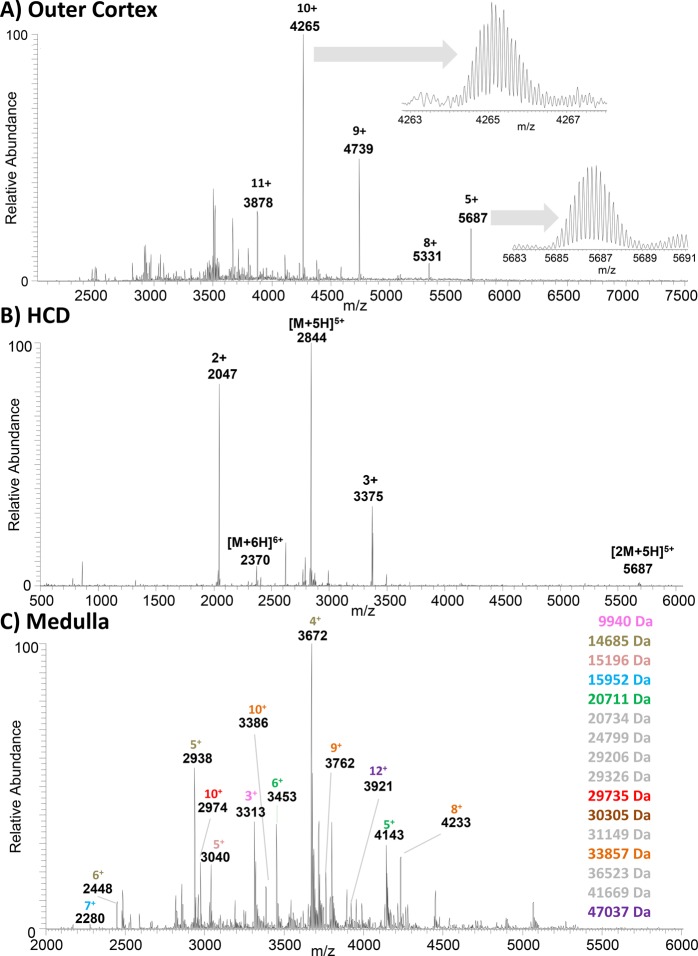
Figure 2A shows a native LESA mass spectrum (sampling solvent, 10 mM ammonium acetate, 0.125% C8E4) obtained from the outer cortex region of rat kidney. Deconvolution of this mass spectrum suggests the presence of a 42.6 kDa protein with peaks at m/z 3878 (11+), 4265 (10+), 4739 (9+), and 5331 (8+) (see Supplemental File 5). In addition, an abundant peak, which was not assigned by the deconvolution algorithm, was observed at m/z 5687. High-resolution scans of the ions at m/z 4265 and m/z 5687 (see inset) confirmed the charge states as 10+ and 5+ and the monoisotopic masses as 42 616 Da and 28 411 Da, respectively. Supplemental Figure 3 shows a native LESA mass spectrum, again obtained from the outer cortex of the kidney, sampled with 10 mM ammonium acetate, 5% MeOH. This mass spectrum also contains a dominant peak at m/z 5687, in addition to peaks at m/z 4739, m/z 4062, and m/z 3555. Deconvolution reveals these peaks as corresponding to the 5+, 6+, 7+, and 8+ charge states of a protein of mass 28.4 kDa (see Supplemental File 6). Figure 2B shows the HCD mass spectrum obtained from the 10+ ions of the 42.6 kDa protein. The results suggest that protein is a homotrimer: the dominant products are 5+ monomer ions observed at m/z 2844 (MW 14.2 kDa). The complementary 5+ dimer ions are observed at m/z 5687 (MW 28.4 kDa). Monomer ions were also observed in the 6+ charge state. Two further abundant fragments were detected, one at m/z 2047 (2+) and one at m/z 3375 (3+). Presumably these fragments derive from backbone cleavage within the monomer; however, it was not possible to identify the monomer based on these fragments. Supplemental Figure 4 shows an HCD mass spectrum obtained from 5+ ions of the 28.4 kDa protein. These results suggest that the 28.4 kDa protein is a homodimer, the dominant product being the monomer in the 3+ charge state. Overall, our findings suggest that both homotrimer and homodimer are observed in the native LESA mass spectra obtained with detergent-containing solvent, whereas the homodimer is dominant when methanol-containing solvent is used, demonstrating that detergent improves protein extraction.
Figure 2.
Native LESA MS of rat kidney tissue. (A) Sampling location, outer cortex; LESA extraction/ionization solvent, 10 mM ammonium acetate, 0.125% C8E4. Inset: high resolution scans of peaks at m/z 4265 and m/z 5687. (B) Native LESA HCD MS/MS spectrum of 10+ ions with m/z 4265 detected in outer cortex. (C) Sampling location, medulla; LESA extraction/ionization solvent, 10 mM ammonium acetate, 0.125% C8E4. Molecular weights of assigned protein are listed. Colors correlate with protein charge states indicated in the mass spectra.
Figure 2C shows a native LESA mass spectrum obtained following sampling of the (inner) medulla region of the kidney. Deconvolution of this mass spectrum (see Supplemental File 7), combined with manual validation, reveals 15 proteins with molecular weights up to 47 kDa. In addition, the peak at m/z 3313 corresponds to the 3+ charge state of a protein of mass 9 940 Da, which has been reported previously in denaturing (acetonitrile based) solvent systems.20 The results obtained following sampling of a second location within the medulla region are shown in Supplemental File 8, and a comparison of the deconvoluted mass spectra is shown in Supplemental Figure 1E. The similarity between the two locations within the medulla region of the kidney is greater than for two locations from the same region of the brain. The proteins detected differ between the medulla and cortex regions of the kidney as illustrated by the comparison of deconvoluted mass spectra shown in Supplemental Figure 1F.
Conclusion
In conclusion, we demonstrate that large intact proteins, >70 kDa, can be analyzed directly from tissue by use of native LESA mass spectrometry on the QE UHMR mass spectrometer. The benefit of including nonionic detergent in the LESA extraction solvent is also demonstrated. For example, while it was possible to obtain native LESA mass spectra from the cerebellum region of the brain in the absence of detergent, the same was not true for the cerebral cortex. These benefits may arise due to improved protein extraction or improved electrospray stability, but further experiments are required to confirm the origin of these improvements. Furthermore, we present the detection of a noncovalent protein complex, a trimer of mass 42.6 kDa, directly from kidney tissue for the first time.
Acknowledgments
R.L.G. and H.J.C. are funded by EPSRC (Grant EP/L023490/1). The authors thank Dr. Richard Goodwin for providing the rat tissue.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.analchem.9b00971.
Supplemental Figure 1, comparison of deconvoluted native LESA mass spectra; Supplemental Figure 2, native LESA MS of rat brain tissue; Supplemental Figure 3, native LESA MS of rat kidney tissue; Supplemental Figure 4, native LESA HCD MS/MS of rat kidney tissue; Supplemental File 1, Biopharma Finder search parameters and outputs for LESA mass spectrum shown in Figure 1A; Supplemental File 2, Biopharma Finder search parameters and outputs for LESA mass spectrum obtained from separate cerebellum location; Supplemental File 3, Biopharma Finder search parameters and outputs for LESA mass spectrum shown in Figure 1B; Supplemental File 4, Biopharma Finder search parameters and outputs for LESA mass spectrum shown in Figure 1C; Supplemental File 5, Biopharma Finder search parameters and outputs for LESA mass spectrum shown in Figure 2A; Supplemental File 6, Biopharma Finder search parameters and outputs for LESA mass spectrum shown in Supplementary Figure 3; Supplemental File 7, Biopharma Finder search parameters and outputs for LESA mass spectrum shown in Figure 2C; Supplemental File 8, Biopharma Finder search parameters and outputs for LESA mass spectrum obtained from a separate medulla location (PDF)
Author Present Address
# R.L.G.: School of Pharmacy, University of Nottingham, University Park, Nottingham NG7 2RD, U.K.
The authors declare the following competing financial interest(s): A.K. and R.V. are employees of Thermo Fisher Scientific.
Notes
Supplementary data supporting this research is openly available from the University of Birmingham data archive at DOI: 10.25500/edata.bham.00000330.
Supplementary Material
References
- Leney A. C.; Heck A. J. R. J. Am. Soc. Mass Spectrom. 2017, 28, 5–13. 10.1007/s13361-016-1545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. J.; Damoc E.; Denisov E.; Makarov A.; Heck A. J. R. Nat. Methods 2012, 9, 1084. 10.1038/nmeth.2208. [DOI] [PubMed] [Google Scholar]
- Li H.; Wolff J. J.; Van Orden S. L.; Loo J. A. Anal. Chem. 2014, 86, 317–320. 10.1021/ac4033214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov M. E.; Damoc E.; Denisov E.; Compton P. D.; Horning S.; Makarov A.; Kelleher N. L. Anal. Chem. 2013, 85, 11163–11173. 10.1021/ac4029328. [DOI] [PubMed] [Google Scholar]
- Dyachenko A.; Wang G.; Belov M. E.; Makarov A.; de Jong R. N.; van den Bremer E. T. J.; Parren P. W. H. I.; Heck A. J. R. Anal. Chem. 2015, 87, 6095–6102. 10.1021/acs.analchem.5b00788. [DOI] [PubMed] [Google Scholar]
- Gault J.; Donlan J. A. C.; Liko I.; Hopper J. T. S.; Gupta K.; Housden N. G.; Struwe W. B.; Marty M. T.; Mize T.; Bechara C.; Zhu Y.; Wu B.; Kleanthous C.; Belov M. E.; Damoc E.; Makarov A.; Robinson C. V. Nat. Methods 2016, 13, 333–336. 10.1038/nmeth.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz V.; Van Berkel G. J. J. Mass Spectrom. 2010, 45, 252–260. 10.1002/jms.1709. [DOI] [PubMed] [Google Scholar]
- Sarsby J.; Martin N. J.; Lalor P. F.; Bunch J.; Cooper H. J. J. Am. Soc. Mass Spectrom. 2014, 25, 1953–1961. 10.1007/s13361-014-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. L.; Creese A. J.; Baumert M.; Griffiths P.; Bunch J.; Cooper H. J. Anal. Chem. 2011, 83, 2265–2270. 10.1021/ac1030804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall E. C.; Bunch J.; Cooper H. J. Anal. Chem. 2014, 86, 10504–10510. 10.1021/ac503349d. [DOI] [PubMed] [Google Scholar]
- Sarsby J.; Griffiths R. L.; Race A. M.; Bunch J.; Randall E. C.; Creese A. J.; Cooper H. J. Anal. Chem. 2015, 87, 6794–6800. 10.1021/acs.analchem.5b01151. [DOI] [PubMed] [Google Scholar]
- Martin N. J.; Griffiths R. L.; Edwards R. L.; Cooper H. J. J. Am. Soc. Mass Spectrom. 2015, 26, 1320–1327. 10.1007/s13361-015-1152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. L.; Cooper H. J. Anal. Chem. 2016, 88, 606–609. 10.1021/acs.analchem.5b03993. [DOI] [PubMed] [Google Scholar]
- Mikhailov V. A.; Griffiths R. L.; Cooper H. J. Int. J. Mass Spectrom. 2017, 420, 43–50. 10.1016/j.ijms.2016.09.011. [DOI] [Google Scholar]
- Griffiths R. L.; Sisley E. K.; Lopez-Clavijo A. F.; Simmonds A. L.; Styles I. B.; Cooper H. J. Int. J. Mass Spectrom. 2019, 437, 23–29. 10.1016/j.ijms.2017.10.009. [DOI] [Google Scholar]
- Randall E. C.; Bunch J.; Cooper H. J. Anal. Chem. 2014, 86, 10504–10510. 10.1021/ac503349d. [DOI] [PubMed] [Google Scholar]
- Fort K. L.; van de Waterbeemd M.; Boll D.; Reinhardt-Szyba M.; Belov M. E.; Sasaki E.; Zschoche R.; Hilvert D.; Makarov A.; Heck A. J. R. Analyst 2018, 143, 100–105. 10.1039/C7AN01629H. [DOI] [PubMed] [Google Scholar]
- Franck J.; Longuespee R.; Wisztorski M.; Van Remoortere A.; Van Zeijl R.; Deelder A.; Salzet M.; McDonnell L.; Fournier I. Med. Sci. Monit. 2010, 16, BR293–BR299. [PubMed] [Google Scholar]
- Wisztorski M.; Desmons A.; Quanico J.; Fatou B.; Gimeno J.-P.; Franck J.; Salzet M.; Fournier I. Proteomics 2016, 16, 1622–1632. 10.1002/pmic.201500508. [DOI] [PubMed] [Google Scholar]
- Griffiths R. L.; Simmonds A. L.; Swales J. G.; Goodwin R. J. A.; Cooper H. J. Anal. Chem. 2018, 90, 13306–13314. 10.1021/acs.analchem.8b02739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.